Abstract
Purpose
We have previously shown that the chemokine CCL2 plays an important role in monocyte trafficking into the retina and alteration of the BRB in an animal model of diabetic retinopathy. In this study, we examined the effect of pharmacologically targeting the chemokine pathway to reduce the increased retinal vascular permeability in this model.
Methods
C57BL/6J mice were made diabetic using streptozotocin. After 4 months of diabetes, mice (n =10) were treated by intraperitoneal injections of TAK-779 (dual CCR2/CCR5 inhibitor) (30 mg/kg) daily for 2 weeks. Retinal vascular permeability and protein expression were done using western blot. The SDF-1 levels were measured by ELISA. Immune cell infiltration in retinas were measured using flow cytometry.
Results
The dual inhibitor significantly decreased retinal vascular permeability in diabetic animals. There was a significant reduction in macrophage/microglia infiltration in retinas of treated animals. Levels of SDF-1 and ICAM-1 were significantly reduced and the tight junction protein ZO-1 level was increased, and phospho VE-Cad was significantly reduced with drug treatment.
Conclusions
A chemokine receptor inhibitor (CCR2/CCR5) can reduce retinal vascular permeability in diabetic animals. Targeting the chemokine pathway pharmacologically may be used as a novel therapeutic strategy in management of diabetic macular edema.
Keywords: Diabetic macular edema, chemokine, CCR2, CCR5, retinal vessels, Vascular permeability, tight junction protein
Introduction
Currently, the first line of treatment of center-involving diabetic macular edema (DME) is the intravitreal injection of anti-VEGF drugs. However, a large number of patients respond poorly to anti-VEGF drugs, and the effect appears to be transient, needing frequent injections. According to several large studies (RIDE/RISE, DRCR, VIVID/ VISTA), only 27% to 45% of patients in the anti-VEGF arm showed a >15-letter vision improvement.[1] Almost one third of DME patients do not respond, or poorly respond (< 5 letters of vision improvement). Thus, there is a big need for development of novel therapeutic strategies in DME. Also, there is the potential of side effects of choriocapillaris atrophy and subsequent photoreceptor damage from long-term use of anti-VEGF drugs.[2] Although steroids work well in these DME patients, they have the potential side effects of cataract formation and increased intraocular pressure.[3]
One of the earlier events in diabetic retinal inflammation is the adhesion of leukocytes to the retinal microvasculature. Increased leukocyte adhesion results in loss of endothelial cells and breakdown of the blood-retinal barrier (BRB). In a recent study, we have demonstrated that chemokines are upregulated in the retinas of diabetic animals, and that the chemokine ligands, CCL2 and CCL5, play an indirect role in mediating the increase in retinal vascular permeability in diabetes.[4] We have also shown evidence for increased monocyte/macrophage trafficking into retinal tissues of diabetic animals. The presence of activated monocytes/macrophages in the extravascular space is evidence of the ability of leukocytes to breach the BRB by diapedesis and establish an inflammatory reaction within the retinal tissues. Furthermore, using CCL2 knockout mice made diabetic, we have shown a significant reduction in retinal vascular leakage and monocyte infiltration following induction of diabetes indicating the importance of this chemokine in alteration of the BRB.[4]
DME is a heterogeneous disease with a wide range of VEGF levels in aqueous and vitreous as well as many other cytokines and chemokines involved in the process.[1] The concentrations of CCL2 and CCL5 have been shown to be increased in the vitreous and aqueous humors of patients with diabetic retinopathy/DME.[1, 4, 5] Thus targeting CCL2 and CCL5 can be an effective potential therapeutic strategy in DME as it addresses the mechanisms of early leukocyte influx, and leukostasis in diabetes.
In the present study, we examined whether the chemokine pathway can be targeted pharmacologically to intervene the retinal vascular leakage and alteration of the BRB in an animal model of diabetic retinopathy. A dual inhibitor of the CCR2/CCR5, TAK-779 was used to target the binding of chemokines to their receptors. This inhibitor is a non-peptide small molecule that has been investigated for the treatment of HIV, rheumatoid arthritis, multiple sclerosis and cancer[6].
Materials and Methods
Animal Model of Diabetes
Diabetes was induced in C57BL/6J mice with five daily consecutive intraperitoneal injections of streptozotocin (50 mg/kg/day). Age-matched non-diabetic control animals received injections of an equal volume of citrate buffer only. Animals with plasma glucose concentrations greater than 250 mg/dL were considered diabetic and were used in the study following 4 months of diabetes.
Blood glucose levels and body weights were monitored regularly. Animals were euthanized by CO2 asphyxiation and eyes enucleated and processed immediately. All animal studies were consistent with and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center.
