Abstract
Alzheimer's disease (AD), a progressive neurodegenerative disorder, is a leading global health concern for individuals and society. However, the potential mechanisms underlying the pathogenesis of AD have not yet been elucidated. Currently, the most widely acknowledged hypothesis is amyloid cascade owing to the brain characteristics of AD patients, including great quantities of extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (NFTs). Nevertheless, the amyloid cascade hypothesis cannot address certain pathologies that precede Aβ deposition and NFTs formation in AD, such as aberrant calcium homeostasis, abnormal lipid metabolism, mitochondrial dysfunction and autophagy. Notably, these earlier pathologies are closely associated with mitochondria-associated membranes (MAMs), the physical structures connecting the endoplasmic reticulum (ER) and mitochondria, which mediate the communication between these two organelles. It is plausible that MAMs might be involved in a critical step in the cascade of earlier events, ultimately inducing neurodegeneration in AD. In this review, we focus on the role of MAMs in the regulation of AD pathologies and the potential molecular mechanisms related to MAM-mediated pathological changes in AD. An enhanced recognition of the preclinical pathogenesis in AD could provide new therapeutic strategies, shifting the modality from treatment to prevention.
Keywords: Alzheimer's disease (AD), Calcium homeostasis, Lipid metabolism, Mitochondria dysfunction, Mitochondria-associated membranes (MAMs)
Introduction
Alzheimer’s disease (AD) is recognized as a global public health priority by the World Health Organization and is the major cause of dementia, accounting for 50–75% of 44 million people the world over suffering from dementia in 2014 [1]. Advancing age is considered as the primary risk factor for AD. Therefore, it is estimated that this number will be more than triple by 2050 due to the rapid growth of the population over 65, and that the annual expense of just dementia in the USA alone will exceed $600 billions [2]. Other AD risk factor include diabetes, hypertension, traumatic brain injury and clinical depression, all of which are believed to render neurons vulnerable to AD via accelerating neuronal energy deficits and enhancing the oxidative stress, are also considered as risk factors for AD [3]. AD, characterized by progressive neuronal loss in the cortex and hippocampus, is primarily manifested as the gradual and irreversible loss of memory and impairment of cognitive function, even the more severe behavioral symptoms that affect the ability to function in daily life [4,5]. AD can develop in both sporadic and familial forms, with the vast majority of AD possessing an apparently sporadic basis. So far, autosomal dominant mutations related to familial AD (FAD) have been identified in three genes: amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2). These genes account only for a minute (<0.5%) proportion of FAD [1]. Generally, the onset age of FAD in individuals who inherit a mutation in any of the three genes (APP, PSEN1 and/or PSEN2) is earlier than sporadic AD (SAD) individuals, typically between their third to fifth decades of life [6,7]. Regrettably, despite the fact that substantial effort has been invested in understanding AD pathogenesis since the first case was reported in 1907 [8], the exact mechanism underlying the pathophysiology and pathogenesis of AD still remain a mystery and no curative treatment for this disease has yet been developed.
Amyloid cascade
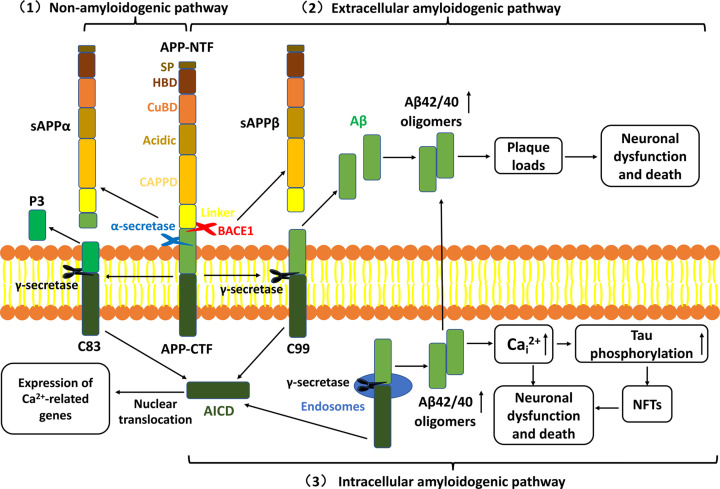
The cardinal positive pathologies of AD are the extracellular accumulation of amyloid plaques mainly composed of β-amyloid (Aβ) and intracellular neurofibrillary tangles (NFTs) containing numerous hyper-phosphorylated tau [9], simultaneously accompanied by astrogliosis and microglial activations [10,11]. Based on the major pathological hallmarks in AD patients’ brain, Hardy and Higgins first proposed the amyloid cascade hypothesis in 1992 [12], which has become the most generally-accepted pathogenetic hypothesis of AD [13]. Aβ is a self-aggregating peptide containing 40–42 amino acids that is generated by the proteolytic processing of APP, a ubiquitous glycoprotein generally expressed in the whole brain. Full-length APP (700 amino acids) is composed of a large transmembrane N-terminal ectodomain including the Aβ sequence and a short intracellular C-terminal domain [14]. APP proteolytic processing occurs via three pathways (Figure 1): (1) The non-amyloidogenic pathway. APP is first cleaved by α-secretase at residues adjacent to the transmembrane domain to produce a large N-terminal fragment (NTF), sAPPα and a short membrane-bound C-terminal fragment (CTF) containing 83 amino acids (C83). C83 is subsequently cleaved at the lipid bilayer by γ-secretase complex to produce a non-amyloidogenic peptide denominated P3 and the APP intracellular domain (AICD), which can be localized to the nucleus to regulate gene expression [15]. (2) The extracellular amyloidogenic pathway. APP is first cleaved of by β-secretase (BACE1), leading to the secretion of sAPPβ NTF and the membrane-tethered 99 amino acids (C99). C99 is then cleaved by γ-secretase to generate the extracellular Aβ as well as AICD. (3) The intracellular amyloidogenic pathway. An alternative route for the C99 generated as in the above amyloidogenic pathway, C99 can also be cleaved by γ-secretase in endosomes to form intracellular Aβ, which accelerates NFTs formation via activating intracellular Ca2+(Cai2+) and extracellular Aβ deposition. The specific cleavage site of γ-secretase plays an essential role in determining the aggregation and toxicity of the Aβ peptide. Aβ42 (containing 42 amino acids) possesses the more toxicity and aggregation than Aβ40 (40 amino acids) and prefers to deposit in vulnerable brain regions primarily related to learning, memory and emotional behaviors such as the entorhinal cortex, hippocampus, inferior parietal cortex and basal forebrain due to its higher rate of fibrillization and insolubility [16]. Additionally, Aβ40 appears to be mostly identified in non-neuronal cells such as cerebral vessels [17]. By analyzing the total Aβ in 27 AD brains, Gravina et al. revealed that the quantity of Aβ42 in AD brains with minimal congophilic angiopathy and minor Aβ40 were equivalent to brains with substantial congophilic angiopathy and plentiful Aβ40, demonstrating that Aβ42 deposition in plaques is critically important for AD pathogenesis with additional deposition of Aβ40 [18]. Their work indicated that the increased ratio of Aβ42/40, rather than the total Aβ, is the exact cause for AD. The transmembrane proteins PSEN1/2 comprise the catalytic subunit of γ-secretase [19]. Significant mutations in APP or/and PSEN1/2 might lead to the excessive generation of Aβ42 and the increased ratio of Aβ42/40 [20]. The serial processes resulting in the Aβ deposition are named ‘amyloid cascade’. The Aβ deposition leads to the progressive neuronal loss in the vulnerable brain regions due to its direct toxic effects on neurons as well as its indirect effects of greatly increasing the vulnerability of neurons to oxidative stress and excitotoxicity, ultimately causing a neurodegenerative disorder [21]. Notably, tau protein in normal brains has an important effect on microtubule stabilization, axonal transport and neurogenesis [22]. However, the high level of phosphorylated tau protein that assemble into paired helical filaments can disturb the physiological activity and aggravate neurotoxicity, which finally leads to apoptosis via interacting with Aβ [23,24].
Figure 1. The amyloid cascade hypothesis.
(1) Non-amyloidogenic pathway: Full-length APP is first cleaved by α-secretase to release sAPPα from the cell membrane and retain C83. C83 is subsequently processed by γ-secretase to produce P3 and AICD, which are shifted toward the nucleus to regulate Ca2+-related gene expression. (2) Extracellular amyloidogenic pathway: APP can also be cleaved by BACE1 in amyloidogenic processing to produce sAPPβ and C99. Subsequent cleavage of C99 by γ-secretase produces Aβ42/40. Mutations that occur in PSEN1, PSEN2 and APP genes can increase the rate of proteolysis of APP by BACE1 and transform the cleavage position of Aβ region by γ-secretase, leading to an increased ratio of Aβ42/40. (3) Intracellular amyloidogenic pathway:C99 can translocate to endosomes and be cleaved by γ-secretase to generate intracellular Aβ, which promotes the NFTs formation via activating the intracellular Ca2+(Cai2+) and extracellular Aβ deposition. Both extracellular Aβ deposition and intracellular NFTs lead to neuronal dysfunction and death; APP, amyloid precursor protein; BACE1, β-secretase; Aβ, β-amyloid; AICD, APP intracellular domain; PSEN1, presenilin 1; PSEN2, presenilin 2; NFTs, neurofibrillary tangles; AD, Alzheimer’s disease.
The amyloid cascade hypothesis consociates many different AD discoveries and explains why mutations in APP and PSEN1/2 result in this disease, making it a persuasive argument. Nevertheless, FAD cases account for only a small percent of all AD individuals and the amyloid cascade hypothesis does not explain the other various features of AD including aberrant calcium homeostasis [16], altered cholesterol and phospholipid metabolism [25,26], mitochondrial dysfunction [27] and autophagy, all of which emerge before the appearance of plaques and tangles but are ostensibly unrelated to the formation of plaques and tangles. Therefore, there may be other cellular mechanisms of mediating Aβ metabolism involved in the AD pathophysiology. Coincidentally, the earlier pathological events of AD are functions concerning to the mitochondria-associated membranes (MAMs), indicating that there is a great possibility that perturbed MAMs functions may be involved in the AD pathogenesis.
