Abstract
Entrainment of the hippocampus by the medial entorhinal area (MEA) in Temporal Lobe Epilepsy (TLE), the most common type of drug-resistant epilepsy in adults, is believed to be mediated primarily through the perforant pathway (PP), which connects stellate cells in layer (L) II of the MEA with granule cells of the dentate gyrus (DG) to drive the hippocampal tri-synaptic circuit. Using immunohistochemistry, high-resolution confocal microscopy and the rat pilocarpine model of TLE, we show here that the lesser known temporoammonic pathway (TAP) plays a significant role in transferring MEA pathology to the CA1 region of the hippocampus independently of the PP. The pathology observed was region-specific and restricted primarily to the CA1c subfield of the hippocampus. As shown previously, daily intracranial infusion of D-serine (100 μm), an antagonist of GluN3-containing triheteromeric N-Methyl D-aspartate receptors (t-NMDARs), into the MEA prevented loss of LIII neurons and epileptogenesis. This intervention in the MEA led to the rescue of hippocampal CA1 neurons that would have otherwise perished in the epileptic animals, and down regulation of the expression of astrocytes and microglia thereby mitigating the effects of neuroinflammation. Interestingly, these changes were not observed to a similar extent in other regions of vulnerability like the hilus, DG or CA3, suggesting that the pathology manifest in CA1 is driven predominantly through the TAP. This work highlights TAP’s role in the entrainment of the hippocampus and identifies specific areas for therapeutic intervention in dealing with TLE.
Keywords: temporoammonic pathway, perforant pathway, Medial Entorhinal Area, hippocampus, neurodegeneration, D-serine intervention, Temporal Lobe Epilepsy
Introduction
TLE is a chronic disease characterized by spontaneous, recurrent seizures and marked degeneration of neurons in LIII of the medial entorhinal area (MEA) and within the hippocampus (Blumcke et al., 2002; Kumar and Buckmaster, 2006; Mathern et al., 1995). This is generally attributed to hyperactivity and hypersynchrony of neurons within the temporal lobe and hippocampus (Santos et al., 2019). Concomitant with neuronal loss, there is infiltration and/or proliferation of reactive astrocytes and microglia, neuroinflammatory cells, in the regions of neurodegeneration as early as 1 to 5 days following initial precipitating injury (pilocarpine administration in the model of TLE used in this study) (Damisah et al., 2020; do Nascimento et al., 2012; Drexel et al., 2012; Shapiro et al., 2008; Wyatt-Johnson et al., 2017). Neuronal loss and mediators of inflammation are further observed throughout the hippocampus and particularly in the CA1 subfield and hilus of the dentate gyrus (Covolan and Mello, 2000; Fujikawa, 1996; Kodam et al., 2019; Morin-Brureau et al., 2018; Upadhya et al., 2019). We believe that the refractory nature of TLE to anti-epileptic medications can be partially attributed to the lack of clear understanding of how pathogenesis in one region of a highly interconnected temporal lobe affects another.
The hippocampus and parahippocampal areas, including the subiculum, presubiculum, parasubiculum, MEA and the lateral entorhinal area (LEA), are interconnected through axonal circuitry (Witter, 2007) and intracellular tracing studies confirm that LII stellate cells (reelinpositive) of the MEA predominately innervate the DG as well as some CA3 neurons, constituting the perforant pathway (PP) (Witter, 2007), whilst pyramidal (calbindin-positive) cells innervate GABAergic neurons in CA1 (Kitamura et al., 2014; Surmeli et al., 2015; Varga et al., 2010). The hippocampal tri-synaptic pathway is composed of axons from the entorhinal cortex to DG via the PP, from DG to CA3 via mossy fibers, and from CA3 to CA1 via Schaffer collaterals (Andersen et al., 1971; Okada et al., 2004), thereby connecting neurons in LII of MEA with those in CA1. A further pathway connects neurons in LIII of MEA with the stratum lacunosum moleculare of CA1 (see Fig. 1B) via the temporoammonic pathway (TAP) (Steward and Scoville, 1976). This pathway is thought to be involved in cognition (Gonzalez et al., 2016; Nakashiba et al., 2008; Remondes and Schuman, 2004; Vago and Kesner, 2008) and input from the TAP to CA1 is considered to modulate CA3 to CA1 synaptic plasticity (Ang et al., 2005; Basu et al., 2016; Dudman et al., 2007; Judge and Hasselmo, 2004; Remondes and Schuman, 2002). In animal models of TLE, disruption of the tri-synaptic pathway failed to prevent CA1 neuronal hyperexcitability originating in the MEA (Behr and Heinemann, 1996; Denslow et al., 2001; Du et al., 1995), suggesting that the TAP might be a major neuronal pathway underlying mesial temporal sclerosis (Laurent et al., 2015; Wozny et al., 2005). The CA1 subfield has been historically partitioned into three subdivisions labeled CA1a, CA1b and CA1c by Lorente de No (Lorente de No, 1934). CA1a (located more distally and closer to the subiculum) has similar pyramidal cells as the adjacent subiculum; CA1b neurons are described as the smallest pyramidal cells in the hippocampus; CA1c is the region adjacent to CA2. However, the border limits of these subdivisions are not easily determined (Lorente de No, 1934). More recent anatomical tracing studies have revealed distinct input zones from the MEA and LEA to the CA1: The MEA projects to proximal CA1c (closer to CA2) and LEA projects to the distal CA1a (closer to the subiculum) (Masurkar et al., 2017; Tamamaki and Nojyo, 1995), with each subdivision of CA1 (CA1a, CA1b and CA1c) being roughly of the same size.