Drug Treatment
After 4 months of diabetes, mice (n =10) were treated by intraperitoneal injections of TAK-779 (a dual CCR2/CCR5 inhibitor) (30 mg/kg) daily for 2 weeks. A control group of diabetic mice (n =10) received IP injections of the vehicle (water). Also, a third group of non-diabetic mice (n =10) were used as controls.
Flow Cytometry
Retinas from mouse eyes were digested with 0.25 mg/mL collagenase D (Roche, Indianapolis, IN) for 30 minutes at 37°C and 100 g/mL DNase I in enzyme free Cell Dissociation Buffer (Life technologies, USA). The tissue digest was then filtered through 70 μm cell strainer, and washed with HBSS buffer with 0.5% BSA for 5 minutes at 400 g at 4°C. The supernatant was carefully removed and the digested tissue pellet was resuspended in FACS buffer to form a single cell suspension. Cell viability was determined by Trypan blue staining method. Viable single cell suspension was then surface stained using fluorescent conjugated monoclonal antibodies CX3CR1-APC and CD11b-PE (eBioscience, San Diego, CA) for 30 minutes at 4°C. Negative controls and single fluorochrome controls were also done for accurate compensation. Flow cytometry data acquisition was done using LSR II (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Ashland, OR).
Stromal cell-derived factor1 (SDF-1) ELISA
SDF-1 levels were measured in the retinal tissues of the study animals by ELISA (MilliporeSigma, St. Louis, MO) according to the manufacturer’s protocol.
Real Time PCR
Total RNA was isolated from retinas (Direct-zol RNA MiniPrep, Zymo Research, Irvine, CA), converted to cDNA (High Capacity cDNA Reverse Transcription; Applied Biosystems, Foster City, CA) and analyzed using the appropriate TaqMan assay probes (VEGF, CCL2, CCL5, CCR2 and CCR5) (Applied Biosystems, Foster City, CA) by quantitative realtime PCR (qRT-PCR). Relative mRNA levels were determined by the comparative Ct method with normalization with GAPDH.
Western blot
Western blot was used to confirm the permeability and also the expression of tight junction proteins in the mice retina. Retinal tissues were lysed using RIPA buffer, sonicated and incubated on ice for 10 min. Lysates were collected by centrifugation at 12000 rpm for 10 min at 4°C. The protein concentration of each sample was estimated using the Bradford assay method. Protein samples were separated in precast TGX gels (Biorad, Hercules, CA) and transferred to blotting membranes and probed with anti-albumin (for measuring retinal vascular permeability), anti-ICAM-1, anti-VE-cadherin, phospho VE-cadherin, anti-β-tubulin, (Abcam, Cambridge, MA) and anti-ZO-1 (Thermofisher Scientific, Waltham, MA), primary antibodies and appropriate secondary antibodies. The band density was measured using Image-J and the ratio of protein of interest and housekeeping protein was plotted as histogram.
Statistical Methods
For all quantitative experiments, statistical analyses of data comparing two separate groups were performed with an unpaired t-test, whereas multiple comparisons were done using a one-way ANOVA (Prism4 software; GraphPad). Differences indicated by ANOVA were compared by the Newman-Keuls test. A value of p<0.05 was considered significant.
Results
Expression of VEGF, CCL2 and CCL5 in diabetic retinas
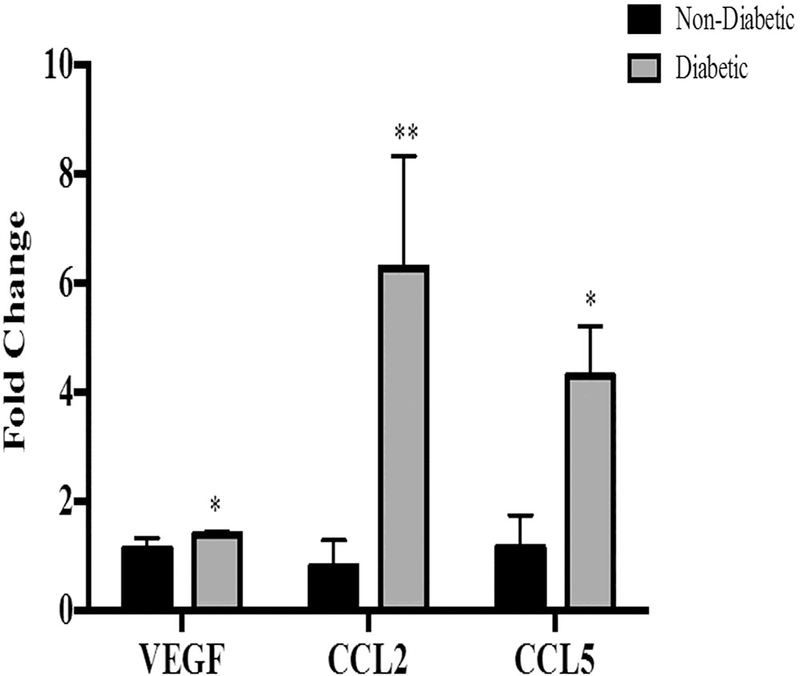
Using real time PCR, we examined the expression of VEGF, CCL2 and CCL5 in mouse retinas from animals with 16 weeks of diabetes compared to age-matched non-diabetic controls (Fig. 1). The expression of all of these genes in retinas was significantly increased with remarkably high levels of CCL2 and CCL5.