MAMs
The multiple biological procedures that are essential for maintaining homeostasis in eukaryotic cells are effectively performed via the compartmentalization of specific biochemical reactions to the corresponding membrane-bound organelles, which fulfills the requirements of transferring many necessary metabolites and signaling molecules between organelles [28]. While the role of the cell membrane in vesicle transport and transcriptional pathways has been well studied, there has been an increase in research focusing on the physical interaction between the endoplasmic reticulum (ER) and mitochondria, referred to as the MAMs. The concept that there is a relationship between the ER and mitochondria was first proposed in the 1960s [29], although the initial morphological evidence for the physical interaction between the ER and mitochondria via an electron microscope did not emerge until the early 1990s [30]. Structurally, MAMs can fluctuate dynamically as they are the highly fluid membranes composed of phospholipids [31]. The distance between the ER and mitochondria contact sites has been measured to be between 10 and 30 nm wide [32]. The closely apposed membranes are sufficient to demonstrate that MAMs are not fusions of membranes but utilizations of proteinaceous tethers, which provide direct, fast, and reciprocal signaling molecules transport between the two compartments [33]. Zhang et al. were the first group to analyzed the MAMs’ proteome in the ‘heavy’ MAMs fraction isolated at lower centrifugal forces rather than the previously standard MAMs isolation measures and identified 991 proteins in this ‘heavy’ MAMs fraction [34]. Afterwards, Poston et al. revealed that there are 1212 high confidence proteins containing weak soluble proteins presenting in brain MAMs [35]. The close gap between the ER and the mitochondria contains numerous proteins and plays a critical role in many crucial metabolic processes, ranging from efficient transfer of calcium [36], phospholipid exchange [37], sterol metabolism, mitochondrial dynamics, autophagy for energy metabolism and even cell survival [38]. Although only a small area of approximately 12% of the outer mitochondrial membrane (OMM) is assessed to interact with the ER [39], this tight ER-mitochondria interaction is extremely stable even when cytoskeletons, including microtubules and intermediate filaments, that are essential for shaping and supporting organelles are undermined [40].
MAMs may play an even more significant role in human neurons, as the distances between the end of axons and the cell body can be up to approximately one meter. The biological procedures in neurons demanding rigorous regulation of both space and time, and have been shown to rely much more on MAMs structures as their particular morphology requirements creates challenges for conventional vesicular transport and transcriptional pathways morphology [41]. Furthermore, emerging evidence exhibits that MAMs are involved in various age-related neurodegenerative disorders, such as AD [42], frontotemporal dementia [43], Parkinson’s disease [44] and amyotrophic lateral sclerosis [45,46]. Previous studies of postmortem AD brains have revealed the increased contact sites between the ER and mitochondria and significant expression of MAMs-associated proteins in primary hippocampal neurons [47]. These findings have been validated in the neuronal cells of APPSwe/Lon mice [48]. In this study, the up-regulation of MAMs-associated proteins could be detected in the 2-, 6- and 10-month-old APPSwe/Lon mice, suggesting that pathophysiologic changes occurred within 2 month of age and significantly ahead of visible plaques and neurofibrillary tangles. Given this early onset, in our examination we systematically highlight the role of MAMs in early AD and the mechanisms underlying the relation between MAMs signaling and AD.
MAM-mediated intracellular Aβ deposit in AD
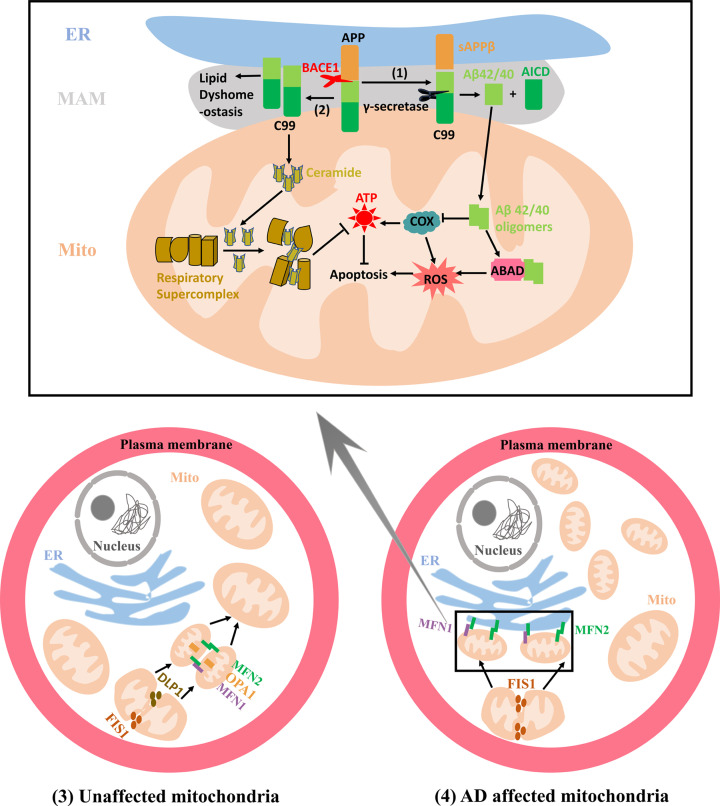
Initial evidence for intracellular Aβ was provided by Wertkin et al. via observing intracellular Aβ in a differentiated neuronal cell line [49]. Previous studies have implicated that intracellular Aβ accumulation induces toxicity in AD [50,51] and that extracellular Aβ deposits could be secondary to the intracellular Aβ accumulation [52–54], where the mitochondria accumulation specifically has proven to play a significant role [55]. Mitochondrial Aβ deposition has been observed in both postmortem AD brains and a transgenic AD mouse model, negatively affecting neuronal functions and ultimately lead to cellular dysfunction [56,57]. Due to the incomplete mitochondrial translocation of APP, it is believed that APP accumulated in the mitochondrial membrane could not be processed to produce Aβ locally [58]. OMM is the connective site to the ER in MAMs. Previous studies have shown that Aβ can be imported into the mitochondria through the translocase of the OMM and subsequently localized to the cristae membranes [59]. The γ-secretase enzyme has been reported to be localized not only at the plasma membrane but also at the different subcellular compartments, predominantly at the MAMs. Both Aβ40 and Aβ42 have been shown to be generated at the MAMs and in immediate proximity to mitochondria [60]. The above findings explain how Aβ accumulates in mitochondria in AD: MAMs are involved in the generation of intracellular Aβ, with the OMM serving as the Aβ transport machinery into mitochondria (Figure 2 (1)). An increase in MAMs may promote mitochondrial Aβ deposition and their interaction with mitochondrial proteins (Figure 2 (1)). For example, Aβ can promote reactive oxygen species (ROS) production via binding to the mitochondrial alcohol dehydrogenase (ABAD), ultimately leading to neuronal apoptosis [57]. Additionally, Aβ impairs the ability of neurons to synthesize adenosine triphosphate (ATP), likely through directly inhibiting the cytochrome c oxidase (COX). Neurons with COX deficiency manifest obvious apoptosis, a feature significantly prevalent in AD [61].
Figure 2. Mitochondrial alterations related to MAMs in AD.
(1) The increased cleavage of C99 by γ-secretase localized at the MAMs promotes Aβ42/40 generation, which are subsequently transferred to mitochondria to form mitochondrial Aβ deposition. Aβ oligomers can inhibit COX and ABAD functions, increasing the production of ROS and ultimately leading to the apoptosis. (2) C99 generated at the MAMs can affect the lipid composition in the MAMs and augment the mitochondrial ceramide concentration by activating the sphingolipid turnover and hydrolysis, which disturbs the assembly and activity of respiratory supercomplexes, ultimately resulting in bioenergetic deficiencies. (3) The mitochondrial fission can be mediated by proteins DLP1 and FIS1. The OMM fusion is regulated by proteins MFN1 and MFN2 that can form MFN1 and MFN2 homo-oligomeric or hetero-oligomeric complexes while IMM fusion respectively utilizes OPA1. In unaffected mitochondria, the mitochondria with relatively equal morphology are uniformly distributed in the cytosol due to the dynamic equilibrium between the mitochondrial fission and fusion processes. (4) In AD affected mitochondria, augmented mitochondrial fission leads to more fragmented mitochondria that tend to form a ‘ring’ around the nucleus. MFN2 is also localized at the ER and the ER MFN2 binds to mitochondrial MFN1 and MFN2 increasing the formation of the interconnection between the ER and mitochondria. DLP1, dynamin-like protein 1; FIS1, fission 1; OMM, outer mitochondrial membrane; OPA1, optic atrophy protein 1; IMM, inner mitochondrial matrix; AD, Alzheimer’s disease; ER, endoplasmic reticulum; MFN1, Mitofusin-1; MFN2, Mitofusin-2; COX, cytochrome c oxidase; Aβ, β-amyloid; MAM, Mitochondria-Associated Membrane; APP, amyloid precursor protein; BACE1, β-secretase; ABAD, alcohol dehydrogenase; ATP, adenosine triphosphate.
MAM-mediated calcium homeostasis in AD
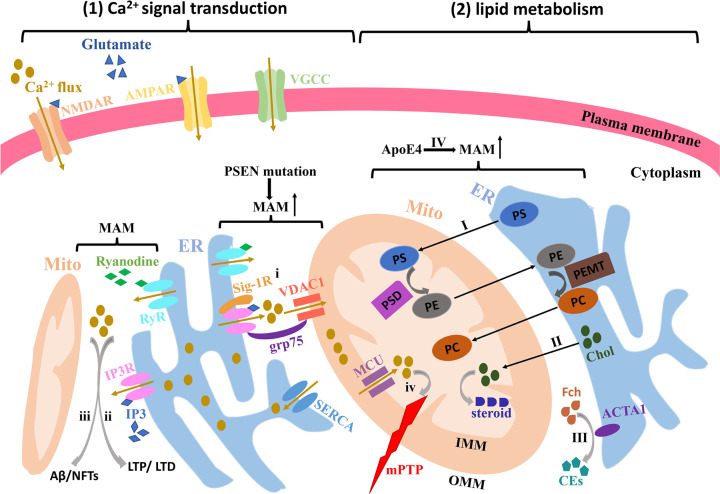
The calcium ion (Ca2+) acts as an intracellular messenger and is fundamental for the regulation of various physiological activities in cells. Ca2+ mediates a variety signaling pathways and is particularly involved in those related to the dynamic changes of neural circuits, regardless of their structure or function [3]. Ca2+ signaling pathways in neurons also play a role in controlling membrane excitability, stimulating the release of neurotransmitters and modulating neuronal biological functions from proliferation, differentiation and apoptosis to gene expression [16]. Factors which all have a connection to how our brains handle received information and stockpile memories [62]. In addition to the Ca2+ influx across the plasma membrane through distinct Ca2+-permeable channels including voltage-gate Ca2+ channel (VGCC), N-methyl-D-aspartic acid (NMDA) receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor and transient receptor potential (TRP) channel, intracellular Ca2+ liberated from ER stores via inositol triphosphate receptor (IP3R) triggered by IP3 [63], and ryanodine receptor (RyR) sensitive to ryanodine [64], also have the prominent effects on intracellular Ca2+ homeostasis (Figure 3(1)). Enhanced cytosolic Ca2+ can also activate IP3R and RyR to trigger the Ca2+-induced Ca2+ release, enabling the interaction between these two pathways [65]. Additionally, due to the powerful Ca2+ buffer function of the mitochondria via the Ca2+ uptake mechanisms, the Ca2+ content in the mitochondrial matrix can achieve > 10 mM despite its regularly values maintaining as low as 0.1 mM [66]. This capacity for rapid Ca2+ influx plays a critical role in neuronal physiology by activating the metabolism and increasing energy productions in the mitochondria [67]. Thereby, both the ER and mitochondria are crucial for maintaining calcium homeostasis [68]. Inadequate intracellular Ca2+ can induce neuronal dysfunction, while excessive Ca2+ can lead to cell death [69,70].
