Abstract
Purpose
To analyze the principal cause for poor vision in eyes with best-corrected visual acuity (BCVA) ≤20/200 two years after a record of neovascular age-related macular degeneration (NV-AMD).
Design
Prospective cohort study of participants enrolled in a clinical trial of oral supplements and receiving anti-VEGF therapy in routine clinical practice.
Participants
Age-Related Eye Disease Study 2 (AREDS2) participants (50–85 years) whose eyes met AREDS2 inclusion criteria at baseline (no late AMD; BCVA ≥20/100; no previous anti-VEGF injections) but began anti-VEGF therapy for incident NV-AMD during follow-up and had data available at two years.
Methods
Participants underwent refracted BCVA testing, ophthalmoscopic examination, and color fundus photography (CFP) at baseline and annual study visits. Self-reports of anti-VEGF injections were collected.
Main outcome measures
Principal cause of BCVA ≤20/200 at two years, detected on CFP grading.
Results
Of the 594 eligible eyes, the number with BCVA ≤20/200 at two years was 56 eyes (9.4%). Mean BCVA was 14.9 letters (SD 12.3; 20/500), versus 70.1 letters (SD 12.8; 20/40) in the other group. Of the 55 eyes with CFP available at two years, 33 (60.0%) were assessed from CFP grading to have central macular atrophy as the principal cause for poor vision. The remaining 22 eyes (40.0%) were assessed to have central subretinal fibrosis. The group with poor BCVA had a higher proportion of non-white participants (8.9% vs 1.7%, p=0.006), lower BCVA two years earlier (mean 38.0, SD 26.7, 20/160 vs 71.8, SD 11.9, 20/40, p<0.0001), higher proportion with macular atrophy two years earlier (26.8% vs 12.3%, p=0.003), higher proportion with macular hemorrhage (25.5% vs 13.2%, p=0.014), and fewer anti-VEGF injections (7.6 vs 10.2, p=0.001).
Conclusions
BCVA data and CFP were obtained in a clinical trial environment but related to anti-VEGF therapy given in routine clinical practice. At two years after starting anti-VEGF therapy, almost one in ten eyes had BCVA at the level of legal blindness. From CFP grading, the cause of poor vision appeared to be macular atrophy in 60% and subretinal fibrosis in 40%. These data may be useful in understanding the long-term limits to good vision in NV-AMD.
Précis
In AREDS2, the principal cause of visual acuity being worse than 20/200 two years after starting anti-VEGF therapy for neovascular age-related macular degeneration was central macular atrophy in 60% and central subretinal fibrosis in 40%.
Introduction
Age-related macular degeneration (AMD) is a multifactorial, degenerative disease of the retina that arises through a complex interplay between increased age, genetics, and environmental factors.1 It represents the leading cause of legal blindness in developed countries.2,3 Late AMD is the stage with highest risk for severe visual loss and can take the form of neovascular or atrophic disease. The defining lesions are macular neovascularization (MNV) and geographic atrophy, respectively.4–6 These can co-exist in the same eye; for example, MNV may be accompanied or followed later by macular atrophy.7 If left untreated, neovascular AMD usually leads to extensive macular damage and irreversible visual loss to the level of legal blindness: the results of one meta-analysis suggested that approximately 75% of eyes will have visual acuity of 20/200 or worse three years after the development of exudative MNV.8 The advent of anti-VEGF therapy led to substantial improvements in visual outcomes for people with neovascular AMD. For the first time, visual acuity (VA) was often improved or stabilized with initial treatment, as demonstrated in landmark randomized clinical trials (RCTs).9,10
However, despite these dramatic improvements in the treatment of neovascular AMD, a proportion of eyes treated with anti-VEGF therapy may still have poor visual outcomes; this is particularly true with longer follow-up time.11–16 Potential reasons might include the progression of neovascular AMD related to relative ‘undertreatment’ with anti-VEGF therapy (e.g., including progression to subretinal fibrosis), the progression of neovascular AMD despite adequate anti-VEGF therapy, the natural progression of AMD to geographic atrophy (i.e., as the final common pathway in disease progression, independent of MNV presence7), or other causes. Hence, central macular atrophy and/or fibrosis may represent the two key features limiting vision over the longer-term for eyes with neovascular AMD undergoing anti-VEGF therapy. Understanding the relative contributions of these two features to poor visual outcomes is important. Therapeutic approaches to prevent poor vision would differ according to the principal cause: a high proportion with central subretinal fibrosis might suggest the importance of earlier and more aggressive anti-VEGF therapy, and/or adjunctive therapy to prevent fibrosis, while a high proportion with central macular atrophy might suggest the importance of other strategies alongside anti-VEGF therapy.
The Age-Related Eye Disease Study 2 (AREDS2) was a multicenter phase III RCT designed to assess the effects of nutritional supplements on the course of AMD in people at moderate to high risk of progression to late AMD.17 All patients who developed neovascular AMD received standard of care treatment by their local ophthalmologists and continued to be followed up at the AREDS2 annual study visits. Detailed information was also collected on the course of the anti-VEGF therapy given by the local ophthalmologists, including refracted best-corrected VA (BCVA) outcomes at multiple time-points, obtained as part of their AREDS2 study visits.
In a previous study of eyes diagnosed with neovascular AMD during the course of AREDS2 and treated with anti-VEGF therapy in routine clinical practice, we observed that, at two years, 9% of eyes had refracted BCVA ≤20/200.11 In this context, the main aim of the current report was to determine the principal cause for poor BCVA in these eyes. The second aim was to analyze the demographic, genetic, and clinical characteristics of these eyes, compared with eyes without poor vision at the same time point.
Methods
The study design for AREDS2 has been described previously.17 In short, 4203 participants aged 50 to 85 years were recruited between 2006 and 2008 at 82 retinal specialty clinics in the USA. Inclusion criteria at enrollment were the presence of either bilateral large drusen or late AMD in one eye and large drusen in the fellow eye. Institutional review board approval was obtained at each clinical site and written informed consent for the research was obtained from all study participants. The research was conducted under the Declaration of Helsinki and complied with the Health Insurance Portability and Accessibility Act. The AREDS2 participants were randomly assigned to placebo, lutein/zeaxanthin, docosahexaenoic acid (DHA) plus eicosapentaenoic acid (EPA), or the combination of lutein/zeaxanthin and DHA plus EPA. At baseline and annual study visits, comprehensive eye examinations were performed by certified study personnel using standardized protocols. The study visits included measurements of BCVA, with refraction, using the electronic Early Treatment Diabetic Retinopathy Study (ETDRS) VA charts, and capture of digital stereoscopic color fundus photographs.
