Abstract
Different neuromodulatory systems are involved in long-term energy balance and body weight and, among these, evidence shows that the endocannabinoid system, in particular the activation of type-1 cannabinoid receptor, plays a key role. We here review current literature focusing on the role of the gene encoding type-1 cannabinoid receptors in the CNS and on the modulation of its expression by food intake and specific eating behaviors. We point out the importance to further investigate how environmental cues might have a role in the development of obesity as well as eating disorders through the transcriptional regulation of this gene in order to prevent or to treat these pathologies.
Keywords: type-1 cannabinoid receptor gene, transcriptional regulation, food intake, eating behaviors
1. Central Regulation of Food Intake and the Role of Cannabinoid Receptor Type-1
Food intake might be considered the integration of humoral and neuronal signals processed by the nervous system for the balance of energy and of sensory cues, as well as of the motivational and emotional state of an individual. Thus, different eating behaviors are finely driven by both homeostatic and hedonic signals, whose functions may vary between individuals according to previous experiences and/or epigenetic variations [1,2,3,4,5,6,7].
Homeostatic and hedonic central circuitries are interconnected, in fact feeding behaviors are affected by brain regions classically viewed as mainly involved in homeostatic feeding; however, these are also influenced by brain corticolimbic and hedonic areas, and vice versa [8,9]. The homeostatic feeding will be terminated once the organism is repleted with energy and nutrients, while hedonic feeding might continue. An imbalance toward the hedonic aspect of feeding without restriction may provoke changes in the food intake with serious consequences on the weight gain/loss [10,11].
The hypothalamus (HYP) is the center for the integration and control of essential bodily functions, such as circadian rhythm, body temperature and plasma-osmolarity, and traditionally recognized as the main brain region regulating food intake. It regulates feeding as a function of caloric and nutritional requirements, by sensing macronutrients and through the action of circulating regulatory hormones, neuropeptides and neuromodulators, such as leptin, cholecystokinin, ghrelin, orexin/hypocretin, insulin, neuropeptide Y, and notably lipid signals like endocannabinoids [12,13,14,15]. The imbalance in hypothalamic function may provoke an altered food intake, potentially leading to eating disorders (EDs) and obesity [16,17,18].
Besides HYP, several limbic brain areas including ventral tegmental area, nucleus accumbens (NAc), amygdala, and hippocampus, as well as cortical brain regions, have also been implicated in the hedonic aspects of feeding [19,20,21].
Studies on the role played by the reward circuits in defining hedonic aspects of feeding allowed to define how common mechanisms are shared by drug abuse and food addiction [22,23,24]. Both are compulsive behavioral disorders that induce alterations in brain mechanisms underlying synaptic plasticity and energy homeostasis, showing common vulnerabilities and pathophysiological aspects [25].
Among the different neuromodulatory systems involved in long-term energy balance and body weight regulation, many preclinical and clinical evidence show the key role of the endocannabinoid system (ECS) [26], and in particular, the activation of type-1 cannabinoid receptors (CB1R) [27,28].
Indeed, several preclinical studies show that orexigenic stimuli induce CB1R activation in the rat brain, specifically in the HYP [29,30] where CB1R positive neurons are present in different nuclei [31], although at low density [32], and support a role in food and energy balance [33,34,35,36]. Brain reward pathways are largely responsible for processing information related to the motivation, expectation, and pursuit of pleasurable experiences, and CB1R signaling was reported to modulate dopaminergic signaling in the ventral tegmental area and NAc to control hedonic eating [37,38,39,40]. CB1R signaling also plays a role in the functional activity of caudal brainstem nuclei: parabrachial nucleus, nucleus of the solitary tract, and dorsal motor nucleus of the vagus nerve. Herein, CB1R mainly controls food preferences, e.g., digestion of fat rich palatable food [37]. Several experimental findings already pointed to CB1R as therapeutic target to treat altered feeding behavior and obesity [30,34,41,42], due to the hyperphagic role of this receptor, and the possible exploitation of its pharmacological blockade, as recently reviewed [43]. It should be recalled that rimonabant, a CB1R antagonist/inverse agonist [44], entered the European mass market, showing weight loss benefits but it was soon withdrawn due to the significant side effects [45]. Here, we focused mainly on the role of type-1 Cannabinoid Receptor gene (CNR1) gene, which encodes for CB1R, and its regulation in food intake and eating behaviors.
2. CNR1 Gene
CB1R is one of the most abundant seven transmembrane G protein-coupled receptor of the class A [46]. It is prominently expressed in the central nervous system (CNS) [47] and has attracted great attention as a modulator of different brain functions including appetite, fear, anxiety and pain [48,49,50]. The ECS, as a whole, is comprised of (1) the endocannabinoids (eCBs) anandamide (N-arachidonoyl-ethanolamine) and 2-arachidonoylglycerol, which are physiological ligands for cannabinoid and non-cannabinoid receptors; (2) the cannabinoid receptors and non-cannabinoid receptors, such as transient receptor potential vanilloid 1 channels [51,52]; and (3) enzymes responsible for the biosynthesis and hydrolysis of eCBs. Biosynthetic routes are mediated by N-acylphosphatidylethanolamines-specific phospholipase D, diacylglycerol lipase, phosphoinositide-specific PLC and lyso-PLC, while termination of eCB signaling is terminated through the action of purported transmembrane transporters, followed by hydrolysis by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase [50].
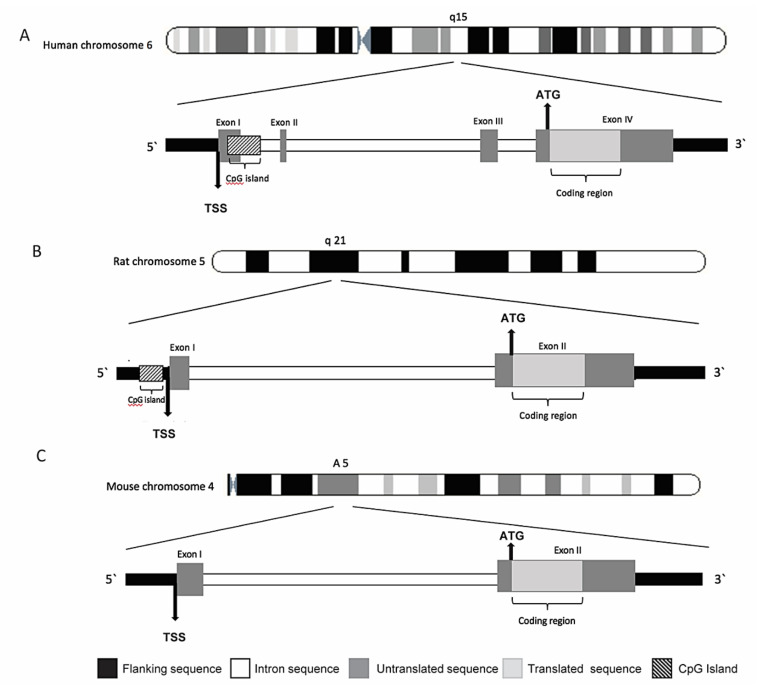
CB1R was first cloned in 1990 and was immediately recognized as the receptor responsible for the effects of marijuana on CNS; it was also reported to be more responsive to psychoactive than non-psychoactive cannabinoids [53]. CB1R is encoded by CNR1 gene, and consists of 472 amino acids in humans, and 473 amino acids in rats and mice, with 97–99% amino acid sequence identity among them [54] (Figure 1). CNR1 gene is located on human chromosome 6q14–15 [55] and its gene sequence is composed of four exons, with exon 4 containing the entire protein coding region.
Figure 1.
Schematic representation of human(A), rat (B) and mouse (C) CNR1 gene, with their cromosomial locations.
Also, in mice and rats the coding region of CNR1 is contained within a single exon. However, the 5′ untranslated region (5′-UTR) and promoter structures differ between mice and humans [56,57], and these structures are not described in rats [58]. Alternative splicing of portions outside the coding region yields six different 5′-UTR splicing variants. In addition, it appears that multiple transcription starting sites exist within the first 60 base pairs (bp) of the first exon [59]. Three CNR1 coding region variants for CB1R protein isoforms have been identified in humans and non-human primates: (1) the intronless 472 amino acid-long protein, the one known as CB1R, (2) the 411 amino acid-long protein, marked as CB1Ra and (3) the 439 amino acid-long protein, marked as CB1Rb [60,61,62]. Some evidence indicated that CB1Ra may also be expressed in the rat brain [59]. Several natural polymorphisms of the human gene have been identified, associated with different responsiveness to cannabinoids [57,63,64,65,66,67]. Alternative splice variants have also been reported, including the canonical long form expressed predominantly in the brain and skeletal muscle and two isoforms with shorter N-terminus, one of which is highly expressed in the liver and pancreatic islet cells where it is involved in metabolic processes [62,68,69]. CB1R displays conserved spatial distribution in the CNS among different mammalian species [70]. In the brain, the majority of CB1R expressing cells are neurons. In the cortex and in the hippocampus high CB1R expressing cells are GABAergic neurons, whereas glutamatergic principal neurons express CB1Rs on a lower level [71], while glial cells and astrocytes exhibit only the marginal expression [72,73]. Localization of CB1R in the brain, correlated with its role in the control of motor function, analgesia, cognition and memory, is abundant in the cortex, hippocampus, basal ganglia nuclei and cerebellum [46,74,75,76,77]. CB1R is also expressed in peripheral tissues like heart, uterus, testis, liver and small intestine, as well as in immune cells [78,79,80] and adipose tissue [81]. In a model of insulin resistance, CNR1 gene was identified as one of the genes with the greatest increase in expression in adipose tissue [82].
