Abstract
Background/Aim: Fusion of histone-lysine N-methyltransferase 2A gene (KMT2A) with the Rho guanine nucleotide exchange factor 12 gene (ARHGEF12), both located in 11q23, was reported in some leukemic patients. We report a KMT2A-ARHGEF12 fusion occurring during treatment of a pediatric acute myeloid leukemia (AML) with topoisomerase II inhibitors leading to a secondary acute lymphoblastic leukemia (ALL). Materials and Methods: Multiple genetic analyses were performed on bone marrow cells of a girl initially diagnosed with AML. Results: At the time of diagnosis with AML, the t(9;11)(p21;q23)/KMT2A-MLLT3 genetic abnormality was found. After chemotherapy resulting in AML clinical remission, a 2 Mb deletion in 11q23 was found generating a KMT2A-ARHGEF12 fusion gene. When the patient later developed B lineage ALL, a t(14;19)(q32;q13), loss of one chromosome 9, and KMT2A-ARHGEF12 were detected. Conclusion: The patient sequentially developed AML and ALL with three leukemia-specific genomic abnormalities in her bone marrow cells, two of which were KMT2A-rearrangements.
Keywords: Pediatric leukemia, acute myeloid leukemia, acute lymphoblastic leukemia, chemotherapy, fusion gene, KMT2A, ARHGEF12, KMT2A-ARHGEF12, KMT2A-MLLT3
The histone-lysine N-methyltransferase 2A (KMT2A, also known as MLL) gene in 11q23 (1,2) may fuse with more than 100 different partners in acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia, myelodysplastic syndromes, lymphomas, and solid tumors (3). Some of the resulting chimeras are common, such as the fusions with the AF4/FMR2 family member 1 (AFF1) and MLLT3 super elongation complex subunit (MLLT3) genes generated by t(4;11)(q21;q23) in ALL (KMT2A-AFF1) and t(9;11)(p21;q23) in AML (KMT2A-MLLT3), while others have only been reported in few or single cases (4,5). The prognostic impact of the frequent KMT2A fusions is well known (6,7); however, knowledge about the clinical consequences of the infrequent chimeras is inadequate. For this reason, not just because of biological curiosity, the reporting of cases involving uncommon KMT2A fusions is important as recently exemplified by the description of rare KMT2A-ELL fusion transcripts in pediatric AML associated with myeloid sarcomas (8-11).
We herein report the first pediatric leukemia, and the sixth case overall, in which fusion of KMT2A with the Rho guanine nucleotide exchange factor 12 (ARHGEF12) gene was detected.
Materials and Methods
Ethics statement. The study was approved by the regional ethics committee (Regional komité for medisinsk forskningsetikk Sør-Øst, Norge, http://helseforskning.etikkom.no), and written informed consent was obtained from the patient’s parents. The Ethics Committee’s approval included a review of the consent procedure. All patient information has been anonymized.
Case report. The patient was a girl diagnosed with AML at an age of 9.5 years (Table I, Sample S0), after a period of three months with fatigue, pallor, headache, and dizziness. There was no history of hematologic diseases, leukemia or frequent cancers in the family. At admittance, she was in good shape. Physical examination was normal except for pallor. Spleen and liver size were normal. Her blood tests were abnormal with hemoglobin 5.8 g/dl, platelet count 95×109/l, white blood cell count 4.9×109/l, and neutrophils 0.1×109/l. Bone marrow investigation revealed findings indicative of acute myelomonocytic leukemia (AML M4), and cytogenetic analysis showed a t(9;11)(p21;q23) chromosome translocation (Table I, sample S0, see below).
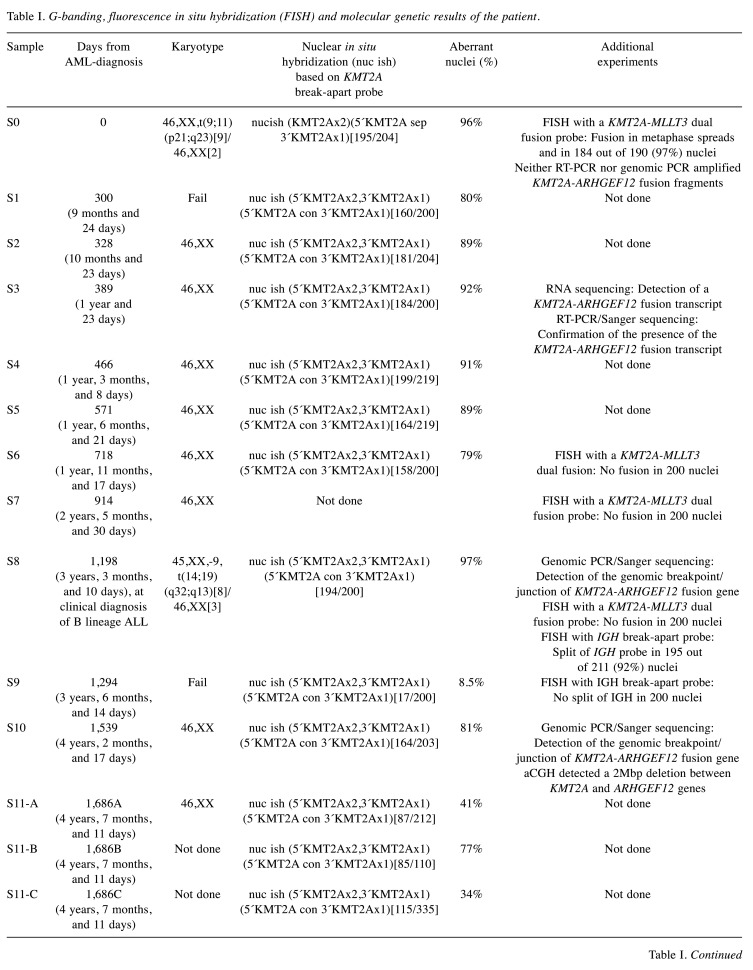
Table I. G-banding, fluorescence in situ hybridization (FISH) and molecular genetic results of the patient.
Clinical diagnosis of acute myeloid leukemia (AML) at time point (sample) S0, diagnosis of B lineage acute lymphoblastic leukemia (ALL) at time point S8. Sample 11 (S11-A, S11-B, and S-11C) represents the last bone marrow before stem cell transplantation. Sample 12 represents bone marrow 3 months after stem cell transplantation. RT-PCR: reverse transcription-polymerase chain reaction. aCGH: array comparative genomic hybridization.A: Bone marrow. B: CD34+/CD117+ cells. C: Negative fraction.
The girl was treated according to the NOPHO-DBH 2012 protocol (12) where she was classified as a standard risk patient. She received five chemotherapy courses including the drugs liposomal daunorubicin, mitoxantrone, etoposide, cytarabine, and fludarabine. Following completion of therapy, she entered a prolonged phase with moderate pancytopenia. During this period which lasted two years and three months, repeated bone marrow examinations were performed (Table I, samples S1-S7). They revealed hematological remission and a normal karyotype but with a persisting KMT2A rearrangement, however one that was different from the original KMT2A-MLLT3 (Table I, samples S1-S7, see below). Flow cytometry showed a small persisting CD34+, CD38+ cell population with aberrant phenotype (reduced HLA-DR, CD33 weak/negative, CD11a weak, CD117 heterogenous), in a proportion decreasing from 1.5% to 0.05%, in addition to the phenotypically normal CD34+ CD38+ myeloid precursors, over eight months. The girl was always in good general health and lived a completely normal life. Following normalization of her peripheral blood values, no more bone marrow examinations were performed until she, two years and nine months after start of the last AML chemotherapy course (or three years and three months after the initial diagnosis of AML), again developed pancytopenia. She was now diagnosed with ALL (Table I, S8). The bone marrow contained 90% blasts of pre-B phenotype, whereas cytogenetic analysis revealed a t(14;19)(q32;q13) chromosome translocation together with loss of one chromosome 9 (Table I, sample S8, see below). Treatment according to the NOPHO-ALL 2008 protocol (13) was begun but, due to increased toxicity, had to be modified. Bone marrow controls during therapy confirmed a good and early treatment response with swift reduction of the lymphoblasts. However, the same small population of abnormal CD34+, CD38+ cells detected shortly after discontinuation of the primary AML treatment and persisting ever since, was still detectable. It was therefore decided to give her one dose of Daratumomab (a CD38-antibody) 16 mg/kg with the aim of eradicating the abnormal clone before stem cell transplantation. After the one dose of Daratumomab CD38 was not detected on any CD34+ cells, using the standard monoclonal anti-CD38. However, using a multiepitope anti-CD38 the antigen could still be detected, indicating down-regulation of the epitope detected by the monoclonal antibody. Other markers demonstrated that the abnormal population was still present in the first controls after Daratumomab. The first bone marrow negative for CD34+ cells was confirmed 11 weeks after initiation of Daratumomab treatment, i.e., more than four years after the clone was first detected. Six weeks after the first negative bone marrow, allogeneic stem cell transplantation was performed with a human leukocyte antigen (HLA) - matched, unrelated donor after standard ALL-conditioning regimen consisting of total body irradiation (TBI) and etoposide. The patient is presently in complete remission, three months after stem cell transplantation. The current bone marrow assessment did not detect abnormal CD34+CD38+ cells or rearrangement of the KMT2A gene (see below).
