Abstract
Simple Summary
Cancer cells adapt to hypoxia, survive, and grow. To that aim, they engage hypoxia-inducible pathways. These pathways are under intense investigation in search of new therapies to interfere with signaling components to kill cancer cells. Nowadays, new technologies enable more in-depth studies of hypoxia-induced signaling including protein–protein interaction and transcriptional processes, as well as the mode of action of different inhibitors. In this review, we give insight into useful techniques for studying the components of the hypoxia-inducible pathway and current inhibitors.
Abstract
Hypoxia is a key characteristic of tumor tissue. Cancer cells adapt to low oxygen by activating hypoxia-inducible factors (HIFs), ensuring their survival and continued growth despite this hostile environment. Therefore, the inhibition of HIFs and their target genes is a promising and emerging field of cancer research. Several drug candidates target protein–protein interactions or transcription mechanisms of the HIF pathway in order to interfere with activation of this pathway, which is deregulated in a wide range of solid and liquid cancers. Although some inhibitors are already in clinical trials, open questions remain with respect to their modes of action. New imaging technologies using luminescent and fluorescent methods or nanobodies to complement widely used approaches such as chromatin immunoprecipitation may help to answer some of these questions. In this review, we aim to summarize current inhibitor classes targeting the HIF pathway and to provide an overview of in vitro and in vivo techniques that could improve the understanding of inhibitor mechanisms. Unravelling the distinct principles regarding how inhibitors work is an indispensable step for efficient clinical applications and safety of anticancer compounds.
Keywords: cancer, hypoxia, hypoxia-inducible factor, HIF, inhibitor, PHD, pVHL, FRET, visualization
1. Introduction
Hypoxia-inducible factors (HIFs) regulate a majority of oxygen-dependent genes and are considered to be the master regulators for cellular adaptation to low oxygen concentrations [1,2]. The dimeric transcription factor HIF is composed of one of three oxygen-regulated α-subunits (HIF-1α, HIF-2α, or HIF-3α) and a constitutively expressed β-subunit, named HIF-1β or ARNT (aryl hydrocarbon nuclear translocator) [3]. HIF-1α is found in all nucleated mammalian cells, while HIF-2α is characterized by a tissue-specific expression. HIF-1α/HIF-2α are highly homologous, with approximately 70% identity in their amino acid sequence and they function in a similar manner [4]. Much less is known about HIF-3α, which can be found in different splice variants and has been suggested to negatively regulate the transcriptional activity of HIF-1α and HIF-2α gene expression [5,6]. HIF-3α has recently been reported to be a target gene of HIF-1α and different HIF-3α isoforms, such as HIF-3α1 or HIF-3α9, were shown to influence the transcription of distinct hypoxia-related genes [7,8]. Herein, we focus on HIF-1α and HIF-2α as oxygen-dependent components of the HIF transcriptional complex.
2. Regulation of the Hypoxia-Inducible Factor (HIF) Pathway
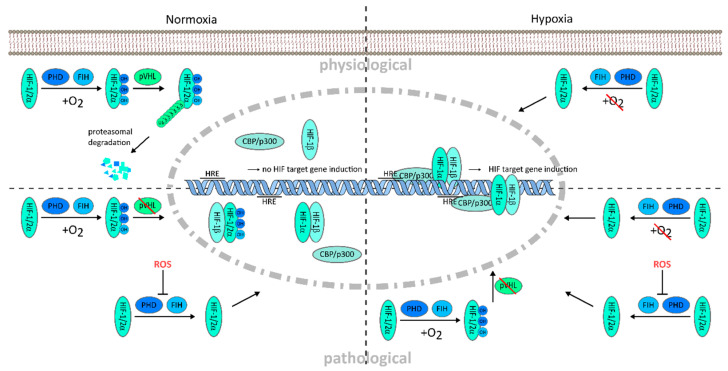
Both HIF-1/2α subunits are regulated on the post-transcriptional level. Under normoxic conditions, two proline residues of the α-subunit are hydroxylated by prolyl-4-hydroxylase domain (PHD) proteins, and thereby marked for proteasomal degradation [9,10]. Since the activity of the three homologous hydroxylases PHD1, PHD2, and PHD3 is strictly dependent on oxygen concentration, they are considered to be the oxygen sensors of the cells [11]. An abundance of HIF-α and PHD isoforms is highly tissue-specific and driven by physiologically different pO2 values in distinct tissues [12]. Upon sufficient oxygen supply, oxygen coordinately binds to one Fe(II) atom in the active center of the PHD enzymes and initiates hydroxylation of HIF protein proline residues. This enables recognition by the von Hippel–Lindau tumor suppressor protein (pVHL) as part of an E3 ubiquitin ligase complex. Lysine residues surrounding these hydroxylated proline residues undergo ubiquitination, which finally labels HIF-α for proteasomal degradation [10,13,14]. Transcriptional activity of the HIF-α subunits depends on oxygen-dependent hydroxylation of asparagine residues in HIF-1/2α by the asparagyl hydroxylase factor-inhibiting HIF (FIH), preventing the binding of transcriptional coactivators (CBP/p300) and subsequent expression of target genes under oxygenated conditions (Figure 1) [9].
Figure 1.
Oxygen dependent hypoxia-inducible factor (HIF) regulation and deregulation of HIF in tumor environment. Normoxic oxygen conditions lead to proteasomal degradation of the hypoxia-inducible factor (HIF) protein and prevent target gene transcription (upper left); Decreased oxygen availability deactivates the hydroxylation of the HIF protein by cofactors, factor-inhibiting HIF (FIH) and prolyl hydroxylases (PHD), which enables HIF protein accumulation and translocation into the nucleus. HIF-α and -β subunits dimerize and form a transcription complex with the cofactors CBP/p300. Complex binding to the hypoxia responsive elements (HRE) on the promoter region of HIF target genes leads to their transcription (upper right); Under pathological conditions, hypoxia can be accompanied by increased reactive oxygen species (ROS) and impaired von Hippel–Lindau protein (pVHL) function, promoting HIF induction to an excessive extent, e.g., driving tumorigenesis under normoxic, as well as hypoxic condition (lower left and right). OH, hydroxylation; Ub, ubiquitination.
Under hypoxic conditions, the lack of oxygen reduces the enzymatic activity of PHDs and FIH and initiation of HIF-α degradation is blocked. The HIF-α subunits accumulate in the cytosol and translocate via a core localization sequence into the nucleus. Nuclear heterodimerization of the HIF-α subunit with the constitutively expressed HIF-1β subunit is mediated by the basic helix–loop–helix (bHLH) and Per-ARNT-Sim (PAS) domains of the proteins [15]. Subsequently, the N- and C-terminal transcription activation domains (N-TAD and C-TAD, respectively) enable transcription complex formation by recruitment of the cofactors CBP/p300 and Src-1 and binding to hypoxia responsive DNA elements (HRE) located in the promoter region of HIF target genes [16]. Thus, transcription by HIF is regulated by the abundance and binding affinity of the α subunits to regulate a variety of genes in physiology and pathophysiology [3,17]. The tumor microenvironment is known to essentially contribute to tumor progression, however, the distinct role of HIF is still under investigation [18]. Deregulation of the HIF pathway can occur on multiple levels in pathological settings. Mutation of the VHL gene, as found in clear cell renal carcinoma, leads to dysfunction of the VHL protein complex. This results in extensive accumulation of the HIF-α protein, even under normoxic conditions, which drives activation of tumorigenic target genes [19]. The inflammatory microenvironment in cancer is further characterized by a high content of reactive oxygen species (ROS). ROS increase the HIF-α protein stability by blocking PHD activity, and thereby protein degradation, independent of the oxygen concentration in the tissue [20,21,22] (Figure 1). HIFs are crucial to overcome potentially fatal hypoxic conditions in tissues, for example, during growth and inflammation, but they also allow malignant cells of multiple cancers to survive the hypoxic tumor environment and to grow outpacing the growth of normal cells. Then, tumor progression can be driven by hypoxia itself (Figure 1).