The inclusion criteria for the current report were study eyes with a baseline AREDS2 severity level of 1 through 9 (i.e. no neovascular AMD or central geographic atrophy), baseline refracted BCVA ≥50 letters (≥20/100), no history of anti-VEGF injections at baseline, the development of neovascular AMD during AREDS2 follow-up that was treated with at least one anti-VEGF injection, and follow-up data at two years after the first study visit when neovascular AMD was recorded. The eligible eyes were then divided into two groups, based on the refracted BCVA at the two-year time-point: those with poor vision (BCVA ≤20/200) and those without poor vision (BCVA >20/200).
In the AREDS2, the digital stereoscopic color fundus photographs were graded centrally by certified graders, without access to the clinical information, at the Fundus Photograph Reading Center of the University of Wisconsin. As described previously, the calibrated stereoscopic images were viewed in a standardized digital viewing platform (ImageNet 2000, Topcon Corp, Tokyo, Japan) after color contrast and illumination adjustment.18 Neovascular AMD was defined by the presence of at least two features (subretinal fluid, serous pigment epithelial detachment, or retinal edema; intraretinal, subretinal pigment epithelium or subretinal blood characteristic of neovascular AMD; hard exudates; subretinal fibrin or fibrosis; and fibrovascular pigment epithelial detachment) or treatment for neovascular AMD (e.g. intravitreal anti-VEGF injection). Geographic atrophy was defined as a lesion equal to or larger than drusen circle I-2 (diameter 433um, area 0.146mm2, i.e. 1/4 disc diameter and 1/16 disc area) in its widest diameter with at least two of the following features present: circular shape, sharp (well-demarcated) edges, and loss of the retinal pigment epithelium (RPE; partial or complete depigmentation of the RPE, typically with exposure of underlying choroidal vessels). However, for eyes receiving a grade of neovascular AMD, the color fundus photographs from subsequent study visits were not graded for macular atrophy at the reading center. For these reasons, the color fundus photographs of the eyes in this report (i.e. those with BCVA ≤20/200) were regraded by a retinal specialist (TDK), without access to the clinical information, to determine the principal cause for poor vision: (i) central macular atrophy without accompanying central subretinal fibrosis, (ii) central subretinal fibrosis (with or without accompanying central macular atrophy, since this is difficult to assess in the presence of fibrosis), or (iii) other cause. The grading definitions used for atrophy and for fibrosis were the same as those used by the reading center graders18, though no specific training or assessment was undertaken for the application of these definitions.