G-banding and fluorescence in situ hybridization (FISH) analyses. Bone marrow cells were short-term cultured, G-banded, and analyzed cytogenetically as previously described (14).
FISH analyses of bone marrow interphase nuclei and metaphase spreads were performed with the Cytocell KMT2A (MLL) and IGH break-apart probes, as well as with KMT2A-MLLT3 translocation dual fusion probes (Cytocell, Oxford Gene Technology, Begbroke, Oxfordshire, UK). Fluorescent signals were captured and analyzed using the CytoVision system (Leica Biosystems, Newcastle, UK).
DNA and RNA isolation and complementary DNA (cDNA) synthesis. Genomic DNA was extracted from the patient’s bone marrow samples at diagnosis as well as 1,198 and 1,539 days after the diagnosis (Table I, samples S0, S8, and S10) using the Maxwell 16 Instrument System and the Maxwell 16 Cell DNA Purification Kit (Promega, Madison, WI, USA). Total RNA was extracted from the patient’s bone marrow at diagnosis and after 389 days (Table I, samples S0, and S3) using the miRNeasy Mini Kit (Qiagen, Hilden, Germany) and the QiaCube automated purification system according to the manufacturer’s instructions (Qiagen). The concentration and purity of DNA and RNA were measured with the QIAxpert microfluidic UV/VIS spectrophotometer (Qiagen). In addition, the Agilent 2100 bioanalyzer and the RNA Integrity Number (RIN) were used to assess the RNA quality (15). RIN of RNA was 6.6. cDNA was synthesized from one µg of total RNA using the iScript Advanced cDNA Synthesis Kit for RT-qPCR according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). The quality of the cDNA synthesis was assessed by amplification of a cDNA fragment of the ABL protooncogene 1, non-receptor tyrosine kinase (ABL1) gene using the primer combination ABL1-91F1/ABL1-404R1 (16).
RNA sequencing. High-throughput paired-end RNA-sequencing was performed at the Genomics Core Facility, Norwegian Radium Hospital, Oslo University Hospital (http://genomics.no/oslo/). For library preparation from total RNA, the Illumina TruSeq RNA Access Library Prep kit was used according to Illuminaʼs protocol (Illumina, San Diego, CA, USA). Sequencing was performed on a NextSeq 550 System (Illumina) and 76 million reads were generated. The FASTQC software was used for quality control of the raw sequence data (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The software FusionCatcher was used to find fusion transcripts (17,18).
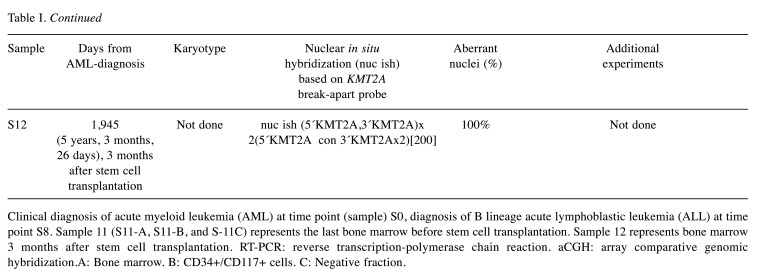
PCR analyses. The primers used for PCR amplification and Sanger sequencing are listed in Table II. For reverse transcription-polymerase chain reaction (RT-PCR) and cycle Sanger sequencing, the BigDye Direct Cycle Sequencing Kit was used (ThermoFisher Scientific, Waltham, MA, USA) according to the company’s recommendations. As template, cDNA corresponding to 20 ng total RNA was used. The primer combinations were M13For-MLL-4010F1/M13Rev-ARHGEF12-1579R1 and M13For-MLL-4115F1/M13Rev-ARHGEF12-1515R1. For the detection of possible KMT2A-ARHGEF12 chimeric cDNA fragments at initial diagnosis, the primer combination MLL-4116-F1/ARHGEF12-1502-R1 was used as described below.
Table II. Primers used for PCR amplification and Sanger sequencing analyses. The M13 forward and reverse primer sequences are in bold and italics.
Genomic PCR amplifications were performed in 25 μl reaction volume which contained 12.5 μl Premix Ex Taq™ DNA Polymerase Hot Start Version (Takara Bio Europe/SAS, Saint-Germain-en-Laye, France), 100 ng of genomic DNA, and 0.4 μM of each of the forward and reverse primers. The primer combinations were MLL-4116-F1/ARHGEF12-1502-R1 and MLL-4202-F1/ARHGEF12-1437-R1. The PCR cycling conditions were an initial denaturation step at 94˚C for 30 sec followed by 35 cycles of 7 sec at 98˚C and 2 min at 68˚C, and a final extension for 5 min at 68˚C. Three μl of the PCR products were stained with GelRed (Biotium, Fremont, CA, USA), analyzed by electrophoresis through 1% agarose gel, and photographed. DNA gel electrophoresis was performed using lithium borate buffer (19). The remaining PCR products were purified using the MinElute PCR Purification Kit (Qiagen) and direct sequenced using the dideoxy procedure with the BigDye terminator v1.1 cycle sequencing kit following the company’s recommendations (ThermoFisher Scientific).
Sequence analyses were performed on the Applied Biosystems SeqStudio Genetic Analyzer system (ThermoFisher Scientific). The basic local alignment search tool (BLAST) software (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used for computer analysis of sequence data (20). The BLAT alignment tool and the human genome browser at UCSC were also used to map the sequences on the Human GRCh37/hg19 assembly (21,22).
Array comparative genomic hybridization (aCGH) analysis. aCGH was performed using the CytoSure array products (Oxford Gene Technology, Begbroke, Oxfordshire, UK) following the company’s protocols. Thus, the CytoSure Genomic DNA Labelling Kit was used for the labelling of 1 μg of patient’s and reference DNAs and the CytoSure Cancer +SNP array for hybridization. The patient’s DNA was that isolated from a sample drawn 1,539 days after the initial diagnosis of AML (Table I, S10). The reference DNA was Promegaʼs human genomic female DNA (Promega, Madison, WI, USA). The slides were scanned by an Agilent scanner using Agilent Feature Extraction Software (version 10.7.3.1). Data were analysed using the CytoSure Interpret analysis software (version 4.9.40). Annotations are based on human genome build 19.
Results
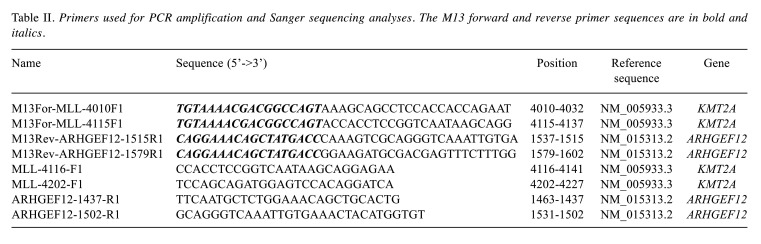
G-banding analyses. The data from G-banding and FISH analyses are summarized in Table I. The G-banding analysis of bone marrow cells at diagnosis yielded the karyotype 46,XX,t(9;11)(p21;q23)[9]/46,XX[2] (Figure 1A). Subse-quently, bone marrow G-banding analyses always showed a normal 46,XX karyotype except for the sample obtained 1,198 days after diagnosis. At that point, the karyotype had become 45,XX,-9, t(14;19)(q32;q13)[8]/ 46,XX[3] and the patient had developed B lineage ALL (Table I, sample S8) (Figure 1B).
