Abstract
OBJECTIVES
The purpose of this study was to determine the relationship between body composition, N-terminal B-type natriuretic peptide (NT-proBNP) levels, and heart failure (HF) phenotypes and outcomes.
BACKGROUND
Abnormalities in body composition can influence metabolic dysfunction and HF severity; however, data assessing fat distribution and skeletal muscle (SM) size in HF with reduced (HFrEF) and preserved EF (HFpEF) are limited. Further, whether NPs relate more closely to axial muscle mass than measures of adiposity is not well studied.
METHODS
We studied 572 adults without HF (n = 367), with HFrEF (n = 113), or with HFpEF (n = 92). Cardiac magnetic resonance was used to assess subcutaneous and visceral abdominal fat, paracardial fat, and axial SM size. We measured NT-proBNP in 334 participants. We used Cox regression to analyze the relationship between body composition and mortality.
RESULTS
Compared with controls, pericardial and subcutaneous fat thickness were significantly increased in HFpEF, whereas patients with HFrEF had reduced axial SM size after adjusting for age, sex, race, and body height (p < 0.05 for comparisons). Lower axial SM size, but not fat, was significantly predictive of death in unadjusted (standardized hazard ratio: 0.63; p < 0.0001) and multivariable-adjusted analyses (standardized hazard ratio = 0.72; p = 0.0007). NT-proBNP levels more closely related to lower axial SM rather than fat distribution or body mass index (BMI) in network analysis, and when simultaneously assessed, only SM (p = 0.0002) but not BMI (p = 0.18) was associated with NT-proBNP. However, both NT-proBNP and axial SM mass were independently predictive of death (p < 0.05).
CONCLUSIONS
HFpEF and HFrEF have distinct abnormalities in body composition. Reduced axial SM, but not fat, independently predicts mortality. Greater axial SM more closely associates with lower NT-proBNP rather than adiposity. Lower NT-proBNP levels in HFpEF compared with HFrEF relate more closely to muscle mass rather than obesity.
Keywords: heart failure, muscle mass, natriuretic peptides, obesity, sarcopenia
It has been traditionally thought that symptom development in heart failure (HF) is predominantly related to low cardiac output and/or elevated left ventricular (LV) filling pressures. Amassing evidence suggests, however, that the pathophysiology of symptoms in HF involves a complex interplay between not only cardiac and vascular organ systems, but also with adipose and skeletal muscle (SM) tissue beds. Obesity is particularly common in HF, both in preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF) populations (1,2). Obesity can worsen HF symptoms through several mechanisms. Adipose tissue, for instance, is metabolically active and is associated with worsening inflammation, hypertension, insulin resistance, abnormal ventricular-vascular coupling, cardiac mechanics, and endothelial dysfunction (3–5). In addition, given that exercise intolerance is a key clinical symptom in patients with HF, it is not surprising that abnormalities in the peripheral skeletal musculature and mitochondrial dysfunction are common and contribute to symptoms (6).
However, not all adipose tissue beds are created equal. Visceral, as opposed to subcutaneous fat, for instance, is thought to be more metabolically toxic. Pericardial fat has paracrine activity and may affect different signaling pathways, including sympathetic nervous system activity (7). Few studies have investigated the distribution of fat in patients with HF and the relative significance of each adipose bed. Further, the prevalence and significance of sarcopenia, often seen in conjunction with adipose accumulation in musculature (so-called “sarcopenic obesity”) in HFpEF and HFrEF remains poorly understood (8,9). Finally, exploring how the relationship between obesity and natriuretic peptides (NPs) is influenced by muscle mass, and thus may help elucidate the obesity paradox in HF, is of interest.
In this study, we sought to determine the distribution of adipose tissue (subcutaneous and visceral abdominal fat as well as paracardial fat) and SM size, and their prognostic significance in adults with HFrEF and HFpEF. Further, we sought to describe the relationship between fat depots, N-terminal B-type natriuretic peptide (NT-proBNP), and muscle mass, and whether the association between adiposity and lower NT-proBNP might more closely relate to greater muscle mass.
METHODS
We enrolled 572 subjects without HF (n = 367), HFrEF (n = 113), or HFpEF (n = 92), referred for a cardiac magnetic resonance (CMR) study at the Corporal Michael J. Crescenz VA Medical Center. The protocol was approved by the Philadelphia VA Medical Center Institutional Review Board, and written informed consent was obtained from all participants. HF definitions and exclusion criteria are available in Supplemental Appendix.
AXIAL SM MASS.
At baseline, a cardiac CMR study was performed. The imaging protocol included sagittal and axial stacks of the chest and upper abdomen, which were used for assessments of axial muscle mass, paracardial, subcutaneous, and abdominal visceral fat. We used a 1.5 Tesla whole-body MRI scanner (Avanto or Espree, Siemens, Malvern, Pennsylvania) equipped with a phase-array cardiac coil. An axial stack of steady state free precession (SSFP) images was obtained, as per our routine cardiac CMR protocol, spanning the entire thorax. Typical acquisition parameters were as follows: repetition time = 30.6 ms; echo time = 1.2 ms; Flip angle = 80; slice thickness = 5 mm; space between slices = 5 mm; matrix size = 256 × 208; parallel image (IPAT) factor = 2.
Measurements of axial SM size were performed as previously described (10). Briefly, CMRs were analyzed using Horos software version 1.2.1. The level of the carina was established as a reference point for measurements of SM cross-sectional area on all axial chest CMRs. Thoracic SM was then manually traced bilaterally for pectoralis major, pectoralis minor, latissimus dorsi, paraspinal and trapezius muscles (Figure 1A). A previous study in this cohort identified a single latent factor that underlies the shared variability in the cross-sectional area of these muscles (10). This underlying factor was used as a continuous measure of axial SM size. We also assessed the pectoralis major cross-sectional area, which was identified as the single muscle with the highest prognostic value in our prior study (10).
FIGURE 1. Methods for Axial Muscle, Pericardial, Subcutaneous, and Visceral Fat Measurements.
