Abstract
Pulmonary arterial hypertension (PAH) is a progressive cardiovascular disease with high mortality. However, there were no efficient medical drugs for PAH to enormously improve the survival and quality of life measures. The present study aimed to explore the protective effect of baicalin against experimental PAH in vivo and vitro. All the experimental rats received intraperitoneal injection of monocrotaline (MCT) to induce PAH model. Baicalin was given by intragastric administration from 2 days after MCT injection. Forty animals were randomly divided into four groups: Control, MCT, saline‐, and baicalin‐treated groups (n = 10 in each). Post‐operation, hemodynamic data, and index of right ventricular hypertrophy (RVHI) were recorded to evaluate the inhibition of baicalin on MCT‐induced PAH. Furthermore, pulmonary artery smooth muscle cells (PASMCs) model induced by tumor necrosis factor‐α (TNF‐α) was used to observe the inhibition of vascular cells proliferation in vitro. The results demonstrated that baicalin significantly attenuated MCT‐induced right ventricular systolic pressure (RVSP), the index of right ventricular hypertrophy, and vessel wall thickness; inhibit inflammatory and cell proliferation induced by MCT or TNF‐α, respectively. In addition, we found that baicalin might protect against experimental PAH via regulating the TNF‐α/BMPR2 signaling pathway.
Keywords: Baicalin, BMPR2, PAH, TNF‐α, Vascular remodeling
Our present study demonstrated that: Baicalin could attenuate MCT‐induced pulmonary vascular remodeling through inhibition inflammatory response. Baicalin could reduce the protein expression of Nuclear factor‐κB (NF‐κB), intercellular cell adhesion molecule‐1 (ICAM1) and vascular cell adhesion molecule‐1 (VCAM1), but increased the vascular remodeling associated protein factors BMPR2, ID1, and Smad1//5/8 in MCT‐PH rats. Baicalin could inhibit TNF‐a‐PAMSC proliferation in vitro through regulation of BMPR2 signaling pathway.

Abbreviations
- BMP
bone morphogenetic proteins
- EndMT
endothelial‐to‐mesenchymal transition
- Exo
exosomes
- hUCMSCs
human umbilical cord mesenchymal stem cells
- ICAM
intercellular cell adhesion molecule
- LV+S
left ventricular plus septal weight
- MCT
monocrotaline
- NF‐κB
nuclear transcription factor‐κB
- PAEC
pulmonary arterial endothelial cell
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary arterial smooth muscle cells
- PCNA
proliferating cell nuclear antigen
- RVHI
right ventricular hypertrophy index
- RVSP
right ventricular systolic pressure
- SBP
systemic blood pressure
- TEM
transmission electron microscope
- TNF‐α
tumor necrosis factor alpha
- VCAM
vascular cell adhesion molecule
- Wnt
Wingless
- WT
wall thickness
- α‐SMA
alpha smooth muscle actin
1. INTRODUCTION
Pulmonary arterial hypertension (PAH) is a life‐threatening disease, characterized by excessive proliferation of pulmonary vascular cells, eventually leading to pulmonary vascular resistance, vascular remodeling, right ventricular hypertrophy, and heart failure. 1 , 2 The annual prevalence of PAH exceed 30–50/million individuals every year, for adults, the average age at presentation ranges from 36 to 50 year.
A large number of reports 3 , 4 , 5 showed that excessive proliferation of pulmonary artery smooth muscle cells (PASMCs) is an important pathogenesis in PAH vascular remodeling process. Bone morphogenetic protein type II receptor (BMPR2) is a key factor in the process of PAH pulmonary remodeling. 6 , 7 BMPR2 levels in pulmonary vasculature are significantly reduced in non‐genetic forms of PAH. More than 70% of heritable and 20% of idiopathic PAH cases have the BMPR2 gene mutations. 8 Current available therapies for PAH have limited efficacy, and new therapeutic strategies need to be developed. 9
Inflammatory is thought to be a powerful trigger factors, promoting the development of PAH in Bmpr2 +/− mice. 10 , 11 The expression of inflammatory markers in clinical PAH patients increased significantly. The inhibitory effects of IL‐1 receptor antagonist and antibodies on monocrotaline (MCT)‐induced experimental PAH model illustrate the importance of anti‐inflammatory treatment. Baicalin, one of several pharmacologically active flavones present in Scutellaria baicalensis Georgi (huang qin), is widely used in traditional Chinese medicinal herbs. Studies 12 , 13 , 14 , 15 found that baicalin has several bio‐pharmacological effects including antioxidant, anti‐inflammatory, antiviral, neuroprotective, anxiolytic, and anti‐cancer activities. Recent studies showed that baicalin has therapeutic potential for PAH through inhibiting pulmonary artery pressure and pulmonary vascular remodeling via anti‐inflammatory response, 15 , 16 however, the underlying mechanism remains elusive.
A recent study showed that inflammatory mediator tumor necrosis factor‐α (TNF‐α) can further promote the development of PAH by reducing BMPR2 expression in PASMCs [Yang et al., 2017]. Our previous studies have shown that baicalin could inhibit inflammation and improve PAH vascular remodeling by reducing TNF‐α factor expression and regulation of BMPR2 signaling pathways in MCT‐induced PAH rats, respectively. 16 , 17 However, it has not been illustrated whether the therapeutic effect of baicalin against PAH is associated with TNF‐α regulation of BMPR2 signaling pathways. Therefore, we tested the hypothesis that baicalin inhibits the pulmonary vascular remodeling via regulating the TNF‐α/BMPR2 signaling pathway. These will provide theoretical foundation for the clinical treatment of PAH.