Figure 1. Cytogenetic analyses of the two pediatric leukemias. (A) Karyogram showing the t(9;11)(p21;q23) found at diagnosis, when the patient had acute myeloid leukemia (AML). (B) Karyogram showing the loss of chromosome 9 and the t(14;19)(q32;q13) found when the patient was diagnosed with acute lymphoblastic leukemia (ALL) 1,198 days after the initial AML. Breakpoint positions are indicated by arrows.
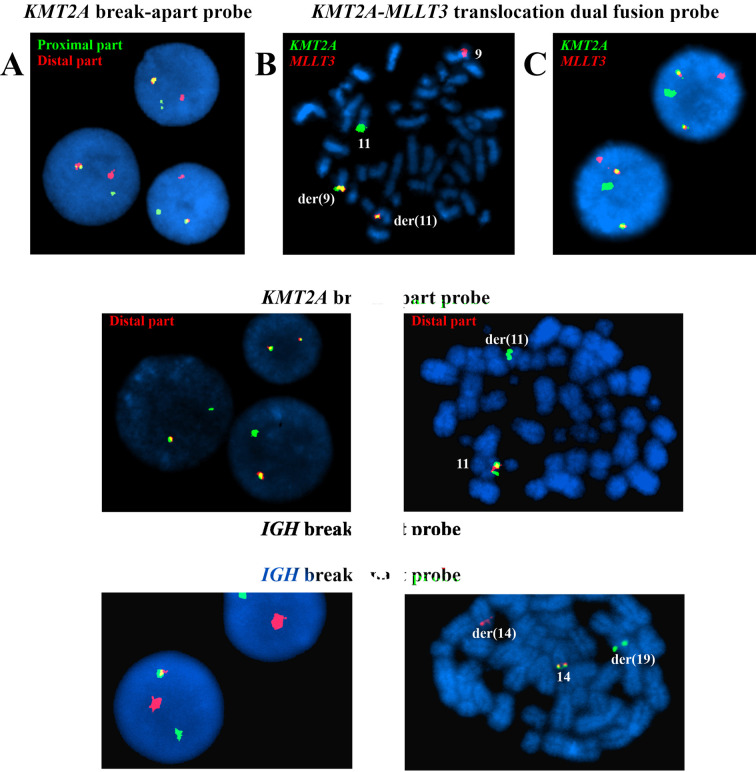
FISH analyses. Three FISH analyses were performed at initial diagnosis of AML. Interphase FISH with the KMT2A break-apart probe showed a normal (yellow) as well as split (separated red and green) signals of the probe in 195 out of 204 examined nuclei (96%) (Table I, sample S0) (Figure 2A). FISH analysis with a KMT2A-MLLT3 translocation dual fusion probe on metaphase spreads showed the KMT2A-MLLT3 and MLLT3-KMT2A fusion genes on chromosomes der(11) and der(9), respectively (Figure 2B). Interphase FISH with the same KMT2A-MLLT3 translocation dual fusion probe showed a normal red signal corresponding to the MLLT3 gene, a normal green signal corresponding to the KMT2A gene, and two yellow fusion signals corresponding to KMT2A-MLLT3 and MLLT3-KMT2A in 184 out of 190 (97%) examined nuclei (Figure 2C). In addition, at another diagnostic laboratory, a KMT2A-MLLT3 fusion transcript was detected (data not shown) which was in an agreement with the G-banding and interphase FISH results.
Figure 2. Fluorescence in situ hybridization (FISH) analyses of pediatric leukemia. (A) Interphase FISH at initial diagnosis of AML with the KMT2A break-apart probe showing a normal (yellow) and split (separated red and green) signals of the probe in 3 nuclei. (B) FISH analysis at initial diagnosis of AML with the KMT2A-MLLT3 translocation dual fusion probe on metaphase spreads showing a normal green signal on chromosome 11, corresponding to KMT2A, a normal red signal on chromosome 9, corresponding to MLLT3, and two yellow fusion signals on der(11) and der(9) corresponding to the KMT2A-MLLT3 and MLLT3-KMT2A fusion genes, respectively. (C) Interphase FISH at initial diagnosis of AML on two nuclei using the KMT2A-MLLT3 translocation dual fusion probe showing a normal green signal, corresponding to KMT2A, a normal red signal, corresponding to MLLT3, and two yellow fusion signals corresponding to the KMT2A-MLLT3 and MLLT3-KMT2A fusion genes. (D) Interphase FISH with the breakapart KMT2A probe on the sample obtained 300 days after diagnosis showing deletion of the distal part of the KMT2A probe (lack of red signal) in two nuclei and two normal (yellow) KMT2A signals in one nucleus. (E) FISH analysis with the break-apart KMT2A probe on metaphase spread from the sample obtained 1,198 days after diagnosis, when a t(14;19)(q32;q13) was seen by karyotyping and the patient had developed ALL. The distal part (red signal) of the probe is absent in one of the two copies of chromosome 11. (F) Interphase FISH with the IGH break-apart probe on the sample obtained 1,198 days after diagnosis, when a t(14;19)(q32;q13) was seen by karyotyping and the patient had developed ALL. A normal (yellow) and split (separated red and green) signals of the probe are shown in 2 nuclei. (G) FISH analysis with the IGH break-apart probe on a metaphase spread from the sample obtained 1,198 days after diagnosis, when a t(14;19)(q32;q13) was seen by karyotyping and the patient had developed ALL. A normal (yellow) signal on chromosome 14 together with separate red, on der(14), and green, on der(19), probe signals are shown.
In the sample obtained 300 days after diagnosis, interphase FISH with a break-apart KMT2A probe for the first time showed loss of the distal part of the KMT2A probe, namely in 160 out of 200 nuclei (80%) (Table I, sample S1, Figure 2D). This pattern with deletion or loss of the distal part of KMT2A has since persisted throughout the entire follow-up period (Table I, samples S1-S11-A) until the patient was transplanted. Deletion of the distal part of the KMT2A probe was also found in 77% of the CD34+CD117+ cells and in 34% of the negative fraction of the sorted cells 1,686 days after the diagnosis (Table I, samples S11-B and S11-C).
In the sample obtained 90 days after allogenic stem cell transplantation the KMT2A break-apart probe showed two normal (yellow) signals in 200 interphase nuclei (Table I, sample S12).
Interphase FISH with a KMT2A-MLLT3 translocation dual fusion probe on samples obtained 718 days and 914 days after the initial diagnosis did not detect any KMT2A-MLLT3 fusion in 200 examined nuclei (Table I, samples S6 and S7).
In the sample obtained 1,198 days after diagnosis, when a t(14;19)(q32;q13) was seen by karyotyping and the patient had developed ALL, the FISH results were as follows: No KMT2A-MLLT3 fusion was seen in 200 nuclei, deletion of the distal part of the KMT2A probe was detected in 194 out of 200 nuclei (97%) and on metaphase spreads (Figure 2E), and splitting of the IGH probe was seen in 195 out of 211 (92%) nuclei as well as on metaphase spreads (Figure 2F and 2G) (Table I, sample S8). In the sample obtained 1,294 days after diagnosis, FISH analyses showed deletion of the distal part of the KMT2A probe in 17 out of 200 nuclei (8.5%) and no split of IGH in 200 nuclei (Table I, sample S9).
RNA-sequencing and molecular genetic confirmation of the fusion. After the G-banding/interphase FISH results were obtained from the sample drawn at day 1,539, we decided to perform retroactive RNA sequencing of the bone marrow sample obtained 389 days after the diagnosis. At that point in time (389 days), the G-banding analysis was normal whereas FISH with the break apart KMT2A probe showed deletion of the distal part of the probe in 184 out of 200 (92%) examined nuclei (Table I, sample S3).
Using FusionCatcher on the raw sequencing data obtained from the Genomics Core Facility, a KMT2A-ARHGEF12 fusion transcript was found in which exon 9 of KMT2A (nt 4241 in sequences with accession numbers NM_005933.3 and NM_001197104.1) was fused to exon 14 of ARHGEF12 (nt 1428 in sequence with accession number NM_015313.2) (AATTCCAGCAGATGGAGTCCACAGGATCAGAGTGGACTTTAAG*ATCAATGGACAGTGCAGCTGTTTCCAGAGCATTGAATTACTAA). No reciprocal ARHGEF12-KMT2A fusion transcript was found.