Questionnaires administered at the baseline and subsequent study visits collected medical and ophthalmologic history information. This comprised details of any treatment for late AMD since the last study visit, including data on anti-VEGF therapy in each eye. Information on anti-VEGF injections was validated by the study coordinators, whenever possible, by verification with the clinical records of the local retinal specialist. The self-reported number of anti-VEGF injections per eye in the two-year study period was computed.
In the AREDS2, 1826 participants consented to genotype analysis. SNPs were analyzed using a custom Illumina HumanCoreExome array.19 The AMD Genetic Risk Score (GRS) is a weighted risk score for late AMD based on 52 independent variants at 34 loci identified in a large genome-wide association study19; it was calculated for each participant according to methods described previously.19 Participants were divided into 3 groups (0–2); group 0 participants were those with a GRS less than the mean GRS of a control population without late AMD, whereas group 1 and 2 participants were those with a higher GRS (less than and more than, respectively, the median GRS of a large population with late AMD). Three individual loci were also selected for analysis, as those with highest attributable risk of late AMD: ARMS2 (rs10490924), CFH (rs10922109 and rs1061170), and C3 (rs2230199).
The demographic, clinical, and genetic characteristics described above were compared between the two group of eyes (i.e. those with and without poor vision at two years). Statistical analyses were performed with SAS 9.4. P values <0.05 were considered statistically significant.
Results
A total of 594 eyes of 549 participants met the inclusion criteria for this study. These eyes were divided into two groups, based on their refracted BCVA at the two-year time point. The number with poor vision (BCVA ≤20/200) was 56 eyes (9.4%) of 56 participants; the remaining 538 eyes (90.6%) of 498 participants had BCVA >20/200. The demographic, genetic, and clinical characteristics of these eyes are shown in Table 1. Mean refracted BCVA was 14.9 ETDRS letters (SD 12.3; Snellen 20/500) in the group with poor vision and 70.1 letters (SD 12.8; 20/40) in the group without.
Table 1.
Demographic, clinical, and genetic characteristics of study eyes, according to the presence or absence of poor visual acuity two years following the first study visit after starting anti-VEGF therapy.
| Refracted BCVA ≤20/200 at two years | Refracted BCVA >20/200 at two years | P | |
|---|---|---|---|
| Eyes, n | 56 | 538 | |
| Female sex, n (%) | 32 (57.1) | 321 (59.7) | 0.71 |
| Age at AREDS2 baseline visit (years), mean (SD) | 76.0 (6.0) | 74.9 (7.2) | 0.48 |
| Age at first post-injection visit (years), mean (SD) | 78.0 (5.9) | 77.1 (7.3) | 0.66 |
| Non-white race, n (%) | 5 (8.9) | 9 (1.7) | 0.006 |
| Education, n (%) | 0.20 | ||
| High school or less | 25 (44.6) | 180 (33.5) | |
| At least some college | 22 (39.3) | 255 (47.4) | |
| Post-graduate | 7 (12.5) | 92 (17.1) | |
| Unknown | 2 (3.6) | 11 (2.0) | |
| Smoking status, n (%) | 0.51 | ||
| Never | 28 (50.0) | 226 (42.0) | |
| Former | 25 (44.6) | 280 (52.0) | |
| Current | 3 (5.4) | 32 (5.9) | |
| Treatment assignment, n (%) | 0.20 | ||
