Patients with tuberculosis and their counsellors were very satisfied with an intervention aimed at addressing alcohol intake, smoking and treatment adherence. However, additional training in counselling and improved SMS-delivery were felt to be necessary.
Keywords: Smoking cessation, Electronic health record, Interoperability, Text message, SmokefreeTXT
Abstract
Too few smokers who present for outpatient healthcare receive evidence-based interventions to stop smoking. Referral to nationally available smoking cessation support may enhance tobacco intervention reach during healthcare visits. This study evaluated the feasibility of outpatient electronic health record (EHR)-enabled, closed-loop referral (eReferral) to SmokefreeTXT, a National Cancer Institute text message smoking cessation program. SmokefreeTXT eReferral for adult patients who smoke was implemented in a family medicine clinic and an allergy and asthma clinic in an integrated Midwestern healthcare system. Interoperable, HIPAA-compliant eReferral returned referral outcomes to the EHR. In Phase 1 of implementation, clinicians were responsible for eReferral; in Phase 2 this responsibility shifted to Medical Assistants and/or nurses. EHR data were extracted to compute eReferral rates among adult smokers and compare demographics among those eReferred versus not referred. SmokefreeTXT data were used to compute SmokefreeTXT enrollment rates among those eReferred. Descriptive analyses of clinic staff surveys assessed implementation context and staff attitudes toward and adaptations of eReferral processes. During clinician implementation, 43 of 299 adult smokers (14.4%) were eReferred. During medical assistant (MA) implementation, 36 of 401 adult smokers (9.0%) were eReferred. Overall, among those eReferred, 25.7% completed SmokefreeTXT enrollment (3.1% of patients eligible for eReferral). Staff survey responses indicated that eReferral was efficient and easy. eReferral rates and relevant attitudes varied meaningfully by clinic. Thus, interoperable eReferral via outpatient EHR to SmokefreeTXT is feasible and acceptable to clinic staff and enrolls roughly 3.0% of smokers. Clinic context and implementation approach may influence reach.
Implications.
Practice: Fully interoperable, electronic referral to text-message support for smoking cessation is feasible in both primary and specialty outpatient care with electronic health record tools.
Policy: Health care systems can extend the reach of evidence-based smoking cessation interventions modestly by adopting electronic health record-enabled, interoperable electronic referral, but must adapt referral processes to local workflows and contexts.
Research: Additional research on reach, effectiveness, adoption, implementation, and maintenance is needed to enhance electronic referral to SmokefreeTXT and to more accurately assess its impact as a smoking cessation treatment extender.
Although adult smoking prevalence has declined to 14% in the U.S. [1], smoking remains the leading preventable cause of death and a major driver of preventable morbidity and healthcare and lost productivity costs [2,3]. In addition, the majority of adults who smoke want to quit [4,5] and most make serious attempts to quit each year [4]. Despite a desire to quit, relatively few smokers take advantage of available treatments, with only 3.7% of adult smokers using any form of counseling, and only 1.6% using a telephone quitline [5]. Although 75.2% of smokers are seen in primary care annually [6], only 57.2% of smokers report receiving healthcare provider advice to quit [5], and even fewer (28–45%) report receiving any form of assistance in quitting (e.g., discussion of medications, brief counseling to motivate cessation, or referral to specialized cessation treatment) as a result of such visits [6–9]. As such, outpatient healthcare settings have untapped potential to enhance the reach of evidence-based smoking cessation interventions.
One way to increase the delivery of smoking cessation treatment in primary care with minimal disruption in workflow is to use the EHR so that the referral of patients who smoke to an external evidence-based smoking cessation treatment is integrated into healthcare visits. This may increase clinician engagement in intervention because it removes some of the burden of intervention from their shoulders. Furthermore, if the external treatment resource imposes few barriers to use by patients, it may attract more smokers than historically underused treatments (e.g., programs requiring attendance at scheduled group sessions [10]). Additionally, providing clinical care teams with feedback about patient referral outcomes may further enhance referral likelihood [11,12].
Mobile interventions, particularly text-based interventions, are viewed as convenient by smokers [13,14] and have the potential for broad reach, given the near ubiquity of cellular telephone use in the U.S. population [15]. These features of text-message-based interventions may attract smokers to treatment, support cessation success, and motivate clinicians to offer assistance in quitting. Text-message-based interventions promote abstinence from tobacco use [16,17] and are now widely available. Such mobile interventions are now recommended by the U.S. Community Preventive Task Force to promote medication adherence, for example [18].
One text-message-based intervention, SmokefreeTXT, is sponsored by the National Cancer Institute and is available nationwide to smokers seeking support in quitting smoking [19]. The program is free of charge for services, but users may be charged for cellular minutes or data use, depending on their mobile carriers and contracts. The general, adult SmokefreeTXT program tailors messages to patient age, gender, time zone, and smoking frequency and heaviness. Proactive messages are sent up to five times per day for up to 2 weeks prior to a target quit date and for 6 weeks following a target quit date. Participants may seek additional, on-demand support by texting key words to receive help with cravings, negative moods, or slips.