Fig. 1. Increased CCL2 and CCL5 in retinas of diabetic mice.
mRNA expression of VEGF (*p=0.05), CCL2 (**p=0.0001) and CCL5 (*p=0.03) were significantly increased in retinas of 16 weeks STZ mice compared with non-diabetic mice. Data are mean±S.D.
Retinal vascular permeability change and chemokine receptors
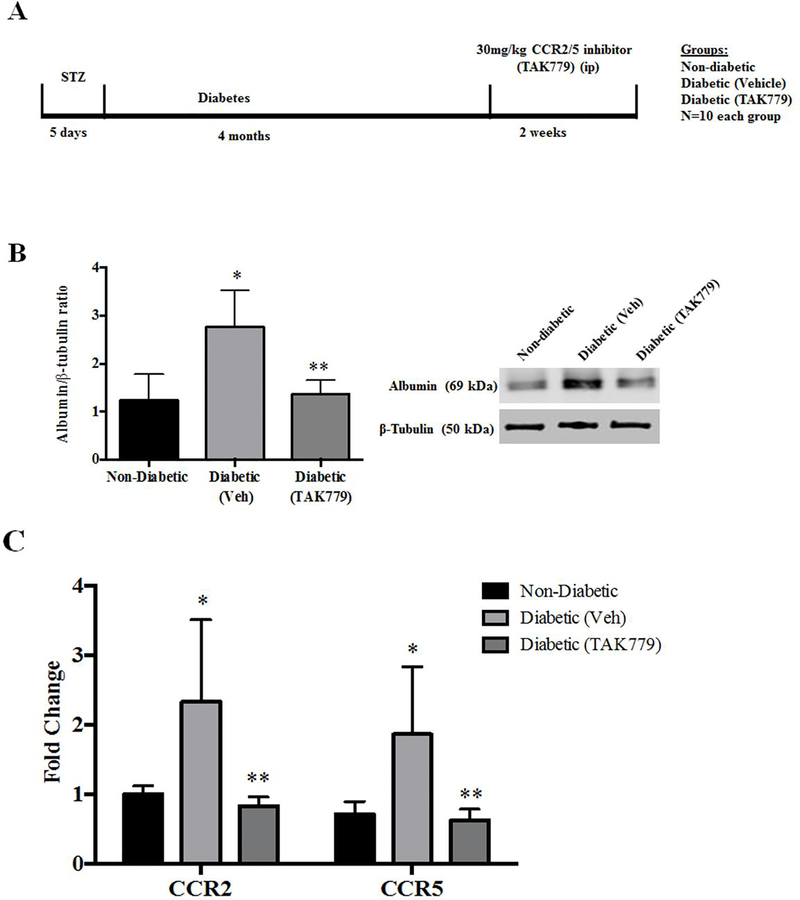
The time line of diabetes induction, drug treatment and the groups are represented in Fig. 2A. The retinal vascular permeability was measured by albumin levels in the mice retina. The albumin protein levels were in significantly elevated levels in retinas of diabetic mice in comparison with the tissues of non-diabetic mice (p=0.01) as expected. The albumin levels were significantly reduced in the retinal tissues of the diabetic mice treated with TAK-779 in comparison with the untreated diabetic group (p=0.01) (Fig.2B). The gene expression levels of both CCR2 and CCR5 receptors were significantly increased in the retinas of diabetic mice compared with non-diabetic mice (p=0.03, p=0.03), and both the receptors expression levels were reduced significantly in drug treated diabetic animals in comparison with the vehicle treated diabetic animals (p=0.02, p=0.02) (Fig.2C).
Fig. 2. Decreased Retinal Vascular Permeability and expression of chemokine receptors in retinas of mice treated with TAK-779.