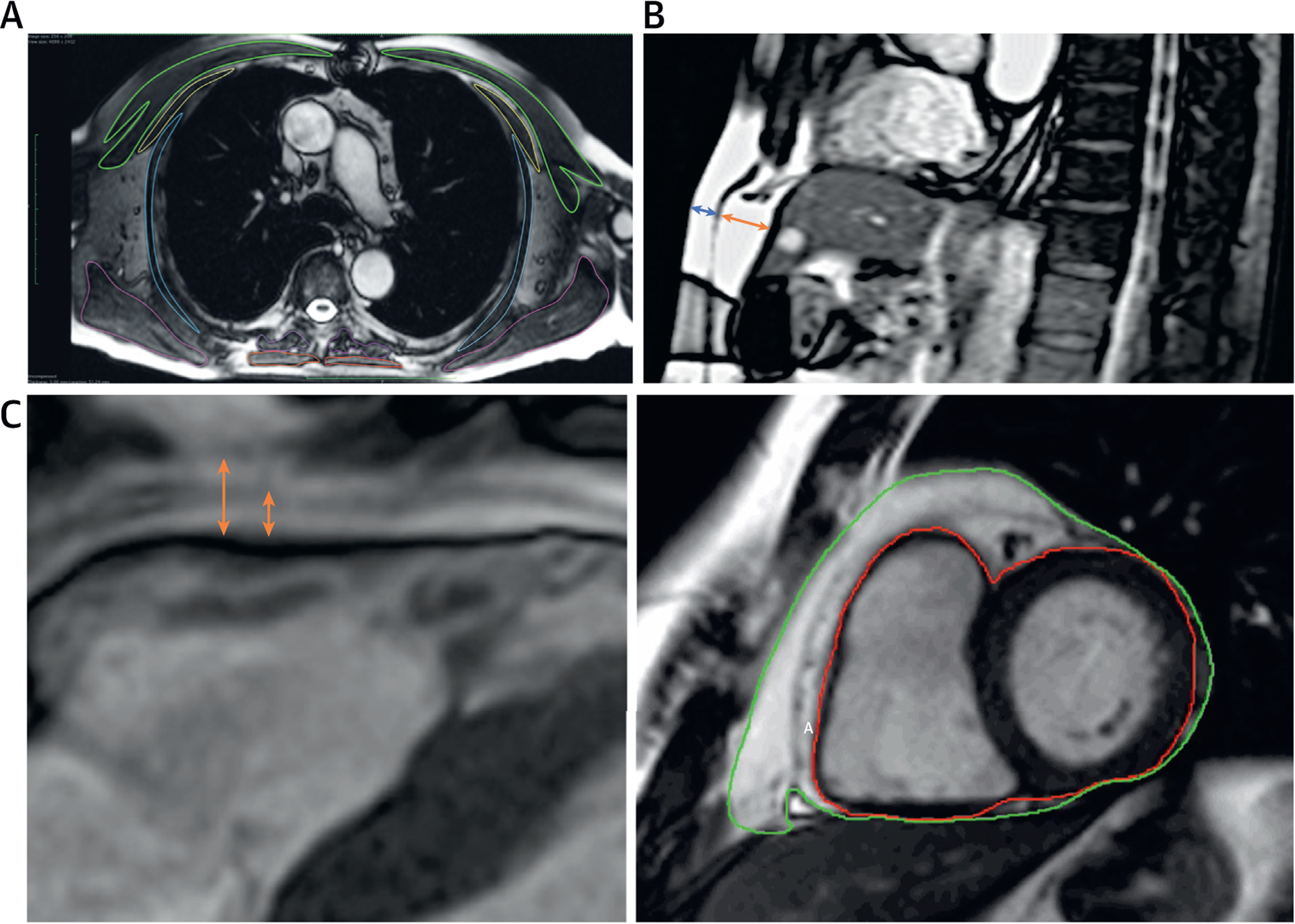
(A) Segmentation of axial muscle groups; (B) measurement of abdominal visceral and subcutaneous fat thickness; (C) segmentation of pericardial fat.
SUBCUTANEOUS AND VISCERAL ABDOMINAL FAT.
Subcutaneous and preperitoneal fat thickness were measured from stored DICOM images in the upper abdomen using axial 2-dimensional SSFP imaging. We measured subcutaneous and visceral fat thickness using a mid-sagittal image (Figure 1B). We measured the upper abdominal preperitoneal fat thickness, which has been shown to strongly correlate with visceral abdominal fat measured by computed tomography (11). The preperitoneal fat thickness was defined as the maximum anteroposterior thickness measurable in the upper abdominal images, anterior to the left lobe of the liver. The subcutaneous fat thickness was defined as the thickness of the fat tissue between the skin-fat interface and the linea alba (11). Adequate measurements for visceral and subcutaneous fat thickness were available in 528 and 531 participants, respectively.
PARACARDIAL FAT THICKNESS AND VOLUME.
We measured epicardial and pericardial adipose tissue thickness anterior to the right ventricle, in enddiastole, in the 3-chamber long axis LV view. Epicardial fat was defined as the fat depot between the outer myocardial border and the visceral pericardium, whereas pericardial fat was defined as the fat depot external to the visceral pericardium (12). However, because epicardial fat is prominent near the interventricular and the atrioventricular grooves and its distribution exhibits considerable individual variability (12), epicardial/pericardial fat thickness measured at a single point may not fully represent the pericardial fat volume. We therefore also measured total epicardial/pericardial fat volume from the atrioventricular groove to the apex, using a stack of SSFP short-axis cardiac cine-images in enddiastole. We contoured the outer myocardial border. A second contour was traced including all the fat surrounding the heart (Figure 1C). Because epicardial and pericardial fat can often not be clearly distinguished in all areas around the heart, these 2 fat depots were measured together as paracardial fat volume, calculated using the summation of disc method, analogous to the method utilized for measurements of LV mass. Adequate images for enddiastolic pericardial fat thickness and volume were available in 552 and 536 participants, respectively.
STATISTICAL ANALYSIS.
We stratified participants into non-HF, HFrEF, and HFpEF groups. Descriptive statistics are presented as mean × SD, median (interquartile range), or counts (percentages) as appropriate. Continuous variables are presented as mean and 95% confidence interval of the mean. Categorical variables were compared using chi-square or Fisher’s exact test. Unadjusted and multivariable-adjusted analyses were performed between groups of participants. We performed linear regression to assess relationships between various measures of body composition and NT-proBNP. We examined residuals via histograms and log-log plots and performed Box Cox transformation of variables, as appropriate, to improve normality in regression models. Beta-coefficients are standardized (i.e., represent the relative risk per standard deviation change in the predictor), to facilitate an intuitive comparison of the association between different indices. We confirmed lack of multicollinearity in linear regression models via inspection of the variance inflation factors. We also performed formal statistical mediation analyses (13) to assess whether an intermediate variable mediates a relationship between body mass index (BMI) and NT-proBNP. Mediating variables are “intermediate” factors that act as a link between a dependent variable and an independent variable. Mediation analyses quantify direct and indirect effect sizes that contribute to an observed relationship between the independent variable (in this case, BMI) and a dependent variable (in this case, NT-proBNP), and examine the role of the potential statistical mediator (such as axial SM (13). Estimates of the total, direct, and indirect effect size were computed. Significant mediation is established when the indirect effect is significantly different from zero. Standardized regression coefficients and effect sizes are presented for easier comparison of the magnitude of the relationships of different predictors. A description of a complementary network analysis is available in Supplemental Appendix.
We used Cox proportional hazards regression to assess the relationship between various muscle mass indicators and latent factors and time to death. We initially adjusted for: 1) age, sex, race, and body height; then 2) additionally adjusted for HF status and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) risk score (14). All hazard ratios are standardized as well. Statistical significance was defined as a 2-tailed p value <0.05. Statistical analyses were performed using the Matlab statistics and Machine Learning toolbox (Matlab 2019b, the Math-works, Natick, Massachusetts) and the M3 mediation toolbox (15,16) within Matlab v2019b.
RESULTS
We enrolled patients without HF patients (n = 367), as well as patients with HFrEF (n = 113) and HFpEF (n = 92) (10). Compared with patients without HF, patients with HF were older, more likely to be African American, had higher NT-proBNP levels, had greater comorbidity burden with use of relevant medications, and had several echocardiographic indices indicating remodeling (larger left atrial volume index) and elevated filling pressures (greater E/e’) (p < 0.05 for all comparisons) (Table 1). BMI was lower in patients with HFrEF (29.0 kg/m2) and higher in patients with HFpEF (35.8 kg/m2) compared with controls (29.5 kg/m2). The EF was 34% in patients with HFrEF, 61% in patients with HFpEF, and 58% in controls.
TABLE 1.