Figure 3. Ca2+ signal transduction and lipid metabolism at the MAMs in AD.
(1) Both Ca2+ influx through the channels NMDAR, AMPAR and VGCC in the plasma membrane and intracellular Ca2+ liberation from ER stores via IP3R and RyR enriched in the MAMs can lead to the toxic amounts of Ca2+ in the cytoplasm. (i) IP3R at the MAMs is linked to VDAC1 at the OMM via grp75 in the cytosol and stabilizes itself by binding to Sig-1R at the ER lumen. (ii and iii) The increased Ca2+ liberation from IP3R and RyR enhances the postsynaptic responses such as LTD and LTP as well as increases the intracellular Aβ deposition and NFTs formation. (iv) Meanwhile, high Ca2+ levels at the MAMs can promote Ca2+ uptake into the mitochondria via triggering the MCU on the IMM. Mitochondrial Ca2+ overload will alter the mitochondrial Ca2+ signaling causing excessive mtPTP opening, which triggers the cell death signaling cascade. (2) MAMs mediate the lipid transport, synthesis and metabolism in AD. I PS is synthesized in the ER via MAM enzymes, while the conversion of PS to PE via PSD occurs in the mitochondria. Ultimately, PEMT catalyzes methylation of PE to PC in the ER. The enzymes of lipid metabolism are highly compartmentalized at the MAMs, which fosters the synthesis and intermembrane transport of phospholipids. II-III Chol transport and metabolism also utilize the interface between the ER and mitochondria. IV ApoE4 can increase the risk of AD through affecting the MAM-mediated lipid metabolism disorders. MAM, Mitochondria-Associated Membrane; AD, Alzheimer's disease; Aβ, β-amyloid; NFTs, neurofibrillary tangles; NMDA, N-methyl-D-aspartic acid; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid; VGCC, voltage-gate Ca2+ channel; IP3R, inositol triphosphate receptor; RyR, ryanodine receptor; VDAC1, voltage-dependent anion channel 1; SERCA, sarco-ER calcium-ATPase; Sig-1R, sigma-1 receptor; LTD, long-term depression; LTP, long-term potentiation; mtPTP, mitochondrial permeability transition pore; MCU, mitochondrial Ca2+ uniporter; IMM, inner mitochondrial matrix; ER, endoplasmic reticulum; PSD, decarboxylation; PEMT, PE-N-methyltransferase; Chol, cholesterol.
The calcium hypothesis in AD was initially proposed in 1982 [71], and a milestone consolidated version in 1994 [72]. The expression levels of proteins that are involved in intracellular Ca2+ signaling pathways such as IP3R and RyR have been shown to be significantly altered in brain tissues acquired from AD patients and AD animal models [73,74]. Moreover, increasing evidence has exhibited that MAMs are highly affluent in IP3R [75] and RyR [76]. Even the voltage-dependent anion channel 1 (VDAC1), sarco-ER calcium-ATPase (SERCA) [77] and sigma-1 receptor (Sig-1R) appear to be linked to MAMs [78] (Figure 3(1)). These Ca2+-related proteins in the MAMs rapidly increase the intracellular Ca2+ and augment Ca2+ uptake into the mitochondria lumen [48,79]. Additionally, mutant PSEN2 has also been shown to have significant impacts on the structural association between the ER and the mitochondria, favoring Ca2+ transfer from the ER to mitochondria. Researchers observed that PSEN1mutations did not induce a similar transformation, despite PSEN1 and PSEN2 being highly homologous [80]. However, this finding is also inconsistent with the observation that PSEN2 mutations are less common than PSEN1 mutations in FAD with a severe clinical course [81]. Additionally, previous researches have shown that PSEN1 mutations heighten the IP3R-mediated calcium trafficking believed to be an MAM-mediated function [3,75]. It is speculated that these two highly homologous proteins induce FAD through the radically different mechanisms. Thus, the conclusion that PSEN2 but not PSEN1 affects MAMs function remains a topic warranting further investigation. Both PSEN1 and PSEN2 mutations have been shown to increase the Ca2+ leakage from the ER and decrease the ER Ca2+ stores, which would accelerate the Ca2+ transfer from the ER to mitochondria and ultimately lead to the mitochondrial Ca2+ overload.
How might the perturbed intercellular Ca2+ concentration mediated by MAMs bring about neurodegeneration and cognitive deficiency in AD? Based on our examination of the relevant studies we postulate this three mechanism: First, the increased release of Ca2+ from the ER stores in presynaptic terminals can undermine synaptic plasticity through consuming the neurotransmitters or enhancing multiple postsynaptic responses such as long-term depression (LTD) and long-term potentiation (LTP) (Figure 3ii) [82], likely leading to a negative impact on the neuronal networks and memory function [83]. Second, the sustained high levels of intracellular Ca2+ in neurons can indirectly contribute to neuronal degeneration in AD by promoting the Aβ deposition and NFTs formation (Figure 3iii). Thirdly, massive mitochondrial Ca2+ uptake will alter the mitochondrial Ca2+ signaling [84], opening the mitochondrial permeability transition pore (mtPTP) (Figure 3iv). Excessive mtPTP openings allow Ca2+ efflux with high conductance from the inner mitochondrial matrix (IMM) lumen, triggering the cell death signaling cascade [85] and ultimately compromising neuronal function and health. Evidence from postmortem analysis of AD brain tissues has revealed increased Ca2+ contents in tangle-bearing neurons compared with healthier neurons [86]. This phenom has also been verified in cultured neurons [87,88]. Aberrant Ca2+ release from the ER can be inhibited by RyR blockage, finally protecting neurons from the impairment of Aβ [89]. Meanwhile, Aβ can also directly induce oxidative stress in neurons, disrupting Ca2+ homeostasis and further exacerbating the amyloidogenic procedures of APP [90]. These events ultimately foster a viscous neurodegenerative cycle. The above discoveries indicate that the increased MAMs in AD individuals could explain the altered intracellular Ca2+ trafficking through IP3Rs and RyRs [80,91], ultimately resulting in the synaptic dysfunction and neuronal degeneration in AD patients and mice models. However, many questions still remain. How does the PSEN mutations influence the physical and functional contact sites? How would the interaction between the ER and mitochondria contribute to the aberrant intracellular Ca2+ in AD. Are other Ca2+-related proteins involved in Ca2+ transport from ER to mitochondria in addition to IP3R and RyR? Does Ca2+ transport unidirectionally? Further studies related to MAMs in AD cellular and animal models will be necessary to identify the role of MAMs-mediated Ca2+ signaling in AD pathogenesis.
MAM-mediated lipid metabolism in AD
Extensive studies have demonstrated that phospholipid synthesis can develop both in the cytoplasm via the Kennedy pathway and in the MAMs through salvaging and recycling [92]. In particular, the latter pathway of phospholipid synthesis has recently garnered significant interest. Mitochondria, as partially autonomous organelles, necessarily rely on the import of specific proteins and lipids to guarantee the cell's structure and survival. The ER, the main ‘lipid factory’ within the cell, is the source of the majority lipids for being imported into mitochondria. The transport of these lipids necessitates interactions between the two organelles such as specific carrier proteins, membrane contact sites and tethering complexes. MAMs were first conformed to be involved in lipid synthesis by Vance et al. in 1990 [93]. The pathway of phospholipids synthesis mediated by MAMs is a typical communication between the ER and mitochondria (Figure 3I). Phosphatidylserine (PS) is synthesized in the MAMs and subsequently translocated from the ER to mitochondria that is considered to be the rate-determining step in phosphatidylethanolamine (PE) synthesis [94]. Under the action of PS decarboxylase (PSD), the mitochondrially localized enzyme in the IMM, PS transferred to mitochondria is converted into PE. PE is then transmitted back to the ER and ultimately converted into phosphatidylcholine (PC) through the enzyme PE-N-methyltransferase (PEMT) [95]. Remarkably, the levels of both PS and PE, synthesis and transport are significantly elevated in PS-mutant cells, demonstrating that there is increased ER-mitochondrial communication in the cells [47]. These observations may help explain the abnormal phospholipids metabolism revealed in AD patients.
In addition to phospholipids synthesis, MAMs are also involved in another key function related to the cholesterol metabolism. Newly synthesized cholesterol in the ER also requires transport into the mitochondria for conversion into pregnenolone (Figure 3II), the precursor for steroids, and MAMs play a key role in this rate-limiting step [96]. Furthermore, MAMs have been shown to possess an abundance of cholesterol acyltransferase 1 (ACAT1), which is involved in the conversion of free cholesterol into cholesteryl esters (CEs) (Figure 3III), generally deposited in lipid droplets [97]. Thus, ACAT1 can be used to indirectly measure MAMs activity. Interestingly, significantly higher ACAT1 activity and more lipid droplets containing CEs have been found in PS-mutant fibroblasts compared with control cells [47]. Remarkably, increased MAMs and altered MAMs-mediated lipid homeostasis have also been observed in fibroblasts from FAD patients with an APP mutation and even in fibroblasts from SAD patients [47]. Activated ACAT1 can directly modulate Aβ formation by regulating the balance between membrane free cholesterol and CEs levels [97,98]. Intriguingly, MAM-mediated lipid synthesis, transport and metabolism can influence the fluidity of the mitochondria membrane and subsequently alter biophysical properties of the membrane environment. The balanced concentration of phospholipids, sterols and sphingolipids seemingly plays an important role in mitochondrial membrane fusion or fission. Thus, two new questions arise: Are MAMs involved in the regulation of lipid transport between the ER and mitochondria? Additionally, can MAM-mediated lipid metabolism in AD affect mitochondrial dynamics and if so, how?