| Placebo | 10 (17.9) | 137 (25.5) | |
| Lutein/zeaxanthin | 11 (19.6) | 142 (26.4) | |
| DHA/EPA | 16 (28.6) | 133 (24.7) | |
| Combination | 19 (33.9) | 126 (23.4) | |
| BCVA at AREDS2 baseline visit (ETDRS letter score), mean (SD) | 73.6 (8.8) Snellen 20/32 | 78.4 (8.1) Snellen 20/25 | 0.0002 |
| BCVA at first post-injection visit (ETDRS letter score), mean (SD) | 38.0 (26.7) Snellen 20/160 | 71.8 (11.9) Snellen 20/40 | <0.0001 |
| BCVA two years after first post-injection visit (ETDRS letter score), mean (SD) | 14.9 (12.3) Snellen 20/500 | 70.1 (12.8) Snellen 20/40 | <0.0001 |
| Geographic atrophy present at first post-injection visit, n (%) | 15 (26.8) | 66 (12.3) | 0.003 |
| Macular hemorrhage present at first post-injection visit, n (%) | 14 (25.5) | 70 (13.2) | 0.014 |
| Lens status at first post-injection visit, n pseudophakic/aphakic (%) | 26 (47.3) | 206 (39.1) | 0.24 |
| Spherical equivalent at first post-injection visit, mean diopters (SD) | −0.5 (2.3) | −0.5 (1.9) | 0.28 |
| Fellow eye BCVA at first post-injection visit (ETDRS letter score), mean (SD) | 62.8 (26.5) Snellen 20/50 | 58.0 (28.1) Snellen 20/63 | 0.46 |
| Fellow eye with neovascular AMD at first post-injection visit, n (%) | 30 (53.6) | 330 (62.1) | 0.21 |
| Fellow eye with geographic atrophy at first post-injection visit, n (%) | 10 (17.9) | 60 (11.3) | 0.15 |
| Time interval between first injection and first post-injection visit (days), mean (SD) | 172 (130) | 169 (116) | 0.89 |
| Number of anti-VEGF injections before first post-injection visit, mean (SD) | 2.7 (1.7) | 2.9 (2.0) | 0.74 |
| Number of anti-VEGF injections over two years from first post-injection visit, mean (SD) | 7.6 (5.4) | 10.2 (6.4) | 0.001 |
| Eyes with genetic data available, n | 24 | 269 | |
| AMD Genetic Risk Score group, n (%) | 0.49 | ||
| 0 | 4 (16.7) | 27 (10.0) | |
| 1 | 7 (29.2) | 78 (29.0) | |
| 2 | 13 (54.2) | 164 (60.9) | |
| ARMS2 rs10490924, n (%) | 0.16 | ||
| G/G | 10 (41.7) | 83 (30.9) | |
| G/T | 6 (25.0) | 122 (45.4) | |
| T/T | 8 (33.3) | 64 (23.8) | |
| CFH rs10922109, n (%) | 0.80 | ||
| C/C | 14 (58.3) | 168 (62.5) | |
| C/A | 9 (37.5) | 95 (35.3) | |
| A/A | 1 (4.2) | 6 (2.2) | |
| CFH rs1061170, n (%) | 0.94 | ||
| T/T | 3 (12.5) | 38 (14.1) | |
| T/C | 12 (50.0) | 139 (51.7) | |
| C/C | 9 (37.5) | 92 (34.2) | |
| C3 rs2230199, n (%) | 0.36 | ||
| C/C | 0 (0.0) | 21 (7.8) | |
| C/G | 10 (41.7) | 104 (38.7) | |
| G/G | 14 (58.3) | 144 (53.5) |
AMD = age-related macular degeneration; AREDS2 = Age-Related Eye Disease Study 2; BCVA = refracted best-corrected visual acuity; CI = confidence interval; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; ETDRS = Early Treatment Diabetic Retinopathy Study; SD = standard deviation
Of the 56 eyes in the group with poor BCVA at two years, 15 (26.8%) already had macular atrophy two years earlier (i.e. present at the first post-injection study visit, co-existing with neovascular AMD); in three of these eyes, the atrophy already involved the central macula.