Target Genes of HIF Transcription Factor
To counteract deadly hypoxia and to ensure cellular survival, cells need to restore their oxygenation by improving oxygen transport and supply on different levels. The most prominent example in this respect, which also leads to the identification of the HIF pathway and its role as the master regulator of hypoxia-induced gene expression, is the hypoxic production of erythropoietin (EPO) [1,13,23]. EPO elevates systemic blood oxygen capacity by increasing erythrocyte numbers, and therefore oxygen transport. Additionally, transcriptional upregulation of the vascular endothelial growth factor (VEGF) stimulates angiogenesis and ensures improved oxygen supply to affected regions by new capillaries. On the cellular level, adaption to ATP shortage is counter regulated via enhanced glucose uptake and glycolysis mediated by increased expression of glucose transporter 1 (GLUT1) [2,3,17]. Glycolytic enzymes such as phosphoglycerate kinase 1, lactate dehydrogenase-A, carbonic anhydrase 9, and aldolase are transcriptionally regulated by HIFs. Here, it is of particular interest that glycolytic enzymes are under the control of HIF-1α, while HIF-2α targets gene transcription of EPO, transforming growth factor alpha, and cyclin D. Nevertheless, there is redundancy in target gene regulation by HIF-1α and HIF-2α under specific conditions. Therefore Holmquist-Mengelbier et al. hypothesized that HIF-1α-dependent target gene transcription could be predominant in the acute hypoxic phase, while HIF-2α driven gene transcription could take over upon chronic hypoxia [24]. Important redundant target genes include VEGF, GLUT1, and adrenomedullin 1 (ADM-1) [25,26,27]. Several reviews have highlighted the differences of HIF-1α and HIF-2α regulation and target gene transcription [17,28,29].
Similar to other master regulators when deregulated, both HIF-1α and HIF-2α can drive pathologies, in particular tumorigenesis and cancer progression. Both are frequently found to be co-expressed in human cancers [25,30,31]. Especially in solid tumors, hypoxic regions develop when tumor cell growth outpaces vascularization; here, the ability to adapt to low oxygen conditions is crucial for survival and proliferation of cancer cells. A broad range of adaptive mechanisms induced by HIF-1/2α is linked to a more aggressive tumor type. The so-called angiogenic switch causes tumor angiogenesis, which often results in chaotic non-functional vessels and does not facilitate blood supply of the tumor; however, tumor cells may enter these capillaries and give rise to metastasis. The role of HIF-1/2α has been well studied for these processes and both isoforms have been identified as potential targets in antitumor therapy [32,33,34]. Recently, the role of HIF-3α has gained more attention from the field. HIF-3α was found to be overexpressed in pancreatic cancer, especially under hypoxic conditions promoting metastasis by activation of the ras homolog family member C/Rho associated coiled-coil containing protein kinase (RhoC-ROCK) pathway [35]. Tolonen et al. showed that HIF-3α directly regulates a subset of hypoxia-inducible genes involved in lipolysis (angiopoietin-like 4) and metabolism (angiopoietin-like 3 and pantothenate kinase 1) and, most interestingly, bound to the promotor region of the EPO gene. The authors suggested that this could indicate synergistic effects of HIF-1/2α and HIF-3α in terms of EPO transcription [36]. Overall, HIF-3 could have a dual role, making it interesting for further research in light of physiological regulation and its deregulation in tumorigenesis.
3. Targeting the HIF Pathway
Deregulation of the HIF pathway in cancers has been intensively addressed [3,28,31]. Hypoxia-related gene regulation is involved in enforcing tumorigenic, invasive, and metastatic potential and in accelerating resistance to chemo- and radio-therapy. Thus, to address the HIF regulatory pathway in therapeutic approaches is very attractive, but a detailed understanding of its components is essential for developing new drugs. In addition to HIF itself, other central components mediating the hypoxic response mechanism present potential drug targets, including the PHDs and pVHL or FIH and CBP/p300.
In recent years, several groups have focused on finding the Achilles’ heel, i.e., weakness, in the HIF pathway to target HIF activity in disease. It is obvious that this is notoriously complicated, since HIF, as a transcription factor, acts in the nucleus making it not easily accessible. Moreover, HIF is important for many physiological processes, thus, one has to be well aware of potential unwanted side effects.
In principle, attempts to modulate the HIF pathway in cancer are driven by the concept of inhibiting HIF, because HIF activity aggravates the disease. In other diseases such as chronic kidney disease with an underlying lack of EPO production, desired drugs can increase HIF activity, preferably by specifically targeting HIF-2α. This approach has been successful for inhibiting PHDs with prolyl hydroxylase inhibitors (PHI) and several drugs are already on the market in China or in advanced clinical trials [37,38,39].
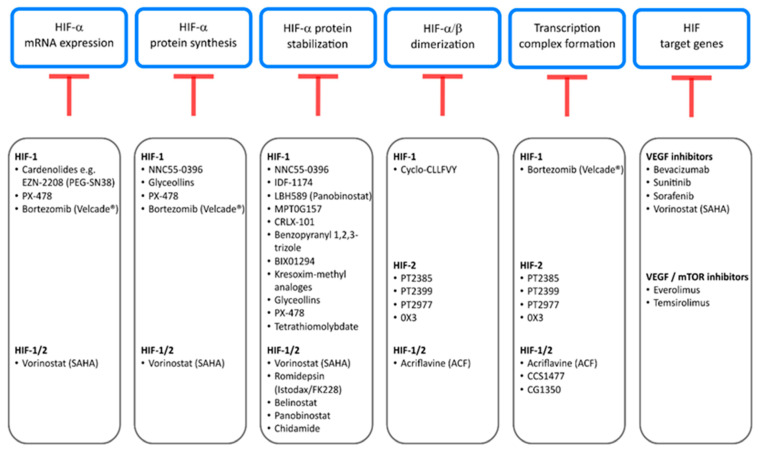
3.1. Inhibitors of HIF-α Transcription, Translation, and Protein Stabilization
HIF inhibitors have quite a broad field of action. They target HIF on multiple levels, ranking from transcription and translation to the transcription of HIF target genes (Figure 2). The so-called cardenolides are steroids that transcriptionally inhibit HIF-1. One example is the compound SN38, the improved version of the metabolite CPT-11 (EZN-2208/PEG-SN38), which has shown effectiveness in preclinical models of glioblastoma and lymphoma [40]. The Ca2+ channel blocker NNC 55-0396 decreases mitochondrial ROS production, thereby blocks HIF-1 activation, and additionally increases HIF-1α protein hydroxylation and degradation and suppresses HIF-1α de novo synthesis [41].
Figure 2.
Inhibitors of the HIF pathway and intervention nodes summarized in this review. HIF inhibitors are broadly classed into their mode of action, targeting different levels of the HIF pathway. Starting from blockage of HIF mRNA expression to, finally, HIF target gene transcription and inhibition of the downstream genes, such as VEGF itself. For detailed information see Section 2.
The HIF-1α inhibitor PX-478 also acts on multiple levels. It decreases HIF-1α mRNA levels, blocks HIF-1α translation, and inhibits de-ubiquitination, which results in enhanced protein degradation [42]. A phase I dose escalation study in patients with solid tumors (NCT00522652) showed good tolerability [43]. Recent studies have reported efficiency against pancreatic ductal adenocarcinoma in combination with chemotherapy (or combination therapy with gemcitabine) and inhibition of esophageal squamous cell tumor growth and immune modulation in mice [44,45].
Similarly, the well-known proteasome inhibitor bortezomib (PS-341/Velcade®, Millenium Pharmaceuticals, Inc., Cambridge, MA, USA) has been shown to repress HIF-1α on transcriptional and translational levels, as well as to inhibit the recruitment of the coactivator p300, blocking the PI3K/Akt/TOR and MAPK pathway (Figure 2) [46,47]. In 2003, bortezomib was approved by FDA for the treatment of multiple myeloma [48]. In addition, bortezomib as a proteasome inhibitor has increased HIF-1α protein level in monocytic leukemia cells [49].
Most current inhibitors focus on the interference with synthesis and stabilization of HIF-α protein, activation of PHDs, or binding of HIF-αs to pVHL. Probably due to its ubiquitous expression, inhibitors for HIF-1α protein have been more intensively investigated than those for HIF-2α. One approach, among several substances under examination, is to directly accelerate HIF-1α protein degradation (Figure 2). This can be achieved via upregulation of pVHL expression by the arytoxy acetylamino benzoic acid analogue IDF-1174 which leads to HIF-1 protein degradation [50]. Likewise, panobinostat (LBH589), developed for solid and hematologic cancers, acts as a histone deacetylase (HDAC) inhibitor and disrupts the Hsp90/HDAC6 complex [51]. Since Hsp90 complexing with HIF-1α, and also acetylation of HIF-1α, have been claimed to prevent degradation through the proteasome/pVHL pathway complex, panobinostat reduces HIF-1α protein. In a similar mode of action the indole-3-ethylsulfamoylphenylacrylamide compound MPT0G157 acts via inhibition of multiple histone deacetylases (1, 2, 3, and 6) and decreased HIF-1α protein in colorectal cancer [52]. Alternatively, HIF-1α protein is reduced by the use of diazepinquinazolin-amine derivate BIX01294 which increases PHD2 and pVHL expression [53] or the drug benzopyranyl 1,2,3-triazole inducing HIF-1α hydroxylation and ubiquitination, leading to increased protein degradation [54]. Finally, kresoxim-methyl analogues have been shown to promote proteasomal degradation of HIF-1α via increased oxygen tension in cancer cells [55].