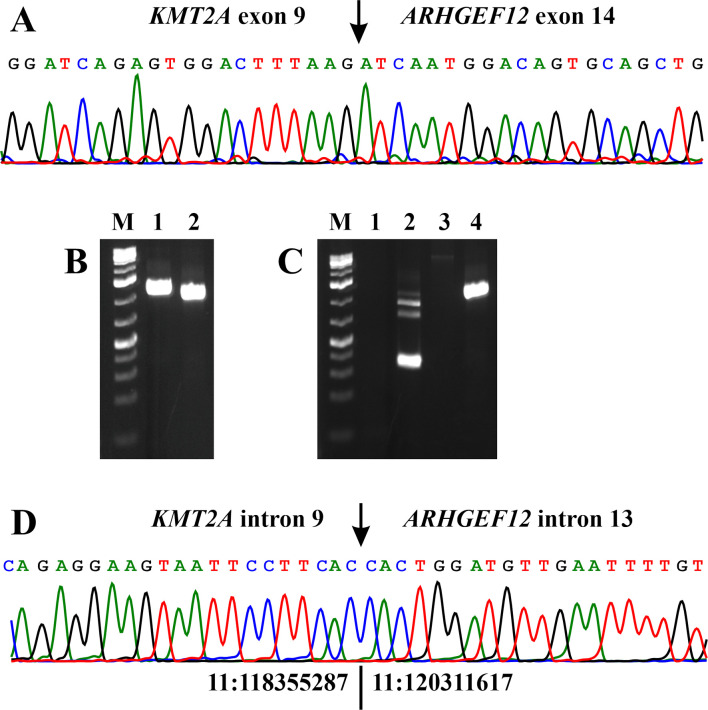
RT-PCR and cycle Sanger sequencing with the primers M13For-MLL-4010F1/M13Rev-ARHGEF12-1579R1 and M13For-MLL-4115F1/M13Rev-ARHGEF12-1515R1 confirmed the results obtained by RNA sequencing/Fusion Catcher analysis (Figure 3A).
Figure 3. PCR analyses of the pediatric leukemias. (A) Partial sequence chromatogram showing the junction position of exon 9 of the KMT2A with exon 14 of ARHGEF12 in the chimeric transcript. (B) Gel electrophoresis showing the amplified KMT2A-ARHGEF12 genomic DNA fragment using two primer combinations and template DNA extracted from the patient’s bone marrow cells 1,539 days after the initial diagnosis. Lane 1: Primer combination MLL-4116F1/ARHGEF12-1502R1. Lane 2: Primer combination MLL-4202-F1/ARHGEF12-1437-R1. (C) Gel electrophoresis showing the absence of amplified KMT2A-ARHGEF12 cDNA (lane 1) and genomic DNA (lane 3) fragments at diagnosis when the leukemic cells had t(9;11)(p21;q23) and a KMT2A-MLLT3 fusion gene, and the presence of KMT2A- ARHGEF12 in the patient’s bone marrow cells 1,198 days after the initial diagnosis, when the patient had developed ALL with t(14;19)(q32;q13) and rearrangement of the IGH locus (lane 4). For both cDNA and genomic DNA amplifications, the primer combination MLL-4116F1/ARHGEF12-1502R1 was used. Lane 2: Assessment of the quality of cDNA synthesis by amplification of a cDNA fragment of ABL1 using the primer combination ABL1-91F1/ABL1-404R1. Lane 4: Amplification of a genomic KMT2AARHGEF12 DNA fragment in the patient’s bone marrow cells 1,198 days after the initial diagnosis. M, GeneRuler 1 Kb Plus DNA ladder (ThermoFisher Scientific). (D) Partial sequence chromatogram showing the junction position of intron 9 of the KMT2A with intron 13 of ARHGEF12 in the chimeric amplified DNA fragment. The junction of positions 11:118355287-11:120311617 is based on the human genome GRCh37/hg19 assembly.
Genomic PCR using as template DNA extracted from the patient’s bone marrow cells sampled 1,198 days and 1,539 days after the initial diagnosis amplified a 1264 bp fragment with the primer combination MLL-4116-F1/ARHGEF12-1502-R1 and a 1133 bp fragment with the primer combination MLL-4202-F1/ARHGEF12-1437-R1 (Figure 3B and C). Direct sequencing showed that they were genomic KMT2A-ARHGEF12 chimeric fragments in which a sequence from intron 9 of KMT2A was fused to a sequence of intron 13 from ARHGEF12 (Figure 3D).
Neither RT-PCR nor genomic PCR detected KMT2A-ARHGEF12 chimeric fragments when the template was RNA (cDNA) or genomic DNA obtained from the diagnostic (day 0) bone marrow (Figure 3C).
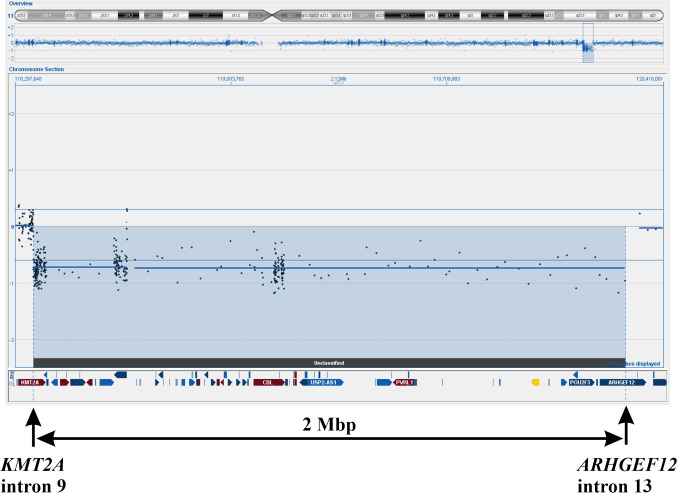
aCGH analysis. aCGH detected a deletion in the q arm of chromosome 11 (Figure 4). Based on the hg19 assembly, the deletion was from the probe at position Chr11:118355288-118355347 in KMT2A to the probe at position Chr11:120290981-120291040 in ARHGEF12 (Figure 4). Thus, the aCGH data agreed with the results of FISH analyses, genomic PCR, and RT-PCR; they suggested that the KMT2A-ARHGEF12 fusion gene was the result of a deletion within chromosome band 11q23.
Figure 4. aCGH showing the deletion in the q arm of chromosome 11. Based on the hg19 assembly, the deletion started from the probe at position Chr11:118355288-118355347 in KMT2A and ended at position Chr11:120290981-120291040 in ARHGEF12. The deletion is approximately 2 Mbp.
Discussion
A fusion of KMT2A with the ARHGEF12 gene was first reported in a 38-year-old male with a history of occupational exposure to herbicides who had developed AML (FAB-M4) and the abnormal karyotype 51,XY,+8,+19,+3mar (23). The patient received standard induction chemotherapy and achieved complete remission by morphological bone marrow analysis, whereupon he received successful allogeneic bone marrow transplantation from an HLA identical sibling. He died 6 months later from interstitial pneumonia. There were no signs of relapse at autopsy (23).
The second case was a 77-year-old female with AML (FAB-M5a) and the karyotype 53,XX,+6,+8,+8,+9,+11,+13,+22 (24). The third patient with KMT2A-ARHGEF12 was an adult female with therapy-related AML (t-AML) (4,5). No information on the treatment and clinical outcome was provided for the second and third patients. The fourth patient with KMT2A-ARHGEF12 fusion was a 69-year-old-male with B-cell ALL and a normal karyotype (25). This patient did not achieve complete remission after two courses of induction therapy and died from the disease (25). The fifth patient was a female with B-cell ALL and the bone marrow karyotype 46,XX,der(9)t(3;9)(p21;q34)/46,idem,der(9)t(3;9) (26). No further information was provided.
The present, sixth patient is the first reported pediatric case with a KMT2A-ARHGEF12 fusion gene. The fusion was detected in the patient’s bone marrow after completion of treatment for an AML with t(9;11)(p21;q23) and a KMT2A-MLLT3 fusion gene. The chemotherapy included, among other drugs, daunorubicin, mitoxantrone and etoposide which all are topoisomerase II inhibitors and associated with DNA double-strand breaks, the generation of KMT2A rearrangements, the formation of KMT2A-fusion genes, and therapy-related acute leukemias (27-33). B-ALL with 11q23 abnormalities/KMT2A rearrangements developing after treatment of a primary malignancy with topoisomerase II inhibitors have been reported (34-40). The majority of cases had the t(4;11)(q21;q23) translocation resulting in formation of a KMT2A-AFF1 fusion gene, but fusions of KMT2A with MLLT1 (19p13.3), FOXO3A (6q21), MAML2 (11q21), ACTN4 (19q13), CEP164 (11q23.3), and PRRC1 (5q23) were also found (5,39). Therefore, we consider the chemotherapy to have caused the KMT2A-ARHGEF12 fusion.