55 eyes had color fundus photographs available at the two-year time-point. Of these, 33 (60.0%) had central macular atrophy (without accompanying central subretinal fibrosis) as the principal cause for poor vision. The remaining 22 eyes (40.0%) had central subretinal fibrosis (with or without central atrophy) as the principal cause for poor vision. Examples for each of these two categories are shown in Figure 1.
Figure 1.
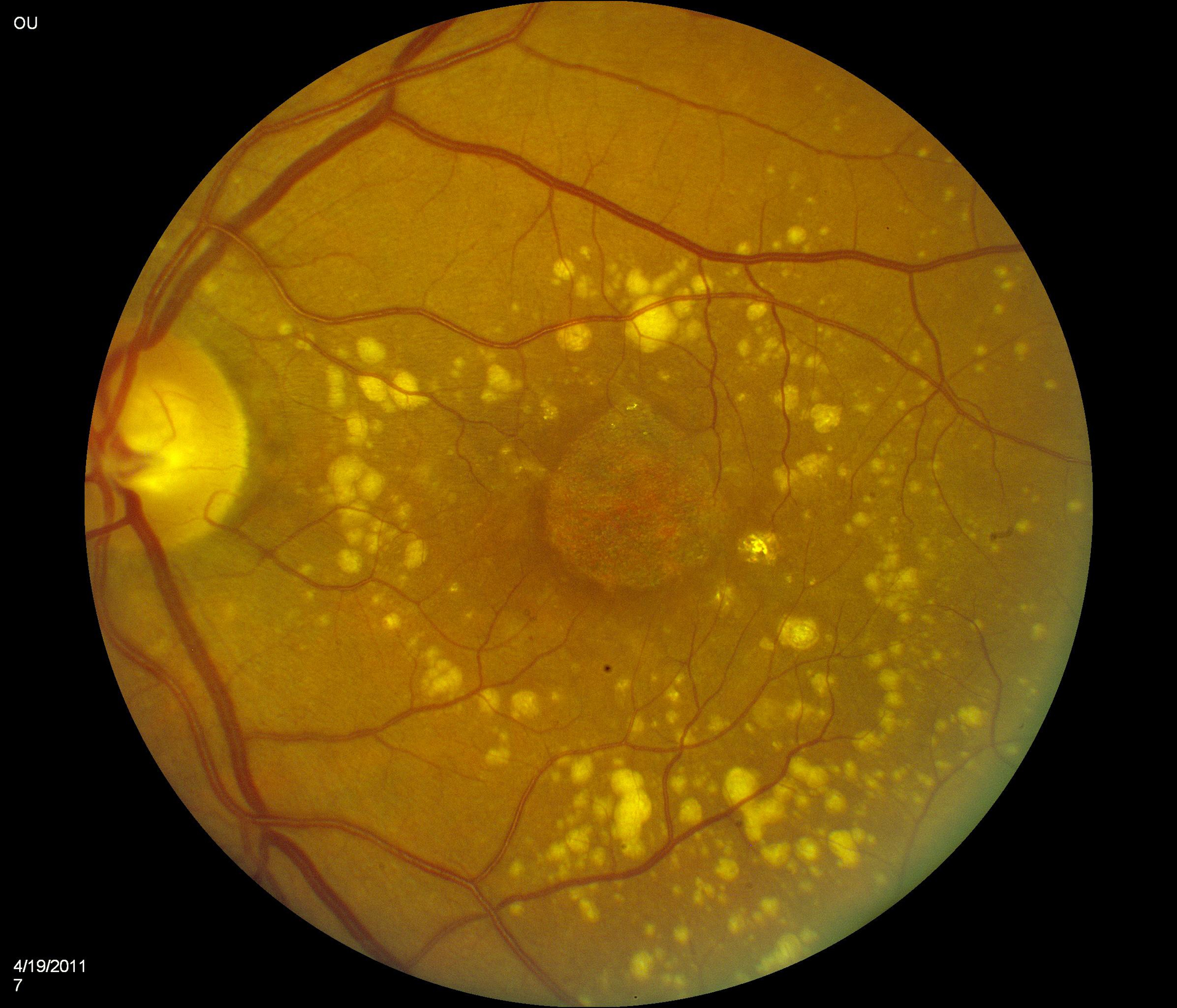
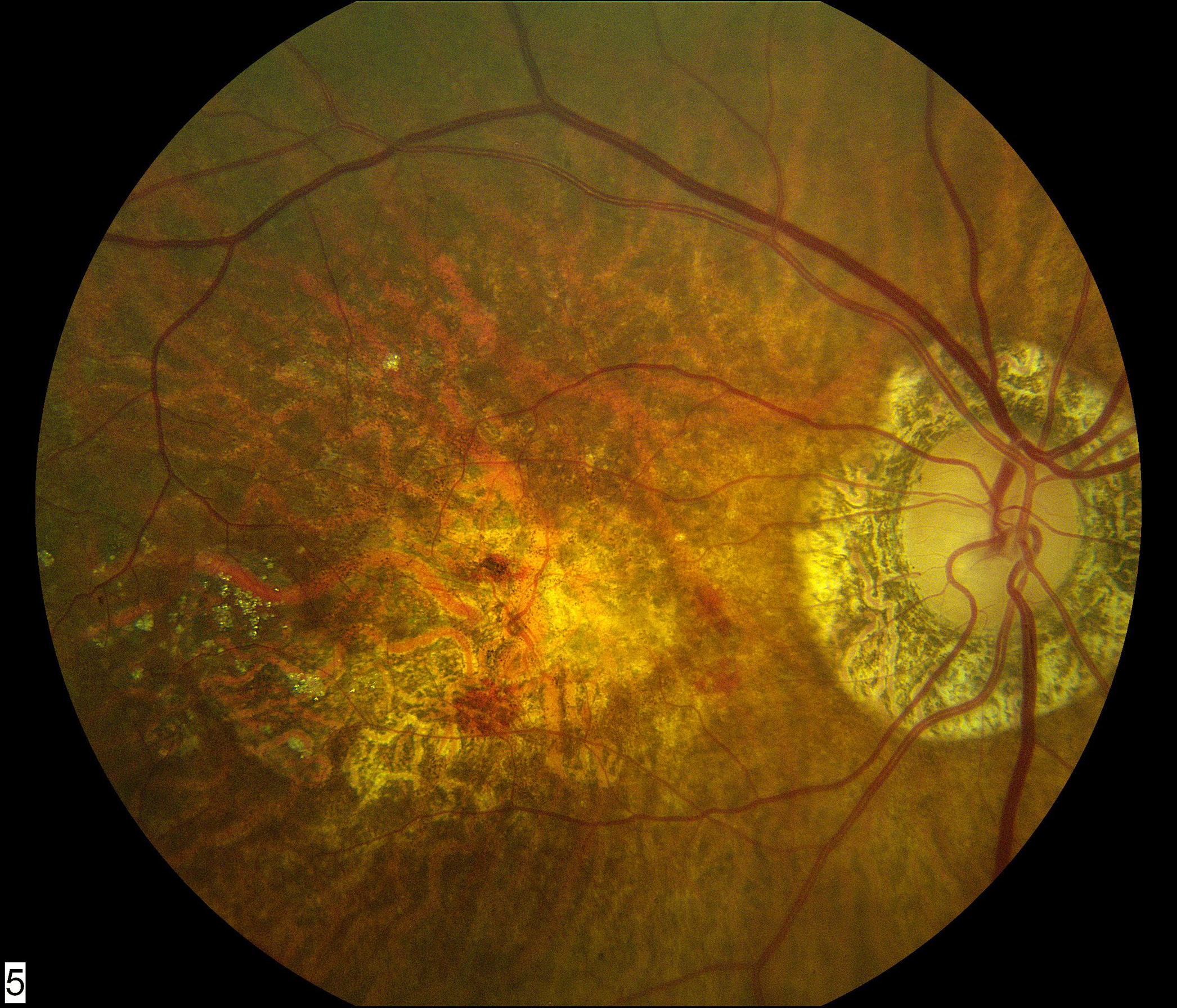
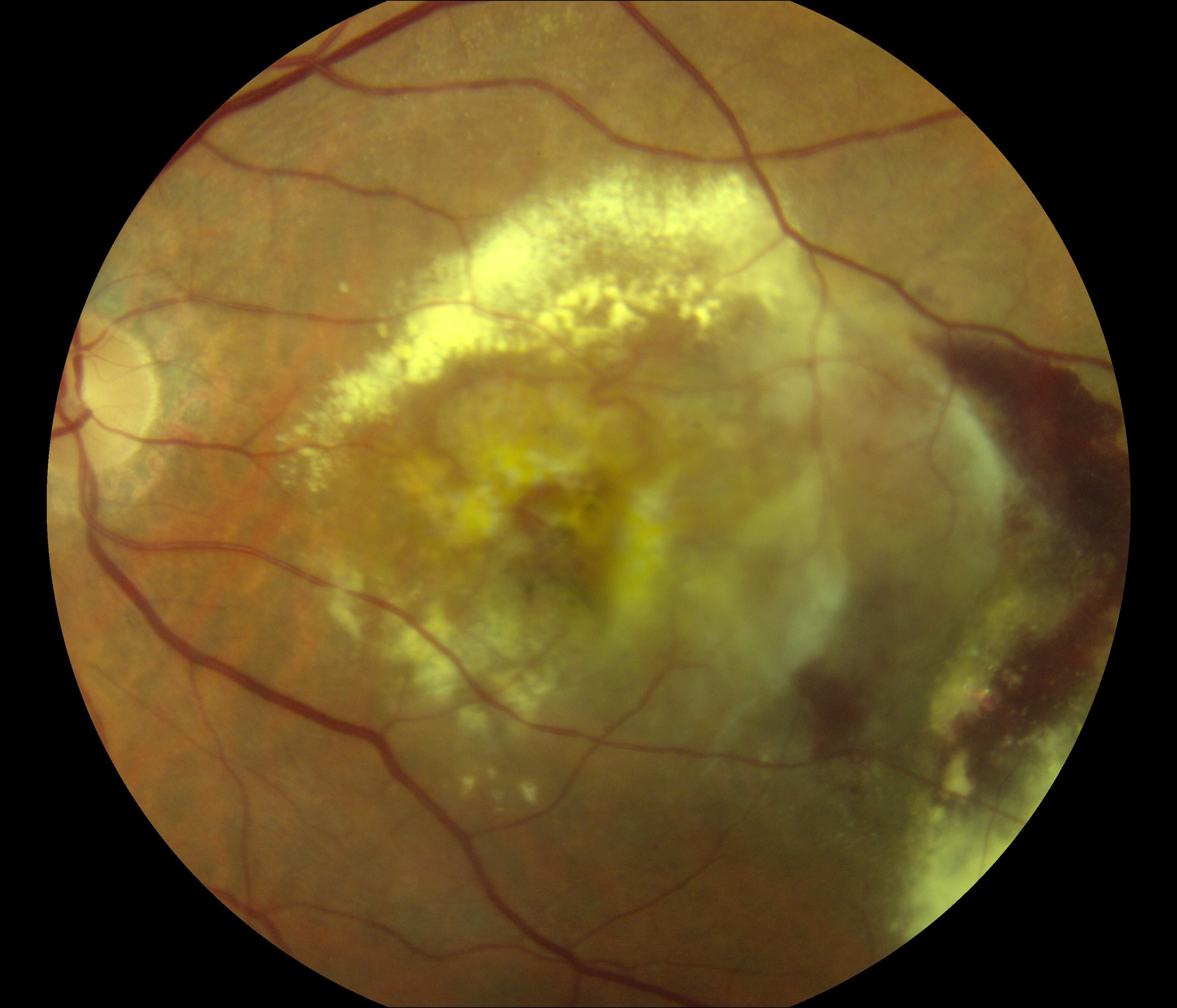
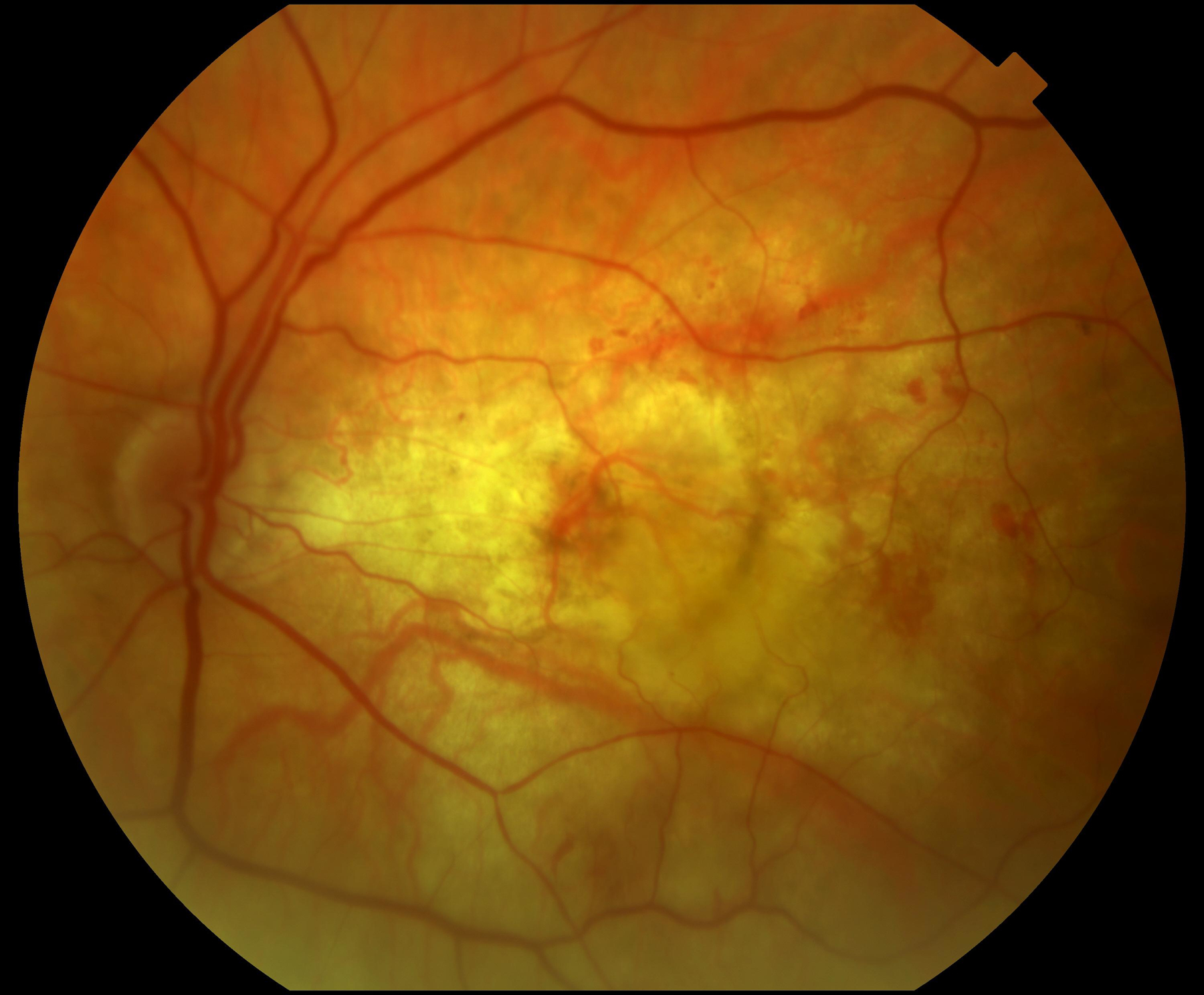
Color fundus photographs of eyes with refracted best-corrected visual acuity below 20/200 at two years after the first study visit where neovascular age-related macular degeneration was recorded. In (A) and (B), the principal cause for poor acuity was central macular atrophy (without accompanying central subretinal fibrosis). In (C) and (D), the principal cause was central subretinal fibrosis.
Of the 15 eyes with macular atrophy already present at the first post-injection visit, 12 had central macular atrophy as the principal cause for poor vision two years later; the remaining three eyes had developed central subretinal fibrosis during the two years. Of the 41 eyes without macular atrophy at the first post-injection visit, 21 had developed central macular atrophy (without fibrosis) as the principal cause for poor vision two years later, and 19 eyes had developed central subretinal fibrosis.
The demographic, genetic, and clinical characteristics of the 56 eyes with poor BCVA at the two-year time-point were analyzed and compared with those of the 538 eyes without poor BCVA at two years (Table 1). The group with poor BCVA had a significantly higher proportion of non-white participants, at 8.9% vs 1.7% (p=0.006). No significant differences were observed between the two groups in terms of age, sex, education level, smoking status, or AREDS2 treatment assignment. In the subset of eyes with genetic information available, no significant differences were observed between the two groups for any of the genetic characteristics (i.e. the AMD GRS, ARMS2, CFH, or C3). The group with poor BCVA had significantly lower BCVA two years earlier (i.e. at the first post-injection visit), at mean 38.0 letters (SD 26.7; Snellen 20/160) vs 71.8 letters (SD 11.9; 20/40; p<0.0001). Similarly, the group with poor BCVA had a significantly higher proportion of eyes with macular atrophy two years earlier, at 26.8% vs 12.3% (p=0.003). In addition, the group with poor BCVA had a significantly higher proportion of eyes with macular hemorrhage characteristic of neovascular AMD (either intraretinal, subretinal, sub-RPE, or a combination) two years earlier, at 25.5% vs 13.2% (p=0.014), though without significant differences by area (p=0.49) or central involvement (p=0.75). No significant differences in fellow eye status two years earlier were observed between the two groups (Table 1). Finally, the group with poor BCVA had a significantly lower self-reported number of anti-VEGF injections over the two-year period, at 7.6 vs 10.2 (p=0.001).