A different approach is to address the accumulation of HIF-1α protein using nanoparticles such as CRLX-101 that suppress HIF-1α protein translation and stability [56] or the substance class of so-called glyceollins from the soybean which block HIF-1α translation via inhibition of the Pi3K/AKT/mTOR pathway and decrease HIF-1α stability by decreasing Hsp90 binding (Figure 2) [57].
Tumorigenesis correlates to irregular histone modifications and genetic instability [18,58,59,60]. HDAC enzymes physiologically tighten the chromatin structure, thereby, repressing gene expression. In addition, HDACs are also known to target non-histone proteins such as heat shock protein 90 (Hsp90). Any defect in HDAC function could, therefore, affect the chromatin structure and genetic stability. To address effects of deregulated HDAC in cancer, HDAC inhibitors are currently under investigation or are already in clinical use as anticancer agents. Vorinostat [46], a HDAC pan inhibitor, was approved for the treatment of cutaneous T cell lymphoma by the FDA, in 2006 [61]. Vorinostat in vitro affected multiple HDACs in glioblastoma, osteosarcoma, and hepatocellular carcinoma cell lines and inhibited hypoxia signaling by lowering HIF-1α and VEGF levels. The distinct mode of action is still not fully understood. The hypotheses rank from direct HIF-1/2 acetylation and degradation by pVHL to interactions with the HsP70/90 chaperone axis and decreased HIF-1/2 nuclear translocation [62]. Other promising and already approved HDAC inhibitors are romidepsin (FK228) [63], belinostat (PXD-101) [64], panobinostat (LBH-589) [65], and chidamide [66], for which effects on HIF have also been described by E. Pojani and D. Barlocco (Figure 2) [67].
HDAC inhibitors seem to be further qualified as a treatment option for neurological diseases characterized by oxidative stress-related cell loss [68], including multiple sclerosis [69], Parkinson’s disease [70], and stroke [71]. HDAC inhibitors act by acetylation of histones and, moreover, via modulation of numerous proteins, such as transcription factors. More specifically, pan-HDAC inhibitors have been shown to protect from oxidative stress-induced cell death (ferroptosis) by increasing DNA binding of the HDAC1-associated transcription factor Sp1 [72]. In line with that, inhibitors against the HIF prolyl hydroxylases could be valuable for fighting neurological diseases, due to the function of PHDs as epigenetic iron sensors, possibly driving oxidative stress-induced cell death, and thereby neurological disease progression [73]. This marks these inhibitor classes as important possible drugs utilizable for therapy of diseases beyond cancer.
Nevertheless, in cancer therapy, one focus could be to target the HIF system using compounds that promote PHD activity. Such drugs might function by strengthening the protein interaction between PHDs and HIF-α or enhancing the enzymatic activity of PHDs by increasing oxygen availability at the enzymatic site. This could also be achieved by pharmacological inhibition of the mitochondrial respiration chain, reducing its oxygen consumption and redirecting oxygen from mitochondria to PHDs [74]. Much less work, so far, has been concentrated on the activation or increased activity of the 2-oxoglutarate dependent oxygenase FIH. One may argue that, as compared with the PHDs, a fundamental role of FIH for the regulation of the hypoxic response has not been found for many HIF-dependent genes and, similarly, with FIH inhibitors regarding their efficacy for boosting the HIF response [75]. In principle, redirecting oxygen from mitochondria to FIH (as described for PHDs above) should increase FIH even more than PHD activity, due to the higher oxygen affinity of FIH as compared with PHDs [76].
3.2. Inhibitors of HIF-α/β Dimerization and Transcription Complex Formation
Essential for the activation of the HIF complex as a transcription factor to induce the expression of HIF target genes is the preceding dimerization of the HIF-α and HIF-β subunit. Obviously, interfering with the dimerization process is an attractive target for inhibitors of HIF because, in contrast to the above-mentioned drugs, such an approach could be highly selective and HIF specific. Thus, dimerization marks a promising target for pharmaceutical interference (Figure 2).
In 2009, Scheuermann et al. identified a cavity within the HIF-2α PAS-B domain, bearing room for small molecules and also, potentially, inhibitors that could interfere with the heterodimerization of the HIF-2 protein complex [77]. Closer investigation of this newly found binding pocket resulted in the development of HIF-2α inhibitors PT2385, PT2399, and later PT2977 [78,79,80]. These inhibitors from the same substance class have been or are currently under investigation in phase II clinical trials targeting clear cell renal cell carcinoma (PT2385: NCT03108066), recurrent glioblastoma (PT2985: NCT03216499), and VHL-associated RCC (PT2977: NCT03401788). A second-generation inhibitor PT2977 was generated by the exchange of a geminal difluoro group with a cis-vicinal difluoro group and it has raised great expectations with respect to efficacy. Another potent specific HIF-2α inhibitor is the compound 0X3, which also binds to the PAS-B domain and possibly alters the dynamics of the domain. Co- and chromatin-immunoprecipitation (ChIP) experiments have demonstrated disruption of HIF heterodimer formation and prevention of HRE binding [81]. Although molecular dynamics simulations revealed an interruption of crucial hydrophobic interactions within the HIF-2 dimer for the inhibitors PT2399 and 0X3, this has to be further verified in vivo [82]. State-of-the-art live cell microscopy could further elucidate mechanisms of action. Unexpectedly, however, first resistance mechanisms have been observed, highlighting the need for a better understanding of the mode of action of these inhibitors [83,84].
In a similar manner, the compound acriflavine (ACF) directly binds to the PAS-B domain of HIF-1α and HIF-2α with nanomolar affinities and blocks heterodimerization with HIF-1β [85]. The FDA-approved drug was studied by using a cell-based luciferase assay, which consisted of a split version of the Renilla luciferase partly coupled to HIF-α and HIF-β fusion proteins. Measurable bioluminescence was only emitted upon dimerization. This method made it possible to scan 200 currently in use drugs for their potential to inhibit HIF dimerization in cells and resulted in the identification of acriflavine that was reported to decrease tumor growth and vascularization [85]. Similar to ACF, the peptide cyclo-CLLFVY was investigated by luciferase reporter plasmids and specifically inhibited HIF-1 dimerization (Figure 2) [86].
Different cancers are associated with mutations or overexpression of HIF coactivators. In these cases, obstruction of transcriptional coactivators CBP and p300 appears to be an attractive strategy to inhibit the HIF pathway. Two domains, the histone acetyltransferase [47] and the bromodomain (BRD), are found in both coactivator proteins (CBP/p300) and present promising targets for pharmacological intervention [87,88]. For further reading about BRD inhibitors classified by their chemotypes, we refer to a recent review [89]. Currently, there are two clinical trials running (phase I/IIa studies) that are investigating the bromodomain inhibitor CCS1477. One trial (NCT03568656) addresses the potential of CCS1477 against advanced solid tumors and metastatic prostate cancer. The other trial focusses on treatment of patients with advanced hematological malignancies [90]. Another BRD inhibitor (CG1350) suppressed multiple myeloma cell proliferation in human cell lines and mice [91]. These (BRD) inhibitors have in common that they target protein–protein or protein–chromatin interactions. Thus, when screening for new drug compounds, fluorescence resonance energy transfer (FRET)-based methods are perfectly suited because they can demonstrate protein–protein interaction (see Section 3). In particular, recently reported time-resolved FRET-based high-throughput assays can be important tools to discover new potent drugs [92]. The BRD inhibitor (CG1350) is an example of a successful application of such methods by taking advantage of chromatin immunoprecipitation and a coupled luminescent-fluorescent method proving the advantages of techniques to study inhibitor binding and protein interaction in vitro. Nevertheless, more profound methods addressing binding dynamics and structural changes in vivo will help to further characterize these compounds.
3.3. Inhibitors of HIF Targets
A recent review from the Nobel prize laureate William G. Kaelin Jr. and his colleague Toni K. Choueiri provided a comprehensive overview of the biological background and clinical development of HIF-2α inhibitors to treat renal cell carcinoma [19]. The majority of clear cell renal carcinoma (ccRCC) tumors are characterized by mutations of the VHL gene, encoding for VHL protein. As described above, pVHL is part of an E3 ubiquitin ligase which recognizes hydroxylated HIF-α proteins, poly-ubiquitinates them, and marks them it for proteasomal degradation under normoxic conditions [10]. With dysfunctional VHL in ccRCC, HIF-α subunits accumulate independent of hypoxia and drive enhanced tumorigenic HIF target genes such as VEGF-A, which is, among epithelial cancers, the highest expressed in ccRCC [93]. Inhibitors against VEGF, including bevacizumab, sunitinib, and sorafenib, rarely trigger complete responses [94,95,96] and most combination therapies fail to improve patient outcome. Nowadays, immune-checkpoint blockers (ICBs) are the main strategy to fight kidney cancer, in addition to the in-use inhibitors against VEGF and the mTOR pathway blockers such as rapamycin analogues, everolimus, and temsirolimus [97]. To improve treatment for metastatic ccRCC, substances are combined in the treatment regime, however, it would be much better to develop new drugs.