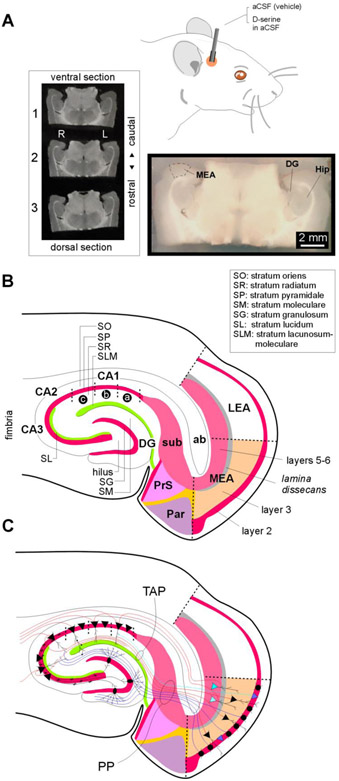
Fig. 1: Details of MEA-hippocampal connectivity in acute brain slices.
A, Schematic of the route and mode of D-serine administration. An actual brain slice (bottom) containing the MEA (area entorhinalis pars medialis) that was micro dissected out to highlight the relative location of the hippocampus (Hip) including the dentate gurus (DG). The three midseries sections (top-left) of a 1-in-6 series of brain sections (50 μm-thick, covering the dorsalventral extent of the MEA) used for histology in this study.
B, Schematic of a MEA-hippocampal brain slice detailing the major subdivisions of the parahippocampal region including MEA, LEA (lateral entorhinal area), presubiculum (PrS) subiculum (sub), parasubiculum (Par) and the hippocampus (DG, hilus, CA1-3). Laminar organization within MEA and hippocampus is also demarcated. ab, angular bundle.
C, Schematic of a MEA-hippocampal brain slice detailing the putative origins and destinations of the perforant pathway (PP, blue) and the temporoammonic pathway (TAP, red) that connect the MEA to the hippocampus. Cell types are designated as follows: pyramidal cells (triangular stomata), stellate cells (circular stomata) and granule cells (oval stomata, whose axons make up the mossy fibers).
Glutamatergic NMDARs are heterotetramers whose three subunits GluN1- GluN3 are derived from distinct gene families. All NMDARs contain one or more of the obligatory GluN1 subunits, which when assembled with GluN2 (A-D) subunits of the same type, give rise to conventional diheteromeric (d-) NMDARs. Triheteromeric NMDARs, on the other hand, contain three different types of subunits, and include receptors that contain one or more subunits from each of the three gene families, designated t-NMDARs (Kumar, 2016). GluN3-containing t-NMDARs can be distinguished from GluN2-containing d-NMDARs electrophysiologically, have reduced affinity for Mg2+ and increased selectivity for Ca2+ over Na+, making them highly Ca2+ permeable (Beesley et al., 2020). These receptors are blocked by the pan-NMDAR antagonist D- (-)-2-Amino-5-phosphonopentanoic acid (D-AP5) and by D-serine, a potential gliotransmitter and a co-agonist of conventional NMDARs (Beesley et al., 2019; Kumar, 2016; Pilli and Kumar, 2012). Given D-serine’s dual role as an agonist of d-NMDARs and an antagonist of t-NMDARs, we applied it focally to the MEA daily via intracranial infusion and found that it mitigated epileptogenesis and TLE-related neuronal loss in LIII by preventing Ca2+-induced excitotoxicity brought about by NMDARs (Beesley et al., 2020). Furthermore, D-serine is well-tolerated, given that it is made in the brain, and specifically antagonizes GluN3-containing t-NMDARs expressed by excitatory neurons in this region (Beesley, et al., 2019).
In this study we examine the effects of this intervention on hippocampal pathogenesis to shed light on the following questions: a) is hyperexcitability and loss of neurons in MEA the cause of CA1 pathogenesis (via the TAP)? b) can intervention within the MEA prevent hippocampal damage? and c) can intervention within the MEA attenuate inflammation in CA1 by reducing infiltration and/or proliferation of astrocytes and microglia?
Experimental Procedures
All experiments were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Florida State University Institutional Animal Care Committee.
Cannulation Surgery
Sprague-Dawley rats (n = 15, male, 160-190 g, age: postnatal day 40-45) were anesthetized with isofluorane (4%, Henry Schein, Melville, New York), prepped for surgery, and secured into place in a stereotaxic instrument with the aid of ear bars. Anesthesia was maintained with isofluorane (2.5%) mixed with oxygen (1.5-2 lit/min) during surgery. The surgical site was sterilized with betadine and EtOH (70%) and an incision made down the midline to expose the skull. Depending on the age and weight of the animal, a hole was drilled between −7.9 and −8.1 mm along the rostral-caudal axis, and 4.2 mm along the medial-lateral axis relative to Bregma, to access the right MEA with a cannula. The precise co-ordinates were confirmed through pilot studies of dye injection. Pilot experiments to triangulate the site of infusion within the MEA gave no indication that the dye spread to any region other than the vicinity of the MEA. Although we confirmed this in multiple sections into which the brain was sliced, we cannot rule out the possibility of extraneous diffusion to neighboring areas. Four additional holes were drilled to place bone screws (Fine Science Tools, Foster City, CA). A custom-made guide cannula (PlasticsOne, Roanoke, VA), cut to a length of 3.2 mm, was lowered carefully and fixed in place with dental cement reinforced with bone screws. An internal cannula inserted through the guide cannula was made to extend 1 mm in depth beyond it such that the site of administration was precisely 4.2 mm ventral to the skull surface. Following placement of the cannulae and drying of the cement, the skin was pulled back together with dissolvable sutures to secure the wound. Animals were carefully monitored post-surgery and given free access to food and water. All animals undergoing surgery were allowed to recover for at least 4 days prior to the pilocarpine procedure.