2.1. CNR1 Gene in the Control of Energy Homeostasis and Obesity
Circuits in the HYP regulate appetite and energy homeostasis [83] and a key role is played by hypothalamic CB1R signaling intertwined with the pathways of metabolic hormones. In fact, for instance, the reduced hypothalamic eCB levels are associated with appetite suppression by leptin [26], while the increased hypothalamic eCB levels are correlated with orexigenic actions of ghrelin, with the involvement of the activation of AMP-activated protein kinase and the inhibition of paraventricular neurons [84].
Using mice lacking CNR1 gene, it has been documented that eCBs actions on food intake and body weight depend on the functional expression and activity of CB1R [85]. In this work, Cota and colleagues demonstrated that germline deletion of CNR1 in male mice resulted in a phenotype characterized by decreased body weight, reduced fat mass, and hypophagia. Moreover, the study highlighted that CNR1 mRNA is co-expressed in the HYP with neuropeptides known to modulate food intake [85].
A significantly reduction in body weight was also reported in mice, where CNR1 gene expression was selectively deleted in the HYP, after 9 weeks of viral-mediated deletion. This effect, without any changes in food intake, suggested an increase in energy expenditure [86]. Further, adult mice, in which CNR1 gene was deleted in adipocytes, resulted to be protected from diet-induced obesity and associated with metabolic alterations [87].
Again, conditional mutant mice, with CNR1 deletion in forebrain and sympathetic neurons, known to control energy balance, are resistant to diet-induced obesity and display a lean phenotype [88].
Moreover, the relevant role of CB1R in the initiation of milk suckling in pups has been observed [89,90] and, in particular, CNR1-knock-out (KO) newborns did not ingest milk on the first day of life, significantly affecting their survival rate [90].
Furthermore, central dysregulation of CNR1 gene expression has been documented in animal models of obesity in different brain areas, implicated in both homeostatic and hedonic aspects of eating [91,92,93].
In particular, the exposure to a palatable diet resulted in tissue and sex-specific changes in the gene expression of both CB1R and type-2 cannabinoid receptor (CB2R) in the HYP of offspring and adults. These results clearly indicate that the maternal diet has long-term effects on the development of pups through multiple alterations of signaling homeostatic pathways that include cannabinoid receptors [93].
Gamelin and colleagues (2016) found in the hippocampus of rats, fed with High Fat Diet (HFD), an increase in the CNR1 mRNA expression compared to rats fed with standard diet. The up-regulation of hippocampal CNR1 expression was increased with exercise training combined with HFD. Indeed, chronic exercise did not appear to counteract ECS overactivation and, in fact, seems even to induce this effect independently from diet. Moreover, the authors showed that CNR1 expression in the HYP is not affected by HFD in rats [91].
It was also reported, in rats exposed to HFD, the reduction in CB1R binding sites in extrahypothalamic brain regions and CB1R density was related to the intake of palatable food, whereas no changes have been observed in the HYP [94]. This does not exclude transient changes in CB1R levels or CNR1 expression over time. Indeed, a transient increase in mouse hypothalamic CB1R density, after 3 weeks of HFD, was normalized at the end of the 20 weeks of HFD, suggesting a temporal CB1R alteration during the development of obesity [95]. A temporal transcriptional regulation of CNR1 gene was also proved in the HYP of rats exposed to diet-induced obesity. The analysis of ECS components gene expression revealed a significant and selective increase in CNR1 mRNA levels at the beginning of obesity development (5 weeks on HFD) as well as after 21 weeks of exposure, when the phenotype was already well-established. Moreover, a consistent selective and significant reduction in DNA methylation at specific Cytosine–phosphate–Guanine (CpG) sites of CNR1 gene promoter in overweight rats was observed just after 5 weeks, but not 21 weeks on HFD [92].
In the same study, the DNA methylation status of CNR1 gene was assessed in peripheral blood mononuclear cells from a subset of obese human subjects. An age-based stratification of DNA methylation levels showed a significant reduction of the epigenetic hallmark at CNR1 promoter in younger (<30 years old) humans with obesity, when compared to age-matching controls. These findings suggest that the regulation of CNR1 gene is altered mainly at early life stage of phenotype development [92].
Considering other epigenetic modifications possibly occurring in the development of obesity, recently a hypothalamic increase in histone acetylation was reported at CNR1 gene promoter and was linked to increased receptor expression [96]. Almeida and colleagues hypothesized that maternal fat enriched diet would up-regulate CNR1 mRNA levels in the HYP of the male offspring at birth [96].
These latter findings support the relevance of environment and lifestyle in the facilitation of diseases progression, including obesity, by engaging epigenetic mechanisms [97], and in meantime could represent an innovative field to produce new strategies of intervention.
Genetic studies have identified several polymorphisms at different locations across the CNR1 gene that have been associated with obesity and related phenotypes, such as metabolic syndrome and dyslipidemia [98,99,100,101,102,103,104,105,106].
Among others, particular attention has been focused on a silent intragenic biallelic polymorphism in codon 435 of CNR1 gene, substitution of G to A at nucleotide position 1359 (1359 G/A rs1049353) [107]. This Single Nucleotide Polymorphism (SNP) was reported to be associated with abdominal adiposity [108], Body Mass Index (BMI) [109], intermuscular fat mass [110], and longitudinal changes from healthy to metabolic syndrome occurrence [111]. However, the literature has been inconsistent with respect to CNR1 polymorphisms and obesity-related markers, with many studies not finding any relevant association with CNR1 gene variants [112,113,114].
2.2. CNR1 Gene in Eating Disorders
EDs, defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-V [115], represent a group of conditions characterized by abnormal appetite and eating patterns, accompanied with other physiological as well as psychological disturbances.
The principal mechanisms implicated in the etiology of EDs involve dysregulation of neuronal circuits regulating homeostatic and hedonic aspects of food intake, thus including the ECS signaling.
Candidate gene association studies revealed the association of ECS genes SNPs in EDs. Specific genetic variants of CNR1, again rs1049353, as well as FAAH genes were identified in individuals with Anorexia Nervosa (AN) and Bulimia Nervosa (BN) [116], even if an earlier study failed to find associations of the same SNPs in a different AN population [114]. Although CNR1 rs1049353 is synonymous or silent, thus, not altering the amino acid sequence of the protein, Monteleone et al. suggested that it might have functional effects by changing mRNA stability or translation as already proposed for other SNPs [117]. Moreover, rs1049353 was found associated with lower BMI with unexplained heterogeneity within the human cohort [107].
A microsatellite polymorphism, namely an AAT (adenine-adenine-thymine) trinucleotide short tandem repeat (AAT)n, is present at CNR1 gene downstream the translation site [57]. It is known that microsatellites might affect transcription efficacy in some genes [118]. This AAT trinucleotide repeat has been found to be associated with restricting and bingeing/purging AN [119].