2. MATERIALS AND METHODS
2.1. Animals experimental design
Male Wistar rats weighing 200–250 g were purchased from animal center of Second Hospital of Shandong University. Male rats were used to minimize hormonal effects (e.g., of estrogen). The animal protocols followed the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Shandong University. All rats received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health.
Baicalin (purity >95%) was purchased from Sigma and was dissolved in dimethyl sulfoxide (DMSO, Sigma‐Aldrich). PAH model was induced by intraperitoneal injection of 50 mg/kg of monocrotaline (MCT) for 6 weeks (Sigma‐Aldrich) as per our previously described with modifications. 16 Blood samples were collected at specific time points, the plasma concentration was determined, 18 and we choose the drug concentration according to the report. 15 In the present study, we have tested the lower dose (20 mg/kg, 50 mg/kg) and higher dose (200 mg/kg), the most effective dose is 100 mg/kg, and the main purpose of this present study is to explore the mechanism, so we only choose 100 mg/kg. Baicalin or the same amount of saline solution was given by intragastric administration from 2 days after MCT injection. Forty animals were randomly assigned to four groups: Control, MCT, saline‐, and baicalin‐treated groups (n = 10 in each).
2.2. Hemodynamic and the right ventricular hypertrophy assessment
Six weeks later, the rats were anesthetized by isoflurane inhalation (1.5%) and then hemodynamic data were recorded as previously described. 16 , 17 Briefly, via femoral vein access, an external diameter 0.9 mm polyethylene plastic (PE) catheter was advanced into the pulmonary artery for determination of heart rate (HR), systemic blood pressure (SBP), and right ventricular systolic pressure (RVSP). For assessment of right ventricular hypertrophy, the left ventricle (LV) and plus the septum (LV+S) were harvested, and the weight ratio of the RV to LV+S weight was calculated to quantify the right ventricular hypertrophy. The right ventricular hypertrophy index (RVHI) was calculated by the formula: RV/(LV + S) × 100.
2.3. Immunological and immunohistochemical analyses
Post‐operation, the lung and heart were quickly harvested and fixed in 4% paraformaldehyde and embedded in paraffin, and the serially sectioned at a thickness of 4–5 µm and then were stained with hematoxylin‐eosin (H&E). Evaluation of pulmonary artery structural remodeling, the vascular wall thickness (WT), vascular external diameter (ED), vascular wall area (WA), and total vascular area (TA), WT% (WT/ED), and WA% (WA/TA) was performed as previously study. 19 Fibrosis area was analyzed by Masson's trichrome staining, and then the sections were captured as digital images. The vascular was counted in blind on 30 sections by using a light microscope at a ×400 magnification. The average of the 10 high‐power fields (hpf) was randomly selected, and positively stained areas were padded with a single color and converted into pixels through optical density (OD) calibration.
Pulmonary artery proliferation was then carried out to analyze the expression of smooth muscle actin (a‐SMA) by immunohistochemistry and immunofluorescence. Briefly, after blocking unspecific protein binding with 5% bovine serum albumin for 30 min at room temperature, the lung sections were incubated overnight at 4°C with relevant antibodies. Images were taken with an Eclipse 90i microscope (Nikon). Staining was quantified using Image Pro Plus (IPP) 6.0 image analysis software (Media Cybernetics). All experiments were performed by two examiners blinded to the treatment assignment.
2.4. Cell preparation and culture
Rats PASMC were purchased from Procell Life Science & Technology Co, Ltd., and cultured in special culture medium (Procell) supplemented with 100 Ug/mL of penicillin, 100 IU/mL streptomycin, and 10% fetal bovine serum at 37°C in an incubator. Cells were passaged after 80% confluence, digested with 0.05% trypsin including 0.04% ethylene diamine tetraacetic acid (EDTA, Sigma‐Aldrich) in PBS. Recombinant rat TNF‐α was purchased from PeproTech (Cat:400‐14). Cells were treated with TNF‐α (5 ng/mL) for 24 h at 37°C in the presence of baicalin (100 μg/mL) for treatment or an equal volume of DMSO in culture medium group, respectively.
2.5. Cells proliferation and migration assay
PASMC proliferation was measured using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) assay kit. PASMCs were seeded in 96‐well culture plates (1–5×104 cells/well), after 80% confluence, cells were pretreatment with TNF‐α for 24 h, and then they were incubated for another 0 h, 24 h, 48 h, and 72 h with baicalin. Following incubation with MTT (5 mg/mL) for 4 h, absorbance was measured at 490 nm.
The migratory function of PASMC was evaluated using a modified Boyden chamber (Transwell; Corning Life Sciences, Inc.) assay with a polycarbonate filter with 8 µm pores placed between the upper and lower chambers. In brief, at 0 h and 24 h following TNF‐α pretreatment, cells were treated with baicalin containing 1% FBS and added to the upper chamber. The lower chamber was filled with complete medium in the presence of 10% FBS. After 48 h incubation at 37˚C under 5% CO2, cells that had not migrated were removed, whereas migrated cells were fixed in 4% paraformaldehyde for 10 min at room temperature and stained with the Crystal Violet Staining Solution kit (Solarbio, Beijing Solarbio Science & Technology Co., Ltd.). The number of migrated cells was counted using a Nikon Eclipse 90i microscope.