The current study tested the feasibility of integrating fully electronic interoperable, Health Insurance Portability and Accountability Act (HIPAA)-compliant, closed-loop referral (eReferral) to the general, adult SmokefreeTXT program through the electronic health record (EHR) into clinical workflows. Integration into workflow is essential to adoption and maintenance of clinical innovations, and EHRs provide a platform for such integration, with great potential to enhance prevention interventions, as highlighted in the Health Information Technology for Economic and Clinical Health (HITECH) Act [20]. In this study, eReferral feasibility was tested by examining eReferral reach, representativeness of reach, and acceptability to staff in both outpatient primary care and specialty care clinics. The approach to implementation and evaluation was informed by the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework for evaluation planning, with engagement from healthcare system leaders in planning processes [21–23].
The study was conducted in two phases to permit evaluation of the feasibility of two eReferral implementation approaches. In Phase 1, treating clinicians were responsible for introducing the SmokefreeTXT option to patients who smoked and obtaining consent for eReferral (the method recommended by the investigators). In Phase 2, eReferral tasks were conducted by the medical assistants (MAs) and nurses (RNs) who ask about smoking status during the clinic rooming process (this was an adaptation suggested by some in the healthcare system). In this way, this pilot project was designed to assess SmokefreeTXT eReferral reach and representativeness of reach under two different clinical workflow models.
Methods
Design
In this pilot project, SmokefreeTXT was implemented in two clinics, and in two phases, each lasting 4 months, within each clinic. Both clinics used an Epic Systems, Inc. (Verona, WI) EHR, an EHR platform that covers roughly 200 million U.S. patients [24]. At baseline and in both phases of implementation, MAs and, less often, RNs were responsible for asking about and documenting tobacco use in the EHR during the rooming assessment process. In implementation Phase 1, treating clinicians (physicians, physician assistants, and nurse practitioners) were responsible for advising quitting, offering assistance in quitting, and assessing interest in referral to SmokefreeTXT, including patient willingness to quit smoking within 30 days based on an EHR prompt that fired for all smokers. Clinicians were responsible for entering the eReferral order to SmokefreeTXT in Phase 1.
In implementation Phase 2, MAs and RNs were responsible for advising quitting, offering assistance in quitting, assessing interest in referral to SmokefreeTXT and willingness to quit within 30 days, and placing the order for eReferral for clinician review and approval. Clinicians were responsible only for approving eReferral orders and offering other cessation interventions, as they deemed fit. This design permits comparison of the reach of SmokefreeTXT eReferral across two workflows that differ in the degree of treating clinician involvement.
Prior to implementation Phase 1, experienced outreach staff from the University of Wisconsin Center for Tobacco Research and Intervention provided in-person group training in addressing tobacco use and healthcare system IT staff demonstrated pertinent EHR modules and tools. A brief follow-up group training was held about 30 days later to engage those who were not able to attend the first training and to enhance implementation fidelity. In implementation Phase 2, in-person training was held (as in Phase 1), supplemented by a 2-min video demonstration of eReferral steps for MAs and RNs to view and refer to for future support.
Participating staff in the two clinics were asked to anonymously and voluntarily complete online surveys regarding their attitudes toward and typical practices in addressing patient smoking just before the launch of SmokefreeTXT eReferral in Phase 1, and again at the end of Phase 2. All aspects of the study were reviewed and approved by an institutional review board.
Clinics
The host healthcare system identified two clinics not involved in other smoking cessation intervention research to serve as pilot test sites. Because the healthcare system was interested in the feasibility of eReferral beyond primary care, a specialty clinic was included. One clinic was a large family medicine clinic that served as a resident training site staffed by 18 resident or attending clinicians, four RNs, and nine MAs. The second clinic was a specialty adult allergy and asthma clinic with two clinicians, five RNs, and one MA.