CNR1 KO mice display significant body weight loss under standard diet, a resistance to the obesogenic effects of the HFD and a reduced food intake on both diet regimens [120] further supporting the specific association of CNR1 gene with hypophagia [121,122]. Furthermore, preclinical studies in the Activity-based anorexia (ABA) rat model, found a reduced density of CB1Rs in lateral HYP and dental gyrus of the hippocampus [123] and, consistently, it was recently reported the reduction in CNR1 gene expression in HYP as well as NAc in ABA rats [124]. It has been suggested that the decrease in CB1R density might be driven by the decrease in eCBs that are necessary for receptor expression [125]. However, others reported increased CB1R availability in ABA rats [126], as well as in AN [127,128] and BN patients [128]. Gerard et al. suggested that in AN, this might act as compensatory mechanisms to chronically hypoactive ECS. Interestingly, short-term starvation increased hypothalamic 2-arachidonoylglycerol concentration in animals [129], whereas a long-term food-restriction (12 days protocol in mice) resulted in whole brain decrease of 2-arachidonoylglycerol [130]. There might occur adaptive strategies for coping with short- and long-lasting food deprivation, as elevated eCB levels might be beneficial to promote food seeking behavior in short term, while down-regulation of this orexigenic signal and reduction of appetite and motivation to eat may aid survival in the conditions of prolonged starvation [131]. Thus, the down-regulation of CNR1 gene expression might be a compensation for a purported reduced sensitivity of the receptor or a physiological consequence of up-regulated eCBs in these disorders [132]. Following to that, a recent study reported that CB1R availability was inversely associated with BMI in homeostatic brain regions of HYP and brainstem both in ED patients and healthy controls, while in the mesolimbic reward system (amygdala, insula, midbrain, striatum, and orbitofrontal cortex), negative correlation was found only in EDs patients [133]. The ECS deviations in homeostatic brain regions most likely present compensatory mechanism aimed at restoring energy balance, while alterations in brain areas implicated in motivation and reward may reflect disordered hedonic eating behavior observed in AN patients.
Endocannabinoids are also implicated in psychiatric comorbidities common in AN, such as anxiety and depression. Chronic stress, anxiety and depression exhibit CB1R deficiency and reduced CB1R-mediated signaling [134,135], while blockade or genetic deletion of CB1Rs has anxiogenic properties [136]. Furthermore, depression in human patients has been linked with several polymorphisms in the CNR1 gene [117,137,138].
The environment, both independently and in interaction with heritable factors, plays a relevant role in the onset of EDs and may influence gene expression via epigenetic mechanisms [139].
A possible transcriptional regulation of CNR1 gene, through DNA methylation of its promoter, was investigated in two animal models of AN (one behavioral and one genetic), in order to gain insight on players involved in AN onset and development [124].
More specifically, as an environmental model, it has been used the ABA model, through which rats are exposed to a restricted feeding schedule combined with physical activity, by giving them free access to a running wheel; a “combo” able to induce to a reduction in food intake, dramatic body weight loss and hyperactivity [140,141,142].
The major outcome of the above-mentioned study is that, among genes of the ECS, the expression of only CNR1 gene resulted to be altered in the ABA group and selectively in the HYP and in the NAc. Moreover, epigenetic analysis on the CNR1 gene promoter showed a consistent and significant increase of DNA methylation in the NAc; whereas, no changes of the epigenetic mark occurred at the earliest time-point (3 days induction) in the same area, nor in the HYP at neither time-points. Moreover, a significant correlation between body weight and both CNR1 expression and DNA methylation was reported. No changes were instead observed in the genetic model of AN, the anx/anx mice [143]. This let the authors to suggest that the selective molecular alterations reported in the ABA model were due to environmental cues (i.e., food restriction and physical activity), and not driven by a genetic predisposition.
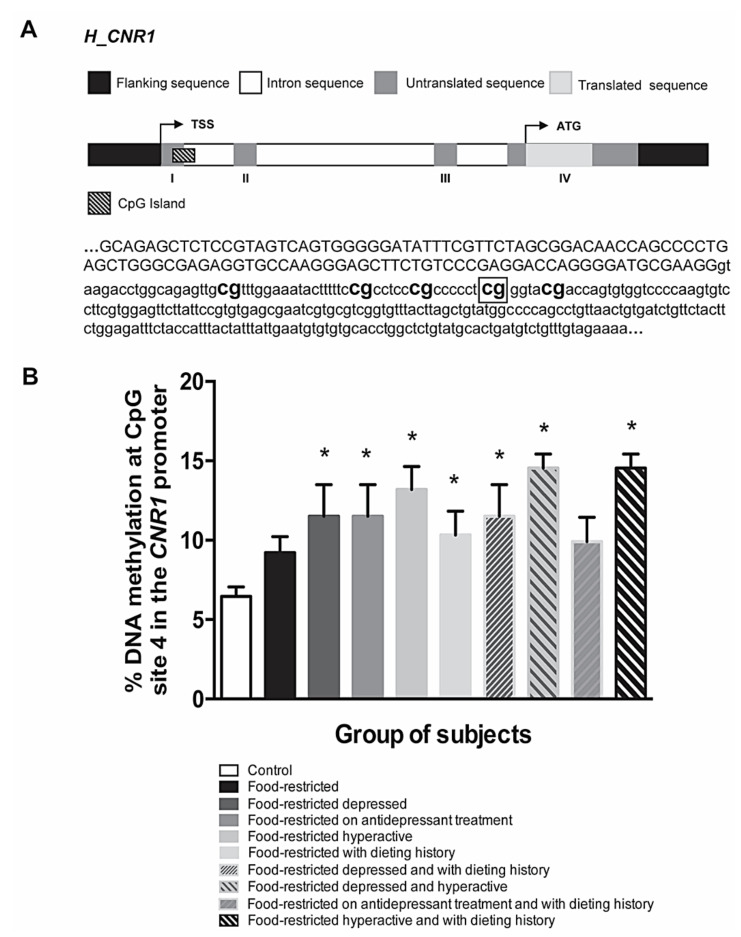
In this respect, we would like to point out that we recently analyzed the transcriptional regulation of CNR1 gene in a small population of subjects with restricted eating habits, mainly resembling AN feature [144]. Recruited subjects were females (n = 9) selected based on the age (18 to 65 years old, excluding pregnant women and people with known genetic mutations) and BMI (BMI ≥18.5–<25 for normal weight subjects with restricted eating habits, BMI <18.5–≥17 for mild malnutrition, BMI <17–≥16 moderate malnutrition, BMI <16–≥15 severe malnutrition and BMI <15 extreme malnutrition), with healthy subjects (n = 21) included as controls. Preliminary findings indicate that, in addition to food restriction, other environmental cues seem to be necessary to alter, in saliva, CNR1 gene DNA methylation patterns, that we analyzed by pyrosequencing as previously described [92] (see Figure 2A for details of the sequence under investigation). These additional environmental inputs include abnormally high levels of physical activity, previously experienced stressful events and/or dieting history. Among the 5 CpG sites under study at CNR1 gene, we have observed the most pronounced methylation differences at CpG site 4 (Figure 2B) (unpublished data ref [144]). Moreover, we identified retinoid X receptor alpha (RXR-α) as the transcription factor (TF) that binds this CpG site. Retinoic acid receptors (RAR) form heterodimers with RXR exerting a broad range of biological effects. For instance, RARs are involved in CB1R up-regulation in both alcohol- and HFD-induced fatty liver and in mediating CB1R expression evoked by eCBs [145]. Moreover, the same CpG site 4 binds another TF, glucocorticoid receptor alpha (GRα), well known for its implication in metabolic conditions of obesity and diabetes and psychiatric illnesses [146]. Agonistic actions on the GR promote fat deposition and central adiposity with adverse metabolic profile, including hyperglycemia, insulin resistance, dyslipidemia and hypertension, observed both in animal models and in human subjects [147,148]. Finally, a synthetic GC was shown to up-regulate peripheral CNR1 expression, suggesting it is involved in GR-regulated lipolysis making it an attractive drug target in type 2 diabetes and dyslipidemia [149].
Figure 2.
(A) Representation of human CNR1 gene and sequence of CpG islands (human GRcH38: crh 6:88165719-88165820) studied for DNA methylation. Flanking sequences are marked in black, UTRs in dark gray, coding regions in light gray, introns in white and CpG islands in lined pattern. Position of ATG, TSS and exons are also reported. Bold text indicates the CpG sites analyzed; framed CG indicates the CpG site number 4. (B) DNA methylation status at the CpG site number 4 in the sequence of the human CNR1 gene under study in food-restricted subjects stratified based on the co-occurrence of different environmental factors. The bars represent the mean of the % of DNA methylation ± the SEM in the different subgroups. Significant differences are indicated as * p < 0.05 vs. Control [144].