2.6. RNA preparation and quantitative reverse transcription‐PCR
Total RNA was extracted using the RNeasy Mini Kit with DNAse digestion (Qiagen) from lung tissues and cultured PASMCs. Quantitative real‐time polymerase chain reaction (RT‐PCR) analysis was performed using a M×3000P System. 1.5% agarose gel electrophoresis in the presence of ethidium bromide (Sigma‐Aldrich) was used to amplification fragments, and β‐actin as the internal control. Primers were designed using the Primer Express software package (Applied Biosystems): TNF‐α: 5’‐AAATGGGCTCCCTCTATCAGTTC‐3’ (forward primer) and 5’‐TCTGCTTGGTGGTTTGCTACGAC‐3’ (reverse primer); IL‐1β, 5’‐CCTTGTGCAAGTGTCTGAAGC‐3’ (forward primer) and 5’‐CCCAAGTCAAGGGCTTGGAA‐3 (reverse primer); IL‐6:5’‐AAGTCGGAGGCTTAATTACACATGT‐3’ (forward primer) and 5’‐AAGTGCATCATCGTTGTTCATACA‐3’ (reverse primer); β‐actin: 5’‐TCTACAATGAGCTGCGTGTG‐3’ (forward primer) and 5’‐GGTCAGGATCTTCATGAGGT‐3’ (reverse primer). The relative gene expression level was determined using the 2−ΔΔCT method.
2.7. Western blot analysis
The protein concentration was detected using a BCA assay kit, lysates were separated by polyacrylamide gel electrophoresis (PAGE) and electrotransferred onto a polyvinylidene fluoride (PVDF), the embranes were blocked in 5% skimmed milk‐Tris‐buffered saline plus Tween‐20 solution, and incubated with primary antibodies, respectively, overnight at 4°C. The primary antibody‐labeled membranes were then treated with the horseradish peroxidase (HRP)‐conjugated goat anti‐rabbit secondary antibody to IgG at room temperature for 1.5 h. The bound antibodies were visualized by using an enhanced chemiluminescence reagent (Millipore) followed by Bio‐Rad Image Lab™. Data were expressed as the relative density of the protein normalized to GAPDH. Primary antibodies of α‐SMA (ab21027), BMPR2 (ab170206), NF‐κB‐p65 (ab16502), p‐NF‐κB‐p65 (ab86299), Smad1/5/8 (sc‐6031), p‐Smad1/5/8 (sc‐12353), ID1 (ab168256), Cyclin D1(MA5‐15512), P27Kip1(ab32034), VCAM‐1 (ab134047), and ICAM (ab171123) were used, respectively.
2.8. Statistical analysis
All data are expressed as mean ± SD. Comparisons of parameters between two groups were made with unpaired Student t test. Comparisons of parameters among three groups were made with one‐way analysis of variance (ANOVA), followed by the Scheffe post hoc test. Statistical analysis was carried out by using the SPSS 19.0 software. p < .05 was regarded as significant statistical difference.
3. RESULTS
3.1. Effect of baicalin on PH vascular remodeling
We evaluated MCT‐induced lung and heart injury by detecting RVSP and right ventricular hypertrophy index RV/(LV+S), as shown in Figure 1A. Compared with control group, the RVSP and RV/(LV+S) in MCT administration group were increased obviously (p < .05), indicating that we successfully established PAH model in rats. However, RVSP and RV/(LV+S) were significantly inhibited in baicalin‐treated rats than that in MCT rats (p < .05). However, there was no significant difference in HR and SBP between groups (p > .05).
FIGURE 1.
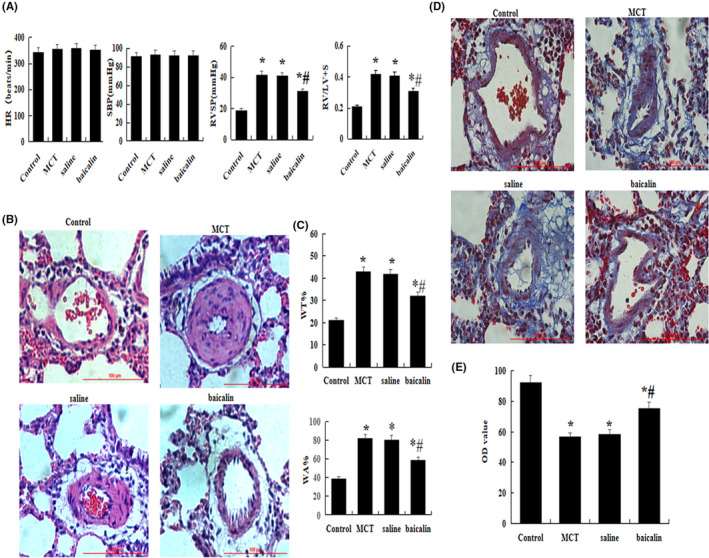
Effect of baicalin on MCT‐induced pulmonary hypertension. A comparison of the HR, SBP, RVSP, and RV/LV+S in each group. (B) A comparison of the medial thickness of the pulmonary arterial walls in each group. (C) Hematoxylin and eosin staining. (D) Massons staining. (E) A comparison of the OD value in each group. The data are present as mean ± SD; *p < .05 and **p < .01 compared with Control group; #p < .05 compared with MCT or Saline. Red bar =100 µm. HR, heart rate; MCT, monocrotaline; OD, optical density calibration; RV/LV+S, the ratio of right ventricular weight to left ventricle plus septum; RVSP, right ventricular systolic pressure; SBP, systemic blood pressure; WA%, the percent of vascular wall area (WA)/total vascular area (TA); WT%, the percent of the vascular wall thickness (WT)/vascular external diameter (ED)
For histological examination, serial lung sections were stained with H&E and Masson's trichrome to analyze the medal thickness of pulmonary arterial walls and the degree of fibrosis in vivo. As shown in Figure 1B and C, WT% and WA% of muscular arteries with an external diameter of 15 to 50 µm were significantly increased in MCT group than that in control, but notably decreased in baicalin group (p < .05). The same results were also showed in Figure 1D and E, the Masson's stained results showed that lung fibrosis was significantly reduced in baicalin group than that in MCT group in vivo (p < .05).