4. Toolbox to Visualize HIF and Its Inhibition
It is fundamental to understand the pharmacological mechanism underlying small molecule inhibitors and their binding to targets for identification of their therapeutic potential, optimal application, and consideration of side effects. Large-scale biochemical analysis of inhibitors often fails to mimic the target engagement in living cells, leaving a gap for more conclusive methods. Qualitative methods to study compound binding are mostly based on ligand-induced protein stabilization with limited stability or need for non-physiological temperatures [98]. Hence, the use of in vivo methods, such as fluorescence resonance energy transfer (FRET) or bioluminescence resonance energy transfer (BRET), can be an asset to determine target engagement in real time, in living cells and tissues.
The use of many HIF inhibitors is dampened by their off-target effects, affecting different pathways in DNA replication, cell division, or cell signaling. Hence, it is important to unveil the distinct mechanism of action to provide efficient and safe compounds for cancer treatment.
A combination of different microscopy methods, such as FRET [99] or fluorescence lifetime imaging microscopy (FLIM) was a significant step forward in understanding protein–protein interactions and HIF complex formation in living cells. In addition to the knowledge gained from conventional protein-biochemical approaches, live cell imaging interaction studies have provided insight into the spatiotemporal regulation of HIF interaction. During the last years, a combination of multiple methods has paved the way for establishing anticancer drugs that target HIF protein dimerization, and thus prevent the activation of deregulated HIF target genes.
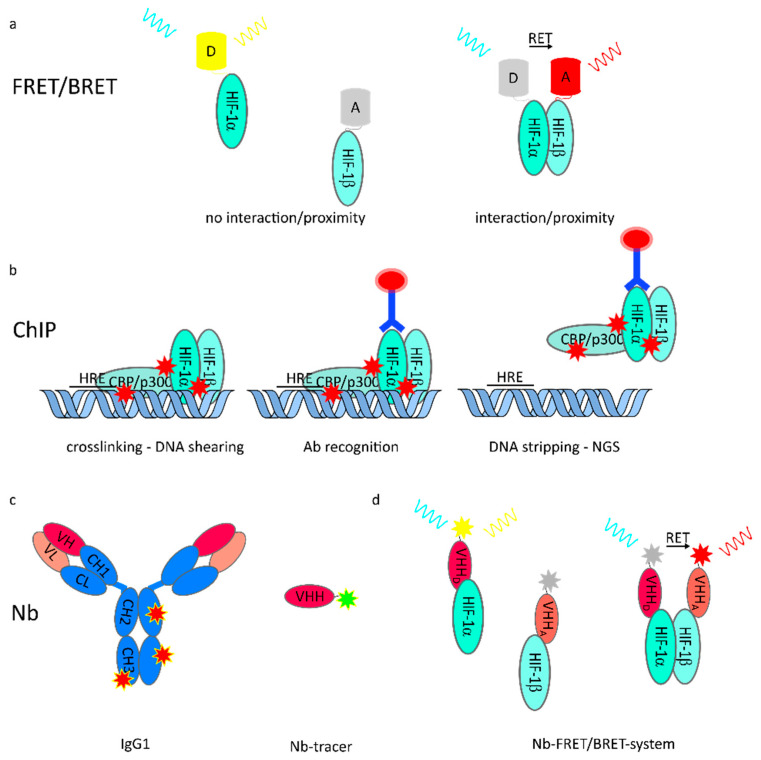
FRET is a non-radiative energy transfer between a donor and an acceptor fluorophore. When in close proximity (< 10 nm), emission energy of the donor may non-radiatively transfer in a dipole–dipole reaction, and thereby excite the acceptor molecule [100]. The emission spectrum of the donor needs to overlap with the excitation spectrum of the acceptor to allow the dipole–dipole interaction of both molecules (Figure 3). To study protein–protein interactions and protein complex formation, labeling of the protein regions of interest with corresponding monomeric fluorophores provides an elegant tool for investigations [101,102,103], since the FRET signal between fluorophores fused to interaction partners correlates strongly with physical interaction. In combination with other fluorescence approaches, this method enables greater insight in the field of hypoxia research and the distinct mechanism of dimerization of the HIF subunits (HIF-1/2/3 α and HIF-1β).
Figure 3.
Methods to unravel effects on HIF protein stability, interaction, and function in vitro and in vivo. (a) Interaction of protein (complexes) is detectable by proximity-depended resonance energy transfer such as fluorescence resonance energy transfer (FRET) or bioluminescence resonance energy transfer (BRET). Protein localization and proximity are directly imaged in vitro or in vivo and recorded with high spatiotemporal resolution; (b) Transcriptional activity of transcription factor is assessed by chromatin immunoprecipitation (ChIP). Precise antibody recognition and accuracy is mandatory for qualitative evaluation of changes upon any perturbation; (c) Nanobodies (Nb), shown in relation to conventionally used IgG1, can be functionalized and used as intravital tracers. Due to their small size, penetration depth of cells and tissue is superior. Stoichiometric labeling of Nb enables quantitative imaging; (d) Nb tracer addressing interaction partners allow in vivo FRET/BRET independent of genetic manipulation, giving rise to a multitude of new life cell/intravital interaction studies.
Immunofluorescence combined with two-photon microscopy unveiled the subnuclear distribution of HIF-1α and interacting factors such as HIF-1β and it has shown that proteins colocalize in the nucleus in speckle-like structures under hypoxic conditions [104]. Additional work using fluorescence recovery after photobleaching (FRAP) have reported faster mobility of HIF-1β as compared with HIF-1α, in cells cotransfected with both fluorescently labeled proteins [105]. Using FRET in combination with enhanced yellow fluorescent protein (EYFP)- or enhanced cyan fluorescent protein (ECFP)-labeled HIF proteins, further analysis of the HIF complex formation in the nucleus in vitro showed a heterogeneous subnuclear distribution of the HIF-1 α/β heterodimers. Moreover, the FRET approach proved the compact assembly of the HIF-1 complex (6.2 to 7.4 nm between the N-and C-termini of the HIF-1 subunits) [106]. Analogous to the interaction of the HIF-1 α/β, the HIF-2 α/HIF1-β heterodimers were proven in living cells with their typical nuclear localization [41,107]. Beyond that, quantification of interacting HIF fractions and potential reduction upon inhibitor application of such interaction has been analyzed using FLIM-FRET microscopy [108]. Detailed analysis of HIF protein regulation and complex formation has enabled the identification of binding sites within the HIF protein, which turned out to be druggable targets within the HIF pathway [77,81]. Testing such drug candidates in live cell FRET microscopy offers a direct observation of their effects, for example, disruption of protein-DNA binding, preventing initial dimerization, and enhancing protein degradation.
Further validation of in vitro results requires studying the HIF pathway in vivo, in appropriate animal models. Near-infrared (NIR) in vivo light imaging has been successfully established [109], although bioluminescent imaging methods with a better signal-to-noise ratio can be used independently from external excitation sources. Fluorescent and bioluminescent proteins can be genetically modified so that close proximity to specific protein areas or enzymes influence their activity or abundance. In these systems, the energy of a bioluminescent donor is transferred to a fluorescent acceptor molecule when in proximity. Similar to in FRET, the spectral overlap of the spectrum of donor emission and that of an acceptor excitation is a prerequisite. Multiple variations of this approach have been developed and have enlightened the understanding the role of hypoxia in disease models. In one example, fusion of gene sequences for the HIF-α oxygen dependent degradation domain (ODD) which causes oxygen lability of HIF-α subunits to luciferase sequences (ODD-Luc) was used to engineer a transgenic reporter mouse. These mice accumulated luciferase protein in all their hypoxic tissues where a lack of PHD dependent hydroxylation of the ODD prevented degradation of the ODD luciferase fusion protein. Imaging the development of tissue hypoxia and also the effect of prolyl hydroxylase inhibitors (PDI) was possible in the mice [110].