Baseline Characteristics of Study Population
| Age, yrs | 63 (57.0–68.8) | 65 (60.0–70.0) | 64 (59.0–71.0) | 0.0174 |
| Male | 340 ± 92.64 | 112 ± 99.12 | 78 ± 84.78 | 0.0005 |
| Race | 0.0118 | |||
| White | 190 ± 51.77 | 49 ± 43.36 | 36 ± 39.13 | |
| African American | 157 ± 42.78 | 63 ± 55.75 | 53 ± 57.61 | |
| Other | 20 ± 5.45 | 1 ± 0.88 | 3 ± 3.26 | |
| BMI, kg/m2 | 29.5 (25.4–33.6) | 29 (24.1–32.7) | 35.8 (30–41.5) | <0.0001 |
| BSA, m2 | 2.13 (1.95–2.32) | 2.1 (1.92–2.27) | 2.36 (2.07–2.56) | <0.0001 |
| Systolic blood pressure, mm Hg | 142 (128–156) | 140 (127–160) | 149 (136–161) | 0.0331 |
| Diastolic blood pressure, mm Hg | 82.8 ± 12.2 | 82.4 ± 13.6 | 85.3 ± 11.2 | 0.1854 |
| Hypertension | 279 ± 76.23 | 94 ± 83.93 | 85 ± 92.39 | 0.0013 |
| Coronary artery disease | 114 ± 31.15 | 65 ± 57.52 | 33 ± 35.87 | <0.0001 |
| Obstructive sleep apnea | 87 ± 23.84 | 24 ± 21.43 | 38 ± 41.30 | 0.0013 |
| Current smoking | 96 ± 26.37 | 42 ± 37.84 | 18 ± 19.78 | 0.0117 |
| Atrial fibrillation/flutter | 23 ± 6.32 | 12 ± 10.71 | 10 ± 10.87 | 0.1673 |
| Medication use | ||||
| Beta-blockers | 173 ± 47.27 | 100 ± 88.50 | 62 ± 67.39 | <0.0001 |
| Aspirin | 214 ± 58.47 | 93 ± 82.30 | 64 ± 69.57 | <0.0001 |
| Clopidogrel | 29 ± 7.95 | 27 ± 23.89 | 11 ± 11.96 | <0.0001 |
| ACE inhibitors | 169 ± 46.17 | 70 ± 61.95 | 52 ± 56.52 | 0.0069 |
| ARBs | 37 ± 10.11 | 17 ± 15.04 | 18 ± 19.57 | 0.0347 |
| Furosemide | 7 ± 1.91 | 72 ± 63.72 | 64 ± 69.57 | <0.0001 |
| Spironolactone | 10 ± 2.73 | 16 ± 14.16 | 8 ± 8.70 | <0.0001 |
| Statins | 233 ± 63.66 | 94 ± 83.19 | 61 ± 66.30 | 0.0005 |
| Long-acting nitrates | 28 ± 7.65 | 19 ± 16.96 | 20 ± 21.74 | 0.0001 |
| Hydralazine | 6 ± 1.63 | 14 ± 12.39 | 10 ± 10.87 | <0.0001 |
| Warfarin | 27 ± 7.38 | 16 ± 14.16 | 4 ± 4.35 | 0.0241 |
| Calcium-channel blockers | 93 ± 25.41 | 24 ± 21.24 | 38 ± 41.30 | 0.0026 |
| Thiazides | 86 ± 23.50 | 18 ± 15.93 | 21 ± 22.83 | 0.2290 |
| eGFR (ml/min per 1.73 m2) | 82 (66.0–101.0) | 77 (58.8–96.2) | 70 (57.0–95.5) | 0.0039 |
| Diabetes mellitus | 155 ± 42.35 | 70 ± 62.50 | 62 ± 68.13 | <0.0001 |
| Triglycerides, mg/dl | 115 (75–196) | 115 (78–167) | 118 (87–185) | 0.6267 |
| HDL-cholesterol, mg/dl | 42 (34.7–50.0) | 41 (32.3–50.0) | 41 (36.0–50) | 0.4986 |
| LDL-cholesterol, mg/dl | 90 (68.0–115.9) | 85 (64.0–110.0) | 85 (68.0–107.3) | 0.5193 |
| NT-proBNP (pg/ml) | 325 (86–950) | 2,748 (1,050–5,450) | 486 (118–1,419) | <0.0001 |
| Left ventricular ejection fraction (%) | 58.4 (51.5–64.3) | 34.3 (26.0–41.3) | 60.6 (55.8–68.8) | <0.0001 |
| Mitral E-wave velocity, cm/s | 67.2 (56.9,83.2) | 61.7 (50.3, 87.1) | 78.4 (63.7, 91.6) | 0.0014 |
| Mitral deceleration time, s | 0.202 (0.170–0.25.0) | 0.2 (0.153–0.260) | 0.217 (0.180–0.251) | 0.3062 |
| E/e’, septal | 9 (7.0–11.3) | 11.6 (8.8–16.1) | 12.1 (9.6–14.4) | <0.0001 |
| E/e’, lateral | 6.9 (5.5–9.4) | 9 (6.6–12.5) | 8.9 (7.2–12.2) | <0.0001 |
| Mean E/e’ | 8 (6.5–10.2) | 10.4 (8.4–14.5) | 10.3 (8.4–13.3) | <0.0001 |
| Left atrial volume index (ml/m2 of BSA) | 29 (21.9–37.6) | 41.6 (30.4–54.9) | 37.7 (29.6–48.4) | <0.0001 |
Values are median (25th–75th percentile) or mean ± SD.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; BSA = body surface area; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LDL = low density lipoprotein.
Table 2 demonstrates unadjusted and adjusted comparisons of body composition by group, with adjusted comparisons and relationships also reflected in Figure 2. Compared with patients without HF, patients with HFrEF had reduced axial muscle mass, whereas patients with HFpEF had more pericardial, subcutaneous, and visceral fat (p < 0.05 for all comparisons). Adjusting for age, sex, race, and height yielded similar findings. A radar plot and heatmap are shown in the Central Illustration demonstrating key differences in body composition between the groups.
TABLE 2.
Comparisons of Body Composition Between the Non-HF and HF Groups
| Subcutaneous fat thickness, mm | 14.1 (13.3–14.8) | 13 (11.7–14.2) | 19.8 (17.7–21.9) | <0.0001†‡ |
| Visceral fat thickness, mm | 13.3 (12.6–14.0) | 11.8 (10.7–13.0) | 15.5 (13.8–17.2) | 0.0007 †‡ |
| Pericardial fat thickness, mm | 14.6 (13.8–15.5) | 15 (13.3–16.6) | 18 (15.9–20.2) | 0.0059 † |
| Epicardial fat thickness, mm | 4.79 (4.53–5.05) | 4.93 (4.43–5.42) | 4.83 (4.28–5.38) | 0.8749 |
| Paracardial fat volume, ml | 151 (142–159) | 188 (168–207) | 175 (154–196) | 0.0003 * |
| Pectoralis major CSA, mm2 | 21.5 (20.7–22.4) | 18.3 (17.1–19.6) | 21.9 (20.2–23.5) | 0.0001 *‡ |
| Axial muscle mass principal component | 2.22 (2.11–2.33) | 1.88 (1.71–2.05) | 2.08 (1.87–2.29) | 0.0046 * |
| Adjusted for Age, Sex, Race and Body Height | No HF | HFrEF | HFpEF | p Value |
|---|---|---|---|---|
| Subcutaneous fat thickness, mm | 14.1 (13.3–14.8) | 13.2 (11.9–14.4) | 19.2 (17.1–21.2) | <0.0001 †‡ |
| Visceral fat thickness, mm | 13.2 (12.5–13.9) | 11.9 (10.7–13.0) | 15.9 (14.1–17.6) | 0.0002 †‡ |
| Pericardial fat thickness, mm | 14.7 (13.9–15.6) | 14.7 (13.1–16.3) | 18 (15.8–20.2) | 0.0076 †‡ |
| Epicardial fat thickness, mm | 4.82 (4.56–5.08) | 4.88 (4.40–5.36) | 4.84 (4.30–5.38) | 0.9770 |
| Paracardial fat volume, ml | 150 (142–158) | 184 (166–203) | 186 (165–207) | <0.0001 *† |
| Pectoralis major CSA, mm2 | 21.2 (20.6–21.9) | 18.3 (17.2–19.4) | 22.9 (21.5–24.3) | <0.0001 *‡ |
| Axial muscle mass principal component | 2.18 (2.08–2.27) | 1.87 (1.72–2.02) | 2.25 (2.05–2.44) | 0.0014 *‡ |
Values are mean (95% confidence interval of the mean).
pairwise comparisons <0.05:
No HF vs. HFrEF;
No HF vs. HFpEF;
HFrEF vs. HFpEF.
