Abstract
Congenital teratoma of oral cavity in a neonate is a rare condition associated with compromised airway and challenges anesthesiologist in airway management. In this report, we describe a scenario of neonate with multiple oral teratoma, cleft palate, and bifid tongue who presented with respiratory distress for surgical excision of mass. The compromised airway can be successfully managed by appropriate prior planning and effective communication between anesthesiologist and surgical team.
Keywords: Congenital teratoma, neonate difficult airway, oral mass
Introduction
Newborn with congenital mass in oral cavity can present as challenges for anesthetist in airway management. The causes of difficult airway in newborn are different from that of adults. It mostly includes congenital anomalies such as laryngomalacia, hemangiomas, epulis, vascular ring, and hypoplastic mandible. Oral teratoma is a rare congenital anomaly occurs in 1:35,000 to 1: 200,000.[1] The clinical presentation depends upon the size and location of mass. It may present as mass protruding from mouth with high chances of respiratory tract obstruction.
Case Report
A 2.8-kg-one-day old female baby was brought to the hospital with respiratory distress and multiple growth protruding from oral cavity [Figure 1]. The child had macrostomia, cleft palate, bifid tongue, and two oral growths one measuring approximately 25 × 20 × 15 mm attached to hard palate and other in the sublingual region measuring approximately 12 × 10 × 15 mm. Computed tomography (CT) finding showed large well defined cystic lesion of size 23 × 17 × 12 mm in left parasagittal palatal region with internal fat fluid density and calcified densities (Hypertrophied palatal bones). Similar small cystic lesion of size approximate 11 × 8 × 13 mm is seen within oral cavity at sublingual region. On examination, the child was vigorous, tachypneic, and there was flaring of ala nasae. Mallampati grading could not be assessed.
Figure 1.
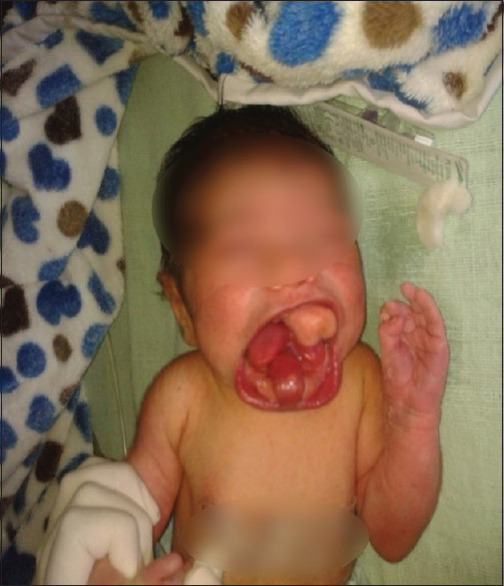
Image of child preoperatively
Excision of the tumor was scheduled on the day 3rd of life of the baby.
On arrival in operating room, routine monitoring (electrocardiogram, pulse oximeter, noninvasive blood pressure, and temperature) were attached. Difficult intubation cart and pediatric bronchoscope were kept ready to encounter any difficulty in intubation, and even surgical team were also standby to perform emergency tracheostomy if required. Oxymetazoline nasal drop was put in both nostril and patency of nare was checked by nasogastric feeding tube to rule out any extension of mass. Injection atropine 0.01 mg/kg was given through already secured intravenous access to limit secretions. For preoxygenation, a bigger size transparent circular silicon face mask was selected along with surgical pads to form a seal around the nose, lower jaw, and the mass to ensure proper bag and mask ventilation without causing any trauma to mass. Preoxygenation was done for 5 min. Once it was established that mask ventilation was possible, anesthesia was induced by sevoflurane and oxygen in gradually increasing concentration. When the patient was anesthetized, the mass was covered with surgical pads to avoid any injury to it and a gentle check laryngoscopy was done to visualize the glottis with Miller's blade 1.5; as glottis was visible, injection succinylcholine 1.5 mg/kg was given. After adequately lubricating the endotracheal tube (ET) with 2% lignocaine jelly, right nasal intubation was done with 3 mm internal diameter uncuffed ET by using Magill's forceps. Tube was fixed on the anterior surface of the nose. Injection fentanyl 2 μg/kg and injection atracurium 0.5 mg/kg were given. Anesthesia was maintained with oxygen, nitrous oxide, and sevoflurane. Pharyngeal packing was done by the surgeon before performing procedure. Sublingual mass was excised completely while mass at hard palate could be excised in piecemeal [Figure 2]. There was not significant blood loss and hemostasis was done. The duration of surgery was 40 min. At the end of surgery, neuromuscular blockade was reversed by injection neostigmine 0.07 mg/kg and injection glycopyrrolate 0.02 mg/kg and extubation could be done smoothly [Figure 3]. Patient was shifted to postoperative care unit. Nasogastric feeding was started on 2nd day and on fourth postoperative day, oral feeding was started and patient was discharged on fifth postoperative day.