The use of genetic bioluminescence reporter assays and bioluminescence resonance energy transfer has further improved the understanding of protein interactions and DNA binding events in vivo. P. Iglesias and J. Costoya used an innovative hypoxia-biosensing system by combining a tracer module with near-infrared fluorescence and bioluminescence (mCherry-luciferase fusion protein), which was activated by HIF-1α. This tool was used to visualize HIF-1α protein accumulation in vivo, in mice with xenograft tumor implants. Obviously, such imaging systems improve our understanding of HIF-1 protein activation and, in particular, the development and role of biological relevant hypoxia in solid tumor development and tumor progression [111]. The detection of HIF-1 activation in vivo is superior to any other hypoxia staining methods or even physical measurements of oxygen tension, since HIF-1 activation reflects the tissue response to low oxygen tension. Other studies have proven the advantages of this system by using injectable imaging probes with a single donor and acceptor (NIR-BRET) for non-invasive detection of the activity of factors regulated by the ubiquitin-proteasome system, such as the HIF-α subunits, in different in vivo cancer models [112]. Among studies in solid tumors, innovations such as self-luminescing BRET-FRET NIR-emitting nanoparticles have enabled in vivo mapping of lymphatic networks and small metastases [113]. Especially for studying difficult-to-access pathologies such as age-related macular degeneration [114] and diabetic retinopathy, biosensors can bring a substantial advantage for treatment decisions. Often, these pathologies are characterized by an inflammatory hypoxic environment that results in the upregulation of the HIF target VEGF and pathological neovascularization in the eye [115]. BRET VEGF biosensors, using Renilla luciferase and extracellular IgG-like domains binding to VEGF, have been shown to quantify VEGF expression in vitro, leading the path to in vivo VEGF measurements in a patient’s eye [116].
In addition to the investigation of hypoxia and related mechanisms, these techniques have also been used to profile binding kinetics and target engagement for inhibitors in living cells. In 2015, M. Robers and colleagues demonstrated a method to quantitatively assess target engagement of the HDAC inhibitor romidepsin (Istodax/FK228) by using BRET in genetically modified cells expressing an intracellular target protein fused to luciferase and a fluorescent compound tracer [117].
Chromatin immunoprecipitation (ChIP) has been widely used to study protein-DNA interactions, and thereby transcriptional initiation of directly activated transcription sites of target genes. Specific proteins, including histones, transcription factors or cofactors, or modifications of these proteins chemically crosslinked to their cognate DNA sequence can be used as targets for precipitation by antibodies, resulting in immune-enriched DNA fragments. These can be subsequently quantified by qPCR or next generation sequencing (NGS) to investigate protein binding to a genomic region/target gene (Figure 3) [118]. Further distinctions are made between ChIP with crosslinking of proteins to proteins and proteins to DNA (X-ChIP) and without crosslinking, named native (N)-ChIP. Whereas N-ChIP is characterized by a predictable antibody specificity and efficient precipitation, X-ChIP has the advantage of being applicable to non-histone proteins with weak binding affinities to DNA with minimal histone rearrangements [119,120]. ChIP can provide valuable information about the binding of nuclear proteins to specific DNA sites, as well as posttranslational modifications of nuclear proteins [121]. However, sometimes the lack of specific and suitable antibodies and quantification of big protein complexes limit this method.
A relatively new approach in cancer theragnostics is the application of so-called nanobodies (Nb) or nanobody-based delivery systems. Nanobodies are, broadly speaking, the variable domain of engineered heavy chain (only) antibodies (HCAbs) in camelidae. Recombinant production of the heavy variable domain (VHH) of the antibody results in the generation of a single domain antibody (sdAB), also known as nanobody (Nb) with full antigen-binding potential [122]. Nanobodies are characterized by high immune compatibility, good solubility, robustness against unsteady pH and temperature values, and thanks to their small size, high infiltration rates into tissues and small cell interfaces or into cells [123,124]. Nanobodies can be further engineered by fusion with another monovalent nanobody, recognizing different epitopes on the same molecule or even binding to different target molecules (Figure 3). In addition, coupling to other molecules (such as albumin for a half-life increase) or conjugation with various drug candidates has displayed wide ranging applicability. For further reading we refer to recent reviews [125,126].
Furthermore, nanobodies serve as an elegant tool for in vitro and in vivo visualization in disease models, as well as in cancer diagnosis and monitoring [127]. To that aim, nanobodies specific for epitopes such as CD20 [128], epidermal growth factor 3 [129], or dipeptidyl-peptidase 6 [130] are fused with the enzyme sortase [131], to create an antigen specific non-invasive imaging probe (Figure 3). During the last years, the spectrum of methods have become divers, including GFP-binding nanobodies coupled to a DNA oligonucleotide-wrapped single-walled carbon nanotube (SWCNT) emitting NIR fluorescence to monitor neurotransmitter and motor proteins in living Drosophila melanogaster embryos [132]. Final imaging is mostly performed with positive electron tomography computed tomography (PET-CT) or single photon emission computed tomography (SPEP-CT). Imaging of tumor and inflammatory tissues with nanobodies takes advantage of the fast penetration of the probes into the target tissue, low background noise, and the possibility to trace distribution in tumors and of invaded immune cells in vivo, which makes it a promising method for future studies.
5. Conclusions
Versatile imaging methods have been combined with classic protein biochemical analysis to enable accurate investigation of HIF protein accumulation, HIF protein–protein interactions, and HIF transcription factor complex assembly. Moreover, the molecular mechanisms underlying the inhibition of these processes can be assessed and optimized. Many compounds target the HIF pathway on multiple levels, making it difficult to differentiate side effects of these compounds from their HIF specific effects. For the safety and optimal usage of inhibitory compounds, it is indispensable to unveil their distinct modes of action by in vitro and in vivo methods. While all inhibitors should finally target HIF-dependent gene expression, they are grouped into classes addressing different levels in the HIF pathway, from gene expression to protein synthesis and protein stabilization to dimerization of the subunits. The imaging methods with luminescence and fluorescence approaches, such as FRET and BRET, in living cells may improve spatiotemporal profiling of the mode of action, unravelling unexpected effects and helping to improve the specificity and efficacy of HIF inhibitors.
Author Contributions
Writing—original draft preparation and visualization, T.S. and K.P.-F.; writing—review and editing, K.P.-F. and J.F.; supervision, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
T.S. was funded by the GRK 2098 from Deutsche Forschungsgemeinschaft (DFG).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992;12:5447–5454. doi: 10.1128/MCB.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greijer A., Van Der Groep P., Kemming D., Shvarts A., Semenza G., Meijer G., Van De Wiel M., Belien J., Van Diest P., van Der Wall E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J. Pathol. A J. Pathol. Soc. G. B. Irel. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 3.Schito L., Semenza G.L. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Ema M., Taya S., Yokotani N., Sogawa K., Matsuda Y., Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makino Y., Cao R., Svensson K., Bertilsson G., Asman M., Tanaka H., Cao Y., Berkenstam A., Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita T., Ohneda O., Nagano M., Iemitsu M., Makino Y., Tanaka H., Miyauchi T., Goto K., Ohneda K., Fujii-Kuriyama Y., et al. Abnormal heart development and lung remodeling in mice lacking the hypoxia-inducible factor-related basic helix-loop-helix PAS protein NEPAS. Mol. Cell. Biol. 2008;28:1285–1297. doi: 10.1128/MCB.01332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T., Wiesener M., Bernhardt W., Eckardt K.-U., Warnecke C. The human HIF (hypoxia-inducible factor)-3 α gene is a HIF-1 target gene and may modulate hypoxic gene induction. Biochem. J. 2009;424:143–151. doi: 10.1042/BJ20090120. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P., Yao Q., Lu L., Li Y., Chen P.-J., Duan C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014;6:1110–1121. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Schofield C.J., Ratcliffe P.J. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 2005;338:617–626. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- 10.Fandrey J., Gorr T.A., Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc. Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 12.Watts E.R., Walmsley S.R. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol. Med. 2019;25:33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell P.H., Pugh C.W., Ratcliffe P.J. Inducible operation of the erythropoietin 3’ enhancer in multiple cell lines: Evidence for a widespread oxygen-sensing mechanism. Proc. Natl. Acad. Sci. USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paltoglou S., Roberts B. HIF-1 α and EPAS ubiquitination mediated by the VHL tumour suppressor involves flexibility in the ubiquitination mechanism, similar to other RING E3 ligases. Oncogene. 2007;26:604–609. doi: 10.1038/sj.onc.1209818. [DOI] [PubMed] [Google Scholar]