The first indication of a KMT2A-ARHGEF12 fusion gene was seen in the patient’s bone marrow cells at days 300 and 328 after the initial AML diagnosis when, in FISH experiments, deletion of the distal part of the KMT2A probe was detected in spite of a normal karyotype (Table I, samples S1 and S2). RNA sequencing performed later detected the KMT2A-ARHGEF12 fusion gene, PCR amplifications verified it both at the transcriptional and genomic level (Figure 3), and aCGH showed that the cause of the fusion was indeed a submicroscopic deletion starting in intron 9 of KMT2A extending to intron 13 of ARHGEF12 (Figure 4). These retrospective findings fit perfectly the early detection of a deleted distal part of the KMT2A probe, linking it to the emergence of the KMT2A-ARHGEF12 fusion. Because the aberration was found in 77% of the CD34+CD117+ cells (Table I, sample S11-B), we concluded that the treatment-induced generation of KMT2A-ARHGEF12 fusion probably took place in a multipotent progenitor cell. Indeed, in vitro experiments have shown that etoposide-induced rearrangements of KMT2A resulting in fusion genes can occur in mouse embryonic stem cells (27), human CD34+ hematopoietic stem cells from fetal liver (41), human CD34+ cells isolated from umbilical cord blood (28,42), and in human embryonic stem cells (43). Furthermore, Libura et al. (42) showed that the etoposide-induced-KMT2A rearrangements were found at high frequency in human primitive hematopoietic stem cells with, in vitro and in vivo, long-term repopulating potential.
During a period of two years and three months (after completion of therapy of the initial AML), repeated bone marrow examinations were performed and in all of them, although there was no sign of malignancy, the genetic pattern was the same: normal karyotype and deletion of the distal part of the KMT2A probe in FISH experiments indicating, as we only later realized, the formation of KMT2A-ARHGEF12 (Table 1, samples S1-S7). Searching the relevant literature, we found two reports describing similar findings to ours (44,45). In the first, a 5-year-old girl was diagnosed with AML carrying a variant of t(8;21) (44). The patient achieved clinical and hematologic remission which, 4 years later when the authors reported the case, was still unquestionable. Bone marrow examination 15 months after the intial diagnosis had revealed a clonal t(11;11)(q13;q23), however, and the translocation gave rise to a KMT2A-ARHGEF17 and the reciprocal ARHGEF17-KMT2A fusion genes (44). Retrospective PCR analysis did not detect any KMT2A-ARHGEF17 chimera in samples drawn at the time of diagnosis suggesting that the fusion was treatment-induced. The KMT2A aberration was found only in myeloid lineage cells and in numerous samples over a 30-month period without simultaneous phenotypic signs of a new malignancy. Because myeloid cells as mature as promyelocytes and even differentiated polymorphonuclear neutrophils carried the KMT2A-ARHGEF17, the authors concluded that the fusion had probably occurred in a myeloid stem cell but without blocking further differentiation (44).
The second case was a 3-year-old boy with B-ALL and a hyperdiploid karyotype: 58<2n>,XY,+X,+4,+6,+10,+15, +17,+18,+18,+20,+21,+21,+22 (45). During chemotherapy, a t(3;11)(p21;q23) developed in bone marrow cells giving rise to a KMT2A–SACM1L fusion gene. The KMT2A–SACM1L fusion was detected in many samples over a 7-year period in which the patient was in hematological remission. A difference between the KMT2A-ARHGEF17 and KMT2A–SACM1L fusion genes was that whereas the first resulted in a KMT2A-ARHGEF17 fusion protein, the second encodes a truncated KMT2A protein (44,45).
Our patient was, two years and nine months after the start of the last AML chemotherapy course (or 3 years and 3 months after the initial diagnosis of AML), diagnosed with B-ALL (Table I, sample S8). The bone marrow contained 90% blasts of the pre-B phenotype, cytogenetic analysis revealed a t(14;19)(q32;q13) translocation together with loss of one chromosome 9 (Figure 1B, Table I, sample S8, see below), and FISH analyses showed splitting of the IGH probe in 92% of the nuclei together with deletion of the distal part of the KMT2A probe in 97% of the nuclei. In addition, later molecular analysis demonstrated presence of the KMT2A-ARHGEF12 fusion gene also at this time. Given the fact that so many bone marrow cells had the aberrations, most of them must have had both, leading us to conclude that the t(14;19)(q32;q13) translocation and loss of one chromosome 9 were secondary to the submicroscopic deletion in 11q23. Three months after treatment for B-ALL had been instituted, the (14;19)(q32;q13)/IGH aberration was no longer detectable while the KMT2A rearrangement was found in only 8.5% of the examined interphase nuclei. Evidently, the treatment given had considerable effect also on the cells carrying KMT2A rearrangement, but not enough to completely eradicate them. Summing up the extensive and somewhat complicating data – the high frequency of t(14;19)(q32;q13)/IGH rearrangement and KMT2A rearrangement in bone marrow cells when the patient developed B-ALL, and the absence of t(14;19)(q32;q13)/IGH rearrangement together with the low frequency of KMT2A rearrangement after treatment for this disease – strongly indicate that (most of) the cells carrying KMT2A-rearrangement were part of the leukemic clone which carried the t(14;19)(q32;q13)/IGH rearrangement when the patient had B-ALL.
Our data are also in agreement with the findings reported by Jeffries et al. (46). Examining 20 B-ALL patients, they showed that translocations involving the IGH locus coexisted with other primary chromosomal rearrangements either in the same or separate clones. In one of the studied patients, KMT2A rearrangement was the primary aberration whereas the IGH-translocation the secondary. Most cells had only KMT2A rearrangement but cells with only IGH-translocation and cells with both KMT2A aberration and IGH-translocation were also found (46).
The case we present has extensive similarities with the case reported by Zuna et al. (47). In brief, a 15-year-old girl with acute promyelocytic leukemia (APL, AML-M3) carrying a PML-RARA fusion gene together with internal tandem duplication of FLT3 (FLT3/ITD) was treated with drugs that included the topoisomerase II inhibitors idarubicin and etoposide. Thirty months after the diagnosis of APL and 13 months after completion of therapy, she was diagnosed with B-ALL. Cytogenetic analysis revealed a t(6;11)(q21;q23) translocation aberration with molecular analyses showing the presence of a KMT2A-FOXO3A fusion gene whereas both PML-RARA fusion gene and FLT3/ITD were absent (47).
Backtracking the B-ALL, just as we did in the present study, the authors found the KMT2A-FOXO3A fusion gene in up to 90% of bone marrow cells, including cells of the myeloid lineage, more than one year before the B-ALL diagnosis which suggested that the generation of the KMT2A-FOXO3A fusion occurred in a multipotent progenitor. During this preleukemic phase, no blasts were found in the bone marrow but a small population of CD19+ B-cells, comprising 0.2% of the bone marrow, was detected (47). In our case, similarly a small persisting aberrant CD34+, CD38+ cell population, decreasing in a proportion from 1.5% to 0.05% over eight months, was found whereas the deletion of the distal part of KMT2A was found in 79-92% of the cells (Table 1, samples S1-S7).
Zuna et al. (47) identified a 10 Mb gain on 19q13.32 as a potential ‘‘second hit’’ for the development of the B-ALL. The gain was present in B-ALL but was not found in the preleukemic specimen. By analogy, in our case t(14;19)(q32;q13) and loss of one chromosome 9 were secondary aberrations found at diagnosis of ALL but not in any previously examined samples (Table I, samples S1-S8).