(A) A schematic representation of the design of the experiment. (B) Representative western blot image of Albumin and β-tubulin protein from the retinas of non-diabetic, diabetic (Vehicle) and diabetic (drug treated) STZ mice (Right). Albumin levels were significantly increased in the retinas of diabetic mice (Vehicle) in comparison with non-diabetic mice (*p=0.01), the drug treated diabetic animals had a significantly less amount of albumin in retinas in comparison with the untreated diabetic animals (**p=0.01) (Left). (C) The gene expression levels of both CCR2 and CCR5 receptors were significantly increased in the retinas of diabetic mice compared with non-diabetic mice (*p=0.03, *p=0.03), and were significantly decreased in the drug treated diabetic mice in comparison with vehicle treated diabetic animals (**p=0.02, **p=0.02). Data are mean ±S.D.
Flow Cytometry Shows decrease in Macrophage/Microglia Numbers in Retinas of drug treated diabetic animals
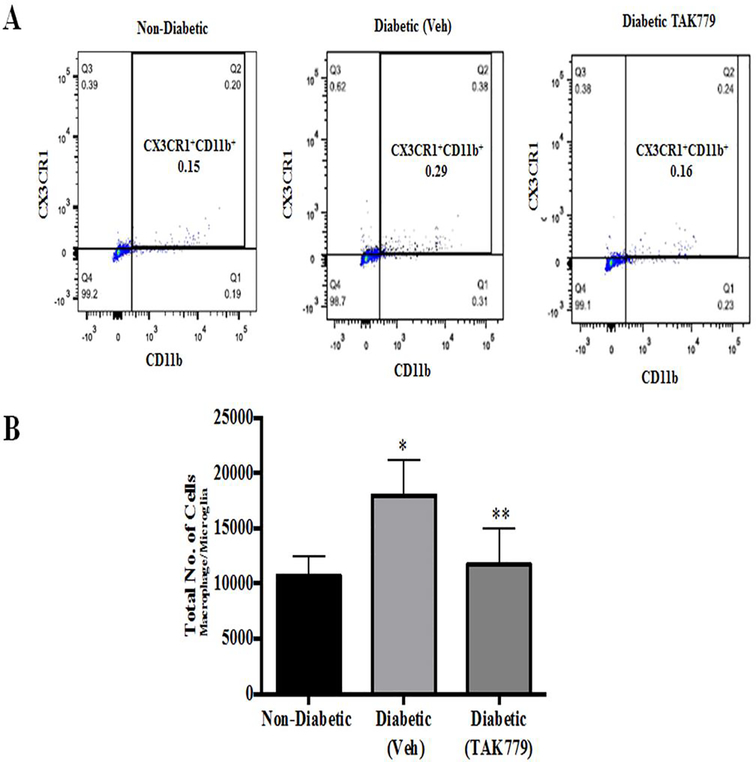
To check the inhibitory effect of the drug on the infiltration of monocytes/macrophages in the retinas of the mice, we did flow cytometry analysis on retinas of the mice. Representative dot plot of the flow cytometry analysis is shown in Fig. 3A. Flow cytometry analysis from diabetic retinas compared to non-diabetic (Ctrl) animals shows a significant increase in the percentage of CX3CR1+/CD11b+ cells (macrophage/microglia), 0.29% versus 0.15% of total cells (p=0.005), respectively and this infiltration of cells was significantly reduced by the drug treatment 0.16% (p=0.02) (Fig. 3B).
Fig. 3. TAK-779 treatment significantly reduces the infiltration of CX3CR1+/CD11b+ macrophage/microglia cells.
(A) Representative dot plots of flow cytometry analysis of control non-diabetic, diabetic (vehicle) and diabetic (drug treated) STZ mice. Gating was done to include macrophage/microglia cells labelled CX3CR1+/CD11b+. (B) Histogram represents the total number of CX3CR1+/CD11b+ cells, with significantly increased cells in the retinas of diabetic mice (vehicle) in comparison with non-diabetic mice (*p=0.05) and there was a significant decrease in the number of cells in the drug treated animals in comparison with vehicle treated diabetic animals (**p=0.02). Data are mean±S.D.