- 15.Wang G.L., Jiang B.-H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang B.-H., Zheng J.Z., Leung S.W., Roe R., Semenza G.L. Transactivation and inhibitory domains of hypoxia-inducible factor 1α modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 17.Wenger R.H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Choueiri T.K., Kaelin W.G. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 2020;26:1519–1530. doi: 10.1038/s41591-020-1093-z. [DOI] [PubMed] [Google Scholar]
- 20.Chandel N., Maltepe E., Goldwasser E., Mathieu C., Simon M., Schumacker P. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad J.J., Land S.C. A non-hypoxic, ROS-sensitive pathway mediates TNF-α-dependent regulation of HIF-1α. FEBS Lett. 2001;505:269–274. doi: 10.1016/S0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- 22.Acker T., Fandrey J., Acker H. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc. Res. 2006;71:195–207. doi: 10.1016/j.cardiores.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R977–R988. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- 24.Holmquist-Mengelbier L., Fredlund E., Löfstedt T., Noguera R., Navarro S., Nilsson H., Pietras A., Vallon-Christersson J., Borg Å., Gradin K., et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 26.Hu C.-J., Wang L.-Y., Chodosh L.A., Keith B., Simon M.C. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol. Cell. Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang V., Davis D.A., Haque M., Huang L.E., Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1α and hypoxia-inducible factor-2α in HEK293T cells. Cancer Res. 2005;65:3299–3306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 28.Keith B., Simon M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai Y.-P., Wu K.-J. Hypoxia-regulated target genes implicated in tumor metastasis. J. Biomed. Sci. 2012;19:102. doi: 10.1186/1423-0127-19-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talks K.L., Turley H., Gatter K.C., Maxwell P.H., Pugh C.W., Ratcliffe P.J., Harris A.L. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. Am. J. Pathol. 2000;157:411–421. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 32.Liao D., Johnson R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 33.Koh M.Y., Powis G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012;37:364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X., Guo X., Chen M., Xie C., Jiang J. HIF-3α Promotes Metastatic Phenotypes in Pancreatic Cancer by Transcriptional Regulation of the RhoC–ROCK1 Signaling Pathway. Mol. Cancer Res. 2018;16:124–134. doi: 10.1158/1541-7786.MCR-17-0256. [DOI] [PubMed] [Google Scholar]
- 36.Tolonen J.-P., Heikkilä M., Malinen M., Lee H.-M., Palvimo J.J., Wei G.-H., Myllyharju J. A long hypoxia-inducible factor 3 isoform 2 is a transcription activator that regulates erythropoietin. Cell. Mol. Life Sci. 2020;77:3627–3642. doi: 10.1007/s00018-019-03387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joharapurkar A.A., Pandya V.B., Patel V.J., Desai R.C., Jain M.R. Prolyl hydroxylase inhibitors: A breakthrough in the therapy of anemia associated with chronic diseases. J. Med. Chem. 2018;61:6964–6982. doi: 10.1021/acs.jmedchem.7b01686. [DOI] [PubMed] [Google Scholar]
- 38.Dhillon S. Roxadustat: First global approval. Drugs. 2019;79:563–572. doi: 10.1007/s40265-019-01077-1. [DOI] [PubMed] [Google Scholar]
- 39.Sakashita M., Tanaka T., Nangaku M. CKD-Associated Complications: Progress in the Last Half Century. Volume 198. Karger Publishers; Basel, Switzerland: 2019. Hypoxia-inducible factor-prolyl hydroxylase domain inhibitors to treat anemia in chronic kidney disease; pp. 112–123. [DOI] [PubMed] [Google Scholar]
- 40.Sapra P., Kraft P., Pastorino F., Ribatti D., Dumble M., Mehlig M., Wang M., Ponzoni M., Greenberger L.M., Horak I.D. Potent and sustained inhibition of HIF-1α and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-2208, results in anti-angiogenic effects. Angiogenesis. 2011;14:245. doi: 10.1007/s10456-011-9209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K.H., Kim D., Park J.Y., Jung H.J., Cho Y.-H., Kim H.K., Han J., Choi K.-Y., Kwon H.J. NNC 55-0396, a T-type Ca 2+ channel inhibitor, inhibits angiogenesis via suppression of hypoxia-inducible factor-1α signal transduction. J. Mol. Med. 2015;93:499–509. doi: 10.1007/s00109-014-1235-1. [DOI] [PubMed] [Google Scholar]
- 42.Koh M.Y., Spivak-Kroizman T., Venturini S., Welsh S., Williams R.R., Kirkpatrick D.L., Powis G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1α. Mol. Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 43.Tibes R., Falchook G.S., Von Hoff D.D., Weiss G.J., Iyengar T., Kurzrock R., Pestano L., Lowe A.M., Herbst R.S. Results from a phase I, dose-escalation study of PX-478, an orally available inhibitor of HIF-1α. J. Clin. Oncol. 2010;28:3076. doi: 10.1200/jco.2010.28.15_suppl.3076. [DOI] [Google Scholar]
- 44.Zhao T., Ren H., Jia L., Chen J., Xin W., Yan F., Li J., Wang X., Gao S., Qian D. Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:2250. doi: 10.18632/oncotarget.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y., Zang Y., Zhao F., Li Z., Zhang J., Fang L., Li M., Xing L., Xu Z., Yu J. Inhibition of HIF-1α by PX-478 suppresses tumor growth of esophageal squamous cell cancer in vitro and in vivo. Am. J. Cancer Res. 2017;7:1198. [PMC free article] [PubMed] [Google Scholar]
- 46.Hideshima T., Mitsiades C., Akiyama M., Hayashi T., Chauhan D., Richardson P., Schlossman R., Podar K., Munshi N.C., Mitsiades N. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood J. Am. Soc. Hematol. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 47.Befani C.D., Vlachostergios P.J., Hatzidaki E., Patrikidou A., Bonanou S., Simos G., Papandreou C.N., Liakos P. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J. Mol. Med. 2012;90:45–54. doi: 10.1007/s00109-011-0805-8. [DOI] [PubMed] [Google Scholar]
- 48.Kane R.C., Bross P.F., Farrell A.T., Pazdur R. Velcade®: US FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 49.Winning S., Splettstoesser F., Fandrey J., Frede S. Acute hypoxia induces HIF-independent monocyte adhesion to endothelial cells through increased intercellular adhesion molecule-1 expression: The role of hypoxic inhibition of prolyl hydroxylase activity for the induction of NF-κB. J. Immunol. 2010;185:1786–1793. doi: 10.4049/jimmunol.0903244. [DOI] [PubMed] [Google Scholar]
- 50.Lee K., Kang J.E., Park S.-K., Jin Y., Chung K.-S., Kim H.-M., Lee K., Kang M.R., Lee M.K., Song K.B. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1α via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010;80:982–989. doi: 10.1016/j.bcp.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs J.J., Murphy P.J., Gaillard S., Zhao X., Wu J.-T., Nicchitta C.V., Yoshida M., Toft D.O., Pratt W.B., Yao T.-P. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y.-C., Huang F.-I., Mehndiratta S., Lai S.-C., Liou J.-P., Yang C.-R. Anticancer activity of MPT0G157, a derivative of indolylbenzenesulfonamide, inhibits tumor growth and angiogenesis. Oncotarget. 2015;6:18590. doi: 10.18632/oncotarget.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh S.Y., Seok J.Y., Choi Y.S., Lee S.H., Bae J.-S., Lee Y.M. The histone methyltransferase inhibitor BIX01294 inhibits HIF-1α stability and angiogenesis. Mol. Cells. 2015;38:528. doi: 10.14348/molcells.2015.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park K., Lee H.E., Lee S.H., Lee D., Lee T., Lee Y.M. Molecular and functional evaluation of a novel HIF inhibitor, benzopyranyl 1, 2, 3-triazole compound. Oncotarget. 2017;8:7801. doi: 10.18632/oncotarget.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S., Kwon O.S., Lee C.-S., Won M., Ban H.S., Ra C.S. Synthesis and biological evaluation of kresoxim-methyl analogues as novel inhibitors of hypoxia-inducible factor (HIF)-1 accumulation in cancer cells. Bioorg. Med. Chem. Lett. 2017;27:3026–3029. doi: 10.1016/j.bmcl.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 56.Pham E., Birrer M.J., Eliasof S., Garmey E.G., Lazarus D., Lee C.R., Man S., Matulonis U.A., Peters C.G., Xu P. Translational impact of nanoparticle–drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin. Cancer Res. 2015;21:808–818. doi: 10.1158/1078-0432.CCR-14-2810. [DOI] [PubMed] [Google Scholar]