The pilocarpine model
Briefly, rats were treated with pilocarpine (380 mg/kg i.p., Sigma) 20 min after scopolamine methylnitrate (1 mg/kg i.p.) (Buckmaster, 2004; Turski et al., 1989). Diazepam (10 mg/kg i.p.) was administered 2 hours after the onset of status epilepticus (continuous seizure activity of severity 3 or above on the Racine scale) and repeated as needed at 20-minute intervals for a maximum of 3 doses. Rats recovering from status epilepticus were given a bolus of saline (0.9%) subcutaneously in the scruff and all animals had ready access to wet food and water. Cannulated animals were infused with 1 μl of either D-serine in aCSF (100 μM, Sigma) or aCSF (vehicle; in mM: 126 NaCl, 26 NaHCO3, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, and 10 D-glucose, pH 7.4) by means of a syringe pump (0.25 μl/min; KD Scientific) daily beginning with the last dose of diazepam for the subsequent 29 days. Following recovery from status epilepticus, rats were video monitored (40 hours/week) for frank spontaneous seizures scored on the Racine scale (Fig. 2) (Racine, 1972). Pilocarpine-treated animals (include both status and non-status) were infused with either D-serine or aCSF (vehicle), of which 82% of aCSF-treated animals and 27% of D-serine-treated animals developed frank seizures that could be observed behaviorally. 46% of post status animals that were infused with D-serine remained seizure-free as opposed to 9% with aCSF.
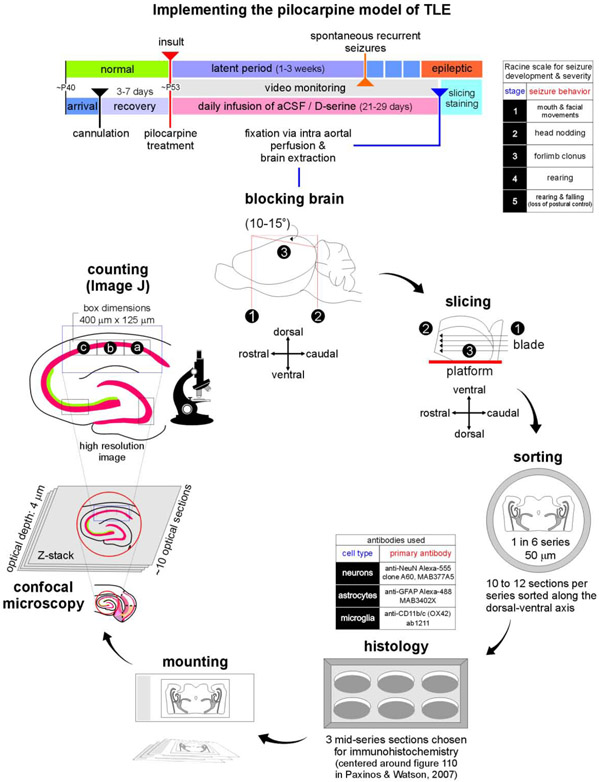
Fig. 2: Outline of the approach used in our investigation.
Timeline of experimental manipulations used in implementing the pilocarpine model of TLE (top). Video monitoring was used to screen animals for frank seizure activity (severity scored on a Racine scale, inset), and confirm animals with spontaneous recurrent seizures as epileptic at the end of the latent period. Sequence of steps beginning with the harvesting of brains and sample preparation, to imaging histologically processed tissue using high resolution confocal microscopy (bottom).
Brain fixation and slicing
Rats were deeply anesthetized with urethane (1.5 mg/kg; i.p.) prior to intra-aortal fixation with 4% paraformaldehyde (PFA) in a 0.1M phosphate buffer solution (PB; pH 7.4; 4°C) following an initial flush with ice-cold saline (0.9%, 4°C). Brains were removed and post-fixed overnight in PFA before being transferred to a 30% sucrose solution in PB until equilibration. They were then transferred to a mold, covered in O.C.T. solution (Tissue-Tek) and stored at −20°C until sectioned. Semi-horizontal slices (50 μm-thick) were cut on a cryostat and the sections (6 series comprising of 12 sections per series) collected in a cryoprotectant solution consisting of 30% ethylene glycol and 25% glycerol in 50 mM PB, which was treated with 0.05% diethylpyrocarbonate (DEPC) to inactivate RNase activity. The cut sections were stored at −20°c until processed or analyzed.