3.2. Effect of baicalin on smooth muscle cells
In this study, smooth muscularization cells marker α‐SMA were analyzed by immunohistochemistry and immunofluorescence. The results showed that the expression of α‐SMA was significantly increased in MCT group than that in control, however, that was significantly dropped in baicalin group (p < .05, Figure 2A–D).
FIGURE 2.
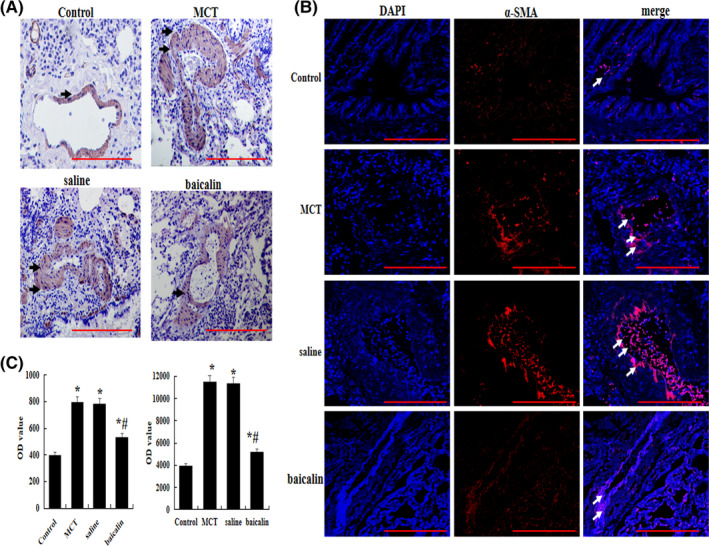
Effects of baicalin on MCT‐induced pulmonary artery smooth muscle. (A) Immunohistochemical analysis of the protein expression of α‐smooth muscle actin (a‐SMA). (B) Immunofluorescence analysis of the protein expression of a‐SMA. (C) A comparison of optical density (OD) value by immunohistochemical and immunofluorescence n = 10 rats per group; *p < 0.05 compared with Control group; # p < 0.05 compared MCT or Saline group; the data are present as mean ± SD. Red bar =100 µm
3.3. Effect of baicalin on inflammatory response
To explore the underlying mechanisms of baicalin against pulmonary vascular remodeling, the mRNA levels of interleukin IL‐1β and IL‐6 were analyzed by qRT‐PCR, and the protein levels of NF‐κB‐p65, p‐NF‐κB‐p65, TNF‐α, VCAM‐1, and ICAM were detected by western blot. The results showed that the inflammatory factors TNF‐α, VCAM‐1, ICAM, and the ratio of phosphorylated to total NF‐κB‐p65 levels were obviously evaluated in lung tissue when the animals were subjected to MCT, but the inflammatory levels were significantly reduced after treatment with baicalin compared with MCT group (p < .05, Figure 3A–C).
FIGURE 3.
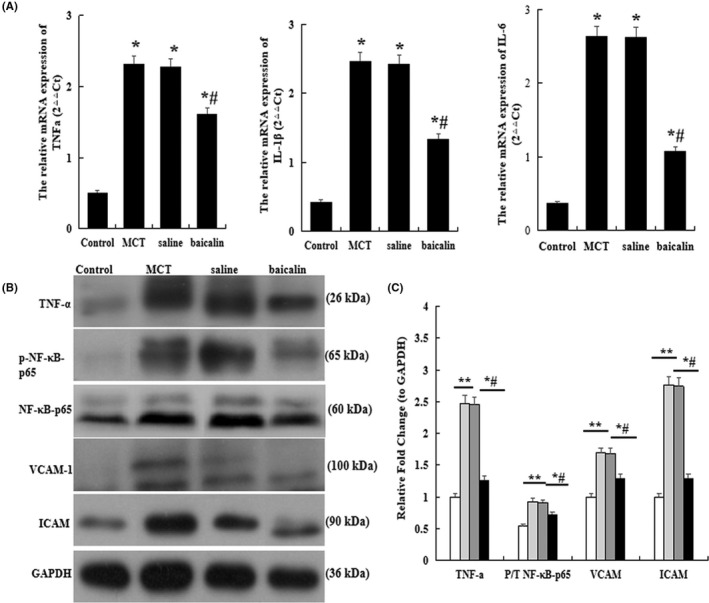
Effect of baicalin on inflammatory in the lung. Quantitative real‐time polymerase chain reaction analysis of the mRNA level of tumor necrosis factor‐α (TNF‐α), interleukin (IL)‐1β and IL‐6. (B) Western blots analysis of the protein expression of TNF‐α, phosphorylated Nuclear factor‐κB‐p65 (p‐NF‐κB‐p65), total NF‐κB‐p65, intercellular cell adhesion molecule‐1 (ICAM1), and vascular cell adhesion molecule (VCAM). (C) A comparison of the fold change of the TNF‐α, the ratio of p‐NF‐κB‐p65 to total NF‐κB‐p65, VCAM‐1 and ICAM in each group. n = 10 rats per group; *p < .05 compared with Control group; # p < .05 compared MCT or Saline group; The data are present as mean ± SD
3.4. Effect of baicalin on BMPR2 signaling pathway in vivo
BMPR2 signaling plays an important role in the pulmonary vascular remodeling. In the present study, we detected the protein levels of BMPR2, Smad1/5/8, p‐Smsd1/5/8, and ID1. The results manifested that the levels of BMPR2, Smad 1/5/8, p‐Smsd1/5/8, and ID1 were upregulated remarkably in baicalin group than that in MCT group (p < .05, Figure 4).