Defects in the endocannabinoid signaling, mediated primarily by CB1R, have been also implicated in development of binge eating disorder (BED), characterized by recurrent episodes of binge eating, with no compensatory behaviors to prevent weight gain, such as vomiting or laxative abuse [150]; therefore, obese individuals are the most commonly affected by BED [151]. CB1R antagonist/inverse agonist rimonabant has been demonstrated to decrease binge eating behavior in female rats by reducing the consumption of the HFD binged, with the accompanying significant body weight loss [152]. It has been also recently demonstrated that female rats under dietary-induced binge eating show a modified central eCB tone in several brain areas within the mesocorticolimbic system, which is the principal neural pathway that drives hedonic eating, as well as reduced CB1R density in the prefrontal cortex, probably related to the development and maintenance of this behavior [153]. Moreover, CB1R-dependent positive reinforcement appears responsible for maintenance of excessive food intake upon withdrawal [154]. With regards to genetic variants, specific allele has been associated with bingeing/purging AN, but not restricting subtype of AN [115], while several polymorphisms in CNR1 gene as well as FAAH gene have been associated with addiction and binge-drinking [155,156,157,158]. Recently, the study of ECS components transcriptional regulation [159] in a rat model of binge-like eating showed altered levels of just FAAH gene in the HYP of binge-eating group. BED also has a complex multifactorial etiology, with both genetic and environmental factors implicated [160]. Evidence on the epigenetic of CNR1 gene in BED are scarce: one report showed a reduced DNA methylation in CNR1 gene promoter in the prefrontal cortex of eating addicted-like animals, correlated with an elevated expression of CB1R protein in the same brain region [161]. More recently, a crucial role of glutamatergic CNR1 gene has been proposed as part of the regulatory mechanisms of relevance in the loss of inhibitory control for palatable food seeking and consumption [162].
3. Conclusions
The ECS is a constitutive signaling system that plays a critical role in energy homeostasis by promoting consumption of palatable food, stimulating fat mass expansion and inhibiting energy expenditure and thermogenesis. Via CB1R, eCBs modulate homeostatic and rewarding neural circuitries, and regulates consequently eating behaviors and energy balance, according to food availability: activation of eCB signaling is favorable when access to food is restricted, whereas it promotes obesity and metabolic diseases when food is abundant. Engagement of ECS occurs in conjunction with other metabolic signals, particularly leptin, that act synergistically through their specific neuronal pathways to maintain body energy homeostasis. We provided an overview of the role of CNR1 gene in EDs and obesity, in order to further stimulate the challenging idea that the modulation of CNR1 gene transcriptional regulation might represent a promising approach to prevent or to treat these pathologies, in addition to existing pharmacological interventions on CB1R [103].
Abbreviations
| HYP | HYPothalamus |
| EDs | Eating Disorder |
| NAc | Nucleus Accumbens |
| ECS | Endocannabinoid System |
| CB1R | Type-1 Cannabinoid Receptor |
| CNR1 | Type-1 Cannabinoid Receptor gene |
| CNS | Central Nervous System |
| eCBs | endogenous Cannabinoids |
| FAAH | Fatty Acid Amide Hydrolase |
| 5′-UTR | 5′-Untranslated Region |
| CpG | Cytosine-phosphate-Guanine |
| TSS | Transcription Start Site |
| ATG | Translation start site |
| bp | base pairs |
| KO | Knock-Out |
| CB2R | Type-2 Cannabinoid Receptor |
| HFD | High Fat Diet |
| SNP | Single Nucleotide Polymorphism |
| BMI | Body Mass Index |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| AN | Anorexia Nervosa |
| BN | Bulimia Nervosa |
| AAT | Adenine-Adenine-Thymine |
| ABA | Activity-Based Anorexia |
| RXR | Retinoid X Receptor |
| TF | Transcription Factor |
| RAR | Retinoic Acid Receptors |
| GR | Glucocorticoid Receptor |
| BED | Binge Eating Disorder |
Author Contributions
Conceptualization: C.D. and M.P.; validation: C.D., C.C., and M.M.; resources, C.D., C.C.; writing—original draft preparation, C.D., E.Z., M.P.; writing—review and editing: C.C., M.M., M.V.M.D.B., E.M.D.B.; supervision, P.D.C.; funding acquisition, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Marie Skłodowska-Curie grant agreement no. 713714 (Rep-Eat—H2020-MSCACOFUND-2015) and partially by a grant from the Italian Ministry of Education, University and Research (PRIN2015KP7T2Y) to C.C. Partial support by the EU-LAC (European LatinoAmerican Countries) Foundation under competitive grant EULAC16/T01-0132 to MM is also gratefully acknowledged. The EU-LAC Foundation had no role in the design or conduct of the study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the preliminary data obtained that we here report.
Data Availability Statement
The data presented in this study reference [144] are available on request from the corresponding author. The data are not publicly available since they are part of an unpublished work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berthoud H.-R. Nutrition Society. Volume 71. Cambridge University Press (CUP); Cambridge, UK: 2012. The neurobiology of food intake in an obesogenic environment; pp. 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson A.W. Eating beyond metabolic need: How environmental cues influence feeding behavior. Trends Neurosci. 2013;36:101–109. doi: 10.1016/j.tins.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Schneeberger M., Gomis R., Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014;220:T25–T46. doi: 10.1530/JOE-13-0398. [DOI] [PubMed] [Google Scholar]
- 4.Yeo G., Heisler L.K. Unraveling the brain regulation of appetite: Lessons from genetics. Nat. Neurosci. 2012;15:1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 5.Cifani C., Di Bonaventura M.V.E., Epucci M., Egiusepponi M.E., Eromano A., Francesco A.E., Emaccarrone M., D’Addario C. Regulation of hypothalamic neuropeptides gene expression in diet induced obesity resistant rats: Possible targets for obesity prediction? Front. Neurosci. 2015;9:187. doi: 10.3389/fnins.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Bonaventura M.V.M., Pucci M., Giusepponi M.E., Romano A., Lambertucci C., Volpini R., Di Bonaventura E.M., Gaetani S., Maccarrone M., D’Addario C., et al. Regulation of adenosine A2A receptor gene expression in a model of binge eating in the amygdaloid complex of female rats. J. Psychopharmacol. 2019;33:1550–1561. doi: 10.1177/0269881119845798. [DOI] [PubMed] [Google Scholar]
- 7.Pucci M., Di Bonaventura M.V.M., Giusepponi M.E., Romano A., Filaferro M., Maccarrone M., Ciccocioppo R., Cifani C., D’Addario C. Epigenetic regulation of nociceptin/orphanin FQ and corticotropin-releasing factor system genes in frustration stress-induced binge-like palatable food consumption. Addict. Biol. 2015;21:1168–1185. doi: 10.1111/adb.12303. [DOI] [PubMed] [Google Scholar]
- 8.Garfield A.S., Shah B.P., Burgess C.R., Li M.M., Li C., Steger J.S., Madara J.C., Campbell J.N., Kroeger D., Scammell T.E., et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 2016;19:1628–1635. doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi M.A., Stuber G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018;27:42–56. doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jager G., Witkamp R.F. The endocannabinoid system and appetite: Relevance for food reward. Nutr. Res. Rev. 2014;27:172–185. doi: 10.1017/S0954422414000080. [DOI] [PubMed] [Google Scholar]