Discussion
In our previous study of eyes diagnosed with neovascular AMD during AREDS2 and treated with anti-VEGF therapy, we found that, at two years, 9% of eyes had refracted BCVA ≤20/200.11 Hence, almost one in ten eyes had vision at the level of legal blindness, despite anti-VEGF therapy. The purpose of this study was to determine the principal cause for poor BCVA in these eyes, as well as explore potential risk factors. The two principal causes were central macular atrophy and central subretinal fibrosis, accounting for 60% and 40% of cases, respectively. No therapies are routinely available to treat either macular atrophy or subretinal fibrosis in AMD, so poor BCVA was clearly irreversible in all these cases (though subretinal fibrosis might potentially have been preventable in some eyes). These data demonstrate that these two elements present the most important barriers to good visual outcomes in the long-term treatment of neovascular AMD.
The development of central subretinal fibrosis may relate to factors including later presentation and/or diagnosis of neovascular AMD, more aggressive disease, relative undertreatment with anti-VEGF therapy, and the possibility that some eyes are more prone to fibrosis. Indeed, MNV type may be particularly important: type 2 lesions, typically exhibiting subretinal hyperreflective material (SHRM) on optical coherence tomography (OCT), have a higher risk for subretinal fibrosis.20–23 Taken together, the results of several large studies have shown the risk factors for subretinal fibrosis to include: (i) presenting characteristics (lower baseline BCVA and longer interval between diagnosis and treatment), (ii) MNV characteristics (larger baseline lesion size and type 2 MNV or a classic pattern on fluorescein angiography), (iii) other anatomical characteristics (SHRM presence, foveal SRF presence, thicker foveal retina, and no RPE elevation), and (iv) ongoing lesion activity (higher proportion of visits with active exudation and the occurrence of large hemorrhages).20,21,23,24
By contrast, the development of central macular atrophy may relate to factors including the presence of non-central atrophy prior to or simultaneous with neovascular AMD, relative undertreatment with anti-VEGF therapy, and the natural progression of AMD to geographic atrophy (i.e. as the final common pathway in disease progression, independent of MNV presence). Additional baseline risk factors for progression to macular atrophy identified by OCT analyses include MNV lesion type (type 3), baseline fluid characteristics (IRF presence and SRF absence), anatomical characteristics (nascent atrophy presence, reticular pseudodrusen presence, lower choroidal thickness, increased foveal thickness, and higher drusen volume), and fellow eye characteristics (geographic atrophy presence).25 Recent analyses suggest that increased anti-VEGF treatment frequency and dose may not be risk factors for atrophy.25–27 On the contrary, regarding potential undertreatment with anti-VEGF therapy, macular atrophy is an important feature of disciform scars (for example, as commonly seen in untreated neovascular AMD in the AREDS7). Hence, relative undertreatment with anti-VEGF therapy might lead to atrophic areas, which may involve the central macula at onset or with time. In particular, persistent or recurrent intraretinal fluid at the central macula is a strong risk factor for poor visual acuity28–31, presumably attributable at least in part to the development of central atrophy.
Understanding the relative contributions of macular atrophy and fibrosis to poor visual outcomes is important, in terms of potential strategies to prevent their occurrence. Regarding subretinal fibrosis, it is possible that this might be prevented or decreased in some cases by early diagnosis and aggressive treatment with anti-VEGF therapy. In addition, the availability of an adjunctive treatment (i.e. given alongside anti-VEGF therapy) to prevent or treat fibrosis would be ideal. This would be particularly helpful in eyes with incipient fibrosis or at high risk of fibrosis. For example, SHRM presence is a strong risk factor for subretinal fibrosis.22 For these reasons, combination anti-VEGF and anti-PDGF therapy has been considered in neovascular AMD. However, three phase III RCTs of the anti-PDGF drug E10030 versus sham (alongside anti-VEGF therapy), for eyes with SHRM, failed their prespecified primary endpoints [NCT01944839, NCT01940900, NCT01940887]. Nevertheless, ongoing pre-clinical and early clinical activity continues in the search for new therapies to address subretinal fibrosis, e.g. targeting endoglin (a co-receptor essential for TGF-β signaling).32
Regarding macular atrophy, as for subretinal fibrosis, it is also possible that atrophy might be prevented or decreased in some cases by early diagnosis and aggressive treatment with anti-VEGF therapy, for the reasons described above. Of course, any atrophy arising through the natural progression of AMD to geographic atrophy (i.e. as the final common pathway in disease progression, independent of MNV presence) would not be amenable to prevention by anti-VEGF therapy. Again, the availability of an adjunctive therapy to prevent atrophy would be ideal.
Risk factors
In this study, poor BCVA at the two-year time-point was significantly associated with non-white race, low baseline BCVA, macular atrophy at baseline, macular hemorrhage at baseline, and fewer anti-VEGF injections. It is intuitive that low BCVA, macular atrophy, and macular hemorrhage at baseline should be associated with poor BCVA two years later. However, the reasons for the association with non-white race are not clear. Although this study is not powered to address this question, potential reasons might include more aggressive disease phenotypes (e.g. higher prevalence of polypoidal choroidal vasculopathy), increased racial predisposition to fibrosis, or differences in treatment characteristics.
Regarding the association with fewer anti-VEGF injections, while undertreatment with anti-VEGF therapy may have led to worse visual outcomes in some eyes (particularly through increased risk of subretinal fibrosis), it is not possible to analyze this question meaningfully in this study; the association may also have arisen through reverse causation, where very poor vision (e.g. from subretinal fibrosis) could have led the patient and physician to decrease treatment frequency or stop altogether due to futility. Interestingly, the genetic risk factors analyzed were not associated with altered risk of poor BCVA at the two-year point, not even the detailed 52-SNP GRS. However, all of the study eyes had already demonstrated the capability of progressing to late AMD; together with low power, this might explain why genotype was not also a distinguishing feature in predicting progression to poor BCVA.
Comparison with literature: rates of poor visual outcomes
The proportion of eyes with very poor BCVA in the current report should be seen in the context of equivalent figures from different studies, including those from clinical trials and from real-world studies. In the MARINA trial of monthly ranibizumab for neovascular AMD, the proportion of eyes with BCVA of 20/200 or worse at two years was 15.0% in the ranibizumab 0.5mg arm.9 The equivalent proportion in the ANCHOR trial of monthly ranibizumab 0.5mg was 20.0%, which was lower than the proportion at baseline (23.0%).10 Similarly, the equivalent proportions at two years in the CATT were 6.7% (monthly ranibizumab 0.5mg) and 5.7% (ranibizumab 0.5mg as required).33 In the HARBOR trial, the equivalent proportions were 10.2% and 10.2%, respectively.34 Overall, the proportion of 9.4% in the current study is closest to that of the HARBOR trial, slightly higher than that of the CATT, and lower than those of the MARINA and ANCHOR trials. Of course, the eligibility criteria and baseline characteristics of the eyes contribute to the differences between these studies and with the current study.