Abbreviations as in Table 1.
FIGURE 2. Differences in Body Composition in Patients With HF Compared With Controls.
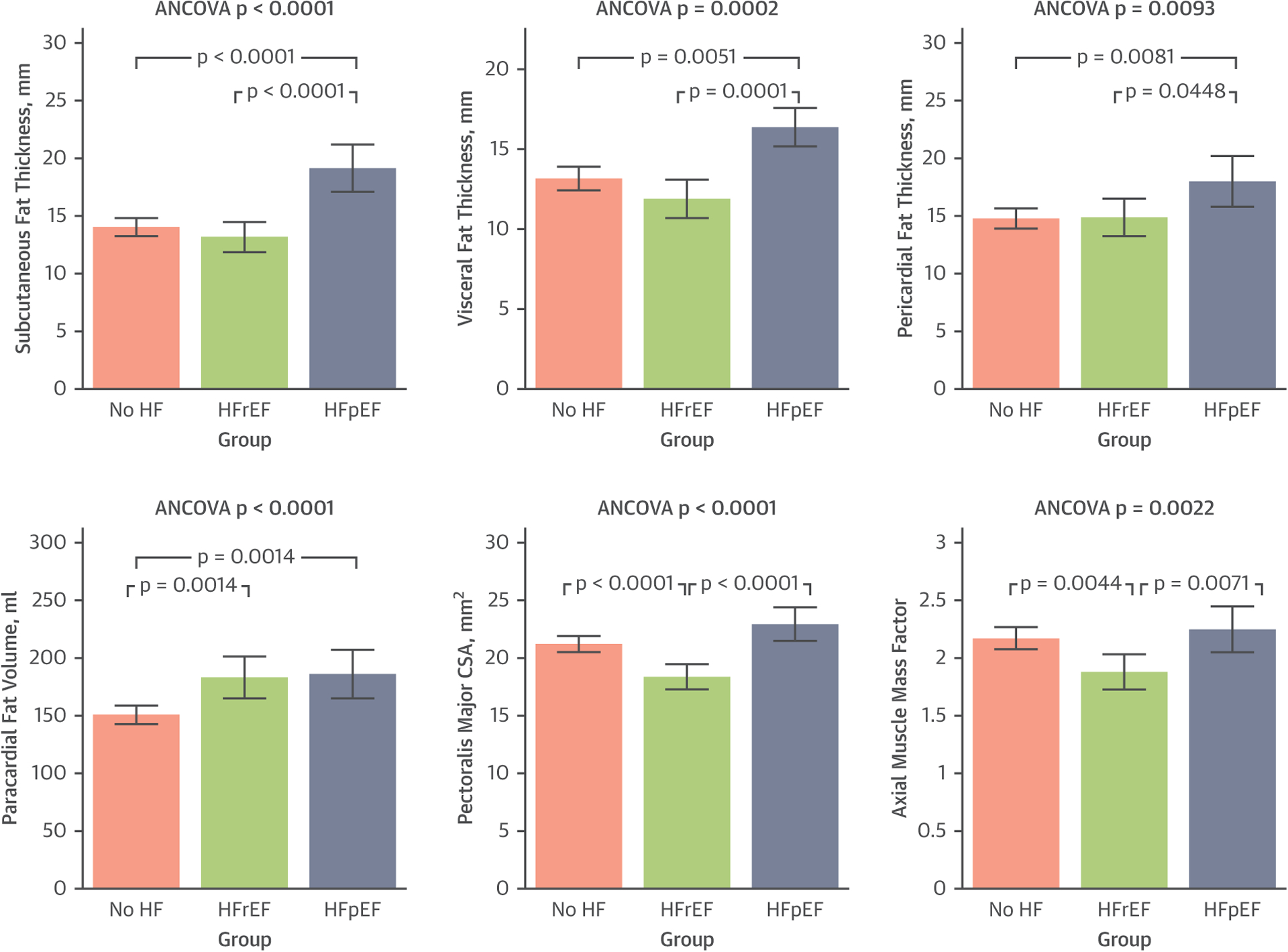
Comparisons are adjusted for sex, age, race, and body height. Compared with patients without heart failure (HF), patients with HF with preserved ejection fraction (HFpEF) had greater pericardial, subcutaneous, and visceral fat, whereas patients with HR with reduced ejection fraction (HFrEF) had reduced axial muscle mass (p values shown in Table 2). CSA = cross-sectional area.
CENTRAL ILLUSTRATION. Relative Differences in Body Composition in Heart Failure Patients Compared With Controls.
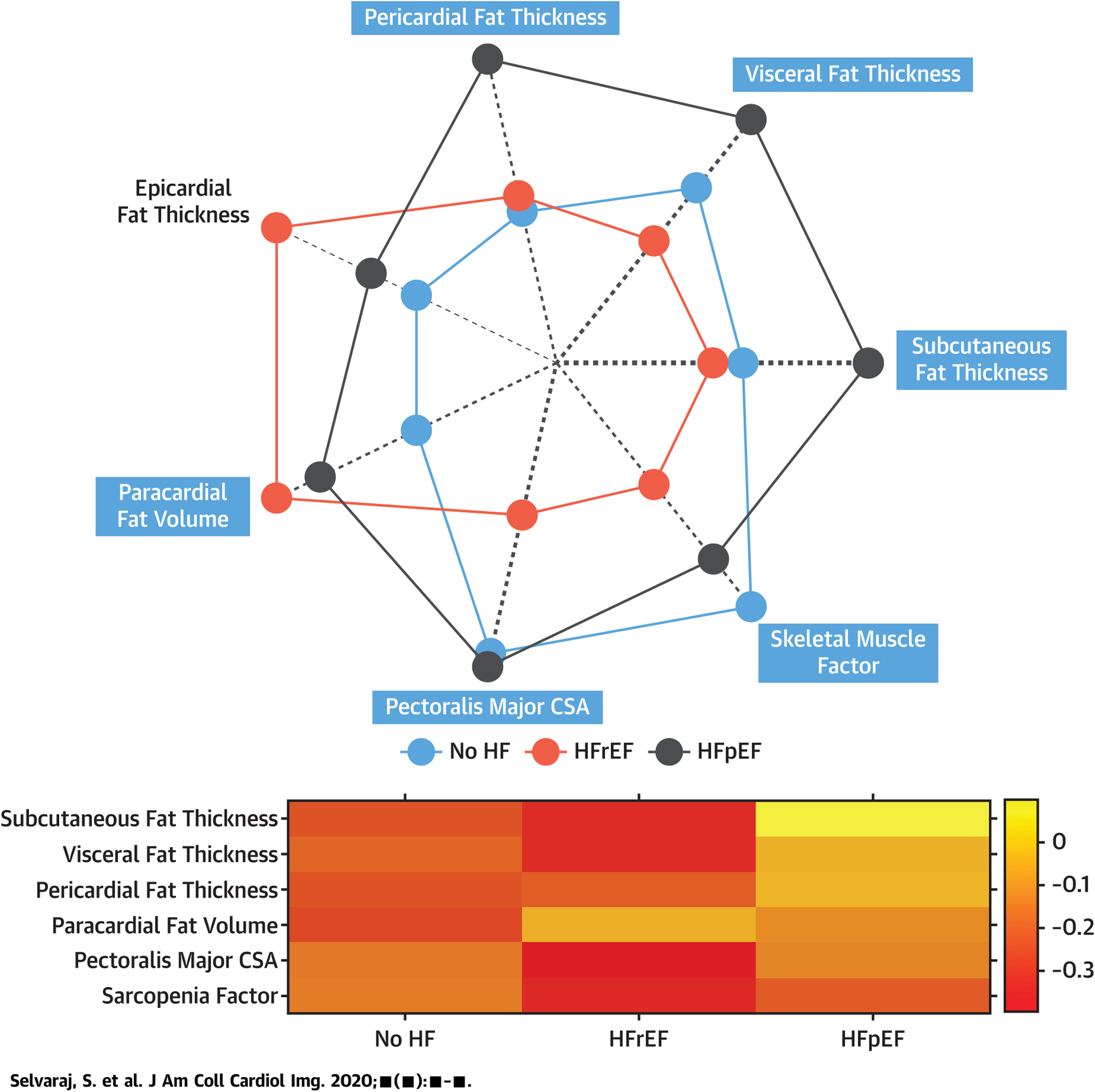
A radar plot (top) and heatmap (bottom) demonstrating key differences in z-scores in various parameters of body composition between the groups is shown. In the radar plot, variables are compared in a normalize scale (z-score) between the groups, and the mean z-score of each group is plotted from low (center) to high (periphery) of the plot. The thickness of the dashed radial lines are proportional to the magnitude of the maximum standardized difference between the groups (maximum minus minimum z-score value) and the shade of the dashed radial lines is proportional to the statistical significance of the differences between the groups (i.e., the −log10 of the analysis of variance p value).