eReferral process in Phase 1: clinician implementation
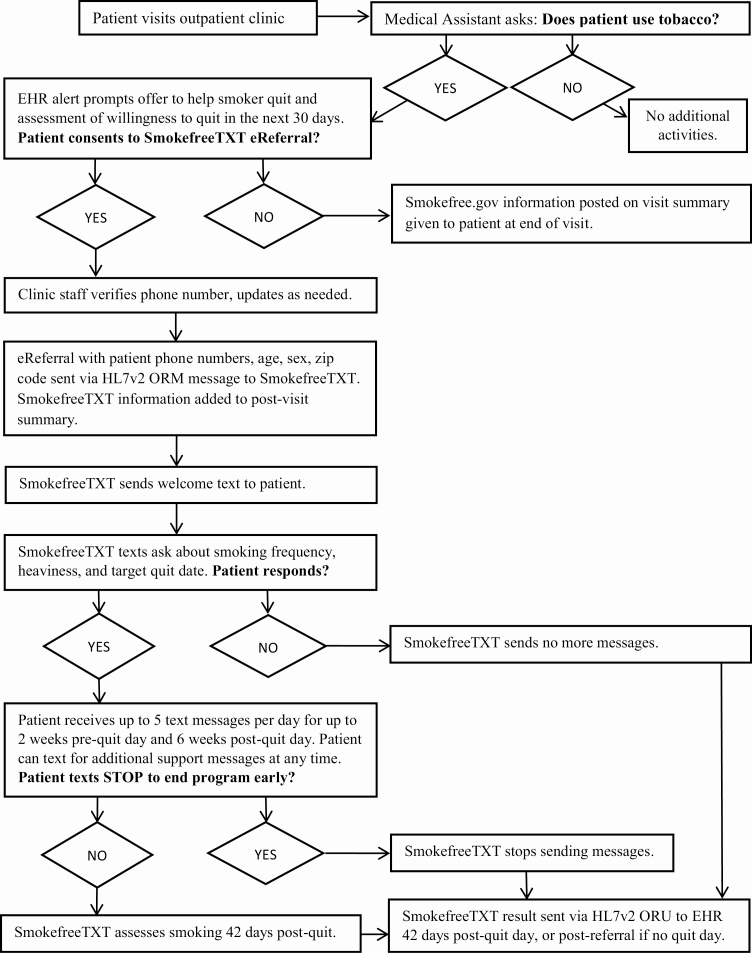
The SmokefreeTXT eReferral workflow is shown in Fig. 1. Referral to SmokefreeTXT in both implementation phases began with assessment of tobacco use during vital sign assessment as part of the clinic rooming process, in accordance with recommended outpatient practice [25,26]. MAs or RNs documented smoking status using existing fields in the Epic EHR. When the clinician opened the encounter record for a current smoker, a hard-stop decision alert appeared to prompt assessment of willingness to quit and consent to eReferral to SmokefreeTXT. The alert provided recommended scripting: “Let’s talk about your smoking. I would like to connect you to the National Cancer Institute’s SmokefreeTXT program. This program will help you set a quit date in the next 30 days and will text your phone quitting tips and support. Are you willing to try to quit with SmokefreeTXT?” The alert presented options to order or not order, and to note the reason for not ordering (patient declined or clinician deferred until later). If the clinician selected “Order,” a referral order set would display the patient’s mobile number for confirmation, and permit entry of a different 10-digit phone number, if the patient’s preferred number was not defaulted. Completing the order set also documented that the clinician provided counseling and the patient consented to the referral by default (unless modified by the clinician). In addition, clinicians were encouraged to prescribe pharmacotherapy to support cessation, as appropriate.
Fig 1.
| Workflow diagram depicting steps in the SmokefreeTXT eReferral process.
For patients who accepted SmokefreeTXT eReferral, their post-visit summary was tailored to include congratulations on their decision to quit, a reminder to expect a text from SmokefreeTXT, and a note that clinic staff were also available to support quitting smoking. For smokers who declined eReferral, their post-visit summary noted that smoking is harmful to health, and encouraged patients to contact their providers for information about quitting and to visit www.smokefree.gov for quitting resources.
Order results from the SmokefreeTXT program returned automatically to the patient’s medical record and the referring clinician’s inbox 42 days following referral (for those who did not answer enrollment questions or set a quit date, and therefore did not complete enrollment) or 42 days following the initial target quit day (for those who set a quit date and fully enrolled). SmokefreeTXT assesses past-week smoking 42 days following the target quit date for all smokers who do not end the program early (by texting STOP to SmokefreeTXT). The eReferral order result returned to the patient’s EHR noted which of several mutually exclusive outcomes occurred. Possible outcomes 42 days post-referral or enrollment included: did not enroll, enrolled but ended messages early, completed program and achieved abstinence, completed program and did not achieve abstinence, completed program but did not respond to queries regarding abstinence, or still in the program (if reset the quit day). The date of enrollment and initial designated quit date were also noted, if present.
eReferral process in Phase 2: rooming staff (MA, RN) implementation
The general SmokefreeTXT eReferral workflow shown in Fig. 1 applies to Phase 2, as well. The key difference in Phase 2 is that clinician involvement was limited to approval of eReferral orders prepared by MAs/RNs and prescription of smoking cessation pharmacotherapy, if deemed appropriate. All other steps (advising to quit, assessing willingness to quit, and arranging help by pending the eReferral order) were handled by the MA or RN. SmokefreeTXT result messages were sent to the inbox of the clinician, but were visible to MAs and RNs as an order result, too.
Measures
Data on SmokefreeTXT eReferral reach were collected from the EHR and from SmokefreeTXT. Data regarding patient volume; patient demographics; and rates of smoking status documentation, smoking, alert firing, and eReferral declines and acceptance were gathered from the EHR. Data regarding SmokefreeTXT enrollment rates among those eReferred were gathered from weekly reports generated by ICF International (Washington, DC), the vendor for SmokefreeTXT. All patient data were collected using existing EHR or SmokefreeTXT fields in a de-identified manner. Selected demographics (age; sex; and insurance payer type) were pulled from the EHR to examine the representativeness of eReferral reach along these dimensions. Age was coded as a continuous variable reflecting the maximum age for the patient, with values above 90 set to 90 to avoid identification of patients. Insurance type, which can change within patients, was coded into mutually exclusive categories. Patients who at any time during the study were uninsured were coded as uninsured; those who were never uninsured and were Medicaid-eligible at any time during the study were coded as having Medicaid; those with Medicare who were never uninsured or Medicaid-eligible during the study were coded as having Medicare; those who only had commercial or employer-based insurance throughout the study period were coded as having commercial insurance.