In most reports from large real-world studies, VA data are often provided in terms of mean acuity and change in acuity, without data on the proportion with VA of 20/200 or worse.14,15,35,36 For example, one of the largest datasets of ranibizumab treatment (created by meta-analysis of 42 observational studies, consisting of over 24,000 eyes), reported mean baseline VA of 55 letters (Snellen equivalent 20/80) and mean VA of 56 letters (20/80) at two years.14 However, a study from the United Kingdom of 12,951 eyes treated with ranibizumab (predominantly using a PRN approach) observed that approximately 20% of eyes had VA of 20/200 or worse at baseline and that approximately 19% (of those eyes continuing therapy at four years) had VA of 20/200 or worse at four years.15
Comparison with literature: causes of poor visual outcomes
In the CATT, 5.9% of eyes developed sustained visual loss (defined as loss of 15 or more letters from baseline to two years).37 Of these eyes, the majority (68.9%) had VA 20/200 or worse. From retrospective review of the color fundus photographs, fluorescein angiograms, and OCT scans, the principal cause of visual acuity loss was attributed to foveal scarring (44.3%), pigmentary abnormalities (27.9%), and foveal GA (11.5%). Hence, despite the substantial differences in inclusion criteria and definitions for visual loss between the CATT and the current study, the proportions caused by subretinal fibrosis were similar. The proportion caused by central macular atrophy was higher in the current study, presumably related in part to the number of eyes with atrophy already present at the first post-injection visit. In the CATT, baseline risk factors independently associated with sustained visual acuity loss were non-foveal GA presence, larger area of MNV, and bevacizumab treatment.37 In a separate report from the CATT, the proportions of eyes with subretinal fibrosis were 32%, 46%, and 56% at years one, two, and five, respectively.23
In the monthly ranibizumab arms of the MARINA and ANCHOR trials, the proportions of eyes with sustained visual acuity loss (again defined as loss of 15 or more letters from baseline to two years) were 9% and 10%, respectively.38 We are unaware of analyses that attributed the visual acuity loss in these eyes to principal causes. However, the baseline characteristics associated with loss at two years included older age, better VA, and larger MNV lesions, while the two year characteristics included increased area of RPE abnormality and increased total lesion area.38
The principal causes of poor visual outcomes in neovascular AMD have also been explored in some real-world studies. A recent real-world study presented the 10-year visual outcomes of patients from Australia/New Zealand (ANZ) and Switzerland.39 The proportion of eyes with VA of 20/200 or worse at 10 years was 14% (ANZ) and 38% (Switzerland), though, of the 795 eyes identified, only 169 completed 10 years of continuous treatment and were included in these analyses. As in the current study, macular atrophy and subretinal fibrosis were the main reasons for poor visual outcomes. Of the eyes that were treated for 10 years and lost more than 10 letters over this time, central macular atrophy was the most common cause in eyes from ANZ (41%), followed by subretinal fibrosis (28%) and endophthalmitis (5%). Interestingly, the proportions were very different in the eyes from Switzerland: subretinal fibrosis was the leading cause (78%), followed by central macular atrophy (6%). The much higher rates of poor VA in Switzerland, caused predominantly by subretinal fibrosis, were thought to relate principally to lower numbers of anti-VEGF injections.39 These results suggest that, in many cases, subretinal fibrosis may be preventable with adequate anti-VEGF therapy. Similarly, in a recent report of neovascular AMD in Asian individuals, subretinal fibrosis was the most important predictor of visual outcomes at one year.40 Between baseline and one year, the prevalence of fibrosis and macular atrophy increased from 13.0% to 37.8% and 9.7% to 17.2%, respectively.
In a real-world study of eyes from the Fight Retinal Blindness! registry that started anti-VEGF therapy between 2007 and 2012, the proportion with sustained VA loss of at least 15 letters at five years was 22.9%.35 We are unaware of analyses that attributed the visual loss in these eyes to principal causes. However, risk factors independently associated with sustained visual acuity loss included older age, fewer injections, and more visits with MNV activity. In addition, in a separate report from the Fight Retinal Blindness! registry, the prevalence of subretinal fibrosis increased from 14.3% at baseline to 26.3% at two years.24 Of course, in all these studies, variation in the rates reported is likely caused in part by differences in patient populations, medical settings (especially clinical trial versus real-world), ascertainment (prospective versus retrospective), imaging modalities, diagnostic definitions, and treatment regimens.
Strengths and limitations
The strengths of this study include its prospective observational nature, very high participant retention, and the use of standardized LogMAR BCVA measurement with refraction in all participants. Also, the large size of the study meant that many eyes with poor vision were available for analysis, with a very large number without poor vision for comparison. Color fundus photography was available in all but one participant at the two-year time point. The availability of genetic data in the majority of participants, including a 52 SNP-based AMD GRS, was a strength.
The limitations of the study include the absence of multimodal imaging, particularly OCT. Hence, atrophy and subretinal fibrosis were graded on color fundus photography alone, which may have led to misclassification between fibrosis and atrophy in a small number of cases. Although this may decrease slightly the confidence in the grading, we consider that color fundus photography was adequate for the task, given the relatively straightforward nature of the assessment. However, it meant that, in eyes where fibrosis was present centrally, no assessment of the co-existence of atrophy could be made. It also meant that OCT-based features could not be assessed as potentially important risk or protective factors, e.g., MNV lesion type, fluid characteristics, reticular pseudodrusen presence, and others. Other limitations included the absence of reading center grading at the two-year time point, follow-up visits being aligned with AREDS2 study visits rather than time since first injection (though this phenomenon has less importance at later time-points), the absence of data on baseline lesion size, and the data on anti-VEGF injections coming from patient self-report (though they were verified wherever possible).
Conclusions
In conclusion, refracted BCVA data and color fundus photographs were obtained in a clinical trial environment but related to anti-VEGF therapy given in normal clinical practice. At two years after the first study visit where neovascular AMD was recorded, almost one in ten eyes had BCVA at the level of legal blindness, despite anti-VEGF therapy. Poor BCVA was associated with non-white race, low baseline BCVA, the presence of macular atrophy at baseline, the presence of macular hemorrhage at baseline, and fewer anti-VEGF injections. Importantly, the principal cause of poor vision from color fundus photograph grading appeared to be central macular atrophy in 60% and central subretinal fibrosis in 40%. No therapies are routinely available to treat either macular atrophy or subretinal fibrosis in AMD, so poor BCVA was clearly irreversible in all these cases. However, subretinal fibrosis might be preventable with adequate anti-VEGF therapy in some eyes. These data may be useful in understanding potential barriers to good visual outcomes in the long-term treatment of neovascular AMD.