Table 3 shows Cox regression models for death using stepwise models. On nonadjusted analyses, only axial muscle mass, but no measurements of fat, was associated with mortality (standardized hazard ratio: 0.63, 95% confidence interval: 0.52 to 0.75; p < 0.0001). Full multivariable adjustment mildly attenuated the association, but it remained robust (hazard ratio: 0.72; p = 0.0007).
TABLE 3.
Unadjusted and Adjusted Hazards Ratios for Mortality for Fat Depots and Axial Muscle Mass
| Subcutaneous fat thickness, mm | 1.05 | 0.88 | 1.25 | 0.5984 |
| Visceral fat thickness, mm | 0.81 | 0.66 | 0.99 | 0.0421 |
| Pericardial fat thickness, mm | 1.05 | 0.87 | 1.27 | 0.6283 |
| Epicardial fat thickness, mm | 0.95 | 0.78 | 1.15 | 0.5877 |
| Paracardial fat volume, ml | 0.91 | 0.76 | 1.09 | 0.3090 |
| Pectoralis major CSA, mm2 | 0.58 | 0.46 | 0.72 | <0.0001 |
| Axial muscle mass principal component | 0.63 | 0.52 | 0.75 | <0.0001 |
| Adjusted for Age, Sex, Race and Body Height | Standardized HR | Standardized HR, 95% CI, Lower Bound | Standardized HR, 95% CI, Upper Bound | p Value |
|---|---|---|---|---|
| Subcutaneous fat thickness, mm | 1.01 | 0.85 | 1.22 | 0.8760 |
| Visceral fat thickness, mm | 0.80 | 0.66 | 0.97 | 0.0236 |
| Pericardial fat thickness, mm | 0.97 | 0.79 | 1.19 | 0.7718 |
| Epicardial fat thickness, mm | 0.90 | 0.74 | 1.10 | 0.3087 |
| Paracardial fat volume, ml | 0.90 | 0.74 | 1.10 | 0.3180 |
| Pectoralis major CSA, mm2 | 0.60 | 0.47 | 0.77 | <0.0001 |
| Axial muscle mass principal component | 0.68 | 0.56 | 0.84 | 0.0003 |
| Additionally Adjusted for HF Status at Baseline and MAGGIC Risk Score | Standardized HR | Standardized HR, 95% CI, Lower Bound | Standardized HR, 95% CI, Upper Bound | p Value |
| Subcutaneous fat thickness, mm | 1.04 | 0.87 | 1.25 | 0.6337 |
| Visceral fat thickness, mm | 0.83 | 0.68 | 1.02 | 0.0766 |
| Pericardial fat thickness, mm | 1.04 | 0.85 | 1.27 | 0.7016 |
| Epicardial fat thickness, mm | 0.95 | 0.78 | 1.16 | 0.6251 |
| Paracardial fat volume, ml | 0.85 | 0.70 | 1.03 | 0.1053 |
| Pectoralis major CSA, mm2 | 0.67 | 0.53 | 0.83 | 0.0004 |
| Axial muscle mass principal component | 0.72 | 0.59 | 0.87 | 0.0007 |
CI = confidence interval; CSA = cross-sectional area; HF = heart failure; HR = hazard ratio; MAGGIC = Meta-Analysis Global Group in Chronic Heart Failure.
RELATIONSHIP BETWEEN FAT DEPOTS, AXIAL MUSCLE MASS, AND NT-proBNP LEVELS.
Figure 3A shows a heatmap representing the correlation between fat depots, muscle mass, and NT-proBNP in the substudy population (N=334). Figure 3B shows a plot of the network connectivity backbone, also representing these relationships. These analyses indicated that NT-proBNP is primarily related to measures of axial muscle mass, rather than adiposity.
FIGURE 3. Heatmap and Network Analysis of Fat Depots, Axial Muscle Mass, and Natriuretic Peptides.