Clinic staff were asked to complete optional, brief, online surveys regarding their beliefs, practices, and attitudes related to addressing tobacco use with patients, perceptions of clinic support and functioning, and familiarity with SmokefreeTXT prior to the launch of Phase 1. All items were rated on 7-point scales ranging from 1 to 7, anchored at 1 (strongly disagree) and 7 (strongly agree). Staff were asked to repeat the survey and answer additional questions regarding their perceptions and use of SmokefreeTXT eReferral at the end of Phase 2. Consent for anonymous survey completion was gathered prior to survey administration.
Data analysis plan
Rates of smoker identification, smoking prevalence, eReferral orders among smokers, and SmokefreeTXT enrollment were computed to characterize the reach and reach representativeness of SmokefreeTXT in terms of sex, age, and insurance type. Descriptive analyses were also conducted to summarize clinic staff beliefs and attitudes relevant to SmokefreeTXT eReferral. Chi-square tests were used to test for differences in reach by clinic, implementation phase, sex, and insurance type; t-tests were used to test for differences in reach by age, at alpha .05.
Results
Rates of asking about tobacco and eReferring to SmokefreeTXT
Patient volume and rates of smoker identification, smoking prevalence, EHR presentation of the alert prompting assessment of interest in SmokefreeTXT among smokers, eReferral orders, and SmokefreeTXT enrollment are shown by clinic and implementation phase in Table 1. Smoking status was documented for at least 95% of patients in both Phases 1 and 2 in both clinics. In both Phases 1 and 2, smoking prevalence was significantly higher (χ2 (N = 6,150) = 97.97, p < .001) among adult patients in the Family Medicine clinic (17% in Phase 1, 19% in Phase 2) than in the Allergy and Asthma clinic (7% in Phase 1, 8% in Phase 2). Smoking prevalence did not differ significantly between Phase 1 and Phase 2 within clinics (χ2 < 1.95, ps > .16). After launch, the alert to prompt clinicians to offer patients who smoke eReferral to SmokefreeTXT occurred consistently once a smoker had been identified in the EHR (Table 1). The alert was suppressed only if the patient had already been eReferred in the past 90 days. During Phase 1 (clinician implementation), the eReferral rate was significantly higher in Allergy and Asthma care than in Family Medicine (χ2(N = 299) = 6.05, p = .01), while there was no significant difference in eReferral by clinic during Phase 2 (MA/RN implementation) (χ2(N = 294) = 0.044, p = .51). There was no significant difference in eReferral rates across phase in Family Medicine (χ2(N = 514) = 0.95, p = .09), but the decline in eReferral rates across phases in the Allergy and Asthma clinic was significant (χ2(N = 79) = 5.29, p = .02). This does not appear to be due to a drop in EHR alerting (100% in both phases at this clinic). Overall, 14.4% of smokers seen during Phase 1, and 9.2% of smokers seen during Phase 2 were eReferred to SmokefreeTXT. Rates of patient engagement in SmokefreeTXT or staff referral to SmokefreeTXT were not documented in the EHR prior to the launch of eReferral in Phase 1 of implementation. Data from ICF International, however, indicated that very few patients eReferred in this study had phone numbers that had previously received messages from SmokefreeTXT (previous registration altered the process of uploading a patient number in the text messaging platform for SmokefreeTXT).
Table 1.
Patient volume, and rates of smoker identification, smoking prevalence, electronic health record (EHR) alerting, and SmokefreeTXT (SFTXT) eReferral and enrollment, by clinic and 4-month study phase
| Family medicine | Allergy and asthma | |||||
|---|---|---|---|---|---|---|
| Implementation | Prea | Clinician | MA/RN | Prea | Clinician | MA/RN |
| Phase | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Patient N | 1,489 | 1,497 | 1,395 | 662 | 597 | 510 |
| Smoking status recorded (% of patients) | 1,424 (95.6%) | 1,444 (96.5%) | 1,353 (97.0%) | 661 (99.8%) | 593 (99.3%) | 506 (99.2%) |
| Smokers (% of patients) | 249 (16.7%) | 268 (17.9%) | 272 (19.5%) | 55 (8.3%) | 52 (8.7%) | 36 (7.1%) |
| EHR alert fired (% of smokers) | 268 (100%) | 261 (96.0%)b | 52 (100%) | 36 (100%) | ||
| eReferred (% of smokers) | 31 (11.6%) | 25 (9.6%) | 12 (23.1%) | 2 (5.6%)c | ||
| Enrolled in SFTXT (% of eReferred) | 8 (25.8%) | 7 (28.0%) | 2 (16.7%) | 1 (50.0%) | ||
MA/RN medical assistants and nurses. Twenty-nine patients were seen in both clinics and were counted as attending in both clinic totals.
aEHR data from the 4 months preceding implementation Phase 1 were extracted for analysis.
bThe EHR alert firing rate can be below 100% for smokers due to a suppression rule that prevents the alert from recurring within 90 days of a previously placed SmokefreeTXT eReferral.
cSmokefreeTXT reported receiving four referrals during this time from the allergy clinic, but only two are recorded in the EHR.