Our patient also has similarities with the case presented by Jonveaux et al (48): A 35-year-old male had an AML (FAB M5b) with the chromosome aberration t(6;11)(q27;q23). FISH analysis showed that presence of a 400-600 kbp deletion downstream of the 11q23 breakpoint. Using Southern blot methodology, the breakpoint of KMT2A was found within intron 7 of the gene. The patient received chemotherapy which, among the drugs, included mitoxantrone and etoposide. Ten and a half months after complete remission, he was diagnosed with B-ALL. The cytogenetic analysis now revealed a t(4;11)(q21;q23) chromosome translocation, FISH showed absence of the deletion, and Southern blot detected a breakpoint in intron 9 of KMT2A (48). The authors considered etoposide as responsible for the development of the secondary B-ALL.
At the transcription level, the fusion of the present case was found to recombine exon 9 of KMT2A with exon 14 of ARHGEF12. This is at odds with what was reported by Kourlas et al. (23) who found fusion between exons 8 of KMT2A and 12 of ARHGEF12. No information on the exact KMT2A-ARHGEF12 fusion point was given for the other three reported patients (4,5,24,25). The KMT2A-ARHGEF12 fusion of the present patient was brought about by a 2Mbp deletion stretching from intron 9 of KMT2A to intron 13 of ARHGEF12. Deletions were also reported as the cause of KMT2A-ARHGEF12 formation in the patients described by Kourlas (23) and Meyer (4,5).
The exact genomic breakpoint position in KMT2A has been shown to correlate with clinical outcome in acute leukemias caused by fusions between KMT2A and some of the more common partner genes (5,49,50). Breakpoints in intron 10 (listed as intron 11 in the references (5,49,50)) interfere with the dimerization capacity of KMT2A plant homeodomains (PHD) 1-3, disabling binding to the BMI1 (B cell-specific Moloney murine leukemia virus integration site 1) repressor complex, and are associated with worse prognosis (5,49-51). Breakpoints within KMT2A introns 8 and 9 (introns 9 and 10 in references (5,49,50), on the other hand, do not affect the three PHD finger domains and are associated with somewhat better clinical outcomes. The breakpoint in intron 9 of the present case could explain the relatively benign clinical behavior observed with long-term survival in spite of the late detection of a KMT2A-ARHGEF12 fusion gene and the therapy-related leukemia it signals.
The ARHGEF12 gene codes for the Rho guanine nucleotide exchange factor 12 which is a guanine nucleotide exchange factor that activates small GTPases of the Rho family (see RHOA, 165390) and catalyzes the exchange of GDP for GTP (52-54). ARHGEF12 was shown to be involved in cell polarization, morphology, invasion, and cytokinetic abscission (55-61). ARHGEF12 together with ARHGEF2 (also known as GEF-H1) were found to be key molecules for cellular adaptation to force and regulate the mechanical response to force on integrins (62). ARHGEF12 was found to regulate human megakaryocyte maturation, is critical for platelet function, regulates erythropoiesis, and is involved in erythroid regeneration after chemotherapy in ALL patients (63,64). ARHGEF12 was also reported as a candidate tumor suppressor gene in breast and colorectal cancers (65). Mutations in the coding region of the gene are registered in COSMIC, the catalogue of somatic mutations in cancer (cancer.sanger.ac.uk), in various disease types (66).
In conclusion, we herein report the complex and sequential genetic steps of a therapy-related pediatric B-ALL. The patient was initially diagnosed with AML carrying a t(9;11)(p21;q23) chromosome translocation and a KMT2A-MLLT3 fusion gene. The first secondary genetic step, resulting from chemotherapy that included treatment with topoisomerase II-inhibitors, was a submicroscopic 2 Mb deletion in 11q23 generating a KMT2A-ARHGEF12 fusion gene in a multipotent progenitor cell. At that point, the patient entered a long ”preleukemic” phase which lasted until yet another genetic aberration occurred, t(14;19)(q32;q13) accompanied by the loss of one chromosome 9. This resulted in the development of B-ALL.
Conflicts of Interest
The Authors declare that they have no potential conflicts of interest in regard to this study.
Authorsʼ Contributions
IP designed and supervised the experiments, performed bioinformatics analysis, molecular genetic experiments, evaluated the data, and drafted the manuscript. KA performed cytogenetic, FISH and molecular experiments and evaluated the data. ME-O evaluated the cytogenetic and FISH data. BZ made clinical evaluations and treated the patient. MCM-K made clinical evaluations and treated the patient. JB made clinical evaluations and treated the patient. LTNO performed the flow cytometric evaluations. FM evaluated the cytogenetic and FISH data. SH evaluated the data and assisted with writing of the manuscript. All Authors read and approved the final manuscript.
Acknowledgements
This work was supported by Grants from Radiumhospitalets Legater.
References
- 1.Daser A, Rabbitts TH. The versatile mixed lineage leukaemia gene MLL and its many associations in leukaemogenesis. Semin Cancer Biol. 2005;15(3):175–188. doi: 10.1016/j.semcancer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Marschalek R. Mechanisms of leukemogenesis by MLL fusion proteins. Br J Haematol. 2011;152(2):141–154. doi: 10.1111/j.1365-2141.2010.08459.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitelman F, Johansson B, Mertens F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer, 2020. Available at: https://mitelmandatabase.isb-cgc.org/
- 4.Meyer C, Hofmann J, Burmeister T, Groger D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A, Villarese P, Macintyre E, Cave H, Clappier E, Mass-Malo K, Zuna J, Trka J, De Braekeleer E, De Braekeleer M, Oh SH, Tsaur G, Fechina L, van der Velden VH, van Dongen JJ, Delabesse E, Binato R, Silva ML, Kustanovich A, Aleinikova O, Harris MH, Lund-Aho T, Juvonen V, Heidenreich O, Vormoor J, Choi WW, Jarosova M, Kolenova A, Bueno C, Menendez P, Wehner S, Eckert C, Talmant P, Tondeur S, Lippert E, Launay E, Henry C, Ballerini P, Lapillone H, Callanan MB, Cayuela JM, Herbaux C, Cazzaniga G, Kakadiya PM, Bohlander S, Ahlmann M, Choi JR, Gameiro P, Lee DS, Krauter J, Cornillet-Lefebvre P, Te Kronnie G, Schafer BW, Kubetzko S, Alonso CN, zur Stadt U, Sutton R, Venn NC, Izraeli S, Trakhtenbrot L, Madsen HO, Archer P, Hancock J, Cerveira N, Teixeira MR, Lo Nigro L, Moricke A, Stanulla M, Schrappe M, Sedek L, Szczepanski T, Zwaan CM, Coenen EA, van den Heuvel-Eibrink MM, Strehl S, Dworzak M, Panzer-Grumayer R, Dingermann T, Klingebiel T, Marschalek R. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27(11):2165–2176. doi: 10.1038/leu.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer C, Burmeister T, Groger D, Tsaur G, Fechina L, Renneville A, Sutton R, Venn NC, Emerenciano M, Pombo-de-Oliveira MS, Barbieri Blunck C, Almeida Lopes B, Zuna J, Trka J, Ballerini P, Lapillonne H, De Braekeleer M, Cazzaniga G, Corral Abascal L, van der Velden VHJ, Delabesse E, Park TS, Oh SH, Silva MLM, Lund-Aho T, Juvonen V, Moore AS, Heidenreich O, Vormoor J, Zerkalenkova E, Olshanskaya Y, Bueno C, Menendez P, Teigler-Schlegel A, Zur Stadt U, Lentes J, Gohring G, Kustanovich A, Aleinikova O, Schafer BW, Kubetzko S, Madsen HO, Gruhn B, Duarte X, Gameiro P, Lippert E, Bidet A, Cayuela JM, Clappier E, Alonso CN, Zwaan CM, van den Heuvel-Eibrink MM, Izraeli S, Trakhtenbrot L, Archer P, Hancock J, Moricke A, Alten J, Schrappe M, Stanulla M, Strehl S, Attarbaschi A, Dworzak M, Haas OA, Panzer-Grumayer R, Sedek L, Szczepanski T, Caye A, Suarez L, Cave H, Marschalek R. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32(2):273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heim S, Mitelman F. Wiley-Blackwell. 2015. Cancer Cytogenetics: Chromosomal and Molecular Genetic Abberations of Tumor Cells. Fourth Edition edn. [Google Scholar]