- 11.Novelle M.G., Dieguez C. Food Addiction and Binge Eating: Lessons Learned from Animal Models. Nutrients. 2018;10:71. doi: 10.3390/nu10010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blouet C., Schwartz G.J. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav. Brain Res. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Coll A.P., Farooqi I.S., O’Rahilly S. The Hormonal Control of Food Intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich M.O., Horvath T.L. Hypothalamic control of energy balance: Insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Volkow N.D., Wang G.-J., Baler R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belgardt B.F., Okamura T., Brüning J.C. Hormone and glucose signalling in POMC and AgRP neurons. J. Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstone A.P. The hypothalamus, hormones, and hunger: Alterations in human obesity and illness. Neural Regen. 2006;153:57–73. doi: 10.1016/s0079-6123(06)53003-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Astarita G., Taussig M.D., Bharadwaj K.G., DiPatrizio N.V., Nave K.-A., Piomelli D., Goldberg I.J., Eckel R.H. Deficiency of Lipoprotein Lipase in Neurons Modifies the Regulation of Energy Balance and Leads to Obesity. Cell Metab. 2011;13:105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Land B.B., Narayanan N.S., Liu R.-J., Gianessi C.A., Brayton C.E., Grimaldi D.M., Sarhan M., Guarnieri D.J., Deisseroth K., Aghajanian G.K., et al. Medial prefrontal D1 dopamine neurons control food intake. Nat. Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovich G.D., Holland P.C., Gallagher M. Amygdalar and Prefrontal Pathways to the Lateral Hypothalamus Are Activated by a Learned Cue That Stimulates Eating. J. Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow N.D., Fowler J., Wang G., Baler R., Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutter M., Nestler E.J. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. J. Nutr. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkow N.D., Wang G.-J., Tomasi D., Baler R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Addario C., Di Bonaventura M.M., Pucci M., Romano A., Gaetani S., Ciccocioppo R., Cifani C., Maccarrone M. Endocannabinoid signaling and food addiction. Neurosci. Biobehav. Rev. 2014;47:203–224. doi: 10.1016/j.neubiorev.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Coccurello R., Maccarrone M. Hedonic Eating and the “Delicious Circle”: From Lipid-Derived Mediators to Brain Dopamine and Back. Front. Neurosci. 2018;12:271. doi: 10.3389/fnins.2018.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G.I., Palmiter R.D., Sugiura T., et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nat. Cell Biol. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 27.Berry E.M., Mechoulam R. Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol. Ther. 2002;95:185–190. doi: 10.1016/S0163-7258(02)00257-7. [DOI] [PubMed] [Google Scholar]
- 28.Pi-Sunyer X., Aronne L.J., Heshmati H.M., Devin J., Rosenstock J., for the RIO-North America Study Group Effect of Rimonabant, a Cannabinoid-1 Receptor Blocker, on Weight and Cardiometabolic Risk Factors in Overweight or Obese Patients. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 29.Engeli S. Dysregulation of the Endocannabinoid System in Obesity. J. Neuroendocrinol. 2008;20:110–115. doi: 10.1111/j.1365-2826.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 30.Mazier W., Saucisse N., Gatta-Cherifi B., Cota D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. 2015;26:524–537. doi: 10.1016/j.tem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Zou S., Somvanshi R.K., Paik S., Kumar U. Colocalization of Cannabinoid Receptor 1 with Somatostatin and Neuronal Nitric Oxide Synthase in Rat Brain Hypothalamus. J. Mol. Neurosci. 2014;55:480–491. doi: 10.1007/s12031-014-0369-5. [DOI] [PubMed] [Google Scholar]
- 32.Moldrich G., Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/S0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 33.Bellocchio L., Cervino C., Pasquali R., Pagotto U. The Endocannabinoid System and Energy Metabolism. J. Neuroendocrinol. 2008;20:850–857. doi: 10.1111/j.1365-2826.2008.01728.x. [DOI] [PubMed] [Google Scholar]
- 34.Di Marzo V., Matias I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- 35.Hillard C.J., Beatka M., Sarvaideo J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2016;7:1–15. doi: 10.1002/cphy.c160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestri C., Di Marzo V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Melis T., Succu S., Sanna F., Boi A., Argiolas A., Melis M.R. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci. Lett. 2007;419:231–235. doi: 10.1016/j.neulet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 38.DiPatrizio N.V., Simansky K.J. Activating Parabrachial Cannabinoid CB1 Receptors Selectively Stimulates Feeding of Palatable Foods in Rats. J. Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seagard J.L., Dean C., Patel S., Rademacher D.J., Hopp F.A., Schmeling W.T., Hillard C.J. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am. J. Physiol. Circ. Physiol. 2004;286:H992–H1000. doi: 10.1152/ajpheart.00870.2003. [DOI] [PubMed] [Google Scholar]
- 40.Derbenev A.V., Stuart T.C., Smith B.N. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J. Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota D., Genghini S., Pasquali R., Pagotto U. Antagonizing the cannabinoid receptor Type 1: A dual way to fight obesity. J. Endocrinol. Investig. 2003;26:1041–1044. doi: 10.1007/BF03348205. [DOI] [PubMed] [Google Scholar]
- 42.Williams C.M., Kirkham T.C. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- 43.Lau B.K., Cota D., Cristino L., Borgland S.L. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology. 2017;124:38–51. doi: 10.1016/j.neuropharm.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Ravula A., Chandasana H., Setlow B., Febo M., Bruijnzeel A.W., Derendorf H. Simultaneous quantification of cannabinoids tetrahydrocannabinol, cannabidiol and CB1 receptor antagonist in rat plasma: An application to characterize pharmacokinetics after passive cannabis smoke inhalation and co-administration of rimonabant. J. Pharm. Biomed. Anal. 2018;160:119–125. doi: 10.1016/j.jpba.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sam A.H., Salem V., Ghatei M.A. Rimonabant: From RIO to Ban. J. Obes. 2011;2011:1–4. doi: 10.1155/2011/432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., De Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hryhorowicz S., Kaczmarek-Ryś M., Andrzejewska A., Staszak K., Korcz A., Słomski R. Allosteric Modulation of Cannabinoid Receptor 1—Current Challenges and Future Opportunities. Int. J. Mol. Sci. 2019;20:5874. doi: 10.3390/ijms20235874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Marzo V., Stella N., Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015;16:30–42. doi: 10.1038/nrn3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiPatrizio N.V., Piomelli D. Intestinal lipid–derived signals that sense dietary fat. J. Clin. Investig. 2015;125:891–898. doi: 10.1172/JCI76302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Begg M., Pacher P., Bátkai S., Osei-Hyiaman D., Offertáler L., Mo F.M., Liu J., Kunos G. Evidence for novel cannabinoid receptors. Pharmacol. Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.-O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nat. Cell Biol. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 54.Ruehle S., Wager-Miller J., Straiker A., Farnsworth J., Murphy M.N., Loch S., Monory K., Mackie K., Lutz B. Discovery and characterization of two novel CB1 receptor splice variants with modified N-termini in mouse. J. Neurochem. 2017;142:521–533. doi: 10.1111/jnc.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonner T. Molecular biology of cannabinoid receptors. J. Neuroimmunol. 1996;69:15–17. [Google Scholar]
- 56.McCaw E.A., Hu H., Gomez G.T., Hebb A.L.O., Kelly M.E.M., Denovan-Wright E.M. Structure, expression and regulation of the cannabinoid receptor gene (CB1) in Huntington’s disease transgenic mice. JBIC J. Biol. Inorg. Chem. 2004;271:4909–4920. doi: 10.1111/j.1432-1033.2004.04460.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P.-W., Ishiguro H., Ohtsuki T., Hess J., Carillo F., Walther D., Onaivi E.S., Arinami T., Uhl G.R. Human cannabinoid receptor 1:5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol. Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 58.Miller L.K., Devi L.A. The Highs and Lows of Cannabinoid Receptor Expression in Disease: Mechanisms and Their Therapeutic Implications. Pharmacol. Rev. 2011;63:461–470. doi: 10.1124/pr.110.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shire D., Calandra B., Delpech M., Dumont X., Kaghad M., Le Fur G., Caput D., Ferrara P. Structural Features of the Central Cannabinoid CB1 Receptor Involved in the Binding of the Specific CB1 Antagonist SR 141716A. J. Biol. Chem. 1996;271:6941–6946. doi: 10.1074/jbc.271.12.6941. [DOI] [PubMed] [Google Scholar]
- 60.Gustafsson K., Wang X., Severa D., Eriksson M., Kimby E., Merup M., Christensson B., Flygare J., Sander B. Expression of cannabinoid receptors type 1 and type 2 in non-Hodgkin lymphoma: Growth inhibition by receptor activation. Int. J. Cancer. 2008;123:1025–1033. doi: 10.1002/ijc.23584. [DOI] [PubMed] [Google Scholar]
- 61.Palermo F.A., Angelini M., Cottone E., Virgili M., Franzoni M.F., Mosconi G., Polzonetti-Magni A.M. Involvement of Endocannabinoid CB1 Receptor in the Modulation of Stress Responses Related to Xenoestrogen Exposure. Ann. N. Y. Acad. Sci. 2009;1163:504–507. doi: 10.1111/j.1749-6632.2009.04455.x. [DOI] [PubMed] [Google Scholar]