Immunofluorescence
Cryo-protected brain slices fixed in PFA were washed in PB (3, 5-minute rinses), main rinse solution (MRS: 0.1M PB, 0.1M glycine, 0.5% Triton X-100; 3, 20-minute rinses) before being exposed to a blocking solution (0.1M PB, 0.5% Triton X-100, 2% goat serum, 2% bovine serum albumin) for a minimum of 1 hour on a shaker. Slices were then exposed to the primary antibody in blocking solution overnight at room temperature. For double staining, we used the following combinations of primary antibodies: anti-NeuN Alexa-555, clone A60 (Millipore, mouse; MAB377A5) and anti-GFAP Alexa-488 (Millipore, mouse; MAB3402X) or anti-NeuN, clone 27-4 (Millipore, rabbit; MABN140) and anti-CD11b/c (OX42) (Abcam, mouse; ab1211). Slices were then washed in PB (3, 5-minute rinses), MRS (3, 20-minute rinses) before being exposed to the secondary antibodies in blocking solution. The following secondary antibodies were used for NeuN clone 27-4 and OX42, respectively: goat anti-rabbit Alexa-405 (Invitrogen, A-31556) and goat anti-mouse Alexa-488 (Invitrogen; A-11001). For the triple stains, we used anti-GFAP Alexa-488, anti-NeuN Alexa-555 and anti-CD11b/c (OX42) as our primary antibodies and goat anti-mouse biotin (Invitrogen, B-2763) and Streptavidin Alexa-594 (Invitrogen, S11227) as our secondary antibodies for OX42. To avoid cross-labeling of the secondary antibodies, as all primary antibodies were raised in mouse, slices were exposed to the OX42, biotin and streptavidin before the rest of the fluorophore-conjugated anti-NeuN and anti-GFAP primary antibodies were added to the mixture overnight. Slices were finally washed in MRS (6, 10-minute rinses) then mounted on slides using vectashield mounting media with or without DAPI (Vector Laboratories, CA). Slices were imaged on a confocal laser-scanning microscope (Zeiss LSM 880) using an EC Plan-Neofluar 10x/0.30 WD =5.2 M27 (GFAP or CD11b/c) or Plan-Apochromat 63x/1.40 Oil DIC M27 (neurons) objectives with appropriate excitation/emission filters. The 63x images were captured using a 35 μm Z-stack with images taken at 5 μm intervals.
Fluoro-Jade C staining
Slices were first immunostained for microglia (OX42) using anti-CD11b/c (OX42) as our primary antibody and goat anti-mouse biotin (Invitrogen, B-2763) and Streptavidin Alexa-594 (Invitrogen, S11227) as our secondary antibody for OX42. Slices were mounted on slides and air dried overnight, at room temperature, prior to Fluoro-Jade C staining (Millipore, AG325). Slides were immersed in 100% ethanol for 5 minutes, 70% ethanol for 2 minutes and then rinsed in deionized water before being re-dried at room temperature. Once dried, they were immersed in a 0.6% KMnO4 solution for 10 minutes (to reduce background staining) and rinsed 2x in deionized water for 1 minute and placed in solution of 0.0002% Fluoro-Jade C diluted in 0.1% acetic acid, for 20 minutes (kept in dark). Slides were rinsed 3x for 1 minute in deionized water and dried overnight in the dark. The slides were subsequently cleared with xylene 3x for 1 minute (in separate containers) then cover slipped using vectashield mounting media.
Neuron and glial cell counting
Neuron (NeuN), astrocyte (GFAP) and microglia/macrophage (OX42) densities were determined using confocal-acquired high resolution images (10x, astrocytes and microglia; 63x stacked-images, neurons) using the Fiji-ImageJ cell counting tool. We did not use stereological analysis for this study because of the size of the areas being imaged and the difficulties of demarcating CA1-CA2 and CA1-subiculum boundaries at high magnifications while maintaining consistency of counts across subdivisions CA1a, CA1b and CA1c in the slices. Neuronal (NeuN) staining was used to demarcate the boundaries between CA1-CA2 and CA1-subiculum. The CA1 was partitioned into 3 subdivisions (400 x 125 μm) and designated as CA1a (proximal to subiculum), CA1b (middle) and CA1c (proximal to CA2) (Masurkar, et al., 2017). To fully resolve individual neurons within CA1, NeuN positive cells were counted using individual Z-stack images (acquired at intervals of 5 μm over 35 μm), to minimize double counting. All other cell types including astrocytes and microglia were counted using the 10x images. Neuronal densities within CA3 and DG were estimated from counts within a 400 x 125 μm boxes centered in these regions. We used the same anatomical landmarks to select regions (of fixed dimensions) for analysis in all slices from the animals. All neurons and astrocytes within the hilus were counted. The density grams (see Figs. 5C, 6B) show the distribution of cell types within three horizontally aligned counting frames covering CA1a-c with each point representing the x- and ycoordinates of a cell relative to origin (bottom-left vertex of the box in CA1c) in a Cartesian plane. All cells within CA1 could be accounted for, based on their location within these boxes. Note that the brain slices used for MEA analysis (not shown) were also the ones used for obtaining data from the hippocampus.
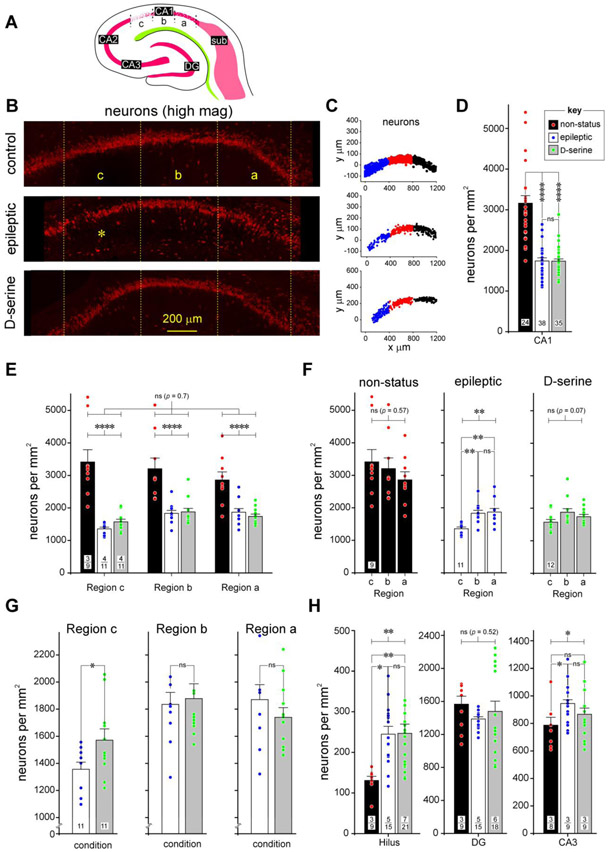
Fig 5: A detailed assessment of neurodegeneration in CA1 of the hippocampus in epileptic and D-serine treated animals.