Figure 2.
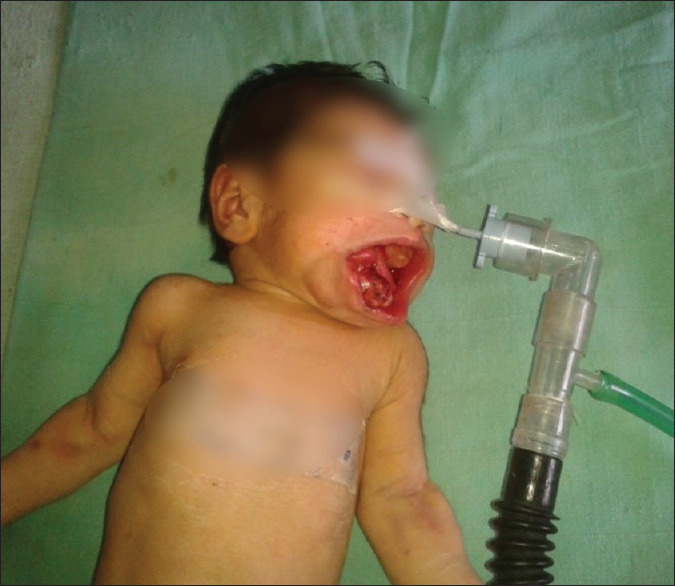
Image of child after excision of mass
Figure 3.
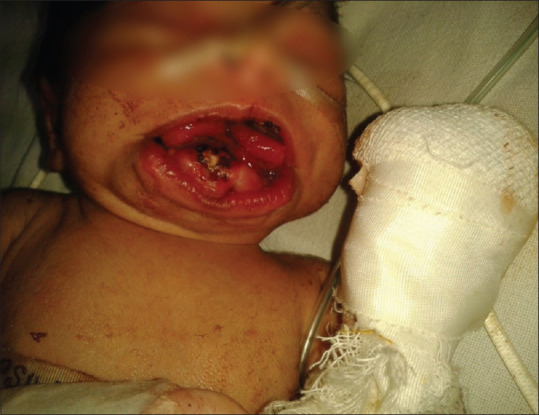
Image of child after extubation
Discussion
Congenital oropharyngeal tumors derived from embryonic germ cells are very rare tumors.[1] Large teratomas can cause respiratory tract obstruction at birth. Child presents with respiratory distress and difficulty in swallowing. Oropharyngeal tumors may require emergent airway management after birth.
The term teratoma is derived from the Greek word teraton, which means monster, and initially was used by Virchow in his first edition of his books on tumors, published in 1863.[2] Teratomas are neoplasms composed of tissue elements foreign to the anatomic site of origin.[3] Epignathus are intraoral malformations present at birth and the point of origin, specifically the alveolar bone or jaw.[4]
The etiology of epignathus is unknown and may arise from pluripotential cells in the region of Rathke's pouch that grow in a disorganized manner.[5] Teratomas contain elements derived from all three embryonic germ layers: ectoderm, mesoderm, and endoderm. The reported incidence is 1:4000 births.[6] They occur most commonly in the sacrococcygeal region, followed by the ovaries.[7] Congenital oral teratoma (epignathus) occurs in 1:35,000 to 1:200,000 live births. This accounts for 2% to 9% of all teratomas.[8] Teratomas are associated with concomitant malformations, with cleft palate being the most commonly associated anomaly.[8] This is thought to be because of mechanical obstruction caused by the neoplasm, preventing closure of the palatal shelves. Other malformations associated with epignathus teratomas are bifid tongues and noses.[9]
This is caused by a delayed swallowing reflex of the fetus and may be associated with a large oral mass. Polyhydramnios is often associated with an epignathus teratoma.[2] Antenatal diagnosis by ultrasound imaging is essential for perioperative care and management. This would allow a team approach in planning the cesarean section and further perioperative care and management, which may include an ex utero intrapartum treatment (EXIT).
Neonatal oral teratomas are benign lesion having potential to cause respiratory obstruction; hence, they should be diagnosed and excised earliest for good prognosis.[5] This rare site accounts for 5% to 6% of teratomas, which generally present in the neonatal period with large tumors. Most are mature or immature teratomas, but up to 20% are malignant. A review of 20 neonates noted that 35% presented with airway obstruction.[5]
The airway management in such type of cases is a challenge for anesthesiologist. An awake flexible fiberoptic intubation is often primary approach for management of difficult airway. Fiberoptic intubation requires considerable skill to perform it. In this case, we planned to isolate the airway by nasotracheal intubation. Prior to induction of anesthesia, all arrangement for emergency surgical tracheostomy and needle cricothyroidotomy is kept ready and necessary consent taken from the parents.
For visualizing the glottis, the approach that we used was different from the conventional one. In this, our blade entered through left side of the mouth, carefully and gently so as to avoid any injury to the mass. This approach helped us visualize the glottis with Miller's blade, and we did not need to use the fiberscope.