FIGURE 4.
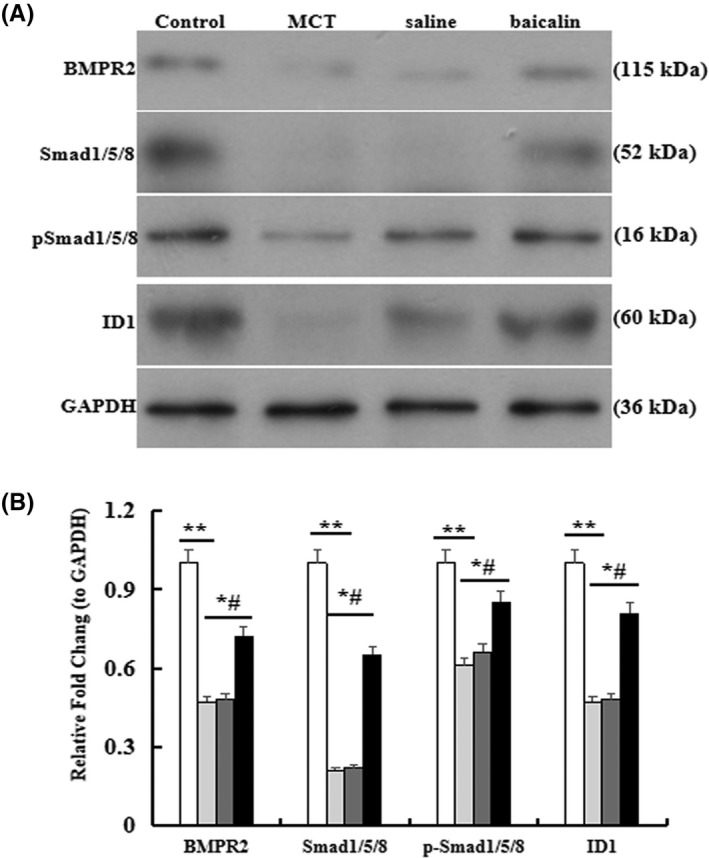
Effect of baicalin on BMPR2 signaling pathway in the lung. (A) Western blots analysis of the protein expression of bone morphogenetic protein type II receptor (BMPR2), p‐Smad1/5/8, and ID1 in lung tissue. (B) A comparison of the fold change of BMPR2, Smad1/5/8, p‐Smad1/5/8, and ID. n = 10 rats per group;*p < .05 compared with Control group; # p < .05 compared MCT or Saline group; The data are present as mean ± SD
3.5. Effect of baicalin on PASMC proliferation and migration
The effect of baicalin on proliferation and migration of PASMC were analyzed in vitro. Briefly, cells were pretreated with TNF‐α for 48 h and then were incubated with 100 μg/mL baicalin for 24, 48, and 72 h. MTT assay displayed that the cells viability rate was significantly increased in TNF‐α group compared with the normal groups. After treatment with baicalin, the cells viability rate was significantly decreased (p < .05, Figure 5A).
FIGURE 5.
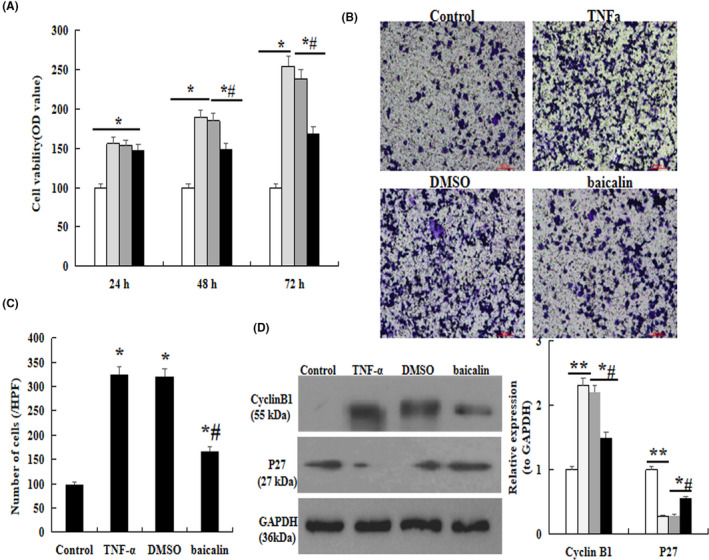
Effect of baicalin on TNF‐α–induced PASMC. MTT assay. (B) Transwell assay. (C) Comparison of number of cells. (D) The protein expression of Cyclin D1 and p27 analysis by western blot. *p < .05 compared with Control group; # p < .05 compared with TNF‐α group; The data are present as mean ± SD. Red bar =100 µm
Transwell assay was performed to observe the effect of baicalin on cells migratory ability. As demonstrated in Figure 5B, the migratory ability was significantly enhanced when the cells were induced by TNF‐α for 48 h, but which was obviously inhibited by baicalin (p < .05, Figure 5B and C).