The hypothesis that perturbed MAMs may be involved in the various events of the lipid metabolism in AD patients has been recently strengthened due to the discovery of the effects of apolipoprotein E (APOE) on MAMs [99]. APOE gene encodes a component of the lipoproteins named APOE that has the ability to ferry cholesterol and lipids throughout the circulation. The APOE gene possesses three variants: ε2, ε3 and ε4. Previous studies have revealed that individuals harboring the ε4 allele are at significantly higher risk for developing SAD than those harboring the common ε3 allele. Compared with non-ε4 carriers, ε4 heterozygotes shows an odds ratio (OR) for AD of ∼3, even rising to ∼12 in homozygotes [1]. There is a possibility that ApoE4 plays a key role in mediating MAMs behavior (Figure 3IV). In the brain, APOE is normally not generated in neurons but produced in astrocytes. MAMs activity has been shown to be significantly upregulated in human neuronal-like SH-SY5Y cells and fibroblasts treated with astrocyte-conditioned media (ACM) from human ApoE4 knock-in mice [99]. Moreover, increased CEs and lipid droplets are observed in ApoE4-ACM-treated cells, implying enhanced cholesterol metabolism at the MAMs [99]. Remarkably, the increase in CEs and phospholipid synthesis were not as statistically significant as in the PS-mutant cells [47], which is compatible with the view that ApoE4 is not a determinative factor, only a risk factor, for developing AD. Mitofusin-2 (MFN2) localized at the MAMs participates in mitochondrial fusion and its ablation has been shown to decrease the connections between the ER and mitochondria [100,101]. To further verify that increased phospholipid production is indeed intermediated by MAMs, mouse embryonic fibroblasts obtained from MFN2 knocked-out mice were treated with ApoE4-ACM. The results demonstrated that the abolition of MFN2 in mouse embryonic fibroblasts led to the down-regulation of the MAMs activity and abrogates the ApoE4-mediated increases in phospholipid synthesis, CEs and cellular lipid droplets [99]. These observations strongly support the view that the ApoE4-mediated effects on phospholipid synthesis and cholesterol metabolism are indeed the results of increased MAMs function. Based on these studies, we propose that altered MAMs behavior can induce the early events in the pathogenesis of AD by participating in lipid transport, synthesis and metabolism.
MAM-mediated mitochondria dysfunction in AD
Over the last few decades, increasing numbers of researchers have focused on the functional impairment of mitochondria in AD. Abundant biochemical evidences has demonstrated that mitochondrial bioenergetic function is clearly attenuated in AD individuals and animal models, characterized by decreased respiratory chain activity and reduced ATP production [102,103]. Moreover, significantly decreased levels of enzymes in the mitochondrial tricarboxylic acid cycle, key to the respiratory chain, have also been observed in AD patients [104]. Impairment of bioenergetic functions in mitochondria leads to the mitochondria generating high levels of ROS and free radicals. As discussed above, the serial processes that predate the appearance of plaques and tangles are responsible for the extensive toxic damage to neuron cells. Therefore, the mitochondrial cascade hypothesis has gained favor as the mechanism for the essential pathogenesis of AD, wherein mitochondrial alterations trigger the cascade of pathologic features [105,106].
Previous studies have revealed that there might be an intriguing connection between the altered mitochondrial function and APP processing [107]. APP processing is reported to occur in lipid raft domains, the membrane regions with abundant cholesterol and sphingolipids [108], such as MAMs. Additionally, Aβ has proven to be formatted in the MAMs [109]. Thus, it is reasonable to speculate that C99 the substrate for the MAM-localized γ-secretase might appear in the MAMs. Consistent with this conjecture, researchers have reported that the increased C99 content emerges not only in endosomes but also at MAMs in AD patients and animal models [110]. The increased C99 localized at the MAMs has been revealed to provoke alterations in mitochondrial lipid composition, membrane potential and permeability [111]. Furthermore, the activation of sphingolipid turnover and hydrolysis would subsequently augment the ceramide concentration, which is a special characteristic in AD individuals [112], particularly in their mitochondrial membranes [113]. The high levels of ceramide are thought to change the properties of the lipid raft domains at the MAMs [114]. Additionally, increased ceramide in mitochondrial membranes disturbs the proper assembly and activity of mitochondrial respiratory supercomplexes (Figure 2 (2)), which are essential for optimal respiratory chain function [115]. Importantly, these alterations of γ-secretase activity and increased C99 content at the MAMs have been observed not only in PS-mutant cells and FAD patients but also in SAD patients [116]. One conclusion from the above findings is that the bioenergetic defects in AD are significantly caused by an increased ceramide concentration in the mitochondria, subsequently hindering the assembly and activity of respiratory supercomplexes, and that this effect is initially stimulated by the unprocessed MAM-localized C99. If true, the critical role of C99 in mitochondrial dysfunction at the early stages of AD pathogenesis is not an instigating factor but a consequence of MAMs dysfunction. Previous studies have also suggested that deficient mitochondrial bioenergy is caused by increased C99 content, rather than elevated Aβ production since it can develop in the absence of Aβ, which differs from the observations that mitochondrial respiration is reduced after the incubation with unphysiologically high concentrations of Aβ [117]. It is conceivable that abnormal mitochondrial respiration is caused by an elevated ratio of C99:Aβ rather than an increased ratio of Aβ42: Aβ40. However, the further research is necessary to fully elucidating this speculation.
Previous studies have revealed that the mitochondrial dynamics in AD cells are changed significantly [27]. Mitochondrial dynamics are the collective processes of the relative physiological balance between fission and fusion. The major dynamin-related GTPases with opposing effects are involved in mediating this physiological balance [118]. In mammals, mitochondrial fission requires dynamin-like protein 1 (DLP1) and fission 1 (FIS1), while mitochondrial fusion is regulated by mitochondrial outer membrane protein MFN1/2 and intermembrane protein optic atrophy protein 1 (OPA1) [119]. The imbalance of mitochondrial fission and fusion ultimately leads to mitochondrial dysfunction via abnormal mitochondrial morphology and distribution [120], which has been reported in AD fibroblasts [121]. Immunoblot analysis of hippocampal tissues from AD patients exhibits significantly decreased expression of DLP1, OPA1, MFN1/2 and increased FIS1 [122], suggesting that the balance of mitochondrial fission and fusion is prone to fission in AD (Figure 2(4)) [123]. MFN1 and MFN2 localized to the OMM can form homo- or hetero-oligomeric complexes via the interactions of their coiled-coil domains to tether adjacent mitochondria together (Figure 2(3)) [124]. While though to be exclusively located in the OMM, MFN2 has also been revealed in the ER membrane [125]. Thus, in addition to its role in mitochondrial fusion, MFN2 may also have an important role in tethering the mitochondria and ER together, eventually forming MAMs [126]. These findings explain why augmented mitochondrial fission in AD is inclined to occur around the MAMs [127] and why the abundant MAMs increases in AD. Additionally, OPA1, MFN1, MFN2 and FIS1, the mitochondrial membrane proteins, are re-distributed to the soma in AD neurons [123]. It has been reported that mitochondria are uniformly distributed in the neuronal cytosol of unaffected individuals (Figure 2(3)) while they form a ‘ring’ around the neuron nucleus in AD patients (Figure 2(4)). Perinuclear localization of the majority of the ER and the increased MAMs in AD patients contribute to the mitochondrial accumulation around the perinuclear in AD. The perinuclear phenotype of mitochondria has also been reproduced in mouse models of AD [128,129]. Interestingly, the previous studies have demonstrated that the compatible ablation of both fusion and fission exhibits a wild-type mitochondrial morphology but also shows significantly massive mitochondrial DNA (mtDNA) loss, suggesting that the mitochondrial fusion and fission play essential roles in maintaining the mitochondrial genome [130]. Genetically, high levels of mtDNA deletions that can result in COX deficiency, a phenotype significantly present in AD patients’ cells and tissues [131]. Future research will be essential to investigate if mtDNA deletions can be induced by MAM-mediated mitochondrial fusion and fission and by what mechanism, ultimately developing the pathogenesis of AD.
Other mechanisms mediated by MAMs in AD pathogenesis
Autophagy can effectively clear the damaged/dead cells and long-lived protein aggregates, in normal neurons [132]. Autophagic regulation is comprised of two complicated signaling transduction pathways including the mTOR-dependent and mTOR-independent mechanisms, both of which are involved in the AD pathology [133,134]. Researchers have hypothesized that impaired autophagy significantly reduces the clearance of Aβ and tau deposition in the brain of AD patients and animal models [135]. In return, both enhanced Aβ deposit and NFTs can promote defective autophagy in AD [136]. Moreover, previous reports have demonstrated that ACAT1 blockage via the ACAT1 inhibitor K604 or ACAT1 gene knockout can promote the autophagosome formation and lysosomal proteolysis in an mTOR signaling-independent manner, ultimately facilitating the degradation of Aβ42 in AD neurons [137,138]. However, ACAT1 blockage has no effect on the total cell cholesterol content, indicating that ACAT1 blockage induces autophagy through a different mechanism [139]. Because MAMs are enriched in ACAT1 protein and cholesterol/sphingolipid, some researchers speculate that ACAT1 blockage might elevate the local cholesterol levels in the MAMs and that this alteration may mediate the enhanced autophagic function [140]. These findings suggest MAMs play an important role in the biogenesis of autophagosome.