Decreased SDF-1 and ICAM-1 levels by the drug treatment
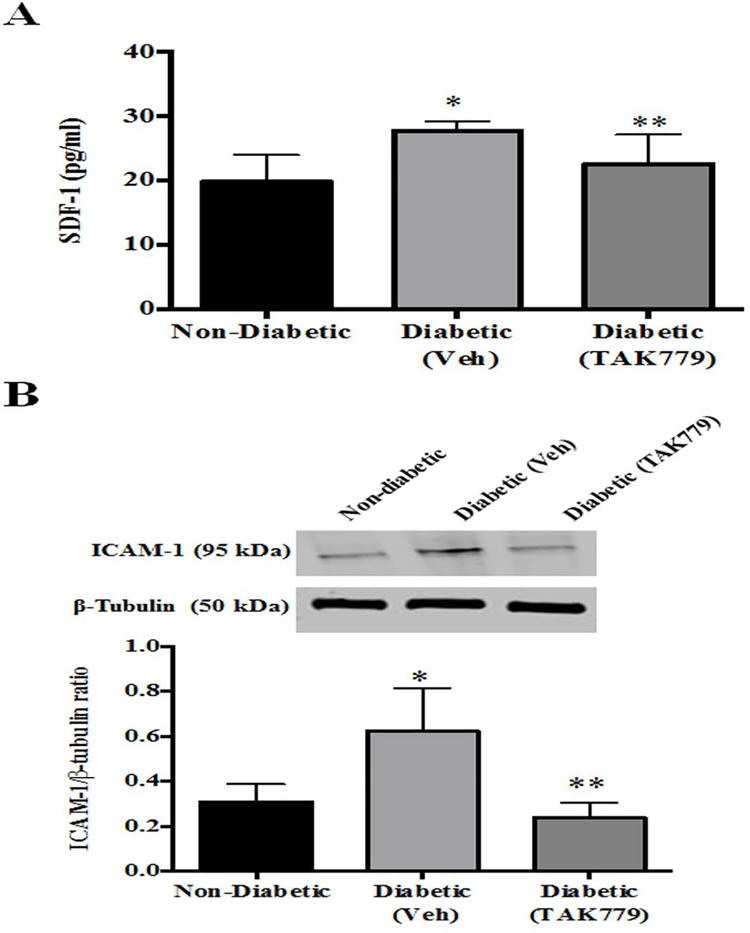
The levels of SDF-1, chemokine which triggers the traffic of immune cells and progenitor cells to the inflamed site was significantly increased in the retinas of diabetic mice (p=0.006) and the SDF-1 levels were reduced with the drug treatment (p=0.05) (Fig.4A).
Fig. 4. SDF-1 and ICAM-1 levels were decreased by the drug treatment:
(A) In comparison with the non-diabetic mice retinas there was a significant increase in the SDF-1 levels (*p=0.006), the levels of SDF-1, was significantly decreased in the retinas of the drug treated diabetic mice (**p=0.05). (B) (Top) Representative western blot image of ICAM-1 and β-tubulin protein from the retinas of non-diabetic, diabetic (Vehicle) and diabetic (drug treated) STZ mice. (Bottom) The levels of ICAM-1 were significantly increased in the retinas of diabetic mice in comparison with the non-diabetic mice retinas (*p=0.04), this level was significantly decreased with drug treatment in comparison with the untreated diabetic mice (**p=0.02).
The infiltration of the immune cells in the retina and the effect of the drug on limiting the effect made us to look into the expression of ICAM-1, the adhesion molecule, which promotes leukocyte infiltration in the inflamed tissue. Here in our study, we observed a significantly increased ICAM-1 protein level in the retina of diabetic mice in comparison with the levels of non-diabetic mice (p=0.04), the drug treatment in the diabetic mice made the ICAM-1 protein level decreased significantly in comparison with the tissues of untreated diabetic mice (p=0.02) (Fig.4B).
Effect of the Drug on Tight Junction Proteins
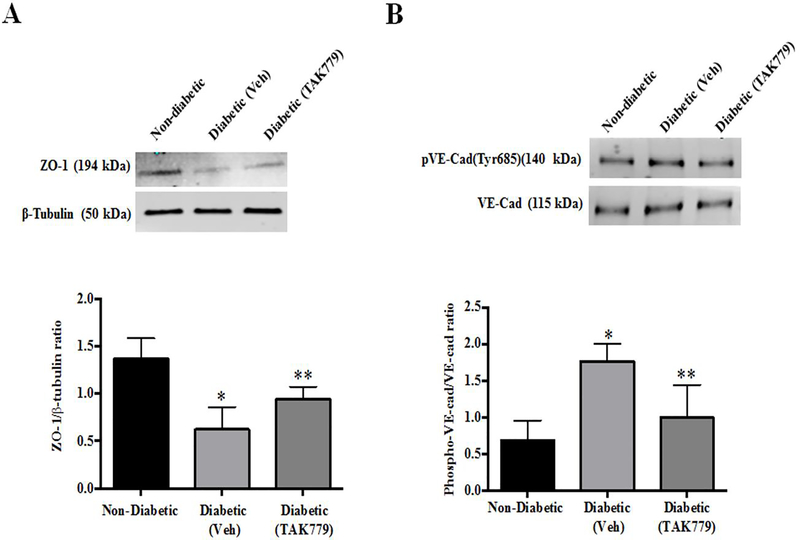
In response to proinflammatory signals, the modification of inter-endothelial junctions is observed, and proteins involved in the formation of tight junctions regulate the permeability for a given macromolecule. The level of tight junction protein ZO-1 expression was significantly decreased in the retinas of diabetic mice (p=0.008) and the levels were significantly increased in the retina of mice which were treated with TAK-779. (p=0.05) (Fig.5A).