- 7.Winters AC, Bernt KM. MLL-rearranged leukemias-an update on science and clinical approaches. Front Pediatr. 2017;5:4. doi: 10.3389/fped.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Braekeleer E, Meyer C, Douet-Guilbert N, Morel F, Le Bris MJ, Marschalek R, Ferec C, De Braekeleer M. A complex 1;19;11 translocation involving the MLL gene in a patient with congenital acute monoblastic leukemia identified by molecular and cytogenetic techniques. Ann Hematol. 2009;88(8):795–797. doi: 10.1007/s00277-008-0656-8. [DOI] [PubMed] [Google Scholar]
- 9.De Braekeleer E, Meyer C, Douet-Guilbert N, Basinko A, Le Bris MJ, Morel F, Berthou C, Marschalek R, Ferec C, De Braekeleer M. Identification of MLL partner genes in 27 patients with acute leukemia from a single cytogenetic laboratory. Mol Oncol. 2011;5(6):555–563. doi: 10.1016/j.molonc.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Braekeleer E, Douet-Guilbert N, Meyer C, Morel F, Marschalek R, De Braekeleer M. MLL-ELL fusion gene in two infants with acute monoblastic leukemia and myeloid sarcoma. Leuk Lymphoma. 2012;53(6):1222–1224. doi: 10.3109/10428194.2011.648632. [DOI] [PubMed] [Google Scholar]
- 11.Panagopoulos I, Gorunova L, Kerndrup G, Spetalen S, Tierens A, Osnes LT, Andersen K, Muller LS, Hellebostad M, Zeller B, Heim S. Rare MLL-ELL fusion transcripts in childhood acute myeloid leukemia-association with young age and myeloid sarcomas. Exp Hematol Oncol. 2015;5:8. doi: 10.1186/s40164-016-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwaan CM, Kolb EA, Reinhardt D, Abrahamsson J, Adachi S, Aplenc R, De Bont ES, De Moerloose B, Dworzak M, Gibson BE, Hasle H, Leverger G, Locatelli F, Ragu C, Ribeiro RC, Rizzari C, Rubnitz JE, Smith OP, Sung L, Tomizawa D, van den Heuvel-Eibrink MM, Creutzig U, Kaspers GJ. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33(27):2949–2962. doi: 10.1200/JCO.2015.62.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, Klausen TW, Jonsson OG, Palk K, Pruunsild K, Quist-Paulsen P, Vaitkeviciene G, Vettenranta K, Asberg A, Frandsen TL, Marquart HV, Madsen HO, Noren-Nystrom U, Schmiegelow K. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. 2018;32(3):606–615. doi: 10.1038/leu.2017.265. [DOI] [PubMed] [Google Scholar]
- 14.Panagopoulos I, Gorunova L, Torkildsen S, Tjonnfjord GE, Micci F, Heim S. DEK-NUP214-fusion identified by RNA-sequencing of an acute myeloid leukemia with t(9;12)(q34;q15) Cancer Genomics Proteomics. 2017;14(6):437–443. doi: 10.21873/cgp.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torkildsen S, Brunetti M, Gorunova L, Spetalen S, Beiske K, Heim S, Panagopoulos I. Rearrangement of the chromatin organizer special AT-rich binding protein 1 gene, SATB1, resulting from a t(3;5)(p24;q14) chromosomal translocation in acute myeloid leukemia. Anticancer Res. 2017;37(2):693–698. doi: 10.21873/anticanres.11365. [DOI] [PubMed] [Google Scholar]
- 17.Kangaspeska S, Hultsch S, Edgren H, Nicorici D, Murumagi A, Kallioniemi O. Reanalysis of RNA-sequencing data reveals several additional fusion genes with multiple isoforms. PLoS One. 2012;7(10):e48745. doi: 10.1371/journal.pone.0048745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicorici D, Satalan H, Edgren H, Kangaspeska S, Murumagi A, Kallioniemi O, Virtanen S, Kikku O. FusionCatcher – a tool for finding somatic fusion genes in paired-end RNA-sequencing data. bioRxiv. 2014 doi: 10.1101/011650. [DOI] [Google Scholar]
- 19.Singhal H, Ren YR, Kern SE. Improved DNA electro-phoresis in conditions favoring polyborates and lewis acid complexation. PLoS One. 2010;5(6):e11318. doi: 10.1371/journal.pone.0011318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 21.Kent WJ. BLAT – the BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, Krahe R, Ruutu T, Knuutila S, Bloomfield CD, Caligiuri MA. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci USA. 2000;97(5):2145–2150. doi: 10.1073/pnas.040569197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih LY, Liang DC, Fu JF, Wu JH, Wang PN, Lin TL, Dunn P, Kuo MC, Tang TC, Lin TH, Lai CL. Characterization of fusion partner genes in 114 patients with de novo acute myeloid leukemia and MLL rearrangement. Leukemia. 2006;20(2):218–223. doi: 10.1038/sj.leu.2404024. [DOI] [PubMed] [Google Scholar]
- 25.Liu YF, Wang BY, Zhang WN, Huang JY, Li BS, Zhang M, Jiang L, Li JF, Wang MJ, Dai YJ, Zhang ZG, Wang Q, Kong J, Chen B, Zhu YM, Weng XQ, Shen ZX, Li JM, Wang J, Yan XJ, Li Y, Liang YM, Liu L, Chen XQ, Zhang WG, Yan JS, Hu JD, Shen SH, Chen J, Gu LJ, Pei D, Li Y, Wu G, Zhou X, Ren RB, Cheng C, Yang JJ, Wang KK, Wang SY, Zhang J, Mi JQ, Pui CH, Tang JY, Chen Z, Chen SJ. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EBioMedicine. 2016;8:173–183. doi: 10.1016/j.ebiom.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevov M, Bunikis I, Häggqvist S, Höglund M, Rosenquist R, Ameur A, Cavelier L. Targeted RNA sequencing assay efficiently identifies cryptic KMT2A (MLL)-fusions in acute leukemia patients. Blood. 2014;124(21):2406–2406. [Google Scholar]
- 27.Blanco JG, Edick MJ, Relling MV. Etoposide induces chimeric Mll gene fusions. FASEB J. 2004;18(1):173–175. doi: 10.1096/fj.03-0638fje. [DOI] [PubMed] [Google Scholar]
- 28.Libura J, Slater DJ, Felix CA, Richardson C. Therapy-related acute myeloid leukemia-like MLL rearrangements are induced by etoposide in primary human CD34+ cells and remain stable after clonal expansion. Blood. 2005;105(5):2124–2131. doi: 10.1182/blood-2004-07-2683. [DOI] [PubMed] [Google Scholar]
- 29.Felix CA, Kolaris CP, Osheroff N. Topoisomerase II and the etiology of chromosomal translocations. DNA Repair. 2006;5(9-10):1093–1108. doi: 10.1016/j.dnarep.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Sung PA, Libura J, Richardson C. Etoposide and illegitimate DNA double-strand break repair in the generation of MLL translocations: new insights and new questions. DNA Repair. 2006;5(9-10):1109–1118. doi: 10.1016/j.dnarep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Cowell IG, Austin CA. Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int J Environ Res Public Health. 2012;9(6):2075–2091. doi: 10.3390/ijerph9062075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ezoe S. Secondary leukemia associated with the anti-cancer agent, etoposide, a topoisomerase II inhibitor. Int J Environ Res Public Health. 2012;9(7):2444–2453. doi: 10.3390/ijerph9072444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evison BJ, Sleebs BE, Watson KG, Phillips DR, Cutts SM. Mitoxantrone, more than just another topoisomerase II poison. Med Res Rev. 2016;36(2):248–299. doi: 10.1002/med.21364. [DOI] [PubMed] [Google Scholar]
- 34.Pagano L, Pulsoni A, Tosti ME, Annino L, Mele A, Camera A, Martino B, Guglielmi C, Cerri R, Di Bona E, Invernizzi R, Castagnola C, Bassan R, Mele L, Todeschini G, Leone G, Mandelli F. Acute lymphoblastic leukaemia occurring as second malignancy: report of the GIMEMA archive of adult acute leukaemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol. 1999;106(4):1037–1040. doi: 10.1046/j.1365-2141.1999.01636.x. [DOI] [PubMed] [Google Scholar]
- 35.Ishizawa S, Slovak ML, Popplewell L, Bedell V, Wrede JE, Carter NH, Snyder DS, Arber DA. High frequency of pro-B acute lymphoblastic leukemia in adults with secondary leukemia with 11q23 abnormalities. Leukemia. 2003;17(6):1091–1095. doi: 10.1038/sj.leu.2402918. [DOI] [PubMed] [Google Scholar]