- 57.Lee S.H., Jee J.G., Bae J.S., Liu K.H., Lee Y.M. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J. Cell. Physiol. 2015;230:853–862. doi: 10.1002/jcp.24813. [DOI] [PubMed] [Google Scholar]
- 58.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 59.Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berdasco M., Esteller M. Aberrant epigenetic landscape in cancer: How cellular identity goes awry. Dev. Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Mann B.S., Johnson J.R., He K., Sridhara R., Abraham S., Booth B.P., Verbois L., Morse D.E., Jee J.M., Pope S. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin. Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 62.Zhang C., Yang C., Feldman M.J., Wang H., Pang Y., Maggio D.M., Zhu D., Nesvick C.L., Dmitriev P., Bullova P. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget. 2017;8:56110. doi: 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prince H.M., Dickinson M. Romidepsin for cutaneous T-cell lymphoma. Clin. Cancer Res. 2012;18:3509–3515. doi: 10.1158/1078-0432.CCR-11-3144. [DOI] [PubMed] [Google Scholar]
- 64.Poole R.M. Belinostat: First global approval. Drugs. 2014;74:1543–1554. doi: 10.1007/s40265-014-0275-8. [DOI] [PubMed] [Google Scholar]
- 65.Cheng T., Grasse L., Shah J., Chandra J. Panobinostat, a pan-histone deacetylase inhibitor: Rationale for and application to treatment of multiple myeloma. Drugs Today (Barc. Spain) 2015;51:491–504. doi: 10.1358/dot.2015.51.8.2362311. [DOI] [PubMed] [Google Scholar]
- 66.Ning Z.-Q., Li Z.-B., Newman M.J., Shan S., Wang X.-H., Pan D.-S., Zhang J., Dong M., Du X., Lu X.-P. Chidamide (CS055/HBI-8000): A new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother. Pharmacol. 2012;69:901–909. doi: 10.1007/s00280-011-1766-x. [DOI] [PubMed] [Google Scholar]
- 67.Pojani E., Barlocco D. Romidepsin (FK228), A Histone Deacetylase Inhibitor and its Analogues in Cancer Chemotherapy. Curr. Med. Chem. 2020 doi: 10.2174/0929867327666200203113926. [DOI] [PubMed] [Google Scholar]
- 68.Emerit J., Edeas M., Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Camelo S., Iglesias A.H., Hwang D., Due B., Ryu H., Smith K., Gray S.G., Imitola J., Duran G., Assaf B. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Aimé P., Karuppagounder S.S., Rao A., Chen Y., Burke R.E., Ratan R.R., Greene L.A. The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson’s disease. Neurobiol. Dis. 2020;136:104725. doi: 10.1016/j.nbd.2019.104725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alim I., Caulfield J.T., Chen Y., Swarup V., Geschwind D.H., Ivanova E., Seravalli J., Ai Y., Sansing L.H., Marie E.J.S. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–1279.e25. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Ryu H., Lee J., Olofsson B.A., Mwidau A., Deodoglu A., Escudero M., Flemington E., Azizkhan-Clifford J., Ferrante R.J., Ratan R.R. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc. Natl. Acad. Sci. USA. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rroji O., Kumar A., Karuppagounder S.S., Ratan R.R. Epigenetic regulators of neuronal ferroptosis identify novel therapeutics for neurological diseases: HDACs, transglutaminases, and HIF prolyl hydroxylases. Neurobiol. Dis. 2020;147:105145. doi: 10.1016/j.nbd.2020.105145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim K.K., Abelman S., Yano N., Ribeiro J.R., Singh R.K., Tipping M., Moore R.G. Tetrathiomolybdate inhibits mitochondrial complex IV and mediates degradation of hypoxia-inducible factor-1α in cancer cells. Sci. Rep. 2015;5:14296. doi: 10.1038/srep14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rose N.R., McDonough M.A., King O.N.F., Kawamura A., Schofield C.J. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 2011;40:4364–4397. doi: 10.1039/c0cs00203h. [DOI] [PubMed] [Google Scholar]
- 76.Koivunen P., Hirsilä M., Günzler V., Kivirikko K.I., Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J. Biol. Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- 77.Scheuermann T.H., Tomchick D.R., Machius M., Guo Y., Bruick R.K., Gardner K.H. Artificial ligand binding within the HIF2α PAS-B domain of the HIF2 transcription factor. Proc. Natl. Acad. Sci. USA. 2009;106:450–455. doi: 10.1073/pnas.0808092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho H., Du X., Rizzi J.P., Liberzon E., Chakraborty A.A., Gao W., Carvo I., Signoretti S., Bruick R.K., Josey J.A. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. 2016;539:107–111. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wallace E.M., Rizzi J.P., Han G., Wehn P.M., Cao Z., Du X., Cheng T., Czerwinski R.M., Dixon D.D., Goggin B.S. A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 2016;76:5491–5500. doi: 10.1158/0008-5472.CAN-16-0473. [DOI] [PubMed] [Google Scholar]
- 80.Courtney K.D., Infante J.R., Lam E.T., Figlin R.A., Rini B.I., Brugarolas J., Zojwalla N.J., Lowe A.M., Wang K., Wallace E.M. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J. Clin. Oncol. 2018;36:867. doi: 10.1200/JCO.2017.74.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scheuermann T.H., Li Q., Ma H.-W., Key J., Zhang L., Chen R., Garcia J.A., Naidoo J., Longgood J., Frantz D.E. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 2013;9:271. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun D.-R., Wang Z.-J., Zheng Q.-C., Zhang H.-X. Exploring the inhibition mechanism on HIF-2 by inhibitor PT2399 and 0X3 using molecular dynamics simulations. J. Mol. Recognit. 2018;31:e2730. doi: 10.1002/jmr.2730. [DOI] [PubMed] [Google Scholar]
- 83.Chen W., Hill H., Christie A., Kim M.S., Holloman E., Pavia-Jimenez A., Homayoun F., Ma Y., Patel N., Yell P. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Courtney K.D., Ma Y., Leon A.D.d., Christie A., Xie Z., Woolford L., Singla N., Joyce A., Hill H., Madhuranthakam A.J. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin. Cancer Res. 2020;26:793–803. doi: 10.1158/1078-0432.CCR-19-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee K., Zhang H., Qian D.Z., Rey S., Liu J.O., Semenza G.L. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Miranda E., Nordgren I.K., Male A.L., Lawrence C.E., Hoakwie F., Cuda F., Court W., Fox K.R., Townsend P.A., Packham G.K., et al. A Cyclic Peptide Inhibitor of HIF-1 Heterodimerization That Inhibits Hypoxia Signaling in Cancer Cells. J. Am. Chem. Soc. 2013;135:10418–10425. doi: 10.1021/ja402993u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dekker F.J., Haisma H.J. Histone acetyl transferases as emerging drug targets. Drug Discov. Today. 2009;14:942–948. doi: 10.1016/j.drudis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Delvecchio M., Gaucher J., Aguilar-Gurrieri C., Ortega E., Panne D. Structure of the p300 catalytic core and implications for chromatin targeting and HAT regulation. Nat. Struct. Mol. Biol. 2013;20:1040. doi: 10.1038/nsmb.2642. [DOI] [PubMed] [Google Scholar]
- 89.He Z.-X., Wei B.-F., Zhang X., Gong Y.-P., Ma L.-Y., Zhao W. Current development of CBP/p300 inhibitors in the last decade. Eur. J. Med. Chem. 2020:112861. doi: 10.1016/j.ejmech.2020.112861. [DOI] [PubMed] [Google Scholar]
- 90.Knurowski T., Clegg K., Brooks N., Ashby F., Pegg N.A., West W., Walter H.S., Somervaille T.C.P., Knapper S., Davies A. An Open-Label Phase I/IIa Study to Evaluate the Safety and Efficacy of CCS1477, a First in Clinic Inhibitor of the p300/CPB Bromodomains, As Monotherapy in Patients with Advanced Haematological Malignancies. Blood. 2019;134:1266. doi: 10.1182/blood-2019-124797. [DOI] [Google Scholar]
- 91.Imayoshi N., Yoshioka M., Chauhan J., Nakata S., Toda Y., Fletcher S., Strovel J.W., Takata K., Ashihara E. CG13250, a novel bromodomain inhibitor, suppresses proliferation of multiple myeloma cells in an orthotopic mouse model. Biochem. Biophys. Res. Commun. 2017;484:262–268. doi: 10.1016/j.bbrc.2017.01.088. [DOI] [PubMed] [Google Scholar]