Fig. 5. Regulation of tight junction protein by the drug treatment:
(A) Representative western blot image of ZO-1 and β-tubulin protein from the retinas of non-diabetic, diabetic (vehicle) and diabetic (drug treated) STZ mice. (Top), the levels of ZO-1 were significantly decreased in the retinas of diabetic mice in comparison with the non-diabetic mice retinas (*p=0.006), this level was significantly increased with drug treatment in comparison with the untreated diabetic mice (**p=0.04) (Bottom). (B) Representative western blot image of phosphor-VE-Cadherin and total VE-Cadherin protein from the retinas of non-diabetic, diabetic (Vehicle) and diabetic (drug treated) STZ mice (Top). The levels of phospho-VE-Cadherin was significantly increased in the retinas of diabetic mice in comparison with the non-diabetic mice retinas (*p=0.006), this level was significantly decreased with drug treatment in comparison with the untreated diabetic mice (**p=0.04) (Bottom).
VE-cadherin is one of the most important proteins for the stability of endothelial junctions and the phosphorylation status of VE-cadherin is essential for vascular leakiness and leukocyte extravasation.[7] In our study we observed a significantly increased phospho-VE-Cadherin (Tyr685) levels in the retinas of diabetic mice (p=0.006) and the treatment with the drug in the diabetic animals decreased the phospho-VE-Cadherin expression in retinas (p=0.04) (Fig.5B).
Discussion
Here in this study we have shown the effect of a dual chemokine receptor (CCR2/CCR5) inhibitor (TAK-779) in inhibiting retinal vaso-permeability and restoring the blood-retinal barrier in the retina of diabetic mice. This inhibitor drug also decreased infiltration of macrophage/microglia, and restored cell-junction molecules in the retina of diabetic mice. Thus, targeting the earlier events of chemokine expression and cellular infiltration in the inflammatory cascade may be an important strategy in reducing the breakdown of the BRB and extravasation of proteins and other solutes from capillaries into the extracellular space (Fig. 6).
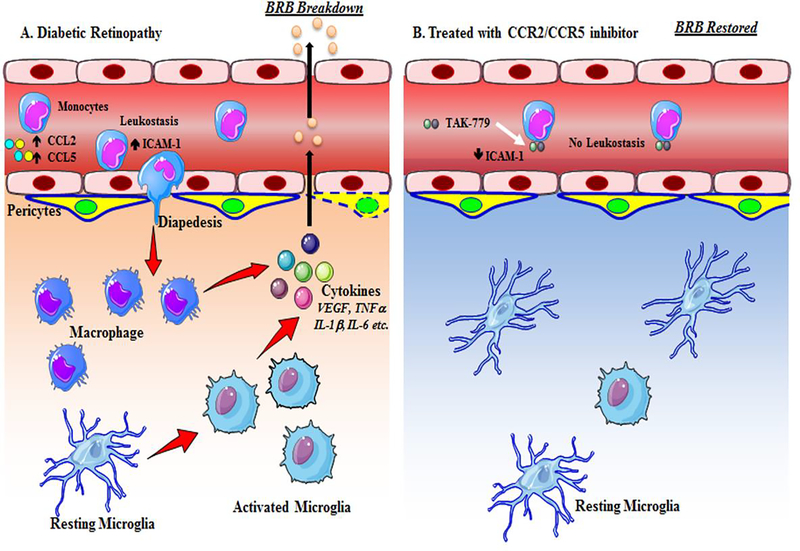
Fig. 6. Schematic diagram of action of CCR2/CCR5 dual inhibitor in diabetic retinopathy.
(A) In diabetes the level of CCL2 and CCL5 goes up resulting in leukostasis and diapedesis of monocytes. Once the monocytes come to the extra vascular space, they are differentiated into macrophages, and also the resting microglia become activated with an ameboid, round configuration. Both macrophages and microglia secrete multiple cytokines that breakdown the cell-cell junctions of the blood retinal barrier resulting in increased vaso-permeability. (B) Treatment with the dual antagonist of CCR2/CCR5 inhibitor results in binding of the drug to the monocytes and preventing leukostasis and diapedesis. This further inhibits the secretion of cytokines and activation of microglia.