- 36.Shivakumar R, Tan W, Wilding GE, Wang ES, Wetzler M. Biologic features and treatment outcome of secondary acute lymphoblastic leukemia – a review of 101 cases. Ann Oncol. 2008;19(9):1634–1638. doi: 10.1093/annonc/mdn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdulwahab A, Sykes J, Kamel-Reid S, Chang H, Brandwein JM. Therapy-related acute lymphoblastic leukemia is more frequent than previously recognized and has a poor prognosis. Cancer. 2012;118(16):3962–3967. doi: 10.1002/cncr.26735. [DOI] [PubMed] [Google Scholar]
- 38.Tang G, Zuo Z, Thomas DA, Lin P, Liu D, Hu Y, Kantarjian HM, Bueso-Ramos C, Medeiros LJ, Wang SA. Precursor B-acute lymphoblastic leukemia occurring in patients with a history of prior malignancies: is it therapy-related. Haematologica. 2012;97(6):919–925. doi: 10.3324/haematol.2011.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douet-Guilbert N, Eveillard JR, Meyer C, Ugo V, Le Bris MJ, Basinko A, Morel F, Marschalek R, De Braekeleer M. MLL partner genes in secondary acute lymphoblastic leukemia: report of a new partner PRRC1 and review of the literature. Leuk Res. 2014;38(11):1316–1319. doi: 10.1016/j.leukres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Aldoss I, Douer D, Pullarkat V. Therapy-related acute lymphoblastic leukemia: Where do we stand with regards to its definition and characterization. Blood Rev. 2019;37:100584. doi: 10.1016/j.blre.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Moneypenny CG, Shao J, Song Y, Gallagher EP. MLL rearrangements are induced by low doses of etoposide in human fetal hematopoietic stem cells. Carcinogenesis. 2006;27(4):874–881. doi: 10.1093/carcin/bgi322. [DOI] [PubMed] [Google Scholar]
- 42.Libura J, Ward M, Solecka J, Richardson C. Etoposide-initiated MLL rearrangements detected at high frequency in human primitive hematopoietic stem cells with in vitro and in vivo long-term repopulating potential. Eur J Haematol. 2008;81(3):185–195. doi: 10.1111/j.1600-0609.2008.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bueno C, Catalina P, Melen GJ, Montes R, Sanchez L, Ligero G, Garcia-Perez JL, Menendez P. Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells. Carcinogenesis. 2009;30(9):1628–1637. doi: 10.1093/carcin/bgp169. [DOI] [PubMed] [Google Scholar]
- 44.Teuffel O, Betts DR, Thali M, Eberle D, Meyer C, Schneider B, Marschalek R, Trakhtenbrot L, Amariglio N, Niggli FK, Schafer BW. Clonal expansion of a new MLL rearrangement in the absence of leukemia. Blood. 2005;105(10):4151–4152. doi: 10.1182/blood-2005-01-0286. [DOI] [PubMed] [Google Scholar]
- 45.Mori T, Nishimura N, Hasegawa D, Kawasaki K, Kosaka Y, Uchide K, Yanai T, Hayakawa A, Takeshima Y, Nishio H, Matsuo M. Persistent detection of a novel MLL-SACM1L rearrangement in the absence of leukemia. Leuk Res. 2010;34(10):1398–1401. doi: 10.1016/j.leukres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Jeffries SJ, Jones L, Harrison CJ, Russell LJ. IGH@ translocations co-exist with other primary rearrangements in B-cell precursor acute lymphoblastic leukemia. Haematologica. 2014;99(8):1334–1342. doi: 10.3324/haematol.2014.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuna J, Burjanivova T, Mejstrikova E, Zemanova Z, Muzikova K, Meyer C, Horsley SW, Kearney L, Colman S, Ptoszkova H, Marschalek R, Hrusak O, Stary J, Greaves M, Trka J. Covert preleukemia driven by MLL gene fusion. Genes Chromosomes Cancer. 2009;48(1):98–107. doi: 10.1002/gcc.20622. [DOI] [PubMed] [Google Scholar]
- 48.Jonveaux P, Hillion J, Bernard O, Le Coniat M, Derre J, Flexor M, Larsen CJ, Berger R. Distinct MLL gene rearrangements associated with successive acute monocytic and lymphoblastic leukemias in the same patient. Leukemia. 1994;8(12):2224–2227. [PubMed] [Google Scholar]
- 49.Emerenciano M, Meyer C, Mansur MB, Marschalek R, Pombo-de-Oliveira MS, Brazilian Collaborative Study Group of Infant Acute Leukaemia The distribution of MLL breakpoints correlates with outcome in infant acute leukaemia. Br J Haematol. 2013;161(2):224–236. doi: 10.1111/bjh.12250. [DOI] [PubMed] [Google Scholar]
- 50.Meyer C, Lopes BA, Caye-Eude A, Cave H, Arfeuille C, Cuccuini W, Sutton R, Venn NC, Oh SH, Tsaur G, Escherich G, Feuchtinger T, Kosasih HJ, Khaw SL, Ekert PG, Pombo-de-Oliveira MS, Bidet A, Djahanschiri B, Ebersberger I, Zaliova M, Zuna J, Zermanova Z, Juvonen V, Grumayer RP, Fazio G, Cazzaniga G, Larghero P, Emerenciano M, Marschalek R. Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL-USP2 fusions. Leukemia. 2019;33(9):2306–2340. doi: 10.1038/s41375-019-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rössler T, Marschalek R. An alternative splice process renders the MLL protein either into a transcriptional activator or repressor. Pharmazie. 2013;68(7):601–607. doi: 10.1055/s-0033-1343653. [DOI] [PubMed] [Google Scholar]
- 52.Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000;485(2-3):183–188. doi: 10.1016/s0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- 53.Booden MA, Siderovski DP, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA. Mol Cell Biol. 2002;22(12):4053–4061. doi: 10.1128/mcb.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277(14):12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 55.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35(1):51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 56.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120(Pt 22):3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 57.Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21(12):1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goulimari P, Knieling H, Engel U, Grosse R. LARG and mDia1 link Galpha12/13 to cell polarity and microtubule dynamics. Mol Biol Cell. 2008;19(1):30–40. doi: 10.1091/mbc.e06-11-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evelyn CR, Ferng T, Rojas RJ, Larsen MJ, Sondek J, Neubig RR. High-throughput screening for small-molecule inhibitors of LARG-stimulated RhoA nucleotide binding via a novel fluorescence polarization assay. J Biomol Screen. 2009;14(2):161–172. doi: 10.1177/1087057108328761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182(6):3837–3845. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 61.Martz MK, Grabocka E, Beeharry N, Yen TJ, Wedegaertner PB. Leukemia-associated RhoGEF (LARG) is a novel RhoGEF in cytokinesis and required for the proper completion of abscission. Mol Biol Cell. 2013;24(18):2785–2794. doi: 10.1091/mbc.E12-07-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13(6):722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou S, Teixeira AM, Yin M, Xiang Y, Xavier-Ferrucio J, Zhang PX, Hwa J, Min W, Krause DS. Leukaemia-associated Rho guanine nucleotide exchange factor (LARG) plays an agonist specific role in platelet function through RhoA activation. Thromb Haemost. 2016;116(3):506–516. doi: 10.1160/TH15-11-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie Y, Gao L, Xu C, Chu L, Gao L, Wu R, Liu Y, Liu T, Sun XJ, Ren R, Tang J, Zheng Y, Zhou Y, Shen S. ARHGEF12 regulates erythropoiesis and is involved in erythroid regeneration after chemotherapy in acute lymphoblastic leukemia patients. Haematologica. 2020;105(4):925–936. doi: 10.3324/haematol.2018.210286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ong DC, Ho YM, Rudduck C, Chin K, Kuo WL, Lie DK, Chua CL, Tan PH, Eu KW, Seow-Choen F, Wong CY, Hong GS, Gray JW, Lee AS. LARG at chromosome 11q23 has functional characteristics of a tumor suppressor in human breast and colorectal cancer. Oncogene. 2009;28(47):4189–4200. doi: 10.1038/onc.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E, Fish P, Harsha B, Hathaway C, Jupe SC, Kok CY, Noble K, Ponting L, Ramshaw CC, Rye CE, Speedy HE, Stefancsik R, Thompson SL, Wang S, Ward S, Campbell PJ, Forbes SA. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019;47(D1):D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]