Moreover, mitochondria and the ER are two the main intracellular locations for ROS generation and these ROS can exchange at the MAMs. As such, multiple oxidative regulators are located at the MAMs, the most common of which is p66Shc. The concentration of p66Shc, a cytosolic adaptor protein, increases at the MAMs, an observation consistent with mitochondrial ROS production in age-related diseases [141]. Previous studies have revealed that p66Shc activation renders central neuron system cells more vulnerable to Aβ toxicity via the promotion of mitochondrial ROS production, ultimately leading to the extensive neuronal death in AD [142]. Additionally, the RNA-dependent protein kinase (PKR)-like ER kinase (PERK) is a major ER stress sensor in the unfolded protein response, which is enriched in the MAMs and critical for maintaining their integrity. Furthermore, PERK has the ability to facilitate ROS exchanges between the ER and mitochondria. PERK deficiency can disturb the ER morphology and reduce ROS exchanges between the ER and mitochondria [143], which has been shown to attenuate neurodegeneration and memory deficits in AD mouse models [144]. Taken together, these findings imply that MAMs play a key role in ROS-based ER stress and the subsequent ROS-mediated mitochondrial apoptosis in AD. However, experiments are necessary for verifying the above findings.
Conclusion
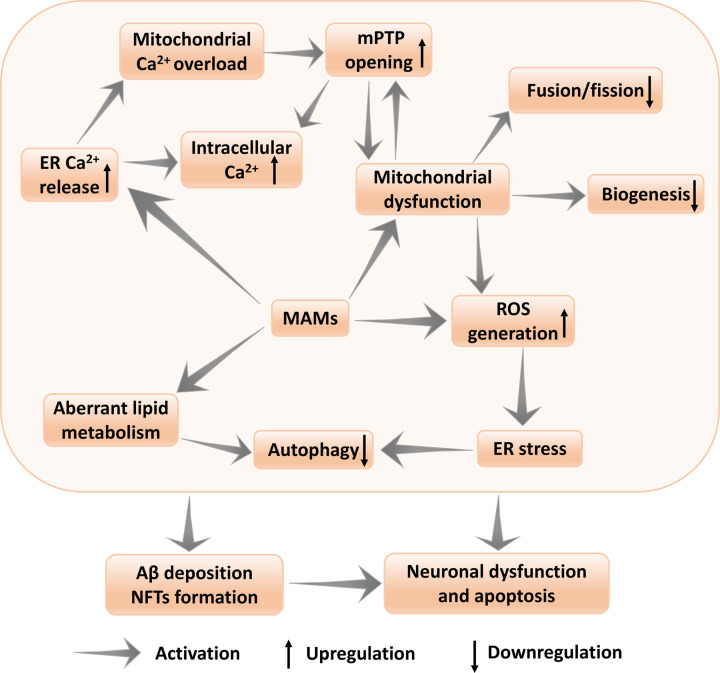
In conclusion, we believe that the evidence supports the MAMs hypothesis as the fundamental for the biochemical and morphological phenotypes in AD as MAMs dysfunction provides an accurate explanation for early phenotypes discovered in both FAD and SAD, including aberrant calcium regulation, elevated lipid levels, mitochondrial dysfunction, autophagy and oxidative stress, all of which precede the appearance of plaques and tangles [145]. Moreover, alterations at the MAMs can be observed in not only neuronal cells but also non-neuronal tissues such as fibroblasts from AD patients [146], further supporting that MAMs play an important role in the pathogenesis of AD. The MAMs hypothesis emphasizes that plaques and tangles are not causes, but indeed, the final consequences of AD pathogenesis. Rather than repudiate the involvement of plaques and tangles in AD pathogenesis due to the fact that the amyloid cascade hypothesis could not explain various events seemingly irrelevant to plaques and tangles, MAMs hypothesis provides such explanations. In practice, perturbed MAMs function can also trigger the amyloid cascade, ultimately contributing to the downstream accumulation of plaques and tangles. Although MAMs regulate the pathogenesis of AD via various mechanisms, there are close interactions among these disperate mechanisms (Figure 4). Elevated intracellular Ca2+ mediated by MAMs can lead to mitochondrial dysfunction by increasing the mPTP opening in the mitochondria, while MAM-mediated mitochondrial dysfunction promotes the intracellular aberrant calcium influx and increased MAMs activity disturbes lipid homeostasis [147]. Additionally, mitochondrial dysfunction can increase the MAMs-mediated ROS generation, which subsequently contributes ER stress. ER stress can decrease the formation of autophagosomes, which significantly reduces the clearance of Aβ and tau deposition in AD. In addition to ER stress, disturbed cholesterol in MAMs via ACTA1 can also impair the autophagy. Ultimately, all of the serial cellular damage events mediated by aberrant MAMs function can directly or indirectly lead to Aβ deposition and NFTs formation, resulting in the neuronal dysfunction and apoptosis [148].
Figure 4. Schematic depiction of MAMs in AD.
Altered MAMs function can directly affect the indicated pathways, including intracellular aberrant calcium, lipid homeostasis, mitochondrial dysfunction, oxidative stress and autophagy. Each of which can have one or more effects that ultimately lead to the pathologic changes in AD. Although the relative one-to-one connection between MAMs functions and AD pathologies has been outlined, there are close interactions among those various MAMs functions. The serial events mediated by aberrant MAMs function can ultimately result in neuronal dysfunction and apoptosis. see text for details. MAM, Mitochondria-Associated Membrane; AD, Alzheimer's disease; mtPTP, mitochondrial permeability transition pore; Aβ, β-amyloid; ER, endoplasmic reticulum; ROS, reactive oxygen species; ACAT1, cholesterol acyltransferase 1.
AD is recognized as the one of the major burden for patients, family and health-care systems in modern medicine. Although extensive efforts have been made into the AD therapeutic research for effective drugs, namely anti-Aβ therapies and anti-tau approaches, initial trials have produced discouraging results and the therapeutic studies are still at an impasse. Alternative targets must be sought. The MAMs hypothesis might provide just such a therapeutic target for the diagnosis and treatment of AD. For instance, the expression levels of ACAT1 can be used to measure the upregulated MAMs activity in AD patients. It may be possible to take advantage of these alterations to diagnose AD far earlier than current methodologies allow. Similarly, increased phospholipid and CEs synthesis mediated by increased MAMs in relatively accessible cells could be used as markers for the diagnosis of AD. Furthermore, methods aimed at recovering normal MAMs function may be more effective therapeutics than trying to eliminate the accumulation of plaques and tangles, since they are secondary to the aberrant MAM function. The availability of reliable biomarkers that track MAMs functions and are associated with the clinical cognitive decline significantly enhances the likelihood of developing the disease-improving drugs.
However, there are still questions that urgently need answers. First, although findings have demonstrated that the functional changes in AD are primarily due to the increased ER-mitochondria communication and up-regulated MAM function, the biochemical upstream causes of ER-mitochondrial hyper-connectivity and perturbed MAM functions still remain a mystery. At time of writing, only a small fraction of the total molecules at the MAMs have been identified. Therefore, further clarification and identification of the unknown molecules at the MAMs will greatly facilitate comprehension of the underlying mechanisms involved in the ER-mitochondria communication and MAMs functions. Conversely, the plethora of as yet unidentified targets reinforces the feasibility that targets for the maintenance and modulation of MAMs function will be identified and be viable as the novel potential drug targets for future AD therapy. Second, in addition to the contacts to the ER, the mitochondria also naturally interact with other organelles, which leads to a high risk of unnecessary organelle cross-contamination when isolating highly purified plasma associated membrane (PAM) and MAMs fractions. Given the difficulty in researching the MAMs’ proteins and lipid compositions, it is essential to combine microscopy visualization techniques with high-throughput proteomics for further elucidation of the molecular compositions of the contacts between the mitochondria and other organelles. Doing so will ultimately enhance the knowledgebase of MAMs and PAM functions in AD. Third, it has been revealed that mitochondria can be linked to both smooth ER (10 nm width) and rough ER (25 nm width, due to the steric impediment of ribosomes) [130]. Intriguingly, although previous studies have showed that up to 80% of the mitochondria are associated with rough ER [149], comparisons of protein profiles between MAMs and smooth or rough ER membranes demonstrate that the proteins at the MAMs are more analogous to the smooth ER than rough ER [93]. Extensive researches related to the MAMs have regularly regarded the MAMs as identical with the diverse ER-mitochondria contact sites. However, there is no strong evidence that purified MAMs consist of all ER-mitochondria contact sites such as rough and smooth ER. Moreover, there is yet no relevant research so far to prove whether mitochondria-smooth ER, mitochondria-rough ER, or both play important roles in AD pathological changes. Therefore, although the MAM hypothesis provides new ways to think about the AD diagnosis and treatment, further studies must be developed to determine the most relevant mechanisms mediated by MAMs.
Abbreviations
- Aβ
β-amyloid
- ABAD
alcohol dehydrogenase
- ACAT1
cholesterol acyltransferase 1
- ACM
astrocyte-conditioned media
- AD
Alzheimer’s disease
- AICD
APP intracellular domain
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid
- APOE4
apolipoprotein E
- APP
amyloid precursor protein
- ATP
adenosine triphosphate
- BACE1
β-secretase
- Ca2+
calcium ion
- CE
cholesteryl ester
- COX
cytochrome c oxidase
- DLP1
dynamin-like protein 1
- ER
endoplasmic reticulum
- FAD
familial AD
- FIS1
fission 1
- IMM
inner mitochondrial matrix
- IP3R
inositol triphosphate receptor
- LTD
long-term depression
- LTP
long-term potentiation
- MAM
mitochondria-associated membrane
- MCU
mitochondrial Ca2+ uniporter
- MFN2
Mitofusin-2
- mtPTP
mitochondrial permeability transition pore
- NFT
neurofibrillary tangle
- NMDA
N-methyl-D-aspartic acid
- OMM
outer mitochondrial membrane
- OPA1
optic atrophy protein 1
- OR
odds ratio
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PEMT
PE-N-methyltransferase
- PERK
RNA-dependent protein kinase (PKR)-like ER kinase
- PS
phosphatidylserine
- PSD
PS decarboxylase
- PSEN1
presenilin 1
- PSEN2
presenilin 2
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SAD
sporadic AD
- SERCA
sarco-ER calcium-ATPase
- Sig-1R
sigma-1 receptor
- TRP
transient receptor potential
- VDAC1
voltage-dependent anion channel 1
- VGCC
voltage-gate Ca2+ channel
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [NSFC: 81971115].