Our previous study has shown both increases in expression of F4/80, a monocyte/macrophage associated marker, increased CX3CR1+/CD11b+ cells (macrophages/microglia), as well as infiltration of activated monocytes/macrophages into the retinas of diabetic animals,[4] and other previous studies has also shown activated monocytes and neutrophils in the retinas of diabetic rats and monkeys.[8, 9] The chemoattractants, CCL2 and CCL5 released by the endothelial cells in diabetes or hyperglycemic condition and binds to CCR2/CCR5 expressed in monocytes, macrophages and microglia, leading to cell recruitment, activation, cytokine production and blood retinal barrier alteration.[4, 10] TAK-779 binds to the CCR2/CCR5 and inhibit the binding of CCL2 and CCL5 to its respective receptors. The inhibition prevents the cascade of events that leads to blood retinal barrier alteration.
CD11b is expressed on neutrophils, monocytes, macrophage, natural killer (NK) cells and a subset of lymphocytes. CD11b has been implicated as having a central role in the migration of leukocytes from peripheral blood to the sites of inflammation.[11] Microglial cells, the resident macrophages of the central nervous system (CNS), are distributed throughout the neural parenchyma of the brain and the retina. These cells play important roles in development, in tissue responses to injury and infection or inflammation.[12] CX3CR1 is a chemokine receptor expressed by microglia in the brain and the retina.[13] Its only known ligand, CX3CL1 (also known as fractalkine or neurotactin), is constitutively expressed by neurons in the CNS[14] and by neurons and endothelial cells in the retina.[15]
We observed a decrease expression of phosphorylated VE-Cadherin levels with TAK-779 treatment. Modulation of VE-cadherin adhesion between endothelial cells induces vascular permeability and can be mediated by phosphorylation of VE-cadherin or components of the adherens complex,[16] by cleavage of the VE-cadherin extracellular domains,[17] or by internalization of VE-cadherin.19 In our study we found the level of ZO-1 was significantly increased in the diabetic retinas following drug treatment. BRB breakdown is also accompanied by the up-regulation of ICAM-1 and decreased expression of tight cell junction protein ZO1.[18]
Our results indicated that the levels of both SDF1 (stromal cell-derived factor1) and ICAM1 (intercellular adhesion molecule 1) were increased in the retinas of diabetic animals, this effect can be reversed with the treatment of TAK779. SDF-1 has been shown to be both necessary and sufficient to promote the incorporation of bone marrow–derived endothelial cells within an ischemic retina.[19] Leukocytes adhesion to the vascular endothelium is an important step in the inflammatory cell emigration from the circulation to the inflamed tissue.[20] ICAM-1 is expressed on vascular endothelial cells and is a counter receptor for integrins in leukocytes.[21] ICAM-1 has been found to mediate retinal leukostasis, vascular permeability and BRB breakdown in diabetes.[18]
Recently, an oral CCR2/CCR5 dual antagonist, PF-04634817 showed a modest improvement in best corrected vision in a phase II, randomized trial in DME patients, but the study did not meet the predefined noninferiority criteria compared with intravitreal ranibizumab injections.[22] The reasons for the modest improvement with this drug could be short duration of treatment, route of administration (oral vs. intravitreal) and lack of bioavailability of the drug in the retina.
Therapeutic targeting approaches have been utilized for at least nine chemokines and their receptors in different diseases including atherosclerosis, multiple sclerosis, diabetic nephropathy and diabetes itself.[23] Targeting CCL2/CCL5 alone or in combination with the current anti-VEGFα therapy or laser, may be a more specific inhibition strategy for reducing vision loss in DME. CCR2 has been shown to alter VEGF production,[24, 25] suggesting that inhibition of CCR2 might also help in reducing VEGF levels.[22] Inhibition of the chemokine pathway (mechanisms of early leukocyte influx and reducing monocyte trafficking into the retina) addresses a critical upstream target in the inflammatory cascade in DME.[1] Our findings of the BRB restoration in diabetic animals with the use of CCR2/CCR5 inhibitors seem promising, and open up the opportunity of a new class of drugs in treatment of DME.
Key Messages.
Chemokines including CCL2 play an important role in the cascade of inflammation in diabetic macular edema.
A dual inhibitor of CCR2/CCR5 receptors, TAK779 can reduce retinal vascular permeability and immune cell infiltration in a diabetic mouse model.
Targeting the chemokine receptors alone or in combination with anti-VEGF therapy may be an important novel therapeutic strategy in management of diabetic macular edema.
Acknowledgements
We acknowledge Amy Lucero for her help in animal care and technical assistance.
Design and conduct of the study: Das, McGuire.
Experiments and interpretation of the data: Monickaraj, Oruganti.
Preparation, review, and approval of the manuscript: Monickaraj, McGuire, Das.
Funding support: NIH EY022327 and VA Merit Review Award IO 1BX001801
Footnotes
Compliance with Ethical Standards:
None of the authors has any financial/conflicting interests to disclose.