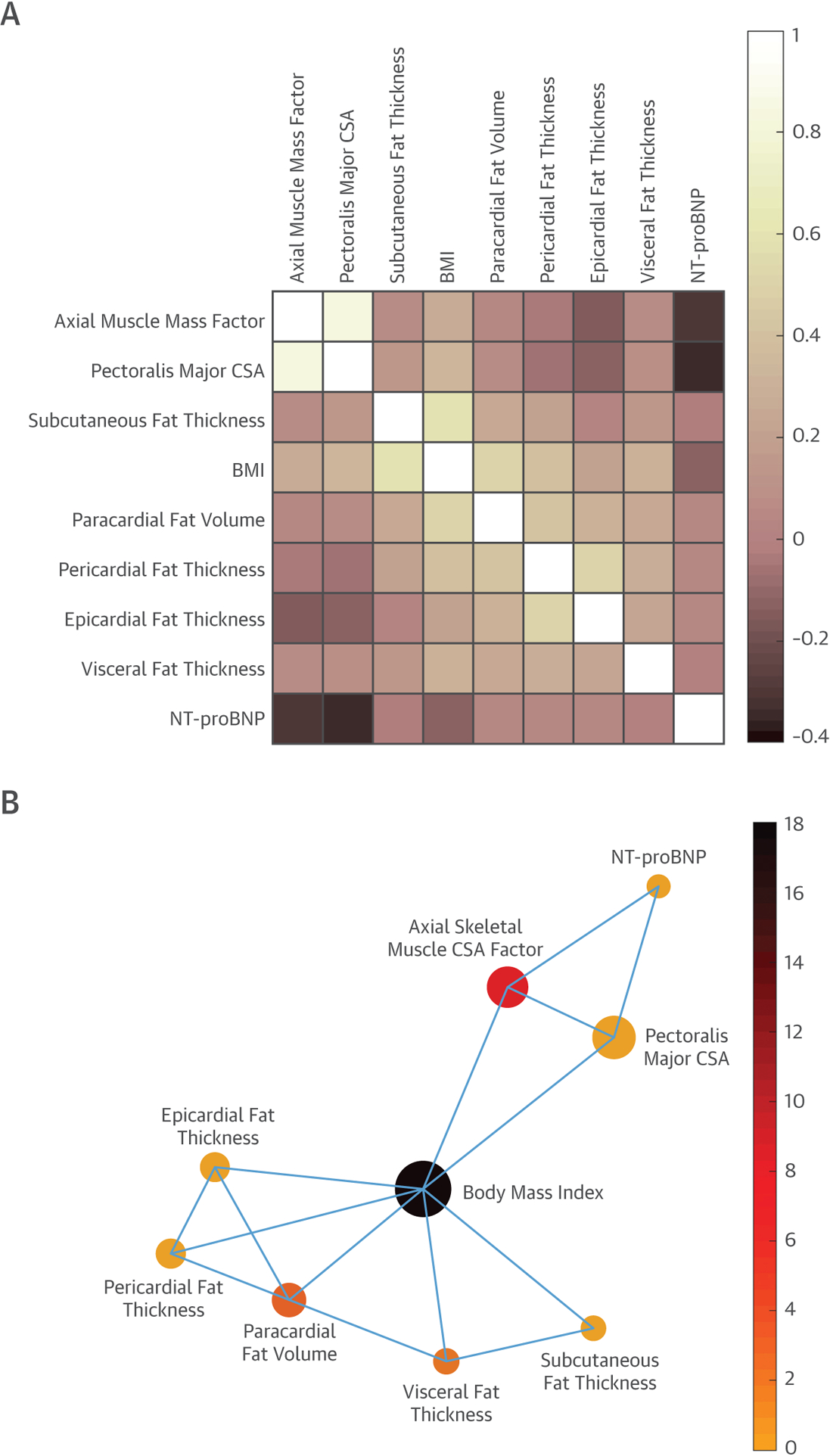
Correlation matrix heatmap (A) and network connectivity backbone (B) of fat depots, measures of axial muscle mass, and NT-proBNP. In B, node size represents eigenvector centrality (which depends both on the number of neighbors and the strength of its connections). Eigenvector centrality measures a node’s importance while giving consideration to the importance (number of connections) of its neighbors. The node color represents betweenness centrality (which quantifies the number of times a node acts as a bridge along the shortest path between 2 other nodes). BMI = body mass index; CSA = cross-sectional area; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
These relationships were also assessed with standard linear regression. In an unadjusted linear model, BMI was significantly associated with NT-proBNP (standardized beta = −0.16; p = 0.0031). This association persisted after adjustment for age and sex (standardized beta = −0.14; p = 0.0046). However, after the addition of axial SM factor, the relationship between BMI and NT-proBNP was not significant (standardized beta = −0.08; p = 0.10), whereas the axial SM factor was independently associated with NT-proBNP (standardized beta = −0.22; p = 0.0002). The addition of other measures of adiposity and HF status did not appreciably change these relationships. In a model that included BMI, HF status, age, sex, and other measures of adiposity (abdominal visceral fat thickness, subcutaneous fat thickness, and paracardial fat volume) (Figure 4), the axial SM factor (standardized beta = −0.19; p = 0.0012), but not BMI or other measures of adiposity, was independently associated with NT-proBNP. The alternative inclusion of other measures of pericardial adiposity (epicardial and/or pericardial fat thickness) did not appreciably change the relationship between the axial SM factor or BMI with NT-proBNP.
FIGURE 4. Adjusted Relationship Between Fat Depots, Muscle Mass, and Heart Failure With NT-proBNP.
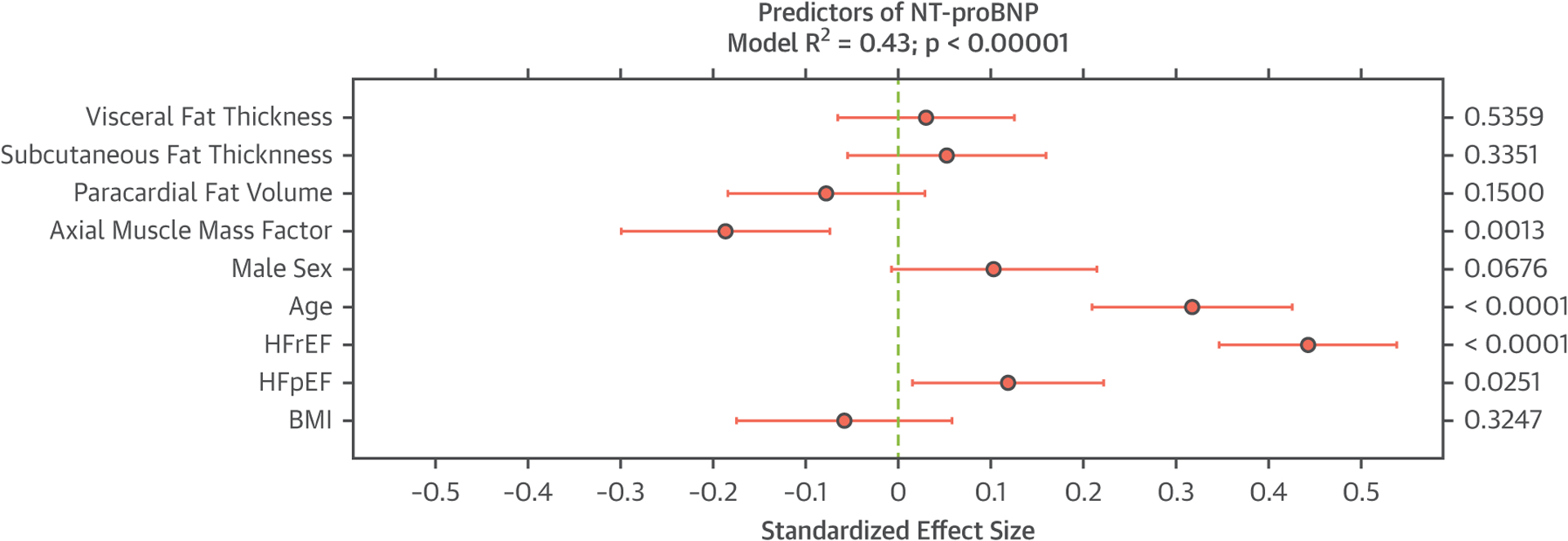
Standardized effect sizes shown for axial muscle mass, various fat depots, HF status, and body mass index (BMI) are shown in relationship to N-terminal pro-brain natriuretic peptide (NT-proBNP). All effect sizes are simultaneously adjusted for the presence of other variables presented in the figure. HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
Figure 5 shows statistical mediation analyses in which the direct and indirect (axial SM–mediated) effects of BMI on NT-proBNP were examined. In this model, BMI demonstrated a significant total effect on NT-proBNP. There was a significant effect of BMI on axial SM, as well as a significant effect of axial SM on NT-proBNP. The direct effect of BMI on NT-proBNP, however, was nonsignificant, whereas its indirect (axial SM-dependent) effect was significant. This analysis was performed solely to assess statistical mediation of cross-sectional associations and does not imply causality.
FIGURE 5. Statistical Mediation Analyses to Quantify Direct and Indirect (Axial SM–mediated) Effects of BMI on NT-proBNP.