- 62.Ryberg E., Vu H.K., Larsson N., Groblewski T., Hjorth S., Elebring T., Sjögren S., Greasley P.J. Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett. 2004;579:259–264. doi: 10.1016/j.febslet.2004.11.085. [DOI] [PubMed] [Google Scholar]
- 63.Agrawal A., Wetherill L., Dick D.M., Xuei X., Hinrichs A., Hesselbrock V., Kramer J., Nurnberger J.I., Jr., Schuckit M., Bierut L.J., et al. Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009;150B:736–740. doi: 10.1002/ajmg.b.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng Q., Vickers K.C., Anderson M., Levin M., Chen W., Harrison D.G., Wilke R.A. A common functional promoter variant links CNR1 gene expression to HDL cholesterol level. Nat. Commun. 2013;4:1–7. doi: 10.1038/ncomms2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gadzicki D., Müller-Vahl K., Stuhrmann M. A frequent polymorphism in the coding exon of the human cannabinoid receptor (CNR1) gene. Mol. Cell. Probes. 1999;13:321–323. doi: 10.1006/mcpr.1999.0249. [DOI] [PubMed] [Google Scholar]
- 66.Hartman C.A., Hopfer C.J., Haberstick B., Rhee S.H., Crowley T.J., Corley R.P., Hewitt J.K., Ehringer M.A. The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug Alcohol Depend. 2009;104:11–16. doi: 10.1016/j.drugalcdep.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruiz-Contreras A.E., Román-López T.V., Caballero-Sánchez U., Rosas-Escobar C.B., Ortega-Mora E.I., Barrera-Tlapa M.A., Romero-Hidalgo S., Carrillo-Sánchez K., Hernández-Morales S., Vadillo-Ortega F., et al. Because difficulty is not the same for everyone: The impact of complexity in working memory is associated with cannabinoid 1 receptor genetic variation in young adults. Memory. 2016;25:335–343. doi: 10.1080/09658211.2016.1172642. [DOI] [PubMed] [Google Scholar]
- 68.González-Mariscal I., Krzysik-Walker S.M., Doyle M.E., Liu Q.-R., Cimbro R., Calvo S.S.-C., Ghosh S., Ciesla L., Moaddel R., Carlson O.D., et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016;6:33302. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shire D., Carillon C., Kaghad M., Calandra B., Rinaldi-Carmona M., Le Fur G., Caput D., Ferrara P. An Amino-terminal Variant of the Central Cannabinoid Receptor Resulting from Alternative Splicing. J. Biol. Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- 70.Elphick M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012;367:3201–3215. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marsicano G., Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 72.Han J., Kesner P., Metna-Laurent M., Duan T., Xu L., Georges F., Koehl M., Abrous D.N., Mendizabal-Zubiaga J., Grandes P., et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 73.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glass M., Faull R., Dragunow M. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 75.Kruk-Slomka M., Dzik A., Budzynska B., Biala G. Endocannabinoid System: The Direct and Indirect Involvement in the Memory and Learning Processes—A Short Review. Mol. Neurobiol. 2017;54:8332–8347. doi: 10.1007/s12035-016-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mackie K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. Handb. Exp. Pharmacol. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- 77.Tsou K., Brown S., Sañudo-Peña M., Mackie K., Walker J. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 78.Klein T.W., Newton C., Larsen K., Lu L., Perkins I., Nong L., Friedman H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 79.Maccarrone M., De Petrocellis L., Bari M., Fezza F., Salvati S., Di Marzo V., Finazzi-Agrò A. Lipopolysaccharide Downregulates Fatty Acid Amide Hydrolase Expression and Increases Anandamide Levels in Human Peripheral Lymphocytes. Arch. Biochem. Biophys. 2001;393:321–328. doi: 10.1006/abbi.2001.2500. [DOI] [PubMed] [Google Scholar]
- 80.Nong L., Newton C., Friedman H., Klein T.W. CB1 and CB2 Receptor mRNA Expression in Human Peripheral Blood Mononuclear Cells (PBMC) from Various Donor Types. Adv. Exp. Med. Biol. 2001;493:229–233. doi: 10.1007/0-306-47611-8_27. [DOI] [PubMed] [Google Scholar]
- 81.Spoto B., Fezza F., Parlongo G., Battista N., Sgro’ E., Gasperi V., Zoccali C., Maccarrone M. Human adipose tissue binds and metabolizes the endocannabinoids anandamide and 2-arachidonoylglycerol. Biochimie. 2006;88:1889–1897. doi: 10.1016/j.biochi.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Pereira M.J., Palming J., Svensson M.K., Rizell M., Dalenbäck J., Hammar M., Fall T., Sidibeh C.O., Svensson P.-A., Eriksson J.W. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metabolism. 2014;63:1198–1208. doi: 10.1016/j.metabol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 83.Timper K., Brüning J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017;10:679–689. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A.B., et al. The Orexigenic Effect of Ghrelin Is Mediated through Central Activation of the Endogenous Cannabinoid System. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M., Auer D., Yassouridis A., Thöne-Reineke C., Ortmann S., et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cardinal P., Bellocchio L., Clark S., Cannich A., Klugmann M., Lutz B., Marsicano G., Cota D. Hypothalamic CB1 Cannabinoid Receptors Regulate Energy Balance in Mice. Endocrinology. 2012;153:4136–4143. doi: 10.1210/en.2012-1405. [DOI] [PubMed] [Google Scholar]
- 87.De Azua I.R., Mancini G., Srivastava R.K., Rey A.A., Cardinal P., Tedesco L., Zingaretti C.M., Sassmann A., Quarta C., Schwitter C., et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Investig. 2017;127:4148–4162. doi: 10.1172/JCI83626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quarta C., Bellocchio L., Mancini G., Mazza R., Cervino C., Braulke L.J., Fekete C., Latorre R., Nanni C., Bucci M., et al. CB1 Signaling in Forebrain and Sympathetic Neurons Is a Key Determinant of Endocannabinoid Actions on Energy Balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 89.Fride E., Foox A., Rosenberg E., Faigenboim M., Cohen V., Barda L., Blau H., Mechoulam R. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: Evidence for a “CB3” receptor. Eur. J. Pharmacol. 2003;461:27–34. doi: 10.1016/S0014-2999(03)01295-0. [DOI] [PubMed] [Google Scholar]
- 90.Fride E., Ginzburg Y., Breuer A., Bisogno T., Di Marzo V., Mechoulam R. Critical role of the endogenous cannabinoid system in mouse pup suckling and growth. Eur. J. Pharmacol. 2001;419:207–214. doi: 10.1016/S0014-2999(01)00953-0. [DOI] [PubMed] [Google Scholar]
- 91.Gamelin F.-X., Aucouturier J., Iannotti F.A., Piscitelli F., Mazzarella E., Aveta T., Leriche M., Dupont E., Cieniewski-Bernard C., Montel V., et al. Effects of chronic exercise on the endocannabinoid system in Wistar rats with high-fat diet-induced obesity. J. Physiol. Biochem. 2016;72:183–199. doi: 10.1007/s13105-016-0469-5. [DOI] [PubMed] [Google Scholar]
- 92.Pucci M., Di Bonaventura M.V.M., Vezzoli V., Zaplatic E., Massimini M., Mai S., Sartorio A., Scacchi M., Persani L., Maccarrone M., et al. Preclinical and Clinical Evidence for a Distinct Regulation of Mu Opioid and Type 1 Cannabinoid Receptor Genes Expression in Obesity. Front. Genet. 2019;10:523. doi: 10.3389/fgene.2019.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramírez-López M.T., Arco R., Decara J., Vázquez M., Rivera P., Blanco R.N., Alén F., De Heras R.G., Suárez J., De Fonseca F.R. Long-Term Effects of Prenatal Exposure to Undernutrition on Cannabinoid Receptor-Related Behaviors: Sex and Tissue-Specific Alterations in the mRNA Expression of Cannabinoid Receptors and Lipid Metabolic Regulators. Front. Behav. Neurosci. 2016;10:241. doi: 10.3389/fnbeh.2016.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrold J.A., Elliott J.C., King P.J., Widdowson P.S., Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: A role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–238. doi: 10.1016/S0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- 95.South T., Huang X.-F. Temporal and Site-Specific Brain Alterations in CB1 Receptor Binding in High Fat Diet-Induced Obesity in C57Bl/6 Mice. J. Neuroendocr. 2008;20:1288–1294. doi: 10.1111/j.1365-2826.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 96.Almeida M.M., Dias-Rocha C.P., Reis-Gomes C.F., Wang H., Atella G.C., Cordeiro A., Pazos-Moura C.C., Joss-Moore L.A., Trevenzoli I.H. Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats. Psychoneuroendocrinology. 2019;103:306–315. doi: 10.1016/j.psyneuen.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Van Djik S., Tellam R.L., Morrison J.L., Mühlhäusler B.S., Molloy P. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin. Epigenetics. 2015;7:1–13. doi: 10.1186/s13148-015-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baye T.M., Zhang Y., Smith E., Hillard C.J., Gunnell J., Myklebust J.B., James R., Kissebah A.H., Olivier M., Wilke R.A. Genetic variation in cannabinoid receptor 1 (CNR1) is associated with derangements in lipid homeostasis, independent of body mass index. Pharmacogenomics. 2008;9:1647–1656. doi: 10.2217/14622416.9.11.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li N., Cao T., Wu X., Tang M., Xiang D., Cai H. Progress in Genetic Polymorphisms Related to Lipid Disturbances Induced by Atypical Antipsychotic Drugs. Front. Pharmacol. 2020;10:1669. doi: 10.3389/fphar.2019.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caruso M.G., Gazzerro P., Notarnicola M., Cisternino A.M., Guerra V., Misciagna G., Laezza C., Bifulco M. Cannabinoid Type 1 Receptor Gene Polymorphism and Macronutrient Intake. J. Nutr. Nutr. 2012;5:305–313. doi: 10.1159/000343563. [DOI] [PubMed] [Google Scholar]