A, Schematic of the hippocampal portion of the MEA-hippocampal brain slice showing subdivisions of the CA1 subfield and the extent of neurodegeneration in the various subdivisions.
B, High magnification images of stratum pyramidale in the CA1 subfields of non-status controls (top), epileptic (middle), and post-status rats treated with D-serine (bottom) immunostained with fluorescently tagged antibodies against NeuN (red), to showcase the significant neurodegeneration in CA1c (*) and its rescue with D-serine intervention in the MEA.
C, Representative examples of density grams for neurons, generated by plotting the precise location of cells counted on a grid in various subdivisions of CA1 (c, blue; b, red and a, black) under the indicated conditions. Note the thinning out of stratum pyramidale in CA1c in epileptic animals.
D, Histogram of averaged neuronal density across the entire CA1 subfield under the indicated conditions. Data within bar plots indicate number of animals used (n) and error bars indicate SEM. The key (inset) pertains to non-status controls, epileptic, and D-serine-treated animals in this and all subsequent histograms. Unless otherwise indicated, in this and subsequent figures, p values are determined with either a parametric or non-parametric (np) 1- or 2-way ANOVA and where appropriate, t-test with Welch’s correction or an unpaired (np) Mann-Whitney test. **** p < 0.0001, ns, not significant.
E, Histograms of averaged neuronal densities across various subdivisions of CA1 under the conditions indicated in the bar plots (non-status controls, epileptic and D-serine-treated; key in D). In this and all subsequent figures, data within bar plots indicates number of animals (n) used (numerator) and the total number of sections assayed (denominator) for each condition. Error bars indicate SEM. **** p < 0.0001, ns, not significant.
F, Histogram of averaged neuronal density across individual subdivisions of the CA1 subfield under the indicated conditions. Data within bar plots indicate number of animals used (n) and error bars indicate SEM. ** p < 0.01, ns, not significant.
G, Histogram of averaged neuronal density in epileptic and D-serine treated animals in the indicated subdivisions of the CA1 subfield. Data within bar plots indicate number of animals used (n) and error bars indicate SEM. * p < 0.05, ns, not significant.
H, Histograms of averaged neuronal densities across the indicate subdivisions of the hippocampus, hilus (left), dentate gyrus (DG, middle) and CA3 (right), under the conditions indicated in the bar plots (non-status controls, epileptic and D-serine-treated; key in D). Data within bar plots indicates number of animals (n) used (numerator) and the total number of sections assayed (denominator) for each condition. Error bars indicate SEM. * p < 0.05, ** p < 0. 01, ns, not significant.
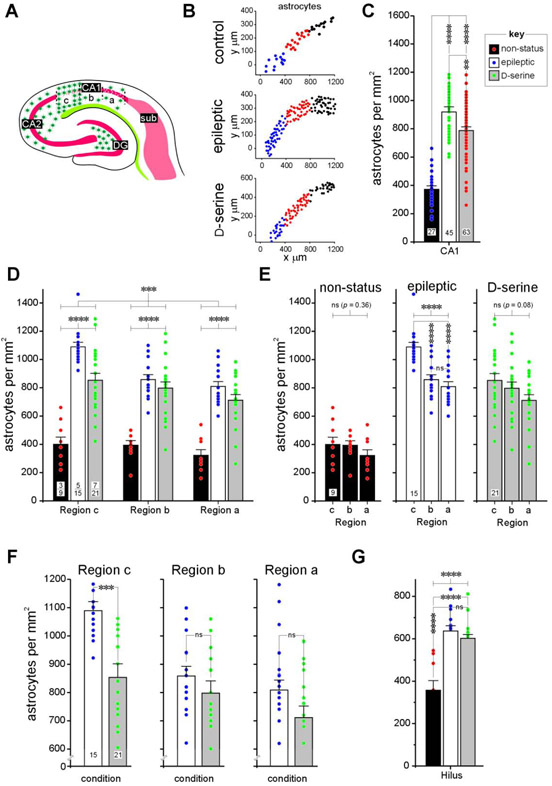
Fig 6: A detailed assessment of astrogliosis in CA1 of the hippocampus in epileptic and D-serine treated animals.
A, Schematic of MEA-hippocampal brain slice showing subdivisions of the CA1 subfield and the extent of astrogliosis in the various subdivisions.
B, Representative examples of density grams for astrocytes, generated by plotting the precise location of cells counted on a grid in various subdivisions of CA1 (c, blue; b, red and a, black) under the indicated conditions. Note the upregulation of astrocytic density in CA1c in epileptic animals.
C, Histogram of averaged astrocytic density across the entire CA1 subfield under the indicated conditions. Data within bar plots indicate number of animals used (n) and error bars indicate SEM. The key (inset) pertains to non-status controls, epileptic and D-serine-treated animals in this and all subsequent histograms. **** p < 0.0001, ** p < 0.01, ns, not significant.
D, Histograms of averaged astrocytic densities across various subdivisions of CA1 under the conditions indicated in the bar plots (non-status controls, epileptic and D-serine-treated; key in C). Data within bar plots indicates number of animals (n) used (numerator) and the total number of sections assayed for each condition (denominator). Error bars indicate SEM. Unless otherwise indicated, in this and subsequent figures, p values are determined with either a parametric or non-parametric (np) 1- or 2-way ANOVA and where appropriate, t-test with Welch’s correction or an unpaired (np) Mann-Whitney test. *** p < 0.001, **** p < 0.0001, ns, not significant.