The MAJOR anesthetic concerns were as follows:
Securing the airway by tracheal intubation and maintaining it perioperatively
Doing intubation without causing any injury to the mass
Maintaining homeostasis so as to prevent dribbling into airway
Prevention of postoperative respiratory difficulties.
Our first major challenge was knowing the fact that the size of the mass was too big to make bag and mask ventilation achievable. To add to this concern was the vascularity of the mass, which needed us to carefully and gently handle the mask, while doing bag and mask ventilation.
To overcome our first hurdle, we chose a bigger size circular transparent mask to achieve a proper seal outside the mass and subsequently, bag and mask ventilation was possible.
Since we could do proper bag and mask ventilation, we ruled out the possibility of an awake technique approach and we carefully induced the patient.
But, since orotracheal intubation seemed remote in this patient, nasotracheal intubation was our first plan of management. And, since the baby was in respiratory difficulty prior to surgery also, we wanted to carefully handle nostrils, as, practically, they were the only possible venue of ventilation for the baby. Keeping this in mind, necessary preparation for use of fiberscope as well as for tracheostomy was done.
Although the possibility of orotracheal intubation seemed remote, we performed a check laryngoscopy by entering from the left side of the mouth and visualization of the glottis was possible.
Adequate premedication with atropine and oxymetazoline drops effectively controlled secretions as well as bleeding while nasotracheal intubation.
Once we accomplished successful nasotracheal intubation using Magill's forceps, maintenance of anesthesia was achieved using oxygen, nitrous, sevoflurane, and atracurium.
Prior pharyngeal packing minimized trickling of blood intraoperatively into the airway. Intraoperatively, another major concern was the problem of maintaining homeostasis during the procedure. A possibility of potential severe hemorrhage was kept in mind and adequate blood was arranged. Fortunately, manipulation and excision of the mass by the surgical team was careful and homeostasis was well maintained throughout and blood loss was minimal.
Postoperatively, smooth extubation was carried out and the baby was comfortable in the postoperative recovery room and manifested no signs and symptoms of respiratory difficulty. The baby was discharged on the fifth postoperative day, after achieving adequate oral feeding. The baby recovered excellently and will be planned up for palate repair at the age of 12 months.
Conclusion
Although airway compromise and its management are infrequent, with appropriate prior planning and effective communication between anesthesiologist and surgical team, it becomes far easier to manage such challenging cases and, therefore, successful outcome and optimal results become easily achievable.
Consent for publication
Written informed consent was obtained from the patient's family for publication of this case report.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient's family have given their consent for clinical information to be reported in the journal. The patient's family understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cohen MM., Jr Syndromes with cleft lip and cleft palate. Cleft Palate J. 1978;15:306–28. [PubMed] [Google Scholar]
- 2.Izadi K, Smith M, Askari M, Hackam D, Hameed AA, Bradley JP, et al. A patient with an epignathus: Management of a large oropharyngeal teratoma in a newborn. J Craniofac Surg. 2003;14:468–72. doi: 10.1097/00001665-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Holt GR, Holt JE, Weaver RG. Dermoids and teratomas of the head and neck. Ear Nose Throat J. 1979;58:320–31. [PubMed] [Google Scholar]
- 4.El-Musa KA, Shehadi RS, Shehadi S. Surgical repair of unidirectional palatopharyngeal epignathus: Case report and review of literature. Cleft Palate Craniofac J. 2006;43:367–9. doi: 10.1597/03-154.1. [DOI] [PubMed] [Google Scholar]
- 5.Azizkhan RG, Haase GM, Applebaum H, Dillon PW, Coran AG, King PA, et al. Diagnosis, management and outcome of cervicofacial teratomas in neonates: A childrens cancer group study. J Pediatr Surg. 1995;30:312–6. doi: 10.1016/0022-3468(95)90580-4. [DOI] [PubMed] [Google Scholar]
- 6.Raines D, Yarington CT., Jr Adult nasopharyngeal teratoma in the newborn. Ann Otol Rhinol Laryngol. 1964;73:957–62. doi: 10.1177/000348946407300408. [DOI] [PubMed] [Google Scholar]
- 7.Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg. 1974;9:389–98. doi: 10.1016/s0022-3468(74)80297-6. [DOI] [PubMed] [Google Scholar]
- 8.Liang CC, Lai JP, Lui CC. Cleft palate with congenital midline teratoma. Ann Plast Surg. 2003;50:550–4. doi: 10.1097/01.SAP.0000037462.83232.1E. [DOI] [PubMed] [Google Scholar]
- 9.Celik M, Akkaya H, Arda I, Hiçsönmez A. Congenital teratoma of the tongue: A case report and review of the literature. J Pediatr Surg. 2006;41:25–8. doi: 10.1016/j.jpedsurg.2006.08.039. [DOI] [PubMed] [Google Scholar]