Supplementary Material
Financial Support
Supported by the intramural program funds and contracts from the National Eye Institute/National Institutes of Health, Department of Health and Human Services, Bethesda Maryland (contract HHS-N-260-2005-00007-C; ADB contract NO1-EY-5-0007). Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements, National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung, and Blood Institute, and National Institute of Neurological Disorders and Stroke. The sponsor and funding organization participated in the design and conduct of the study; data collection, management, analysis and interpretation; and the preparation, review and approval of the manuscript.
Abbreviations
- AMD
age-related macular degeneration
- AREDS2
Age-Related Eye Disease Study 2
- BCVA
best-corrected visual acuity
- CATT
Comparison of AMD Treatments Trial
- DHA
docosahexaenoic acid
- EMR
electronic medical record
- EPA
eicosapentaenoic acid
- ETDRS
Early Treatment Diabetic Retinopathy Study
- FDA
Food and Drug Administration
- GA
geographic atrophy
- PRN
pro re nata
- RCT
randomized controlled trial
- RPE
retinal pigment epithelium
- VA
visual acuity
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of Interest:
No conflicting relationship exists for any author.
References
- 1.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quartilho A, Simkiss P, Zekite A, Xing W, Wormald R, Bunce C. Leading causes of certifiable visual loss in England and Wales during the year ending 31 March 2013. Eye (Lond). 2016;30(4):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 4.Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan TD, Agron E, Domalpally A, et al. Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number 16. Ophthalmology. 2018;125(12):1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christakis PG, Agron E, Klein ML, et al. Incidence of Macular Atrophy after Untreated Neovascular Age-Related Macular Degeneration: Age-Related Eye Disease Study Report 40. Ophthalmology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115(1):116–126. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65 e55. [DOI] [PubMed] [Google Scholar]
- 11.Keenan TD, Vitale S, Agron E, et al. Visual Acuity Outcomes after Anti-Vascular Endothelial Growth Factor Treatment for Neovascular Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report Number 19. Ophthalmol Retina. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. [DOI] [PubMed] [Google Scholar]
- 13.Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Maguire MG, Martin DF, et al. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123(8):1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of Real-World Outcomes of Intravitreal Ranibizumab for the Treatment of Neovascular Age-Related Macular Degeneration. Retina. 2016;36(8):1418–1431. [DOI] [PubMed] [Google Scholar]
- 15.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092–1101. [DOI] [PubMed] [Google Scholar]
- 16.Gillies MC, Campain A, Barthelmes D, et al. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration: Data from an Observational Study. Ophthalmology. 2015;122(9):1837–1845. [DOI] [PubMed] [Google Scholar]
- 17.AREDS2 Research Group, Chew EY, Clemons T, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Invest Ophthalmol Vis Sci. 2013;54(7):4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel E, Toth CA, Grunwald JE, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloch SB, Lund-Andersen H, Sander B, Larsen M. Subfoveal fibrosis in eyes with neovascular age-related macular degeneration treated with intravitreal ranibizumab. Am J Ophthalmol. 2013;156(1):116–124 e111. [DOI] [PubMed] [Google Scholar]
- 22.Roberts PK, Zotter S, Montuoro A, et al. Identification and Quantification of the Angiofibrotic Switch in Neovascular AMD. Invest Ophthalmol Vis Sci. 2019;60(1):304–311. [DOI] [PubMed] [Google Scholar]
- 23.Daniel E, Pan W, Ying GS, et al. Development and Course of Scars in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2018;125(7):1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teo KYC, Joe AW, Nguyen V, et al. Prevalence and Risk Factors for the Development of Physician-Graded Subretinal Fibrosis in Eyes Treated for Neovascular Age-Related Macular Degeneration. Retina. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Sadda SR, Abdelfattah NS, Lei J, et al. Spectral-Domain OCT Analysis of Risk Factors for Macular Atrophy Development in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Bailey C, Scott LJ, Rogers CA, et al. Intralesional Macular Atrophy in Anti-Vascular Endothelial Growth Factor Therapy for Age-Related Macular Degeneration in the IVAN Trial. Ophthalmology. 2019;126(1):75–86. [DOI] [PubMed] [Google Scholar]
- 27.Grunwald JE, Pistilli M, Daniel E, et al. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2017;124(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldstein SM, Philip AM, Leitner R, et al. Correlation of 3-Dimensionally Quantified Intraretinal and Subretinal Fluid With Visual Acuity in Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 2016;134(2):182–190. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Erfurth U, Vogl WD, Jampol LM, Bogunovic H. Application of Automated Quantification of Fluid Volumes to Anti-VEGF Therapy of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Wickremasinghe SS, Sandhu SS, Busija L, Lim J, Chauhan DS, Guymer RH. Predictors of AMD treatment response. Ophthalmology. 2012;119(11):2413–2414 e2415. [DOI] [PubMed] [Google Scholar]
- 31.Kodjikian L, Decullier E, Souied EH, et al. Predictors of One-Year Visual Outcomes after Anti-Vascular Endothelial Growth Factor Treatment for Neovascular Age-Related Macular Degeneration. Retina. 2018;38(8):1492–1499. [DOI] [PubMed] [Google Scholar]
- 32.Shen W, Lee SR, Yam M, et al. A Combination Therapy Targeting Endoglin and VEGF-A Prevents Subretinal Fibro-Neovascularization Caused by Induced Muller Cell Disruption. Invest Ophthalmol Vis Sci. 2018;59(15):6075–6088. [DOI] [PubMed] [Google Scholar]
- 33.Comparison of Age-related Macular Degeneration Treatments Trials Research G, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181–2192. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen CL, Gillies MC, Nguyen V, et al. Characterization of Poor Visual Outcomes of Neovascular Age-related Macular Degeneration Treated with Anti-Vascular Endothelial Growth Factor Agents. Ophthalmology. 2019;126(5):735–742. [DOI] [PubMed] [Google Scholar]
- 36.Holz FG, Figueroa MS, Bandello F, et al. RANIBIZUMAB TREATMENT IN TREATMENT-NAIVE NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: Results From LUMINOUS, a Global Real-World Study. Retina. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying GS, Kim BJ, Maguire MG, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol. 2014;132(8):915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. [DOI] [PubMed] [Google Scholar]
- 39.Gillies M, Arnold J, Bhandari S, et al. Ten-Year Treatment Outcomes of Neovascular Age-Related Macular Degeneration from Two Regions. Am J Ophthalmol. 2020;210:116–124. [DOI] [PubMed] [Google Scholar]
- 40.Cheung CMG, Grewal DS, Teo KYC, et al. The Evolution of Fibrosis and Atrophy and Their Relationship with Visual Outcomes in Asian Persons with Neovascular Age-Related Macular Degeneration. Ophthalmol Retina. 2019;3(12):1045–1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