SmokefreeTXT enrollment rate
SmokefreeTXT enrollment rates among those eReferred (Table 1) did not differ significantly across phases, or across clinics within phases (all χ2 < 1.13, all ps > .29). The overall enrollment rate across clinics and phases was 18 out of 70 eReferrals (25.7%).
Demographics associated with eReferral
Summary statistics for available demographics (age, sex, and insurance type) for adult patients who smoked are shown in Table 2, by eReferral status (eReferred vs. not eReferred). Although there was a tendency for male and Medicaid-eligible patients to be over-represented among those eReferred versus not eReferred (in all but Phase 1 in Family Medicine), these effects were not significant in this small sample, and no substantial age differences were noted between those eReferred versus not. On average, Family Medicine patients were significantly younger than Allergy and Asthma patients across Phases.
Table 2.
Patient age, sex, and insurance type by clinic and phase for smokers who were not eReferred versus those who were eReferred
| Phase 1: clinician implementation | Phase 2: MA/RN implementation | |||
|---|---|---|---|---|
| Family medicine clinic | Not eReferred | eReferred | Not eReferred | eReferred |
| Patient N | 237 | 31 | 236 | 25 |
| Age in years up to 90 M (SD) | 42.6 (13.9) | 41.5 (11.8) | 42.0 (14.1) | 41.1 (11.2) |
| Male n (%) | 90 (38.0%) | 8 (25.8%) | 100 (42.4%) | 11 (44.0%) |
| Commercial Insurance n (%) | 74 (31.2%) | 12 (38.7%) | 83 (35.2%) | 8 (32.0%) |
| Medicaid n (%) | 86 (36.3%) | 13 (41.9%) | 85 (36.0%) | 7 (28.0%) |
| Medicare n (%) | 42 (17.7%) | 3 (9.7%) | 42 (17.8%) | 4 (16.0%) |
| Uninsured n (%) | 35 (14.8%) | 3 (9.7%) | 26 (11.0%) | 6 (24.0%) |
| Allergy and asthma clinic | Not eReferred | eReferred | Not eReferred | eReferred |
| Patient N | 40 | 12 | 34 | 2 |
| Age in years up to 90 M (SD) | 45.5 (15.5) | 40.7 (13.5) | 47.2 (15.7) | 49.0 (2.8) |
| Male n (%) | 10 (25.0%) | 4 (33.3%) | 9 (26.5%) | 1 (50.0%) |
| Commercial Insurance n (%) | 16 (40.0%) | 2 (16.7%) | 13 (38.2%) | 0 |
| Medicaid n (%) | 12 (30.0%) | 6 (50.0%) | 8 (23.5%) | 2 (100.0%) |
| Medicare n (%) | 10 (25.0%) | 2 (16.7%) | 9 (26.5%) | 0 |
| Uninsured n (%) | 2 (5.0%) | 2 (16.7%) | 4 (11.8%) | 0 |
MA/RN medical assistants and nurses.
Staff surveys
Mean staff ratings to survey items are shown along with the text of the items administered in Table 3. Pre-implementation, 15 of 22 staff (68%) at the Family Medicine clinic and 7 of 8 staff (88%) at the Allergy and Asthma clinic completed the survey. Staff strongly endorsed the importance of addressing tobacco use, agreed they had the knowledge and clinic support to address tobacco use, and agreed they are part of an effective team that works well together. Ratings of patient receptivity to tobacco intervention, the effectiveness of tobacco intervention, and having time to address tobacco use well were lower. Views of existing tobacco use intervention protocols and EHR tools were slightly positive, as were attitudes toward mobile interventions to address tobacco use. There were no significant differences across clinics in pre-implementation ratings (collected just before Phase 1 launch).
Table 3.