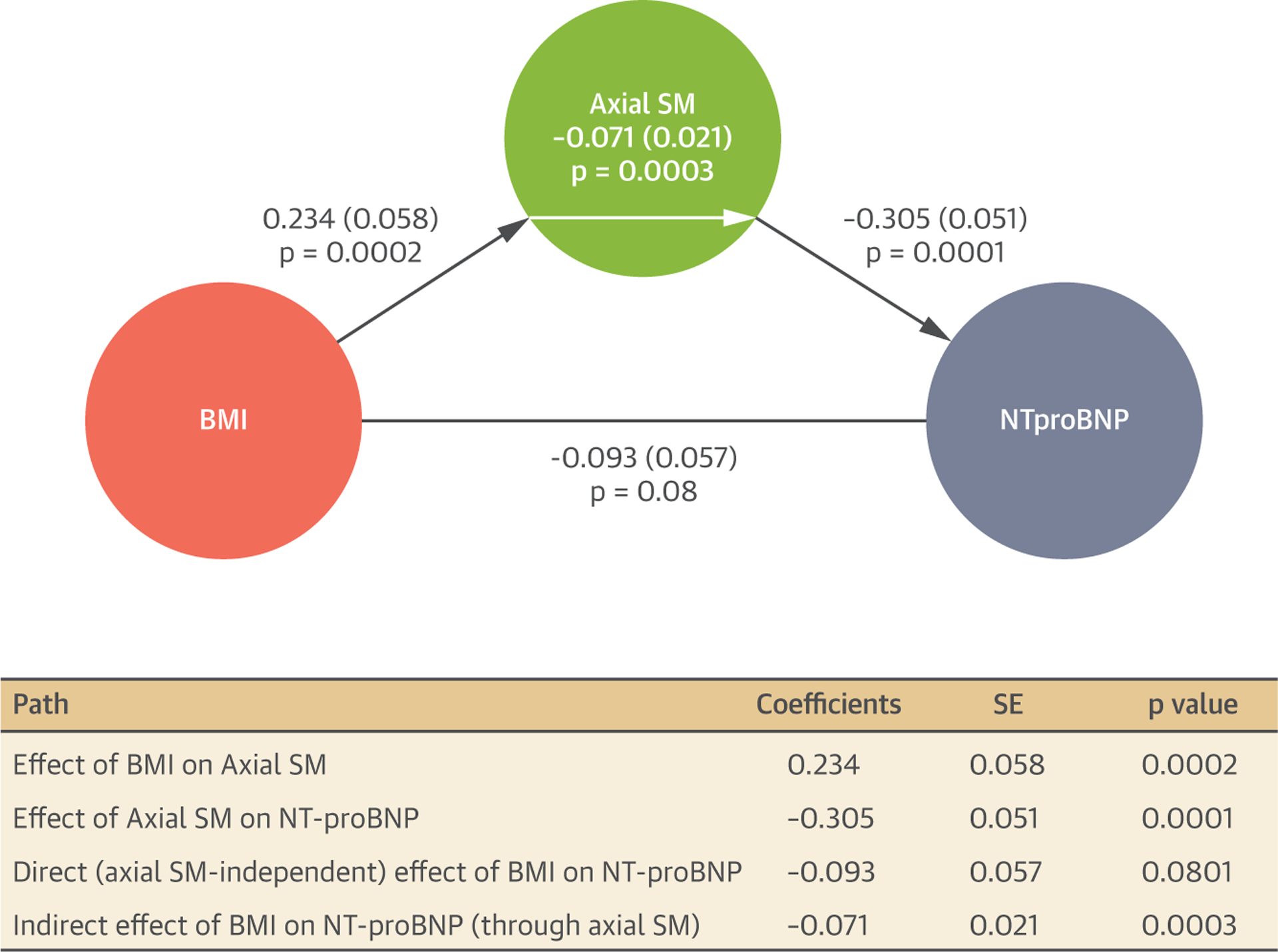
Regression coefficients, SE, and p values are shown in the path graph as well as in the mediation table. BMI = body mass index; NT-proBNP = N-terminal pro-B-type natriuretic peptide; SM = skeletal muscle.
DISCUSSION
In a large study using CMR to quantify measures of adiposity and axial SM mass in patients with HF compared with unselected controls, we found a significant differential distribution of adipose tissue in HFrEF and patients with HFpEF. After multivariable adjustment for a significant number of potentially confounding variables, only low muscle mass was associated with an increased mortality risk. We also report a significant independent inverse relationship between axial muscle size and NT-proBNP, which subsequently demonstrated that the resulting relationship between adiposity (both BMI and various fat depots) and NT-proBNP was nonsignificant. These data highlight: 1) the feasibility of gathering significant prognostic data in patients with HF using readily available images from standard CMR protocols; 2) the importance of low muscle mass in HF; and 3) an important relationship between axial muscle mass and NPs, which may help elucidate the “obesity paradox” in HF and may partially explain the important clinical paradox of lower NT-proBNP levels in patients HFpEF, who exhibit less sarcopenia/cachexia than patients with HFrEF.
We found patients with HFpEF had higher amounts of subcutaneous, visceral, and pericardial fat than both HFrEF and control patients, whereas patients with HFrEF exhibited a marked reduction in SM compared to HFpEF and control patients. Although the increase in adipose tissue is not a surprise in HFpEF (2), we show that HFpEF is characterized by diffuse adipose deposition without specific predilection for 1 tissue bed. Thus, each tissue bed via its specific metabolic and endocrine effects may contribute to the pathophysiology of HFpEF. Our findings are largely concordant with a recent analysis in HFpEF compared to healthy controls, though in the previous study, HFpEF participants exhibited lower epicardial fat (17). Interestingly, increasing amounts of adiposity in each tissue bed, however, was not associated with an increased risk for death. Only axial SM mass was associated with mortality in multivariable analysis, similar to other findings showing the importance of muscle mass in relation to prognosis (18). These findings may partially underlie the obesity paradox in HF, whereby overweight or obese individuals with HF tend to exhibit a lower mortality (19,20). Larger muscle mass in obese individuals may allow for a more active lifestyle and also provide metabolic substrate in times of stress. Alternatively, muscle degradation precedes adipose loss in chronic HF, and therefore muscle mass may be a more sensitive indicator of adverse prognosis (21,22). It is possible, moreover, that adipose tissue still partially mediates the relationship between sarcopenia and adverse events, given that adipose infiltration of the muscle beds is common in HF (8,9) and sarcopenic obesity may be more malignant than nonsarcopenic obesity. Adipose infiltration can exert paracrine and vasocrine effects on the adjacent muscle bed, including impairment of perfusion, reduction of systemic blood from muscle through a “steal phenomenon,” reduction of capillary density, and impaired mitochondrial function (3,8,9).
Notably, we demonstrated a strong inverse relationship between axial muscle mass and NT-proBNP, whereas no relationship between BMI or fat depots with adverse events was found when adjusted for axial muscle size. Because BMI does not discriminate between lean and fat mass (23,24), further work has clarified that lean mass (assessed by dual-energy X-ray absorptiometry scanning), but not fat mass, is associated with lower NPs among non-HF participants (25). Our study extends these findings of the interaction between muscle mass, fat composition, and NPs to HF participants. The mechanisms underpinning how muscle mass may relate to NPs are unclear. However, it has been postulated that sex steroid hormones, which influence body composition, also influence NPs (25). Further research regarding potential mechanisms linking muscle mass to NPs is needed.
Metrics of body composition (muscle mass and fat depots) can be obtained from thoracic images obtained routinely in cardiac CMR studies (26). Therefore, although dedicated imaging of the lower extremities has been performed for this purpose previously (9), we demonstrate that it is feasible to obtain significant prognostic information from CMR of the thoracic musculature alone. Given the lack of validated cutoffs for thoracic axial muscle mass in a healthy population, we did not define low muscle mass in our study according to specific cutpoints, but rather used muscle areas as a continuous measure of axial muscle mass.