Furthermore, western blot results showed that the expression of Cyclin D1 was significantly increased, but the P27kip1 was decreased remarkably in MCT group compared with the control. However, baicalin administration could significantly restore these results compared with that in MCT rats (p < .05, Figure 5D).
3.6. Effect of baicalin on TNF‐α induces BMPR2 signaling
To further explore the underlying mechanisms, the protein expression of BMPR2 signaling was analyzed by immunofluorescence and western blot in vitro. The results showed that BMPR2, ID1, and p‐Smad1/5/8 were significantly suppressed in TNF‐α‐induced cells. However, the protein expression was restored when the cells were treated with baicalin (p < .05, Figure 6).
FIGURE 6.
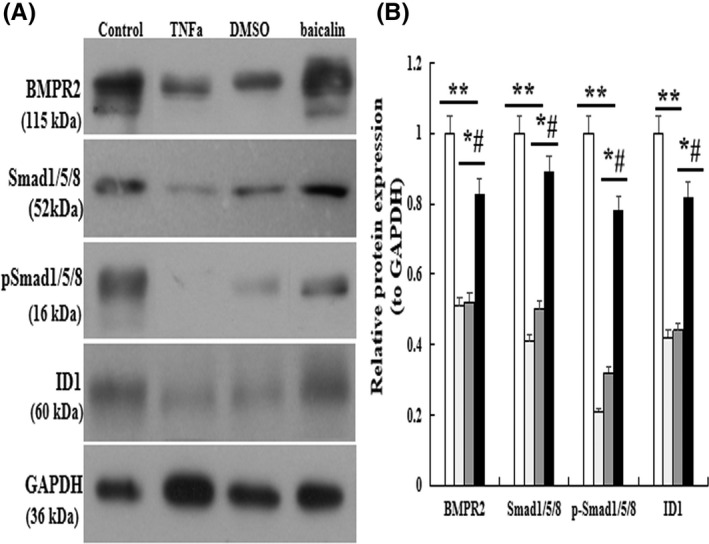
Effect of baicalin on BMPR2 signaling pathway in vitro. The protein expression of BMPR2, Smad1/5/8, p‐Smad1/5/8, and ID1 analysis by western blot. (B) Normalized band intensity quantification showing the fold change of BMPR2, Smad1/5/8, p‐Smad1/5/8, and ID. *p < .05 compared with Control group; # p < 0.05 compared with TNF‐α group; The data are present as mean ± SD
4. DISCUSSION
Pulmonary arterial hypertension (PAH) is a kind of refractory rare lung diseases, possessing the characteristic of the distal pulmonary arterial remodeling. 20 , 21 The pathogenesis of PAH is not very clear, so no effective therapy is available for it. Previous studies 16 , 17 suggested that baicalin has potential to inhibit the vascular remodeling in PAH. In the present study, our data confirmed that administration of baicalin could significantly reduce RVSP and RV/(LV + S) as compared with the MCT‐stressed rats (p < .05), warranting baicalin as a novel potential.
Inflammation 11 , 22 plays a key role in initiating and maintaining vascular remodeling in PAH animal models. Therefore, to alleviate inflammatory response might attenuate the development of PAH. 23 Earlier reports have shown that TNF‐α level was significantly higher in heritable and idiopathic PAH cases as compared with healthy people. 11 , 24 , 25 In our present study, the results showed significantly decreased mRNA and protein levels of TNF‐α, IL‐1β, IL‐6, and NF‐κB in baicalin group than that in MCT‐induced PAH group (p < .05). These indicate that baicalin could suppress the inflammatory response in MCT‐PAH rats. Therefore, we further analyzed the protein expressions of the adhesion molecules VCAM‐1, ICAM, and the ratio of phosphorylated‐to‐total NF‐κB‐p65 levels, which were involved in inflammatory processes. 26 , 27 Collectively, our results indicated that baicalin attenuated MCT‐induced inflammation by decreased expressions of adhesion molecules.
Bone morphogenetic proteins (BMPs) and their receptors are required for PAH‐induced right ventricular hypertrophy, which play an important role in the remodeling of pulmonary resistance vessels in the process of PAH. 10 , 28 , 29 BMPR2 was significantly decreased in MCT and chronic hypoxic induced rat PAH models. 30 , 31 BMP signaling regulates the occurrence of pulmonary fibrosis process through receptor‐mediated phosphorylation and p‐Smad1/5/8 transcription factors and alterations in gene transcription. In addition to BMPR2, BMP2, BMP4, BMP6, and BMP9 also have regulatory role for pulmonary vascular cell proliferation. 32 , 33 , 34 , 35 In the present study, we found that the protein expression of BMPR2, Smad1/5/8, p‐Smad1/5/8, and ID1 was significantly upregulated by 100 mg/kg baicalin (p < .05). Taken together, these results confirmed that baicalin could significantly repair the MCT‐induced PAH pulmonary vascular remodeling through regulation of the BMP signaling pathways.