Author Contribution
Yining Huang and Weiwei Yu were involved in devising the concept. Weiwei Yu contributed to drafting this manuscript. Yining Huang and Haiqiang Jin were involved in revising the manuscript and all authors approved the final version of the manuscript.
References
- 1.Lane C.A., Hardy J. and Schott J.M. (2018) Alzheimer's disease. Eur. J. Neurol. 25, 59–70 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- 2.Baumgart M., Snyder H.M., Carrillo M.C., Fazio S., Kim H. and Johns H. (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 3.Mattson M.P. (2010) ER calcium and Alzheimer's disease: in a state of flux. Sci. Signal 3, pe10 10.1126/scisignal.3114pe10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(2020) 2020 Alzheimer's disease facts and figures. Alzheimer's & Dementia: J. Alzheimer's Assoc. 16, 391–460 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- 5.Liang J., Kulasiri D. and Samarasinghe S. (2015) Ca2+ dysregulation in the endoplasmic reticulum related to Alzheimer's disease: A review on experimental progress and computational modeling. Biosystems 134, 1–15 10.1016/j.biosystems.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Hardy J. (2006) A hundred years of Alzheimer's disease research. Neuron 52, 3–13 10.1016/j.neuron.2006.09.016 [DOI] [PubMed] [Google Scholar]
- 7.Bateman R.J., Aisen P.S., De Strooper B., Fox N.C., Lemere C.A., Ringman J.M. et al. (2011) Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res. Ther. 3, 1 10.1186/alzrt59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzheimer A., Stelzmann R.A., Schnitzlein H.N. and Murtagh F.R. (1995) An English translation of Alzheimer's 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 8, 429–431 [DOI] [PubMed] [Google Scholar]
- 9.Serrano-Pozo A., Frosch M.P., Masliah E. and Hyman B.T. (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itagaki S., McGeer P.L., Akiyama H., Zhu S. and Selkoe D. (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 24, 173–182 10.1016/0165-5728(89)90115-X [DOI] [PubMed] [Google Scholar]
- 11.Masliah E., Mallory M., Hansen L., Alford M., Albright T., Terry R. et al. (1991) Immunoreactivity of CD45, a protein phosphotyrosine phosphatase, in Alzheimer's disease. Acta Neuropathol. 83, 12–20 10.1007/BF00294425 [DOI] [PubMed] [Google Scholar]
- 12.Hardy J.A. and Higgins G.A. (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- 13.Karran E., Mercken M. and De Strooper B. (2011) The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712 10.1038/nrd3505 [DOI] [PubMed] [Google Scholar]
- 14.Boix C.P., Lopez-Font I., Cuchillo-Ibañez I. and Sáez-Valero J. (2020) Amyloid precursor protein glycosylation is altered in the brain of patients with Alzheimer's disease. Alzheimers Res Ther 12, 96 10.1186/s13195-020-00664-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leissring M.A., Murphy M.P., Mead T.R., Akbari Y., Sugarman M.C., Jannatipour M. et al. (2002) A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc. Natl. Acad. Sci. U. S. A. 99, 4697–4702 10.1073/pnas.072033799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bezprozvanny I. and Mattson M.P. (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 31, 454–463 10.1016/j.tins.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welander H., Frånberg J., Graff C., Sundström E., Winblad B. and Tjernberg L.O. (2009) Abeta43 is more frequent than Abeta40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 110, 697–706 10.1111/j.1471-4159.2009.06170.x [DOI] [PubMed] [Google Scholar]
- 18.Gravina S.A., Ho L., Eckman C.B., Long K.E., Otvos L. Jr, Younkin L.H. et al. (1995) Amyloid beta protein (A beta) in Alzheimer's disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43). J. Biol. Chem. 270, 7013–7016 10.1074/jbc.270.13.7013 [DOI] [PubMed] [Google Scholar]
- 19.De Strooper B. (2003) Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 38, 9–12 10.1016/S0896-6273(03)00205-8 [DOI] [PubMed] [Google Scholar]
- 20.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N. et al. (1996) Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2, 864–870 10.1038/nm0896-864 [DOI] [PubMed] [Google Scholar]
- 21.Mattson M.P. (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77, 1081–1132 10.1152/physrev.1997.77.4.1081 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y. and Mandelkow E. (2016) Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 10.1038/nrn.2015.1 [DOI] [PubMed] [Google Scholar]
- 23.Iqbal K., Liu F., Gong C.X. and Grundke-Iqbal I. (2010) Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 7, 656–664 10.2174/156720510793611592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan S.S. and Bloom G.S. (2016) Tau: The Center of a Signaling Nexus in Alzheimer's Disease. Front. Neurosci. 10, 31 10.3389/fnins.2016.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefani M. and Liguri G. (2009) Cholesterol in Alzheimer's disease: unresolved questions. Curr. Alzheimer Res. 6, 15–29 10.2174/156720509787313899 [DOI] [PubMed] [Google Scholar]
- 26.Pettegrew J.W., Panchalingam K., Hamilton R.L. and McClure R.J. (2001) Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem. Res. 26, 771–782 10.1023/A:1011603916962 [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Su B., Zheng L., Perry G., Smith M.A. and Zhu X. (2009) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J. Neurochem. 109, 153–159 10.1111/j.1471-4159.2009.05867.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabaldón T. and Pittis A.A. (2015) Origin and evolution of metabolic sub-cellular compartmentalization in eukaryotes. Biochimie 119, 262–268 10.1016/j.biochi.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copeland D.E. and Dalton A.J. (1959) An association between mitochondria and the endoplasmic reticulum in cells of the pseudobranch gland of a teleost. J. Biophys. Biochem. Cytol. 5, 393–396 10.1083/jcb.5.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bereiter-Hahn J. (1990) Behavior of mitochondria in the living cell. Int. Rev. Cytol. 122, 1–63 10.1016/S0074-7696(08)61205-X [DOI] [PubMed] [Google Scholar]
- 31.Yang M., Li C. and Sun L. (2020) Mitochondria-Associated Membranes (MAMs): A Novel Therapeutic Target for Treating Metabolic Syndrome. Curr. Med. Chem. [DOI] [PubMed] [Google Scholar]
- 32.Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J. and Voeltz G.K. (2011) ER tubules mark sites of mitochondrial division. Science 334, 358–362 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowland A.A. and Voeltz G.K. (2012) Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 13, 607–625 10.1038/nrm3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang A., Williamson C.D., Wong D.S., Bullough M.D., Brown K.J., Hathout Y. et al. (2011) Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol. Cell. Proteomics 10, M111.009936 10.1074/mcp.M111.009936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poston C.N., Krishnan S.C. and Bazemore-Walker C.R. (2013) In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J. Proteomics 79, 219–230 10.1016/j.jprot.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 36.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M. et al. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- 37.Achleitner G., Gaigg B., Krasser A., Kainersdorfer E., Kohlwein S.D., Perktold A. et al. (1999) Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 264, 545–553 10.1046/j.1432-1327.1999.00658.x [DOI] [PubMed] [Google Scholar]
- 38.Paillusson S., Stoica R., Gomez-Suaga P., Lau D.H.W., Mueller S., Miller T. et al. (2016) There's Something Wrong with my MAM; the ER-Mitochondria Axis and Neurodegenerative Diseases. Trends Neurosci. 39, 146–157 10.1016/j.tins.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F. et al. (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921 10.1083/jcb.200604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soltys B.J. and Gupta R.S. (1992) Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules–a quadruple fluorescence labeling study. Biochem. Cell. Biol. 70, 1174–1186 10.1139/o92-163 [DOI] [PubMed] [Google Scholar]
- 41.Krols M., van Isterdael G., Asselbergh B., Kremer A., Lippens S., Timmerman V. et al. (2016) Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol. 131, 505–523 10.1007/s00401-015-1528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Area-Gomez E., de Groof A.J., Boldogh I., Bird T.D., Gibson G.E., Koehler C.M. et al. (2009) Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 175, 1810–1816 10.2353/ajpath.2009.090219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoica R., De Vos K.J., Paillusson S., Mueller S., Sancho R.M., Lau K.F. et al. (2014) ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 5, 3996 10.1038/ncomms4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottolini D., Cali T., Negro A. and Brini M. (2013) The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum. Mol. Genet. 22, 2152–2168 10.1093/hmg/ddt068 [DOI] [PubMed] [Google Scholar]
- 45.Watanabe S., Ilieva H., Tamada H., Nomura H., Komine O., Endo F. et al. (2016) Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol. Med. 8, 1421–1437 10.15252/emmm.201606403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoica R., Paillusson S., Gomez-Suaga P., Mitchell J.C., Lau D.H., Gray E.H. et al. (2016) ALS/FTD-associated FUS activates GSK-3beta to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations. EMBO Rep. 17, 1326–1342 10.15252/embr.201541726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Area-Gomez E., Del Carmen Lara Castillo M., Tambini M.D., Guardia-Laguarta C., de Groof A.J., Madra M. et al. (2012) Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31, 4106–4123 10.1038/emboj.2012.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hedskog L., Pinho C.M., Filadi R., Ronnback A., Hertwig L., Wiehager B. et al. (2013) Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer's disease and related models. Proc. Natl. Acad. Sci. U. S. A. 110, 7916–7921 10.1073/pnas.1300677110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wertkin A.M., Turner R.S., Pleasure S.J., Golde T.E., Younkin S.G., Trojanowski J.Q. et al. (1993) Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular beta-amyloid or A4 peptides. Proc. Natl. Acad. Sci. U. S. A. 90, 9513–9517 10.1073/pnas.90.20.9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández-Vizarra P., Fernández A.P., Castro-Blanco S., Serrano J., Bentura M.L., Martínez-Murillo R. et al. (2004) Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer's disease. Histol. Histopathol. 19, 823–844 [DOI] [PubMed] [Google Scholar]