E, Histogram of averaged astrocytic density across individual subdivisions of the CA1 subfield under the indicated conditions. Data within bar plots indicate number of animals used (n) and error bars indicate SEM. **** p < 0.0001, ns, not significant.
F, Histogram of averaged astrocytic density in epileptic and D-serine treated animals in the indicated subdivisions of the CA1 subfield. Data within bar plots indicate number of animals used (n) and error bars indicate SEM. *** p < 0.001, ns, not significant.
G, Histograms of averaged astrocytic densities in the hilus under the indicated conditions (non-status controls, epileptic and D-serine-treated; key in C). Data within bar plots indicates number of animals (n) used (numerator) and the total number of sections assayed for each condition (denominator). Error bars indicate SEM. **** p < 0.0001, ns, not significant.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (v8). Data were first tested for normality using one of four tests available through GraphPad (Anderson-Darling, D'Agostino & Pearson, Shapiro-Wilk and Kolmogorov-Smirnov). Upon confirmation of normality, the data were subsequently analyzed using parametric tests: 1- or 2-way ANOVA with Tukey’s multiple correction (≥ 3 groups) or an unpaired t-test with Welsh’s correction (2 groups; not assuming equal standard deviations). Data that did not pass the normality test were analyzed using non-parametric (np) tests: Kruskal-Wallis test (np 1- or 2-way ANOVA, on ranks) with Dunn’s multiple correction (≥ 3 groups) and the unpaired Mann-Whitney test (2 groups). We also analyzed significance based on cell counts (neurons, astrocytes and microglia) per animal (as opposed to sections) using a One-Way nested ANOVA. Except for neuron counts in CA3 (see results), this statistical analysis yielded similar conclusions.
Results
Distinct TLE-related pathologies in the MEA and Hippocampus
To characterize how TLE-related pathology in the MEA influences pathology in the hippocampus, we queried the long-term effects of the chemoconvulsant pilocarpine in an animal model of TLE on neurons, astrocytes, and microglia in both regions using acute brain slices from animals (Fig. 1A) whose epileptogenic status was confirmed through frank seizure activity. As confirmed through previous studies, TLE causes hyperexcitability of stellate cells (LII) and loss of both excitatory and inhibitory neurons (LIII) in the MEA (Fig. 1B) (Kumar and Buckmaster, 2006; Kumar et al., 2007). Previous studies also posited that the bulk of excitatory input to the hippocampus arises from stellate cells in LII of the MEA, constituting the perforant pathway (PP), which drives the hippocampal tri-synaptic circuit, via the dentate gyrus (MEA→DG→CA3→CA1, Figs. 1B, 1C). To determine the role, if any, of the lesser known temporoammonic pathway (TAP, Fig. 1C), which connects LIII of the MEA with CA1, in the pathogenesis of the hippocampus, we a) characterized the neuronal (CA1, CA3, DG and hilus) and glial (CA1 and hilus) pathology in the hippocampus in brain sections from control and epileptic animals and b) studied what effect, if any, D-serine intervention in the MEA (to prevent neuron loss and epileptogenesis), has on the pathology in these regions. Our approach to this investigation is outlined in Figure 2.
Immunostaining with antibodies against NeuN and GFAP revealed a conspicuous loss of neurons and marked upregulation of astrocytes throughout layer 3 of the MEA (Fig. 3A) in epileptic animals compared with the controls (for a representative example see Fig. 3B). While this pathology was observed consistently in tissue samples from epileptic animals, treating post-status animals with D-serine curtailed neuron loss and upregulation of astrocytic signal in the MEA to a significant extent and confined it to just the midmost portions of the MEA (top-down panels to the right, Fig. 3B). Post-status animals treated with D-serine resembled controls with only a moderate loss of neurons (top-down panels to the left, Fig. 3B). D-serine-mediated rescue of neurons was observed bilaterally in spite of its unilateral infusion into the right hemisphere presumably via ventricular transport and/or cross hemispheric connectivity of neuron between the left and right MEAs (Honda and Ishizuka, 2004; Steward and Scoville, 1976; Tang et al., 2015).
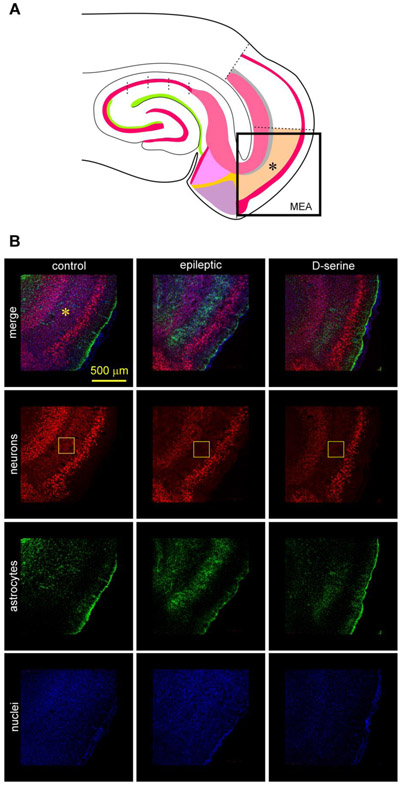
Fig 3: TLE-related pathology in the MEA.
A, Schematic of the MEA-hippocampal brain slice showing the area imaged in B using high resolution confocal microscopy.