A recent study showed that the expression of BMPR2 was inhibited by TNF‐α in pulmonary vascular cells 36 and pulmonary artery endothelial cells. 37 Many cytokines such as TNF‐α, IL‐1β, IL‐6, and IL‐8 are involved in the pathogenesis of PAH. In our study, only TNF‐α selectively reduced BMPR2 expression in distal PASMCs and PAECs. On the other hand, TNF‐α could promote pulmonary vascular remodeling in the setting of BMPR2 deficiency, increase BMP6 expression, and cause upregulation of transient p‐Smad1/5/8 responses in PASMCs. 38
To further explore the underlying mechanism of baicalin for PAH, we used TNF‐α‐induced PASMC injury model in vitro. The effect of baicalin on proliferation and migration abilities of PASMC were analyzed, MTT and Transwell results showed that the viability and proliferation rate were significantly increased after cells were treated with TNF‐α compared with the normal cells, but baicalin could significantly inhibit the cells proliferation and migration. Furthermore, the PASMC proliferation induced by TNF‐α was also observed through detecting the protein expression of Cyclin D1 and P27. Our results showed that the expression of CyclinD1 was significantly higher, P27 was significantly lower in baicalin group than that in TNF‐α group. Moreover, the expression of BMPR2 signaling was significantly upregulated in baicalin group compared to that in TNF‐α group. Thus, our data provide a strong evidence that baicalin inhibited the PAH vascular remodeling through suppressing the BMPR2 signaling derived by TNF‐α.
In summary, the present study demonstrated for the first time that the protective effect of baicalin against PAH vascular remodeling was via regulating the TNF‐α/BMPR2 signaling pathway.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health. Also, all experiments were approved by the Institutional Animal Care and Use Committee of Shandong University. All animals were anesthetized by isoflurane inhalation (1.5%–2%) and then euthanized by cervical dislocation. The work was done in accordance with the Helsinki Declaration's guidelines.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
DISCLOSURE
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Participated in research design: Xue, Zhang, Jiang, Luan.
Conducted experiments: Xue, Zhang, Jiang, Wang, Li, Xin, Luan.
Contributed new reagents or analytic tools: Xue, Jiang, Qi.
Performed data analysis: Xue, Jiang, Qi, Luan.
Wrote or contributed to the writing of the manuscript: Xue, Zhang, Jiang, Xin, Li, Luan.
Xue X, Zhang S, Jiang W, et al. Protective effect of baicalin against pulmonary arterial hypertension vascular remodeling through regulation of TNF‐α signaling pathway. Pharmacol Res Perspect.2021;9:00703 10.1002/prp2.703
These authors contributed equally to this work and should be considered co‐first authors.
Funding information
This project was supported by the Natural Science Foundation of Shandong Province (ZR201910230371), Youth Interdisciplinary Innovation Science Fund of Shandong University [2020QNQT019], the Natural Science Foundation of China (82073872), the Science and Technology Development Project of Shandong Province [2019GSF107093], and Science and Technology Development Project of Jinan Medical and Health [201907001, 202000358].
DATA AVAILABILITY STATEMENT
All data generated in this study are included in this manuscript.
REFERENCES
- 1. Lambert M, Capuano V, Boet A, et al. Characterization of kcnk3‐mutated rat, a novel model of pulmonary hypertension. Circ Res. 2019;125:678‐695. [DOI] [PubMed] [Google Scholar]
- 2. Wang J, Yu M, Xu J, et al. Glucagon‐like peptide‐1 (GLP‐1) mediates the protective effects of dipeptidyl peptidase IV inhibition on pulmonary hypertension. J Biomed Sci. 2019;26:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Man FS, Handoko ML, Vonk‐Noordegraaf A. The unknown pathophysiological relevance of right ventricular hypertrophy in pulmonary arterial hypertension. Eur Respir J. 2019;53:1900255. [DOI] [PubMed] [Google Scholar]
- 4. Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen‐associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156‐163. [DOI] [PubMed] [Google Scholar]
- 5. Kurakula K, Sun XQ, Happé C, et al. Prevention of progression of pulmonary hypertension by the Nur77 agonist 6‐mercaptopurine: role of BMP signaling. Eur Respir J. 2019;54:1802400. [DOI] [PubMed] [Google Scholar]
- 6. Hurst LA, Dunmore BJ, Long L, et al. TNF‐α drives pulmonary arterial hypertension by suppressing the BMP type‐II receptor and altering NOTCH signalling. Nat Commun. 2017;8:14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, Wei C, Kim IK, Janssen‐Heininger Y, Gupta S. Inhibition of nuclear factor‐κB in the lungs prevents monocrotaline‐induced pulmonary hypertension in mice. Hypertension. 2014;63:1260‐1269. [DOI] [PubMed] [Google Scholar]
- 8. Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia‐induced pulmonary hypertension. Circulation. 2012;126:2601‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma RK, Oliveira AC, Kim S, et al. Involvement of neuroinflammation in the pathogenesis of monocrotaline induced pulmonary hypertension. Hypertension. 2018;71:1156‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Hui H, Yang H, et al. Wogonoside induces cell cycle arrest and differentiation by affecting expression and subcellular localization of PLSCR1 in AML cells. Blood. 2013;121:3682‐3691. [DOI] [PubMed] [Google Scholar]