Clinic staff attitude and belief rating means (standard deviations) pre-Phase 1 and post-Phase 2 of eReferral implementation
| Pre-Phase 1 | Post-Phase 2 | |||
|---|---|---|---|---|
| Family medicine (n = 15) | Allergy and asthma (n = 7) | Family medicine (n = 11) | Allergy and asthma (n = 6) | |
| Addressing tobacco use with patients is a very important use of my time. | 6.5 (0.7) | 6.7 (0.5) | 6.6 (0.5) | 7.0 (0.0) |
| I know what to do to address my patients’ tobacco use well. | 6.1 (0.9) | 5.4 (1.3) | 5.5 (1.4) | 6.7 (0.5) |
| I have enough time to address my patients’ tobacco use well. | 4.6 (1.9) | 5.4 (1.3) | 4.8 (1.3) | 6.7 (0.5)* |
| My clinic supports me in my attempts to address patients’ tobacco use. | 6.4 (1.1) | 6.9 (0.4) | 6.2 (1.0) | 7.0 (0.0) |
| I feel that I am part of a good healthcare team that is working well together. | 6.7 (0.5) | 6.9 (0.4) | 6.6 (0.5) | 6.8 (0.4) |
| Patients seem to welcome my efforts to address their tobacco use with them. | 5.1 (1.1) | 4.6 (1.3) | 5.1 (1.4) | 5.7 (1.0) |
| Very few patients will stop using tobacco even with treatment. | 3.6 (1.4) | 3.9 (1.2) | 3.1 (1.4) | 3.7 (1.8) |
| I often get feedback on whether my patients got tobacco treatment if they wanted it. | 3.4 (1.3) | 3.7 (1.5) | 4.2 (1.9) | 4.2 (0.8) |
| The steps I need to take to address my patients’ tobacco use are efficient/well designed. | 5.5 (0.9) | 5.6 (1.0) | 6.0 (0.9) | 6.5 (0.5) |
| The EHR helps me address my patients’ tobacco use. | 5.1 (1.3) | 5.6 (1.0) | 5.5 (1.4) | 6.7 (0.5) |
| I am familiar with text-to-quit programs such as SmokefreeTXT | 3.5 (1.9) | 4.2 (1.7) | 6.2 (0.8) | 6.6 (0.5) |
| I have referred my patients to a text-to-quit program like SmokefreeTXT | 2.7 (2.2) | 2.2 (1.3) | 6.2 (0.8) | 6.3 (1.0) |
| I believe mobile interventions like texting programs can help my patients stop using tobacco | 5.1 (1.1) | 4.6 (1.5) | 5.1 (1.2) | 5.0 (2.3) |
| The SmokefreeTXT text message program is an effective aid to my patients who want to quit | 4.9 (1.4) | 5.3 (2.0) | ||
| I understand how to refer my patients to SmokefreeTXT | 6.3 (0.7) | 6.7 (0.5) | ||
| The method to refer patients to SmokefreeTXT is easy | 6.1 (0.8) | 6.7 (0.5) | ||
| The method to refer patients to SmokefreeTXT is effective | 4.0 (1.4) | 6.0 (1.3)* | ||
| I regularly receive feedback regarding the outcome of the patients I refer to SmokefreeTXT | 4.4 (2.1) | 5.8 (1.3) | ||
| How often do you read the script provided in the EHR when assessing patient willingness to accept eReferral to SmokefreeTXT?a | 2.9 (1.5) | 2.5 (1.2) | ||
All items rated from 1 = strongly disagree to 7 = strongly agree, except.
aWhich was rated from 1 = never to 5 = always.
*Clinic difference t test significant at p < .05, not assuming equal variances.
Eight months later, at the end of Phase 2, 15 of 25 (60%) Family Medicine staff and 6 of 8 (75%) Allergy and Asthma staff completed the survey. Differences across pre-Phase 1 and post-Phase 2 measurements were not tested formally due to the unknown degree of overlap in samples in this anonymous survey. Ratings of having enough time to address tobacco use well increased from pre- to post-implementation in both clinics, and ended significantly higher in the Allergy and Asthma clinic than in the Family Medicine post-Phase 2. Confidence in addressing tobacco use well and perception of patient receptivity to intervention increased markedly in the Allergy and Asthma clinic from before Phase 1 to the end of Phase 2. Staff reported greater familiarity with and referral experience with SmokefreeTXT after versus before implementation. Ratings of SmokefreeTXT effectiveness as a cessation aid were moderately positive, confidence in using eReferral was high, and eReferral was rated as easy to use. The effectiveness of the eReferral method was rated substantially and significantly lower in the Family Medicine clinic than in the Allergy and Asthma clinic. It is also worth noting that staff in both clinics reported rarely or only sometimes following the script in the EHR when introducing SmokefreeTXT. Qualitative responses indicated that some staff first assess willingness to quit before offering SmokefreeTXT, whereas others introduce the texting aspect of the program first, before assessing interest. Two noted that they stressed program novelty, but not the use of text messages. One noted that he or she tells patients that other patients have found the program beneficial.