Sarcopenia is common in HF (27) and has been shown to occur with the development of HF, particularly in men (28). Sarcopenia is closely associated with reduced strength and independently predicts reduced exercise capacity (27). Muscle wasting in HF is likely the result of several insults, including sympathetic activation, renin-angiotensin-aldosterone system upregulation, and release of proinflammatory cytokines (29). Our findings demonstrating the importance of sarcopenia in HF are concordant with the results of a randomized controlled trial to increase muscle mass and strength in HFpEF. Exercise training improved peak VO2 and was significantly correlated with improvement in lean body mass and thigh muscle:intermuscular fat ratio (30). Therefore, exercise training remains an important and effective part of the HF treatment armamentarium (31), and exercise training in HF remains a class I indication in professional society guidelines (32).
STUDY STRENGTHS AND LIMITATIONS.
Strengths of the current study include a detailed assessment of different compartments of adiposity, use of CMR (the noninvasive gold standard of LV EF and mass), and comprehensive collection and adjustment for important, potentially confounding variables. There are some limitations. First, we used an alternative index for assessing muscle mass in our study than has been traditionally used in the published reports, studying axial as opposed to appendicular muscle mass. However, lower extremity muscle mass may be more subject to deconditioning, whereas axial muscle mass may be more representative of underlying neurohumoral pathology. Further, a recent study in patients with advanced HF undergoing LV assist device implantation also showed a powerful relationship between axial muscle mass and adverse outcomes (33). In addition, our method of quantification using CMR is readily available in individuals undergoing scanning for other purposes and still shows significant prognostic information. Second, we do not have information on alteration of cellular morphology (such as the density of type I and II fibers or reduction in functioning mitochondria) that can be analyzed on muscle biopsy nor are functional data (i.e., grip strength, exercise capacity) available. Finally, consistent with population of patients cared for in the Veterans Affairs medical system, our population was predominantly male.
CONCLUSIONS
HFpEF and HFrEF are associated with distinct abnormalities in body composition. Patients with HFpEF have an overall increase in adipose tissue throughout all beds, whereas patients with HFrEF have reduced axial muscle mass. Reduced axial muscle mass, but not subcutaneous, pericardial, or visceral fat, independently predicted death. We observed a strong, inverse relationship between muscle mass and NT-proBNP that explained the relationship between BMI and NPs. Reducing muscle wasting in HF, either through exercise training or therapeutic inhibition of pathogenic mediators, may not only produce significant symptomatic benefit, but may have an impact on neurohormonal regulation and the risk of death. These important issues need to be addressed in future studies.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Patients with HFrEF and HFpEF have distinct abnormalities in body composition. NT-proBNP levels relate more strongly to muscle mass rather than obesity.
TRANSLATIONAL OUTLOOK:
Future research into mechanisms by which NT-proBNP relates to sarcopenia is warranted.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants R01 HL 121510–01A1 (Dr. Chirinos), 5-R21-AG-043802–02 (Dr. Chirinos), and a VISN-4 research grant from the department of Veterans Affairs (Dr. Chirinos). NT-proBNP measurements were supported by a research grant from Bristol-Myers Squibb. Dr. Selvaraj has received research grant support the National Institutes of Health (Training Grant 5-T32HL007843–23), the Doris Duke Charitable Foundation (Physician Scientist Fellowship Award 2020061), the Measey Foundation, Institute for Translational Medicine and Therapeutics (Junior Investigator Preliminary/Feasibility Grant Program award), and the American Society of Nuclear Cardiology (Institute for the Advancement of Nuclear Cardiology award). Dr. Chirinos has been supported by NIH grants R01-HL 121510–01A1, R61-HL-146390, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874–01, and R56-HL136730; has received consulting honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck, Edwards Lifesciences, Bayer, and JNJ and has received research grants from the NIH, Microsoft, Fukuda-Denshi, and Bristol-Myers Squibb and has been named as inventor in a UPenn patent for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction and a patent application for the use of novel neo-epitope biomarkers of tissue fibrosis in heart failure. All other authors have reported that they have no relationships relevant to the contents of the paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LV
left ventricular
- CMR
cardiac magnetic resonance
- NP
natriuretic peptide
- NT-proBNP
N-terminal B-type natriuretic peptide
- SM
skeletal muscle
- SSFP
steady state free precession
Footnotes
APPENDIX For supplemental methods, please see the online version of this paper.
REFERENCES
- 1.Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res 2014;164:345–56. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 2017;70:2739–49. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 2016;68:200–3. [DOI] [PubMed] [Google Scholar]
- 4.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvaraj S, Martinez EE, Aguilar FG, et al. Association of central adiposity with adverse cardiac mechanics: findings from the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging 2016;9:e004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parisi V, Rengo G, Perrone-Filardi P, et al. Increased epicardial adipose tissue volume correlates with cardiac sympathetic denervation in patients with heart failure. Circ Res 2016;118:1244–53. [DOI] [PubMed] [Google Scholar]
- 8.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci 2013;68:968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113: 1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Ansari BA, Kim J, et al. Axial muscle size as a strong predictor of death in subjects with and without heart failure. J Am Heart Assoc 2019; 8:e010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SK, Kim HJ, Hur KY, et al. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr 2004;79:593–9. [DOI] [PubMed] [Google Scholar]
- 12.Schneiter SM, Warrier R, Lefkovits L, Laurie C, O’Brien PE, Taylor AJ. Effects of weight loss on pericardial fat and left ventricular mass assessed with cardiac magnetic resonance imaging in morbid obesity. Int J Clin Med 2011;02:360–6. [Google Scholar]
- 13.Hayes A Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 14.Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–13. [DOI] [PubMed] [Google Scholar]
- 15.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009;47:821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008;59:1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haykowsky MJ, Nicklas BJ, Brubaker PH, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiya K, Masuda T, Matsue Y, et al. Complementary role of arm circumference to body mass index in risk stratification in heart failure. JACC Heart Fail 2016;4:265–73. [DOI] [PubMed] [Google Scholar]
- 19.Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2011;4:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streng KW, Voors AA, Hillege HL, et al. Waist-to-hip ratio and mortality in heart failure. Eur J Heart Fail 2018;20:1269–77. [DOI] [PubMed] [Google Scholar]
- 21.Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J 1999;20:683–93. [DOI] [PubMed] [Google Scholar]
- 22.von Haehling S The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc 2015;74:367–77. [DOI] [PubMed] [Google Scholar]
- 23.Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis 2018; 61:142–50. [DOI] [PubMed] [Google Scholar]
- 24.Lavie CJ, Forman DE, Arena R. Bulking up skeletal muscle to improve heart failure prognosis. JACC Heart Fail 2016;4:274–6. [DOI] [PubMed] [Google Scholar]
- 25.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 2005;112:2163–8. [DOI] [PubMed] [Google Scholar]
- 26.Neeland IJ, Yokoo T, Leinhard OD, Lavie CJ. Twenty-first century advances in multimodality imaging of obesity for care of the cardiovascular patient. J Am Coll Cardiol Img 2020. April 10 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 2013;34:512–9. [DOI] [PubMed] [Google Scholar]
- 28.Forman DE, Santanasto AJ, Boudreau R, et al. Impact of incident heart failure on body composition over time in the health, aging, and body composition study population. Circ Heart Fail 2017;10:e003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017;14:323–41. [DOI] [PubMed] [Google Scholar]
- 30.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1506–31. [DOI] [PubMed] [Google Scholar]
- 32.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016;68: 1476–88. [DOI] [PubMed] [Google Scholar]
- 33.Teigen LM, John R, Kuchnia AJ, et al. Preoperative pectoralis muscle quantity and attenuation by computed tomography are novel and powerful predictors of mortality after left ventricular assist device implantation. Circ Heart Fail 2017;10: e004069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