- 92.Zhang F.-c., Sun Z.-y., Liao L.-p., Zuo Y., Zhang D., Wang J., Chen Y.-t., Xiao S.-h., Jiang H., Lu T., et al. Discovery of novel CBP bromodomain inhibitors through TR-FRET-based high-throughput screening. Acta Pharmacol. Sin. 2020;41:286–292. doi: 10.1038/s41401-019-0256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jubb A.M., Pham T.Q., Hanby A.M., Frantz G.D., Peale F.V., Wu T.D., Koeppen H.W., Hillan K.J. Expression of vascular endothelial growth factor, hypoxia inducible factor 1α, and carbonic anhydrase IX in human tumours. J. Clin. Pathol. 2004;57:504–512. doi: 10.1136/jcp.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., Chevreau C., Filipek M., Melichar B., Bajetta E. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 95.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O., Oudard S., Negrier S., Szczylik C., Kim S.T. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 96.Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M., Negrier S., Chevreau C., Solska E., Desai A.A. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 97.Hua H., Kong Q., Zhang H., Wang J., Luo T., Jiang Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019;12:71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jafari R., Almqvist H., Axelsson H., Ignatushchenko M., Lundbäck T., Nordlund P., Molina D.M. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 2014;9:2100. doi: 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 99.Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948;437:55–75. doi: 10.1002/andp.19484370105. [DOI] [Google Scholar]
- 100.Lakowicz J., Fu Y. Modification of single molecule fluorescence near metallic nanostructures. Laser Photonics Rev. 2009;3:221–232. doi: 10.1002/lpor.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bastiaens P.I., Squire A. Fluorescence lifetime imaging microscopy: Spatial resolution of biochemical processes in the cell. Trends Cell Biol. 1999;9:48–52. doi: 10.1016/S0962-8924(98)01410-X. [DOI] [PubMed] [Google Scholar]
- 102.Majoul I., Straub M., Duden R., Hell S.W., Söling H.-D. Fluorescence resonance energy transfer analysis of protein–protein interactions in single living cells by multifocal multiphoton microscopy. Rev. Mol. Biotechnol. 2002;82:267–277. doi: 10.1016/S1389-0352(01)00042-3. [DOI] [PubMed] [Google Scholar]
- 103.Clayton A., Hanley Q., Verveer P. Graphical representation and multicomponent analysis of single-frequency fluorescence lifetime imaging microscopy data. J. Microsc. 2004;213:1–5. doi: 10.1111/j.1365-2818.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 104.Berchner-Pfannschmidt U., Wotzlaw C., Merten E., Acker H., Fandrey J. Visualization of the three-dimensional organization of hypoxia-inducible factor-1 alpha and interacting cofactors in subnuclear structures. Biol. Chem. 2004;385:231–237. doi: 10.1515/BC.2004.017. [DOI] [PubMed] [Google Scholar]
- 105.Wotzlaw C., Otto T., Berchner-Pfannschmidt U., Metzen E., Acker H., Fandrey J. Optical analysis of the HIF-1 complex in living cells by FRET and FRAP. FASEB J. 2007;21:700–707. doi: 10.1096/fj.06-6280com. [DOI] [PubMed] [Google Scholar]
- 106.Berchner-Pfannschmidt U., Frede S., Wotzlaw C., Fandrey J. Imaging of the hypoxia-inducible factor pathway: Insights into oxygen sensing. Eur. Respir. J. 2008;32:210–217. doi: 10.1183/09031936.00013408. [DOI] [PubMed] [Google Scholar]
- 107.Konietzny R., König A., Wotzlaw C., Bernadini A., Berchner-Pfannschmidt U., Fandrey J. Molecular Imaging: Into In Vivo Interaction of HIF-1α and HIF-2α with ARNT. Ann. N. Y. Acad. Sci. 2009;1177:74–81. doi: 10.1111/j.1749-6632.2009.05029.x. [DOI] [PubMed] [Google Scholar]
- 108.Prost-Fingerle K., Hoffmann M.D., Schützhold V., Cantore M., Fandrey J. Optical analysis of cellular oxygen sensing. Exp. Cell Res. 2017;356:122–127. doi: 10.1016/j.yexcr.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 109.Frangioni J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 110.Safran M., Kim W.Y., O’Connell F., Flippin L., Günzler V., Horner J.W., DePinho R.A., Kaelin W.G. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: Assessment of an oral agent that stimulates erythropoietin production. Proc. Natl. Acad. Sci. USA. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iglesias P., Costoya J.A. A novel BRET-based genetically encoded biosensor for functional imaging of hypoxia. Biosens. Bioelectron. 2009;24:3126–3130. doi: 10.1016/j.bios.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 112.Kuchimaru T., Suka T., Hirota K., Kadonosono T., Kizaka-Kondoh S. A novel injectable BRET-based in vivo imaging probe for detecting the activity of hypoxia-inducible factor regulated by the ubiquitin-proteasome system. Sci. Rep. 2016;6:34311. doi: 10.1038/srep34311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiong L., Shuhendler A.J., Rao J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat. Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nikooharf A., Arezumand R., Mansouri K., Khoshi A.H., Ahmadabad H.N. Development of a Recombinant Monospecific Anti-PLGF Bivalent Nanobody and Evaluation of it in Angiogenesis Modulation. Mol. Biotechnol. 2020;62:580–588. doi: 10.1007/s12033-020-00275-7. [DOI] [PubMed] [Google Scholar]
- 115.Campochiaro P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stumpf C., Wimmer T., Lorenz B., Stieger K. Creation of different bioluminescence resonance energy transfer based biosensors with high affinity to VEGF. PLoS ONE. 2020;15:e0230344. doi: 10.1371/journal.pone.0230344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robers M.B., Dart M.L., Woodroofe C.C., Zimprich C.A., Kirkland T.A., Machleidt T., Kupcho K.R., Levin S., Hartnett J.R., Zimmerman K. Target engagement and drug residence time can be observed in living cells with BRET. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuo M.-H., Allis C.D. In Vivo Cross-Linking and Immunoprecipitation for Studying Dynamic Protein: DNA Associations in a Chromatin Environment. Methods. 1999;19:425–433. doi: 10.1006/meth.1999.0879. [DOI] [PubMed] [Google Scholar]
- 119.Orlando V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem. Sci. 2000;25:99–104. doi: 10.1016/S0968-0004(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 120.O’Neill L.P., Turner B.M. Immunoprecipitation of native chromatin: NChIP. Methods. 2003;31:76–82. doi: 10.1016/S1046-2023(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 121.Collas P. The current state of chromatin immunoprecipitation. Mol. Biotechnol. 2010;45:87–100. doi: 10.1007/s12033-009-9239-8. [DOI] [PubMed] [Google Scholar]
- 122.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hammers C., Songa E.B., Bendahman N., Hammers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 123.Dolk E., van Vliet C., Perez J.M.J., Vriend G., Darbon H., Ferrat G., Cambillau C., Frenken L.G.J., Verrips T. Induced refolding of a temperature denatured llama heavy-chain antibody fragment by its antigen. Proteins Struct. Funct. Bioinform. 2005;59:555–564. doi: 10.1002/prot.20378. [DOI] [PubMed] [Google Scholar]
- 124.Harmsen M.M., Haard H.J.d. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu Y., Liu C., Muyldermans S. Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Front. Immunol. 2017;8:1442. doi: 10.3389/fimmu.2017.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chanier T., Chames P. Nanobody engineering: Toward next generation immunotherapies and immunoimaging of cancer. Antibodies. 2019;8:13. doi: 10.3390/antib8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schoonooghe S., Laoui D., van Ginderachter J.A., Devoogdt N., Lahoutte T., Baetselier P.D., Raes G. Novel applications of nanobodies for in vivo bio-imaging of inflamed tissues in inflammatory diseases and cancer. Immunobiology. 2012;217:1266–1272. doi: 10.1016/j.imbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 128.Krasniqi A., D’Huyvetter M., Xavier C., van der Jeught K., Muyldermans S., van der Heyden J., Lahoutte T., Tavernier J., Devoogdt N. Theranostic radiolabeled anti-CD20 sdAb for targeted radionuclide therapy of non-Hodgkin lymphoma. Mol. Cancer Ther. 2017;16:2828–2839. doi: 10.1158/1535-7163.MCT-17-0554. [DOI] [PubMed] [Google Scholar]
- 129.Warnders F.J., van Scheltinga A.G.T., Knuehl C., van Roy M., de Vries E.F.J., Kosterink J.G.W., de Vries E.G.E., Lub-de Hooge M.N. Human epidermal growth factor receptor 3–specific tumor uptake and biodistribution of 89Zr-MSB0010853 visualized by real-time and noninvasive PET imaging. J. Nucl. Med. 2017;58:1210–1215. doi: 10.2967/jnumed.116.181586. [DOI] [PubMed] [Google Scholar]
- 130.Balhuizen A., Massa S., Mathijs I., Turatsinze J.-V., Vos J.d., Demine S., Xavier C., Villate O., Millard I., Egrise D. A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-15417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Massa S., Vikani N., Betti C., Ballet S., Vanderhaegen S., Steyaert J., Descamps B., Vanhove C., Bunschoten A., van Leeuwen F.W.B. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging. 2016;11:328–339. doi: 10.1002/cmmi.1696. [DOI] [PubMed] [Google Scholar]
- 132.Mann F.A., Lv Z., Großhans J., Opazo F., Kruss S. Nanobody-Conjugated Nanotubes for Targeted Near-Infrared In Vivo Imaging and Sensing. Angew. Chem. Int. Ed. 2019;58:11469–11473. doi: 10.1002/anie.201904167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.