Discussion
In this pilot study of eReferral to SmokefreeTXT in one primary care and one specialty adult outpatient clinic, results indicated that fully electronic, closed-loop referral to the nationally available SmokefreeTXT program is feasible, acceptable to staff, and has the potential to effectively connect patients who smoke with evidence-based cessation support. Overall, 12.0% of patients identified as eligible smokers at clinic visits were eReferred to SmokefreeTXT and 25.7% of those eReferred fully enrolled with SmokefreeTXT by setting a quit date. This rate of connection is highly similar to quitline connection rates reported in studies of fax referral to telephone tobacco quitlines (e.g., [27], in which 23.6% of fax-referred patients connected with the quitline). This translates into 3.1% of all patients who smoked being effectively connected with the SmokefreeTXT mobile intervention. These SmokefreeTXT rates are also similar to rates of referral and connection observed with EHR-based quitline referral methods when used in adult outpatient settings; for example, referral rates of 14% [14] and an enrollment rate of 2.3% [12]. The connection rate is also similar to that observed with a feedback intervention designed to enhance provider adherence to guideline recommendations (3.9% counseling connection rate among adult patients in [28]). Thus, although only 3.1% of unselected adult primary care patients who smoke set a quit date with SmokefreeTXT, this level of reach is comparable to that of other forms of counseling and referral in healthcare settings. SmokefreeTXT has the added benefit of being available nationwide, 24 hr per day, 365 days per year, without added costs and with few barriers to access. Because roughly 75% smokers are seen in primary care annually [5], the potential reach of SmokefreeTXT via eReferral in primary care, even with a modest 3.1% rate of enrollment, could approach 800,000 adults in the U.S. [1].
Results showed, however, that eReferral rates varied across clinics, and there was some evidence that clinician-based eReferral in Phase 1 was somewhat more effective than MA/RN implementation in Phase 2 in the Allergy & Asthma clinic. Reach rates did not vary markedly by sex or age, and were not significantly different across insurance types. Survey responses indicated that, after SmokefreeTXT eReferral implementation (post-Phase 2), staff provided positive evaluations of both the efficiency of this method and their confidence in addressing tobacco use. However, ratings of eReferral effectiveness were lower in the Family Medicine clinic than in the specialty clinic.
Taken together, these results suggest that EHR-enabled SmokefreeTXT eReferral has the potential to serve as a useful extender of in-clinic interventions to support smoking cessation, so long as eReferral is well-integrated into clinic workflows. As noted above, there was some evidence that SmokefreeTXT eReferral was more positively received by staff in the specialty clinic than in the Family Medicine clinic. This difference may be related to clinic workflow differences pertaining to resident training. Residents and attending clinicians at Family Medicine engage in collaborative care that requires them to re-enter patient encounter records multiple times during care delivery. Staff at the Family Medicine clinic reported that they were frustrated by having to address the hard-stop EHR alert every time they entered an encounter record. This underscores the importance of ensuring that implementation is tailored to clinic workflows and procedures. It would be informative to evaluate eReferral reach and acceptability without a hard-stop, and with other adaptations (e.g., modified suppression rules to reduce presentation of the EHR alert for repeat patients) in future research to see if this enhances acceptability without reducing reach. The decline in eReferral rates from Phase 1 to Phase 2 may reflect differences in practice, workflow, or practitioner, and we are not able to parse these alternatives in this pilot study. The lack of substantial sex, age, and insurance differences in eReferral reach and enrollment rates is reassuring, although tests of significance are underpowered in this pilot study.
Clinic surveys suggested that clinic MAs, RNs, and clinicians viewed tobacco use intervention as an important part of their duties and viewed the clinic climate and team positively in terms of tobacco use intervention support and resources. Post-implementation assessment showed that eReferral to SmokefreeTXT was generally viewed as an easy process to use, but staff, especially in the Family Medicine clinic, expressed concern about the effectiveness of the SmokefreeTXT eReferral method. In addition, free responses indicated that clinical staff frequently adapted the treatment invitation, in varied ways. Future eReferral implementation research may identify adaptations that enhance eReferral reach and effectiveness.
This proof-of-concept, pilot study supports the feasibility of SmokefreeTXT eReferral, but is limited in terms of the small number of clinics used and because the participating clinics might not be representative; that is, the healthcare system selected amongst volunteer clinics. The study is also limited in that it did not examine representativeness of reach in terms of race and ethnicity, diagnoses, or health status. In addition, the sample was too small to permit exploration of sources of variance in eReferral implementation across staff, and anonymous staff surveys could not be linked to eReferral activity at the individual staff level. Despite these limitations, this pilot study suggests that EHR-enabled, closed-loop eReferral to SmokefreeTXT is feasible in primary and specialty outpatient settings. With buy-in and engagement among healthcare system information technology and clinical care teams, roughly 12% of smokers were referred to this cessation treatment service and 3% set a quit date and received tailored supportive text messages.
Acknowledgments
We would like to acknowledge our partners at the Gundersen Health System, ICF International (the vendor that implements SmokefreeTXT for the National Cancer Institute), and Epic Systems Corporation (including Brad Fox, Thanos Tsiolis, and Aaron Beal) for their contributions to this study. This study was funded by the National Cancer Institute (R35CA197573).
Compliance with Ethical Standards
Conflict of Interest: All authors declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: This study does not involve human participants and informed consent was therefore not required; staff surveys were conducted anonymously and all patient-level data were fully de-identified by the healthcare system.
Welfare of Animals: This article does not contain any animal studies performed by any author.