- 13. Lin L, Wu XD, Davey AK, Wang J. The anti‐inflammatory effect of baicalin on hypoxia/reoxygenation and TNF‐alpha induced injury in cultural rat cardiomyocytes. Phytother Res. 2010;24:429‐437. [DOI] [PubMed] [Google Scholar]
- 14. Woo AY, Cheng CH, Waye MM. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc Res. 2005;65:244‐253. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Pu Z, Wang J, Zhang Z, Hu D, Wang J. Baicalin inhibits hypoxia‐induced pulmonary artery smooth muscle cell proliferation via the AKT/HIF‐1α/p27‐associated pathway. Int J Mol Sci. 2014;15:8153‐8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Z, Zhang L, Sun C, et al. Baicalin attenuatesmonocrotaline‐induced pulmonary hypertension through bone morphogenetic protein signaling pathway. Oncotarget. 2017;8:63430‐63441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luan Y, Chao S, Ju ZY, et al. Therapeutic effects of baicalin on monocrotaline‐induced pulmonary arterial hypertension by inhibiting inflammatory response. Int Immunopharmacol. 2015;26:188‐193. [DOI] [PubMed] [Google Scholar]
- 18. Hwang YH, Yang HJ, Kim DG, Ma JY. Inhibitory effects of multiple‐dose treatment with Baicalein on the pharmacokinetics of ciprofloxacin in rats. Phytother Res. 2017;31:69‐674. [DOI] [PubMed] [Google Scholar]
- 19. Mitani Y, Maruyama K, Sakurai M. Prolonged administration of L‐arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation. 1997;96:689‐697. [PubMed] [Google Scholar]
- 20. Han JC, Guild SJ, Pham T, et al. Left‐ventricular energetics in pulmonary arterial hypertension‐induced right‐ventriculhypertrophic failure. Front Physiol. 2018;8:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pham T, Nisbet L, Taberner A, Loiselle D, Han JC. Pulmonary arterial hypertension reduces energy efficiency of right, but not left, rat ventricular trabeculae. J Physiol. 2018;596:1153‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hemnes AR, Rathinasabapathy A, Austin EA, et al. A potential therapeutic role for angiotensin‐converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018;51:1702638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen‐Kaminsky S, Hautefort A, Price L, Humbert M, Perros F. Inflammation in pulmonary hypertension: what we know and what we could logically and safely target first. Drug Discov Today. 2014;19:1251‐1256. [DOI] [PubMed] [Google Scholar]
- 24. Montani D, Humbert M, Souza R. ,Letter by Montani et al. regarding article, "Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension". Circulation. 2011;123:e614. [DOI] [PubMed] [Google Scholar]
- 25. Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920‐927. [DOI] [PubMed] [Google Scholar]
- 26. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678‐689. [DOI] [PubMed] [Google Scholar]
- 27. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803‐815. [DOI] [PubMed] [Google Scholar]
- 28. Hautefort A, Mendes‐Ferreira P, Sabourin J, et al. BMPR2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation. 2019;139:932‐948. [DOI] [PubMed] [Google Scholar]
- 29. Yan L, Cogan JD, Hedges LK, Nunley B, Hamid R, Austin ED. The Y chromosome regulates BMPR2 expression via SRY: a possible reason "Why" fewer males develop pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;198:1581‐1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crosby A, Soon E, Jones FM, et al. Hepatic shunting of eggs and pulmonary vascular remodeling in Bmpr2(+/‐) mice with schistosomiasis. Am J Respir Crit Care Med. 2015;192:1355‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murphy N, Gaynor KU, Rowan SC, et al. Altered expression of bone morphogenetic protein accessory proteins in murine and human pulmonary fibrosis. Am J Pathol. 2016;186:600‐615. [DOI] [PubMed] [Google Scholar]
- 32. Cai P, Kovacs L, Dong S, Wu G, Su Y. BMP4 inhibits PDGF‐induced proliferation and collagen synthesis via PKA‐mediated inhibition of calpain‐2 in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2017;312:L638‐L648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morrell NW, Bloch DB, ten Dijke P, et al. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016;13:106‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tielemans B, Delcroix M, Belge C, Quarck R. TGF‐β and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov Today. 2019;24:703‐716. [DOI] [PubMed] [Google Scholar]
- 35. Tu L, Desroches‐Castan A, Mallet C, et al. Selective BMP‐9 inhibition partially protects against experimental pulmonary hypertension. Circ Res. 2019;124:846‐855. [DOI] [PubMed] [Google Scholar]
- 36. Singhatanadgit W, Salih V, Olsen, I . Bone morphogenetic protein receptors and bone morphogenetic protein signaling are controlled by tumor necrosis factor‐alpha in human bone cells. Int J Biochem Cell Biol. 2006;38:1794‐1807. [DOI] [PubMed] [Google Scholar]
- 37. Kim CW, Song H, Kumar S, et al. Anti‐inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1350‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu PB, Deng DY, Beppu H, et al. Bone morphogenetic protein (BMP) type II receptor is required for BMP‐mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem. 2008;283:3877‐3888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated in this study are included in this manuscript.