All animal studies were consistent with and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center.
Availability of data and materials:
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Das A, McGuire PG, Rangasamy S (2015) Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology 122: 1375–1394 DOI 10.1016/j.ophtha.2015.03.024 [DOI] [PubMed] [Google Scholar]
- 2.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M (2012) Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 122: 4213–4217 DOI 10.1172/JCI65157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangasamy S, McGuire PG, Das A (2012) Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol 19: 52–59 DOI 10.4103/09749233.92116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A (2014) Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 9: e108508 DOI 10.1371/journal.pone.0108508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen LA, Hartnett ME (2013) Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema. Curr Diab Rep 13: 476–480 DOI 10.1007/s11892-013-0382-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q (2010) Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol 88: 41–55 DOI 10.1189/jlb.1009671 [DOI] [PubMed] [Google Scholar]
- 7.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D (2014) Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol 15: 223–230 DOI 10.1038/ni.2824 [DOI] [PubMed] [Google Scholar]
- 8.Schröder S, Palinski W, Schmid-Schönbein GW (1991) Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 139: 81–100 [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA (2005) Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes 54: 1534–1542 DOI: 10.2337/diabetes.54.5.1534 [DOI] [PubMed] [Google Scholar]
- 10.Bogdanski P, Pupek-Musialik D, Dytfeld J, Jagodzinski PP, Jablecka A, Kujawa A, Musialik K (2007) Influence of insulin therapy on expression of chemokine receptor CCR5 and selected inflammatory markers in patients with type 2 diabetes mellitus. Int J Clin Pharmacol Ther 45: 563–567 DOI 10.5414/cpp45563 [DOI] [PubMed] [Google Scholar]
- 11.Springer TA (1990) Adhesion receptors of the immune system. Nature 346: 425–434 DOI 10.1038/346425a0 [DOI] [PubMed] [Google Scholar]
- 12.Falsig J, van Beek J, Hermann C, Leist M (2008) Molecular basis for detection of invading pathogens in the brain. J Neurosci Res 86: 1434–1447 DOI 10.1002/jnr.21590 [DOI] [PubMed] [Google Scholar]
- 13.Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, Debré P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F (2007) CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 117: 2920–2928 DOI 10.1172/JCI31692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1expressing microglia. Proc Natl Acad Sci U S A 95: 10896–10901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman MD, Zamora DO, Pan Y, Texeira PV, Baek SH, Planck SR, Rosenbaum JT (2003) Constitutive and inflammatory mediator-regulated fractalkine expression in human ocular tissues and cultured cells. Invest Ophthalmol Vis Sci 44: 1608–1615 DOI 10.1167/iovs.02-0233 [DOI] [PubMed] [Google Scholar]
- 16.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J (2008) Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 121: 29–37 DOI 10.1242/jcs.022681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaratna D, McGuire PG, Menicucci G, Das A (2007) Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 56: 2380–2387 DOI 10.2337/db06-1694 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB (2011) Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res 2: 96–103 DOI 10.4103/0975-3583.83035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW (2005) SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 115: 86–93 DOI 10.1172/JCI22869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nourshargh S, Williams TJ (1990) Evidence that a receptor-operated event on the neutrophil mediates neutrophil accumulation in vivo. Pretreatment of 111In-neutrophils with pertussis toxin in vitro inhibits their accumulation in vivo. J Immunol 145: 2633–2638 [PubMed] [Google Scholar]
- 21.Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76: 301–314 [DOI] [PubMed] [Google Scholar]
- 22.Gale JD, Berger B, Gilbert S, Popa S, Sultan MB, Schachar RA, Girgenti D, Perros-Huguet C (2018) A CCR2/5 Inhibitor, PF-04634817, Is Inferior to Monthly Ranibizumab in the Treatment of Diabetic Macular Edema. Invest Ophthalmol Vis Sci 59: 2659–2669 DOI 10.1167/iovs.17-22731 [DOI] [PubMed] [Google Scholar]
- 23.Koenen RR, Weber C (2010) Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov 9: 141–153 DOI 10.1038/nrd3048 [DOI] [PubMed] [Google Scholar]
- 24.Krause TA, Alex AF, Engel DR, Kurts C, Eter N (2014) VEGF-production by CCR2-dependent macrophages contributes to laser-induced choroidal neovascularization. PLoS One 9: e94313 DOI 10.1371/journal.pone.0094313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seok SJ, Lee ES, Kim GT, Hyun M, Lee JH, Chen S, Choi R, Kim HM, Lee EY, Chung CH (2013) Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant 28: 1700–1710 DOI 10.1093/ndt/gfs555 [DOI] [PubMed] [Google Scholar]