References
- 1. National Health Interview Survey. Prevalence of current cigarette smoking among adults aged 18 and over; United States, 2006-March 2018 2018. Available from: https://public.tableau.com/profile/tina.norris#!/vizhome/FIGURE8_1/Dashboard8_1. Accessibility verified May 17, 2019
- 2. U.S. Department of Health and Human Services. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services; 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessibility verified May 17, 2019. [Google Scholar]
- 3. Ma J, Siegel RL, Jacobs EJ, Jemal A. Smoking-attributable Mortality by State in 2014, U.S. Am J Prev Med. 2018;54(5):661–670. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Smoking and tobacco use 2018. Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm. Accessibility verified May 17, 2019.
- 5. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting SMOKING AMONG ADULTS - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. [DOI] [PubMed] [Google Scholar]
- 6. King BA, Dube SR, Babb SD, McAfee TA. Patient-reported recall of smoking cessation interventions from a health professional. Prev Med. 2013;57(5):715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vijayaraghavan M, Yuan P, Gregorich S, et al. Disparities in receipt of 5As for smoking cessation in diverse primary care and HIV clinics. Prev Med Rep. 2017;6:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kruger J, O’Halloran A, Rosenthal AC, Babb SD, Fiore MC. Receipt of evidence-based brief cessation interventions by health professionals and use of cessation assisted treatments among current adult cigarette-only smokers: National Adult Tobacco Survey, 2009-2010. BMC Public Health. 2016;16:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papadakis S, Gharib M, Hambleton J, Reid RD, Assi R, Pipe AL. Delivering evidence-based smoking cessation treatment in primary care practice: experience of Ontario family health teams. Can Fam Physician. 2014;60(7):e362–e371. [PMC free article] [PubMed] [Google Scholar]
- 10. West R, May S, West M, Croghan E, McEwen A. Performance of English stop smoking services in first 10 years: analysis of service monitoring data. Bmj. 2013;347:f4921. [DOI] [PubMed] [Google Scholar]
- 11. Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentz CJ, Bayley KB, Bonin KE, et al. Provider feedback to improve 5A’s tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine Tob Res. 2007;9(3):341–349. [DOI] [PubMed] [Google Scholar]
- 13. Naughton F, Jamison J, Sutton S. Attitudes towards SMS text message smoking cessation support: a qualitative study of pregnant smokers. Health Educ Res. 2013;28(5):911–922. [DOI] [PubMed] [Google Scholar]
- 14. Pechmann C, Delucchi K, Lakon CM, Prochaska JJ. Randomised controlled trial evaluation of Tweet2Quit: a social network quit-smoking intervention. Tob Control. 2017;26(2):188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pew Research Center. Mobile fact sheet2018, 10 Oct 2018 Available from: http://www.pewinternet.org/fact-sheet/mobile/. Accessibility verified May 17, 2019.
- 16. Palmer M, Sutherland J, Barnard S, et al. The effectiveness of smoking cessation, physical activity/diet and alcohol reduction interventions delivered by mobile phones for the prevention of non-communicable diseases: a systematic review of randomised controlled trials. PLoS One. 2018;13(1):e0189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD006611. [DOI] [PubMed] [Google Scholar]
- 18. The Community Guide. CPSTF findings for health communication and health information technology 2018. Available from: https://www.thecommunityguide.org/content/cpstf-findings-health-communication-and-health-information-technology. Accessibility verified May 17, 2019.
- 19. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. SmokefreeTXT 2017. Available from: https://smokefree.gov/smokefreetxt-about. Accessibility verified May 17, 2019.
- 20. Hesse BW. Time to reboot: resetting health care to support tobacco dependency treatment services. Am J Prev Med. 2010;39(6 Suppl 1):S85–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glasgow RE, Estabrooks PE. Pragmatic applications of RE-AIM for health care initiatives in community and clinical settings. Prev Chronic Dis. 2018;15:170271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glasgow RE, Dickinson P, Fisher L, et al. Use of RE-AIM to develop a multi-media facilitation tool for the patient-centered medical home. Implement Sci. 2011;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Educ Couns. 2001;44(2):119–127. [DOI] [PubMed] [Google Scholar]
- 24. Cohen JK. Epic, Cerner make up 50% of hospital EHR market share, ONC data shows 2018. Available from: https://www.beckershospitalreview.com/ehrs/epic-cerner-make-up-50-of-hospital-ehr-market-share-onc-data-shows.html.
- 25. Boyle R, Solberg LI. Is making smoking status a vital sign sufficient to increase cessation support actions in clinical practice? Ann Fam Med. 2004;2(1):22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rothemich SF, Woolf SH, Johnson RE, et al. Effect on cessation counseling of documenting smoking status as a routine vital sign: an ACORN study. Ann Fam Med. 2008;6(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willett JG, Hood NE, Burns EK, et al. Clinical faxed referrals to a tobacco quitline: reach, enrollment, and participant characteristics. Am J Prev Med. 2009;36(4):337–340. [DOI] [PubMed] [Google Scholar]
- 28. Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Haas JS. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med. 2009;169(8):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]