- 51.Schmitz C., Rutten B.P., Pielen A., Schäfer S., Wirths O., Tremp G. et al. (2004) Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer's disease. Am. J. Pathol. 164, 1495–1502 10.1016/S0002-9440(10)63235-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masters C.L., Multhaup G., Simms G., Pottgiesser J., Martins R.N. and Beyreuther K. (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 4, 2757–2763 10.1002/j.1460-2075.1985.tb04000.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanchard V., Moussaoui S., Czech C., Touchet N., Bonici B., Planche M. et al. (2003) Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp. Neurol. 184, 247–263 10.1016/S0014-4886(03)00252-8 [DOI] [PubMed] [Google Scholar]
- 54.Takahashi R.H., Milner T.A., Li F., Nam E.E., Edgar M.A., Yamaguchi H. et al. (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161, 1869–1879 10.1016/S0002-9440(10)64463-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Su B., Perry G., Smith M.A. and Zhu X. (2007) Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radic. Biol. Med. 43, 1569–1573 10.1016/j.freeradbiomed.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 56.Caspersen C., Wang N., Yao J., Sosunov A., Chen X., Lustbader J.W. et al. (2005) Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 19, 2040–2041 10.1096/fj.05-3735fje [DOI] [PubMed] [Google Scholar]
- 57.Lustbader J.W., Cirilli M., Lin C., Xu H.W., Takuma K., Wang N. et al. (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 304, 448–452 10.1126/science.1091230 [DOI] [PubMed] [Google Scholar]
- 58.Devi L., Prabhu B.M., Galati D.F., Avadhani N.G. and Anandatheerthavarada H.K. (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J. Neurosci. 26, 9057–9068 10.1523/JNEUROSCI.1469-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I. et al. (2008) The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. U. S. A. 105, 13145–13150 10.1073/pnas.0806192105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreiner B., Hedskog L., Wiehager B. and Ankarcrona M. (2015) Amyloid-beta peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 43, 369–374 10.3233/JAD-132543 [DOI] [PubMed] [Google Scholar]
- 61.Cottrell D.A., Borthwick G.M., Johnson M.A., Ince P.G. and Turnbull D.M. (2002) The role of cytochrome c oxidase deficient hippocampal neurones in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 28, 390–396 10.1046/j.1365-2990.2002.00414.x [DOI] [PubMed] [Google Scholar]
- 62.Berridge M.J., Bootman M.D. and Lipp P. (1998) Calcium–a life and death signal. Nature 395, 645–648 10.1038/27094 [DOI] [PubMed] [Google Scholar]
- 63.Piegari E., Villarruel C. and Ponce Dawson S. (2019) Changes in Ca(2+) Removal Can Mask the Effects of Geometry During IP3R Mediated Ca(2+) Signals. Front. Physiol. 10, 964 10.3389/fphys.2019.00964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanner J.T., Georgiou D.K., Joshi A.D. and Hamilton S.L. (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2, a003996 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neymotin S.A., McDougal R.A., Bulanova A.S., Zeki M., Lakatos P., Terman D. et al. (2016) Calcium regulation of HCN channels supports persistent activity in a multiscale model of neocortex. Neuroscience 316, 344–366 10.1016/j.neuroscience.2015.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarasov A.I., Griffiths E.J. and Rutter G.A. (2012) Regulation of ATP production by mitochondrial Ca(2+). Cell Calcium 52, 28–35 10.1016/j.ceca.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Popugaeva E. and Bezprozvanny I. (2013) Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front. Mol. Neurosci. 6, 29 10.3389/fnmol.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malli R. and Graier W.F. (2017) The Role of Mitochondria in the Activation/Maintenance of SOCE: The Contribution of Mitochondrial Ca(2+) Uptake, Mitochondrial Motility, and Location to Store-Operated Ca(2+) Entry. Adv. Exp. Med. Biol. 993, 297–319 10.1007/978-3-319-57732-6_16 [DOI] [PubMed] [Google Scholar]
- 69.Filadi R., Theurey P. and Pizzo P. (2017) The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 62, 1–15 10.1016/j.ceca.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 70.Danese A., Patergnani S., Bonora M., Wieckowski M.R., Previati M., Giorgi C. et al. (2017) Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim. Biophys. Acta Bioenerg. 1858, 615–627 10.1016/j.bbabio.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 71.Khachaturian Z.S. (1994) Calcium hypothesis of Alzheimer's disease and brain aging. Ann. N. Y. Acad. Sci. 747, 1–11 10.1111/j.1749-6632.1994.tb44398.x [DOI] [PubMed] [Google Scholar]
- 72.(2017) Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 13, 178.e117–182.e117 [DOI] [PubMed] [Google Scholar]
- 73.Poon H.F., Shepherd H.M., Reed T.T., Calabrese V., Stella A.M., Pennisi G. et al. (2006) Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol. Aging 27, 1020–1034 10.1016/j.neurobiolaging.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 74.Emilsson L., Saetre P. and Jazin E. (2006) Alzheimer's disease: mRNA expression profiles of multiple patients show alterations of genes involved with calcium signaling. Neurobiol. Dis. 21, 618–625 10.1016/j.nbd.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 75.Hayashi T., Rizzuto R., Hajnoczky G. and Su T.P. (2009) MAM: more than just a housekeeper. Trends Cell Biol. 19, 81–88 10.1016/j.tcb.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.García-Pérez C., Hajnóczky G. and Csordás G. (2008) Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J. Biol. Chem. 283, 32771–32780 10.1074/jbc.M803385200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Meis L., Ketzer L.A., da Costa R.M., de Andrade I.R. and Benchimol M. (2010) Fusion of the endoplasmic reticulum and mitochondrial outer membrane in rats brown adipose tissue: activation of thermogenesis by Ca2+. PLoS ONE 5, e9439 10.1371/journal.pone.0009439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayashi T. and Fujimoto M. (2010) Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol. 77, 517–528 10.1124/mol.109.062539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D'Eletto M., Rossin F., Occhigrossi L., Farrace M.G., Faccenda D., Desai R. et al. (2018) Transglutaminase Type 2 Regulates ER-Mitochondria Contact Sites by Interacting with GRP75. Cell Rep. 25, 3573.e3574–3581.e3574 [DOI] [PubMed] [Google Scholar]
- 80.Zampese E., Fasolato C., Kipanyula M.J., Bortolozzi M., Pozzan T. and Pizzo P. (2011) Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. U. S. A. 108, 2777–2782 10.1073/pnas.1100735108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jayadev S., Leverenz J.B., Steinbart E., Stahl J., Klunk W., Yu C.E. et al. (2010) Alzheimer's disease phenotypes and genotypes associated with mutations in presenilin 2. Brain 133, 1143–1154 10.1093/brain/awq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unni V.K., Zakharenko S.S., Zablow L., DeCostanzo A.J. and Siegelbaum S.A. (2004) Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J. Neurosci. 24, 9612–9622 10.1523/JNEUROSCI.5583-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cuestas Torres D.M. and Cardenas F.P. (2020) Synaptic plasticity in Alzheimer's disease and healthy aging. Rev. Neurosci. 31, 245–268 10.1515/revneuro-2019-0058 [DOI] [PubMed] [Google Scholar]
- 84.Sanz-Blasco S., Valero R.A., Rodriguez-Crespo I., Villalobos C. and Nunez L. (2008) Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE 3, e2718 10.1371/journal.pone.0002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giacomello M., Drago I., Pizzo P. and Pozzan T. (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 14, 1267–1274 10.1038/sj.cdd.4402147 [DOI] [PubMed] [Google Scholar]
- 86.Nixon R.A., Saito K.I., Grynspan F., Griffin W.R., Katayama S., Honda T. et al. (1994) Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease. Ann. N. Y. Acad. Sci. 747, 77–91 10.1111/j.1749-6632.1994.tb44402.x [DOI] [PubMed] [Google Scholar]
- 87.Mattson M.P. (1990) Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron 4, 105–117 10.1016/0896-6273(90)90447-N [DOI] [PubMed] [Google Scholar]
- 88.Pierrot N., Santos S.F., Feyt C., Morel M., Brion J.P. and Octave J.N. (2006) Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J. Biol. Chem. 281, 39907–39914 10.1074/jbc.M606015200 [DOI] [PubMed] [Google Scholar]
- 89.Guo Q., Sopher B.L., Furukawa K., Pham D.G., Robinson N., Martin G.M. et al. (1997) Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J. Neurosci. 17, 4212–4222 10.1523/JNEUROSCI.17-11-04212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jo D.G., Arumugam T.V., Woo H.N., Park J.S., Tang S.C., Mughal M. et al. (2010) Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer's disease. Neurobiol. Aging 31, 917–925 10.1016/j.neurobiolaging.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M. and Parker I. (2006) Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 26, 5180–5189 10.1523/JNEUROSCI.0739-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flis V.V. and Daum G. (2013) Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb. Perspect. Biol. 5, 10.1101/cshperspect.a013235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vance J.E. (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265, 7248–7256 [PubMed] [Google Scholar]
- 94.Vance J.E. (2014) MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta 1841, 595–609 10.1016/j.bbalip.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 95.Voelker D.R. (2005) Bridging gaps in phospholipid transport. Trends Biochem. Sci. 30, 396–404 10.1016/j.tibs.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 96.Prasad M., Kaur J., Pawlak K.J., Bose M., Whittal R.M. and Bose H.S. (2015) Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J. Biol. Chem. 290, 2604–2616 10.1074/jbc.M114.605808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puglielli L., Konopka G., Pack-Chung E., Ingano L.A., Berezovska O., Hyman B.T. et al. (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat. Cell Biol. 3, 905–912 10.1038/ncb1001-905 [DOI] [PubMed] [Google Scholar]
- 98.Puglielli L., Ellis B.C., Ingano L.A. and Kovacs D.M. (2004) Role of acyl-coenzyme a: cholesterol acyltransferase activity in the processing of the amyloid precursor protein. J. Mol. Neurosci. 24, 93–96 10.1385/JMN:24:1:093 [DOI] [PubMed] [Google Scholar]
- 99.Tambini M.D., Pera M., Kanter E., Yang H., Guardia-Laguarta C., Holtzman D. et al. (2016) ApoE4 upregulates the activity of mitochondria-associated ER membranes. EMBO Rep. 17, 27–36 10.15252/embr.201540614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Brito O.M. and Scorrano L. (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- 101.Filadi R., Greotti E., Turacchio G., Luini A., Pozzan T. and Pizzo P. (2015) Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl. Acad. Sci. U. S. A. 112, E2174–E2181 10.1073/pnas.1504880112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Atamna H. and Frey W.H. 2nd (2007) Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion 7, 297–310 10.1016/j.mito.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 103.Bosetti F., Brizzi F., Barogi S., Mancuso M., Siciliano G., Tendi E.A. et al. (2002) Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol. Aging 23, 371–376 10.1016/S0197-4580(01)00314-1 [DOI] [PubMed] [Google Scholar]