B, Immunofluorescence images of the MEA (*, top down panels) in a non-status control (left), epileptic (middle), and post-status rat treated with D-serine (right). Triple immunostaining of neurons (red), astrocytes (green) and nuclei (blue) highlighting neuronal and astroglial pathology within MEA (topmost horizontal panel). Neurons immunoassayed with fluorescently tagged antibodies against NeuN (red, second horizontal panel from top), astrocytes with antibodies against GFAP (green, third horizontal panel from top), and nuclei with DAPI (blue, fourth horizontal panel from top) shown separately [for quantification of data in MEA see (Beesley, et al., 2020)]. The core of the pathology, including cell loss and astrogliosis occurs in layer III of the MEA (rectangular boxes in yellow).
When the hippocampus within the same sections was examined (Fig. 4A), we were surprised to see an intact granule cell layer (stratum granulosum) in the dentate gyrus, and an intact pyramidal cell layer (stratum pyramidale) in CA3 and CA2 of the epileptic animals. However, there was serious disruption in the CA1 region, particularly in the segment closest to CA2 (top-down panels in the middle, Fig. 4B). This region also saw an upregulation of astrocytes that were manifest as a “curtain,” extending through all layers from stratum oriens into the stratum radiatum (third horizontal panel, Fig. 4B). To confirm this disruption, we also immunostained sections with a fluorescently tagged antibody specific to microglia, that are known for their phagocytic activity and generally upregulated during neuroinflammation. We noted a conspicuous signal in stratum pyramidale throughout the entire CA1. A relatively weaker signal was also observed in the adjoining CA2 and CA3 (see Fig. 7a). This pattern of neuronal loss, astro- and microgliosis was mitigated to a large extent with infusion of D-serine into the MEA, suggesting that rescue of neurons upstream in the entorhinal cortex rescues the pathology in the hippocampus in epileptic animals.
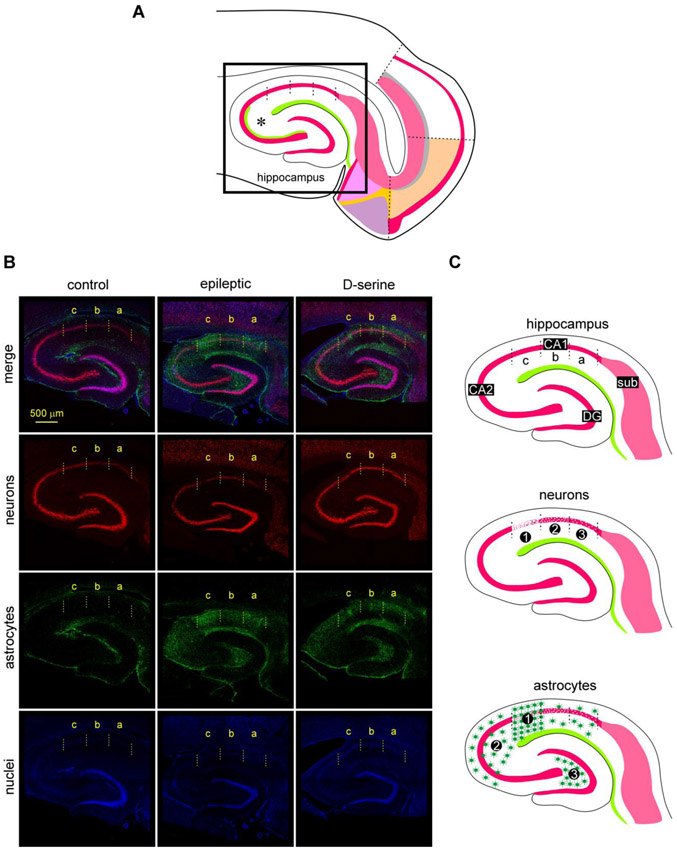
Fig 4: TLE-related pathology in the hippocampus.
A, Schematic of the MEA-hippocampal brain slice showing the area imaged in B using high resolution confocal microscopy.
B, Triple immunofluorescence images of the hippocampus (top down panels) in non-status controls (left), epileptic (middle), and post-status rats treated with D-serine (right) highlighting changes in neuronal (red) and astroglial (green) cell densities in the various subdivisions of CA1 and the hilus (nuclei, blue). Neurons immunoassayed with fluorescently tagged antibodies against NeuN (red, second horizontal panel from top), astrocytes with antibodies against GFAP (green, third horizontal panel from top) and nuclei with DAPI (blue, fourth horizontal panel from top) shown separately. Note that significant pathology, including cell loss, astrogliosis can be found in the CA1c subdivision.
C, Schematic of the hippocampal portion of brain slices showing subdivisions of the CA1 subfield (top) that are affected (1, most effected, to 3, least effected) with respect to neurodegeneration (middle) and astrogliosis (bottom) in epileptic animals. Note that astrocytic density is markedly increased in CA1c (1), CA3 (2) and the hilus (3) of the hippocampus.