- 101.De Luis D.A., Sagrado M.G., Aller R., Izaola O., Conde R. Relation of G1359A polymorphism of the cannabinoid receptor (CB1) gene with metabolic syndrome by ATP III classification. Diabetes/Metab. Res. Rev. 2011;27:506–511. doi: 10.1002/dmrr.1200. [DOI] [PubMed] [Google Scholar]
- 102.Jaeger J.P., Mattevi V.S., Callegari-Jacques S.M., Hutz M.H. Cannabinoid Type-1 Receptor Gene Polymorphisms Are Associated with Central Obesity in a Southern Brazilian Population. Dis. Markers. 2008;25:67–74. doi: 10.1155/2008/841490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maccarrone M., Gasperi V., Catani M.V., Diep T.A., Dainese E., Hansen H.S., Avigliano L. The Endocannabinoid System and Its Relevance for Nutrition. Annu. Rev. Nutr. 2010;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- 104.Russo P., Strazzullo P., Cappuccio F.P., Tregouet D.A., Lauria F., Loguercio M., Barba G., Versiero M., Siani A. Genetic Variations at the Endocannabinoid Type 1 Receptor Gene (CNR1) Are Associated with Obesity Phenotypes in Men. J. Clin. Endocrinol. Metab. 2007;92:2382–2386. doi: 10.1210/jc.2006-2523. [DOI] [PubMed] [Google Scholar]
- 105.Schleinitz R., Carmienke S., Böttcher Y., Tönjes A., Berndt J., Klöting N., Enigk B., Müller I., Dietrich K., Breitfeld J., et al. Role of genetic variation in the cannabinoid type 1 receptor gene (CNR1) in the pathophysiology of human obesity. Pharmacogenomics. 2010;11:693–702. doi: 10.2217/pgs.10.42. [DOI] [PubMed] [Google Scholar]
- 106.Zhuang M., Yang Y., Cao F., Lu M., Wang X., Zhang J., Chen X., Cheng P., Zhang N., Ye W., et al. Associations of variants of CNR1 with obesity and obesity-related traits in Chinese women. Gene. 2012;495:194–198. doi: 10.1016/j.gene.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 107.Sadeghian M., Rahmani S., Mansoori A. G1359A Variant of the Cannabinoid Receptor Gene (rs1049353) and Obesity-Related Traits and Related Endophenotypes: A Meta-Analysis. Ann. Nutr. Metab. 2018;73:76–85. doi: 10.1159/000490668. [DOI] [PubMed] [Google Scholar]
- 108.Peeters A., Beckers S., Mertens I., Van Hul W., Van Gaal L. The G1422A variant of the cannabinoid receptor gene (CNR1) is associated with abdominal adiposity in obese men. Endocrine. 2007;31:138–141. doi: 10.1007/s12020-007-0022-y. [DOI] [PubMed] [Google Scholar]
- 109.Gazzerro P., Caruso M.G., Notarnicola M., Misciagna G., Guerra V., Laezza C., Bifulco M. Association between cannabinoid type-1 receptor polymorphism and body mass index in a southern Italian population. Int. J. Obes. 2006;31:908–912. doi: 10.1038/sj.ijo.0803510. [DOI] [PubMed] [Google Scholar]
- 110.Frost M., Nielsen T.L., Wraae K., Hagen C., Piters E., Beckers S., De Freitas F., Brixen K., Van Hul W., Andersen M.S. Polymorphisms in the endocannabinoid receptor 1 in relation to fat mass distribution. Eur. J. Endocrinol. 2010;163:407–412. doi: 10.1530/EJE-10-0192. [DOI] [PubMed] [Google Scholar]
- 111.Kvaløy K., Holmen J., Hveem K., Holmen T.L. Genetic Effects on Longitudinal Changes from Healthy to Adverse Weight and Metabolic Status—The HUNT Study. PLoS ONE. 2015;10:e0139632. doi: 10.1371/journal.pone.0139632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Luis D.A., Pacheco D., Aller R., Sagrado M.G., Conde R., Izaola O., Cuellar L., Terroba M.C., Martin T., Ventosa M. G 1359A polymorphism of the cannabinoid receptor gene (CNR1) and clinical results of biliopancreatic diversion. Eur. Rev. Med. Pharmacol. Sci. 2010;14:197–201. [PubMed] [Google Scholar]
- 113.Łaczmański Ł., Milewicz A., Dunajska K., Jędrzejczuk D., Pawlak M., Lwow F. Endocannabinoid type 1 receptor gene (CNR1) polymorphisms (rs806381, rs10485170, rs6454674, rs2023239) and cardiovascular risk factors in postmenopausal women. Gynecol. Endocrinol. 2011;27:1023–1027. doi: 10.3109/09513590.2011.569796. [DOI] [PubMed] [Google Scholar]
- 114.Müller T.D., Reichwald K., Brönner G., Kirschner J., Nguyen T.T., Scherag A., Herzog W., Herpertz-Dahlmann B., Lichtner P., Meitinger T., et al. Lack of association of genetic variants in genes of the endocannabinoid system with anorexia nervosa. Child Adolesc. Psychiatry Ment. Health. 2008;2:33. doi: 10.1186/1753-2000-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; Arlington, VA, USA: 2013. [Google Scholar]
- 116.Monteleone P., Bifulco M., Di Filippo C., Gazzerro P., Canestrelli B., Proto M.C., Di Genio M., Grimaldi C., Maj M. Association of CNR1 and FAAH endocannabinoid gene polymorphisms with anorexia nervosa and bulimia nervosa: Evidence for synergistic effects. Genes Brain Behav. 2009;8:728–732. doi: 10.1111/j.1601-183X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 117.Domschke K., Dannlowski U., Ohrmann P., Lawford B.R., Bauer J., Kugel H., Heindel W., Young R., Morris P., Arolt V., et al. Cannabinoid receptor 1 (CNR1) gene: Impact on antidepressant treatment response and emotion processing in Major Depression. Eur. Neuropsychopharmacol. 2008;18:751–759. doi: 10.1016/j.euroneuro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Li T., Liu X., Zhu Z.H., Zhao J., Hu X., Ball D.M., Sham P.C., Collier D.A. No association between (AAT)n repeats in the cannabinoid receptor gene (CNR1) and heroin abuse in a Chinese population. Mol. Psychiatry. 2000;5:128–130. doi: 10.1038/sj.mp.4000670. [DOI] [PubMed] [Google Scholar]
- 119.Siegfried Z., Kanyas K., Latzer Y., Karni O., Bloch M., Lerer B., Berry E. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: Differences between restricting and bingeing/purging subtypes. Am. J. Med. Genet. 2004;125B:126–130. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- 120.Trillou C.R., Delgorge C., Menet C., Arnone M., Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 121.Jbilo O., Trillou C.R., Arnone M., Buisson I., Bribes E., Péleraux A., Pénarier G., Soubrié P., Le Fur G., Galiègue S., et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- 122.Nogueiras R., López M., Diéguez C. Regulation of lipid metabolism by energy availability: A role for the central nervous system. Obes. Rev. 2010;11:185–201. doi: 10.1111/j.1467-789X.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 123.Collu R., Scherma M., Piscitelli F., Giunti E., Satta V., Castelli M.P., Verde R., Fratta W., Bisogno T., Fadda P. Impaired brain endocannabinoid tone in the activity-based model of anorexia nervosa. Int. J. Eat. Disord. 2019;52:1251–1262. doi: 10.1002/eat.23157. [DOI] [PubMed] [Google Scholar]
- 124.D’Addario C., Ms E.Z., Giunti E., Pucci M., Di Bonaventura M.V.M., Scherma M., Dainese E., Maccarrone M., Nilsson I.A.K., Cifani C., et al. Epigenetic regulation of the cannabinoid receptor CB1 in an activity-based rat model of anorexia nervosa. Int. J. Eat. Disord. 2020;53:432–446. doi: 10.1002/eat.23271. [DOI] [PubMed] [Google Scholar]
- 125.Romero J., Garcia L., Fernández-Ruiz J., Cebeira M., Ramos J. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to δ9-tetrahydrocannabinol. Pharmacol. Biochem. Behav. 1995;51:731–737. doi: 10.1016/0091-3057(95)00023-P. [DOI] [PubMed] [Google Scholar]
- 126.Casteels C., Gérard N., Van Kuyck K., Pottel L., Nuttin B., Bormans G., Van Laere K. Small animal PET imaging of the type 1 cannabinoid receptor in a rodent model for anorexia nervosa. Eur. J. Nucl. Med. Mol. Imaging. 2013;41:308–321. doi: 10.1007/s00259-013-2522-8. [DOI] [PubMed] [Google Scholar]