- 104.Gibson G.E., Chen H.L., Xu H., Qiu L., Xu Z., Denton T.T. et al. (2012) Deficits in the mitochondrial enzyme α-ketoglutarate dehydrogenase lead to Alzheimer's disease-like calcium dysregulation. Neurobiol. Aging 33, 1121.e1113–1124.e1113 10.1016/j.neurobiolaging.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moreira P.I., Carvalho C., Zhu X., Smith M.A. and Perry G. (2010) Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim. Biophys. Acta 1802, 2–10 10.1016/j.bbadis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 106.Swerdlow R.H., Burns J.M. and Khan S.M. (2014) The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim. Biophys. Acta 1842, 1219–1231 10.1016/j.bbadis.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lopez Sanchez M.I.G., Waugh H.S., Tsatsanis A., Wong B.X., Crowston J.G., Duce J.A. et al. (2017) Amyloid precursor protein drives down-regulation of mitochondrial oxidative phosphorylation independent of amyloid beta. Sci. Rep. 7, 9835 10.1038/s41598-017-10233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cordy J.M., Hooper N.M. and Turner A.J. (2006) The involvement of lipid rafts in Alzheimer's disease. Mol. Membr. Biol. 23, 111–122 10.1080/09687860500496417 [DOI] [PubMed] [Google Scholar]
- 109.Schreiner B., Hedskog L., Wiehager B. and Ankarcrona M. (2015) Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 43, 369–374 10.3233/JAD-132543 [DOI] [PubMed] [Google Scholar]
- 110.Pera M., Larrea D., Guardia-Laguarta C., Montesinos J., Velasco K.R., Agrawal R.R. et al. (2017) Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. EMBO J. 36, 3356–3371 10.15252/embj.201796797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kogot-Levin A. and Saada A. (2014) Ceramide and the mitochondrial respiratory chain. Biochimie 100, 88–94 10.1016/j.biochi.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 112.He X., Huang Y., Li B., Gong C.X. and Schuchman E.H. (2010) Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol. Aging 31, 398–408 10.1016/j.neurobiolaging.2008.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kennedy M.A., Moffat T.C., Gable K., Ganesan S., Niewola-Staszkowska K., Johnston A. et al. (2016) A Signaling Lipid Associated with Alzheimer's Disease Promotes Mitochondrial Dysfunction. Sci. Rep. 6, 19332 10.1038/srep19332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dinkla S., Wessels K., Verdurmen W.P., Tomelleri C., Cluitmans J.C., Fransen J. et al. (2012) Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death. Dis. 3, e410 10.1038/cddis.2012.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Acin-Perez R. and Enriquez J.A. (2014) The function of the respiratory supercomplexes: the plasticity model. Biochim. Biophys. Acta 1837, 444–450 10.1016/j.bbabio.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 116.Pera M., Alcolea D., Sánchez-Valle R., Guardia-Laguarta C., Colom-Cadena M., Badiola N. et al. (2013) Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol. 125, 201–213 10.1007/s00401-012-1062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Casley C.S., Canevari L., Land J.M., Clark J.B. and Sharpe M.A. (2002) Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 80, 91–100 10.1046/j.0022-3042.2001.00681.x [DOI] [PubMed] [Google Scholar]
- 118.McBride H.M., Neuspiel M. and Wasiak S. (2006) Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560 10.1016/j.cub.2006.06.054 [DOI] [PubMed] [Google Scholar]
- 119.Okamoto K. and Shaw J.M. (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 39, 503–536 10.1146/annurev.genet.38.072902.093019 [DOI] [PubMed] [Google Scholar]
- 120.Chen H., Chomyn A. and Chan D.C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 10.1074/jbc.M503062200 [DOI] [PubMed] [Google Scholar]
- 121.Wang X., Su B., Fujioka H. and Zhu X. (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am. J. Pathol. 173, 470–482 10.2353/ajpath.2008.071208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang P., Sheng D., Guo Q., Wang P., Xu S., Qian K. et al. (2020) Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer's disease. Biomaterials 238, 119844 10.1016/j.biomaterials.2020.119844 [DOI] [PubMed] [Google Scholar]
- 123.Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H., Wang Y. et al. (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. U. S. A. 105, 19318–19323 10.1073/pnas.0804871105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ishihara N., Eura Y. and Mihara K. (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117, 6535–6546 10.1242/jcs.01565 [DOI] [PubMed] [Google Scholar]
- 125.Basso V., Marchesan E., Peggion C., Chakraborty J., von Stockum S., Giacomello M. et al. (2018) Regulation of ER-mitochondria contacts by Parkin via Mfn2. Pharmacol. Res. 138, 43–56 10.1016/j.phrs.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 126.Ainbinder A., Boncompagni S., Protasi F. and Dirksen R.T. (2015) Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium 57, 14–24 10.1016/j.ceca.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Brito O.M. and Scorrano L. (2010) An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 29, 2715–2723 10.1038/emboj.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manczak M., Calkins M.J. and Reddy P.H. (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum. Mol. Genet. 20, 2495–2509 10.1093/hmg/ddr139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manczak M., Kandimalla R., Yin X. and Reddy P.H. (2018) Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 27, 1332–1342 10.1093/hmg/ddy042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giorgi C., De Stefani D., Bononi A., Rizzuto R. and Pinton P. (2009) Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 41, 1817–1827 10.1016/j.biocel.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krishnan K.J., Ratnaike T.E., De Gruyter H.L., Jaros E. and Turnbull D.M. (2012) Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer's disease. Neurobiol. Aging 33, 2210–2214 10.1016/j.neurobiolaging.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 132.Wu M.Y., Song J.X., Wang S.F., Cai C.Z., Li M. and Lu J.H. (2018) Selective autophagy: The new player in the fight against neurodegenerative diseases? Brain Res. Bull. 137, 79–90 10.1016/j.brainresbull.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 133.Li X., Song J. and Dong R. (2019) Cubeben induces autophagy via PI3K-AKT-mTOR pathway to protect primary neurons against amyloid beta in Alzheimer's disease. Cytotechnology 71, 679–686 10.1007/s10616-019-00313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang L., Lin M., Zhong X., Yang H. and Deng M. (2019) Galangin decreases p-tau, Aβ42 and β-secretase levels, and suppresses autophagy in okadaic acid-induced PC12 cells via an Akt/GSK3β/mTOR signaling-dependent mechanism. Mol. Med. Rep. 19, 1767–1774 [DOI] [PubMed] [Google Scholar]
- 135.Lonskaya I., Hebron M.L., Desforges N.M., Schachter J.B. and Moussa C.E. (2014) Nilotinib-induced autophagic changes increase endogenous parkin level and ubiquitination, leading to amyloid clearance. J. Mol. Med. (Berl.) 92, 373–386 10.1007/s00109-013-1112-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reddy P.H. and Oliver D.M. (2019) Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease. Cells 8, 10.3390/cells8050488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boland B., Kumar A., Lee S., Platt F.M., Wegiel J., Yu W.H. et al. (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J. Neurosci. 28, 6926–6937 10.1523/JNEUROSCI.0800-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neely K.M., Green K.N. and LaFerla F.M. (2011) Presenilin is necessary for efficient proteolysis through the autophagy-lysosome system in a γ-secretase-independent manner. J. Neurosci. 31, 2781–2791 10.1523/JNEUROSCI.5156-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shibuya Y., Chang C.C., Huang L.H., Bryleva E.Y. and Chang T.Y. (2014) Inhibiting ACAT1/SOAT1 in microglia stimulates autophagy-mediated lysosomal proteolysis and increases Aβ1-42 clearance. J. Neurosci. 34, 14484–14501 10.1523/JNEUROSCI.2567-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shibuya Y., Niu Z., Bryleva E.Y., Harris B.T., Murphy S.R., Kheirollah A. et al. (2015) Acyl-coenzyme A:cholesterol acyltransferase 1 blockage enhances autophagy in the neurons of triple transgenic Alzheimer's disease mouse and reduces human P301L-tau content at the presymptomatic stage. Neurobiol. Aging 36, 2248–2259 10.1016/j.neurobiolaging.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lebiedzinska M., Duszynski J., Rizzuto R., Pinton P. and Wieckowski M.R. (2009) Age-related changes in levels of p66Shc and serine 36-phosphorylated p66Shc in organs and mouse tissues. Arch. Biochem. Biophys. 486, 73–80 10.1016/j.abb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 142.Lone A., Harris R.A., Singh O., Betts D.H. and Cumming R.C. (2018) p66Shc activation promotes increased oxidative phosphorylation and renders CNS cells more vulnerable to amyloid beta toxicity. Sci. Rep. 8, 17081 10.1038/s41598-018-35114-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Verfaillie T., Rubio N., Garg A.D., Bultynck G., Rizzuto R., Decuypere J.P. et al. (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 19, 1880–1891 10.1038/cdd.2012.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma V., Ounallah-Saad H., Chakraborty D., Hleihil M., Sood R., Barrera I. et al. (2018) Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties. J. Neurosci. 38, 648–658 10.1523/JNEUROSCI.0628-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Area-Gomez E. and Schon E.A. (2017) Alzheimer Disease. Adv. Exp. Med. Biol. 997, 149–156 10.1007/978-981-10-4567-7_11 [DOI] [PubMed] [Google Scholar]
- 146.Schon E.A. and Area-Gomez E. (2013) Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 55, 26–36 10.1016/j.mcn.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 147.Duce J.A., Zhu X., Jacobson L.H. and Beart P.M. (2019) Therapeutics for dementia and Alzheimer's disease: New directions for precision medicine. Br. J. Pharmacol. 176, 3409–3412 10.1111/bph.14767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X. and Zheng W. (2019) Ca(2+) homeostasis dysregulation in Alzheimer's disease: a focus on plasma membrane and cell organelles. FASEB J. 33, 6697–6712 10.1096/fj.201801751R [DOI] [PubMed] [Google Scholar]
- 149.Szymański J., Janikiewicz J., Michalska B., Patalas-Krawczyk P., Perrone M., Ziółkowski W. et al. (2017) Interaction of Mitochondria with the Endoplasmic Reticulum and Plasma Membrane in Calcium Homeostasis, Lipid Trafficking and Mitochondrial Structure. Int. J. Mol. Sci. 18, 10.3390/ijms18071576 [DOI] [PMC free article] [PubMed] [Google Scholar]