- 127.Gérard N., Pieters G., Goffin K., Bormans G., Van Laere K. Brain Type 1 Cannabinoid Receptor Availability in Patients with Anorexia and Bulimia Nervosa. Biol. Psychiatry. 2011;70:777–784. doi: 10.1016/j.biopsych.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Kaye W.H. Neurobiology of anorexia and bulimia nervosa. Physiol. Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kirkham T.C., Williams C.M., Fezza F., Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hanus L.O., Avraham Y., Ben-Shushan D., Zolotarev O., Berry E.M., Mechoulam R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003;983:144–151. doi: 10.1016/S0006-8993(03)03046-4. [DOI] [PubMed] [Google Scholar]
- 131.Fride E., Bregman T., Kirkham T.C. Endocannabinoids and Food Intake: Newborn Suckling and Appetite Regulation in Adulthood. Exp. Biol. Med. 2005;230:225–234. doi: 10.1177/153537020523000401. [DOI] [PubMed] [Google Scholar]
- 132.Frieling H., Albrecht H., Jedtberg S., Gozner A., Lenz B., Wilhelm J., Hillemacher T., De Zwaan M., Kornhuber J., Bleich S. Elevated cannabinoid 1 receptor mRNA is linked to eating disorder related behavior and attitudes in females with eating disorders. Psychoneuroendocrinology. 2009;34:620–624. doi: 10.1016/j.psyneuen.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 133.Ceccarini J., Weltens N., Ly H.G., Tack J., Van Oudenhove L., Van Laere K. Association between cerebral cannabinoid 1 receptor availability and body mass index in patients with food intake disorders and healthy subjects: A [(18)F]MK-9470 PET study. Transl. Psychiatry. 2016;6:e853. doi: 10.1038/tp.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hillard C.J. Stress regulates endocannabinoid-CB1 receptor signaling. Semin. Immunol. 2014;26:380–388. doi: 10.1016/j.smim.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koethe D., Llenos I.C., Dulay J.R., Hoyer C., Torrey E.F., Leweke F.M., Weis S. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J. Neural Transm. 2007;114:1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- 136.Patel S., Hillard C.J. Role of Endocannabinoid Signaling in Anxiety and Depression. Curr. Top. Behav. Neurosci. 2009;1:347–371. doi: 10.1007/978-3-540-88955-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Agrawal A., Nelson E.C., Littlefield A.K., Bucholz K.K., Degenhardt L., Henders A.K., Madden P.A.F., Martin N.G., Montgomery G.W., Pergadia M.L., et al. Cannabinoid Receptor Genotype Moderation of the Effects of Childhood Physical Abuse on Anhedonia and Depression. Arch. Gen. Psychiatry. 2012;69:732–740. doi: 10.1001/archgenpsychiatry.2011.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kong X., Miao Q., Lu X., Zhang Z., Chen M., Zhang J., Zhai J. The association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression. Medicine. 2019;98:e17403. doi: 10.1097/MD.0000000000017403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huebel C., Marzi S.J., Breen G., Bulik C.M. Epigenetics in eating disorders: A systematic review. Mol. Psychiatry. 2019;24:901–915. doi: 10.1038/s41380-018-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chowdhury T.G., Chen Y.-W., Aoki C. Using the Activity-based Anorexia Rodent Model to Study the Neurobiological Basis of Anorexia Nervosa. J. Vis. Exp. 2015;10:e52927. doi: 10.3791/52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klenotich S.J., Dulawa S.C. The Activity-Based Anorexia Mouse Model. Methods Mol. Biol. 2011;829:377–393. doi: 10.1007/978-1-61779-458-2_25. [DOI] [PubMed] [Google Scholar]
- 142.Scherma M., Satta V., Collu R., Boi M.F., Usai P., Fratta W., Fadda P. Cannabinoid CB1/CB2receptor agonists attenuate hyperactivity and body weight loss in a rat model of activity-based anorexia. Br. J. Pharmacol. 2017;174:2682–2695. doi: 10.1111/bph.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nilsson I.A.K. The anx/anx Mouse—A Valuable Resource in Anorexia Nervosa Research. Front. Neurosci. 2019;13:59. doi: 10.3389/fnins.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.D’Addario C. University of Teramo; Teramo, Italy: 2020. Unpublished work. [Google Scholar]
- 145.Mukhopadhyay B., Liu J., Osei-Hyiaman D., Godlewski G., Mukhopadhyay P., Wang L., Jeong W.-I., Gao B., Duester G., Mackie K., et al. Transcriptional Regulation of Cannabinoid Receptor-1 Expression in the Liver by Retinoic Acid Acting via Retinoic Acid Receptor-γ. J. Biol. Chem. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moraitis A.G., Block T., Nguyen D., Belanoff J.K. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. J. Steroid Biochem. Mol. Biol. 2017;165:114–120. doi: 10.1016/j.jsbmb.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 147.Geer E.B., Islam J., Buettner C. Mechanisms of Glucocorticoid-Induced Insulin Resistance. Endocrinol. Metab. Clin. N. Am. 2014;43:75–102. doi: 10.1016/j.ecl.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Majer-Łobodzińska A., Adamiec-Mroczek J. Glucocorticoid receptor polymorphism in obesity and glucose homeostasis. Adv. Clin. Exp. Med. 2017;26:143–148. doi: 10.17219/acem/41231. [DOI] [PubMed] [Google Scholar]
- 149.Sidibeh C.O., Pereira M.J., Börjesson J.L., Kamble P.G., Skrtic S., Katsogiannos P., Sundbom M., Svensson M.K., Eriksson J.W. Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance. Endocrine. 2017;55:839–852. doi: 10.1007/s12020-016-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hilbert A. Binge-Eating Disorder. Psychiatr. Clin. N. Am. 2019;42:33–43. doi: 10.1016/j.psc.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 151.De Zwaan M. Binge eating disorder and obesity. Int. J. Obes. 2001;25:S51–S55. doi: 10.1038/sj.ijo.0801699. [DOI] [PubMed] [Google Scholar]
- 152.Chen B., Hu N. Rimonabant improves metabolic parameters partially attributed to restoration of high voltage-activated Ca2+ channels in skeletal muscle in HFD-fed mice. Braz. J. Med. Biol. Res. 2017;50:e6141. doi: 10.1590/1414-431x20176141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Satta V., Scherma M., Piscitelli F., Usai P., Castelli M.P., Bisogno T., Fratta W., Fadda P. Limited Access to a High Fat Diet Alters Endocannabinoid Tone in Female Rats. Front. Neurosci. 2018;12:40. doi: 10.3389/fnins.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parylak S.L., Koob G.F., Zorrilla E.P. The dark side of food addiction. Physiol. Behav. 2011;104:149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marcos M., Pastor I., De La Calle C., Laso F.-J., Barrio-Real L., González-Sarmiento R. Cannabinoid Receptor 1 Gene is Associated with Alcohol Dependence. Alcohol. Clin. Exp. Res. 2011;36:267–271. doi: 10.1111/j.1530-0277.2011.01623.x. [DOI] [PubMed] [Google Scholar]
- 156.Proudnikov D., Kroslak T., Sipe J.C., Randesi M., Li D., Hamon S., Ho A., Ott J., Kreek M.J. Association of polymorphisms of the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes with heroin addiction: Impact of long repeats of CNR1. Pharm. J. 2010;10:232–242. doi: 10.1038/tpj.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schmidt L.G., Samochowiec J., Finckh U., Fiszer-Piosik E., Horodnicki J., Wendel B., Rommelspacher H., Hoehe M.R. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/S0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- 158.Zhou Y., Huang T., Lee F., Kreek M.J. Involvement of Endocannabinoids in Alcohol “Binge” Drinking: Studies of Mice with Human Fatty Acid Amide Hydrolase Genetic Variation and After CB1 Receptor Antagonists. Alcohol. Clin. Exp. Res. 2016;40:467–473. doi: 10.1111/acer.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pucci M., Di Bonaventura M.V.M., Zaplatic E., Bellia F., Maccarrone M., Cifani C., D’Addario C. Transcriptional regulation of the endocannabinoid system in a rat model of binge-eating behavior reveals a selective modulation of the hypothalamic fatty acid amide hydrolase gene. Int. J. Eat. Disord. 2018;52:51–60. doi: 10.1002/eat.22989. [DOI] [PubMed] [Google Scholar]
- 160.Bulik C.M., Yilmaz Z., Hardaway J.A. Genetics and epigenetics of eating disorders. Adv. Genom. Genet. 2015;5:131–150. doi: 10.2147/AGG.S55776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mancino S., Burokas A., Gutiérrez-Cuesta J., Gutiérrez-Martos M., Martín-García E., Pucci M., Falconi A., D’Addario C., Maccarrone M., Maldonado R. Epigenetic and Proteomic Expression Changes Promoted by Eating Addictive-Like Behavior. Neuropsychopharmacology. 2015;40:2788–2800. doi: 10.1038/npp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Domingo-Rodriguez L., De Azua I.R., Dominguez E., Senabre E., Serra I., Kummer S., Navandar M., Baddenhausen S., Hofmann C., Andero R., et al. A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-14458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study reference [144] are available on request from the corresponding author. The data are not publicly available since they are part of an unpublished work.