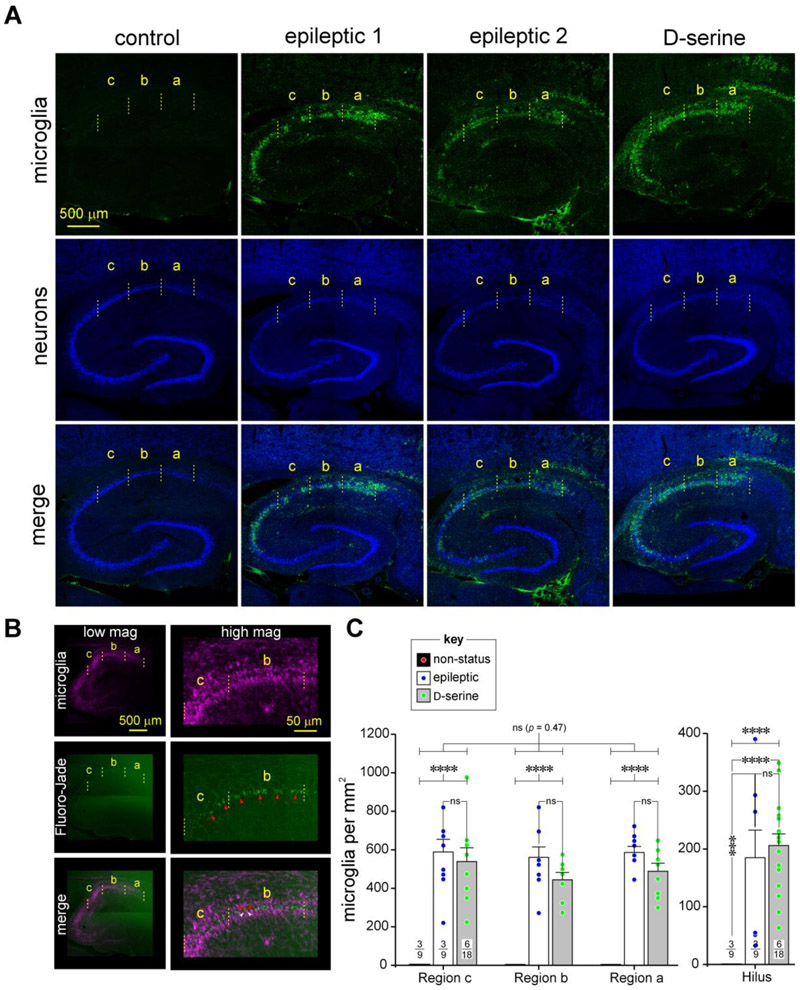
Fig 7: An assessment of microgliosis in CA1 of the hippocampus in epileptic and D-serine treated animals.
A, Immunofluorescence images of the hippocampus (top down panels) in non-status controls (left), epileptic (middle two), and post-status rats treated with D-serine (right) highlighting changes in microglial cell densities in the various subdivisions of CA1 and the hilus. Microglia immunoassayed with fluorescently tagged anti-CD11b antibody, OX-42 (green, topmost horizontal panel) and neurons with antibodies against NeuN (blue, second horizontal panel from top) shown separately (top two panels) and merged images (bottommost panel from top). Note the conspicuous infiltration and/or proliferation of microglia in the CA1 subfield of the hippocampus in the epileptic animals.
B, Co-staining of sections histologically processed for microglia (purple, topmost horizontal panel) with FluroJade (green, second horizontal panel from top), a marker of neural degeneration. Note spatial colocalization of the purple and green signals (merged images, bottom panel) in the low (left) and high (right) magnification images indicate that manifestation of microglia in the CA1 region of epileptic animals is associated with neurodegeneration.
C, Histograms of averaged microglia densities across various subdivisions of CA1 (left) and the hilus (right) under the conditions indicated in the bar plots (key: non-status controls, epileptic and D-serine-treated). Note that microglia are essentially absent in non-status control animals. Data within bar plots indicates number of animals (n) used (numerator) and the total number of sections assayed for each condition (denominator). Error bars indicate SEM. *** p < 0.001, **** p < 0.0001, ns, not significant.
To gauge the extent and degree of damage to the CA1 region during TLE and to quantitate our observations above, we obtained cell density measurements of neurons, astrocytes (Figs. 5 through 6) and microglia (Fig. 7) using high resolution confocal microscopy of the three midseries sections of a 1-in-6 series of sections (50 μm thick; centered around figure 110 in The Rat Brain in Stereotaxic Coordinates, 6th edition) (Paxinos and Watson, 2007) from control, epileptic and D-serine-treated animals as described in Figure 2. The choice of sections assumed their high likelihood of retaining MEA→CA1 connectivity via the TAP. The CA1 region was partitioned into three 0.05 mm2 counting boxes (designated CA1c, CA1b and CA1a; 400 μm x 125 μm; Fig. 4B) based roughly on historical anatomical demarcations of the CA1 region. Each of these counting boxes was centered around and encompassed stratum pyramidale. Cell density measurements were also obtained from the hilus, the cell vulnerable region of the dentate gyrus, as a control and to estimate the relative impact of the PP on hippocampal pathology.
Loss of LIII neurons in MEA is correlated with neurodegeneration in CA1c of the hippocampus
As can be seen in schematic form (Figs. 5A, 4C, severity scored on a scale of 1, high to 3, moderate) and from the high resolution images of immunostained sections (Fig. 5B, high-mag; Fig. 4B, low-mag), hippocampal CA1c appears to be the most affected region of the CA1 subfield with a noticeable disruption of the pyramidal cell layer (*, Fig. 5B). This thinning out of stratum pyramidale in CA1 in epileptic animals can be easily gauged through visual inspection of the density grams (Fig. 5C), generated by plotting the precise location of the neurons counted on a grid in various subdivisions of CA1. Note that the action-at-a-distance effect of D-serine intervention in the MEA is clearly restorative from the perspectives of neurons.
To gain a more in-depth understanding of TLE-related pathology in CA1 (Figs. 5D-5G) and a more detailed assessment of the effects of D-serine intervention in the MEA, we assayed its region and condition-specific effects on neuronal densities within CA1. Our analysis of the MEA (Beesley, et al., 2020) showed no differences in neuron, astrocyte or microglia counts between D-serine-treated animals that developed seizures and those that were seizure-free following D-serine infusion. These observations were also true of neurons (p = 0.24, t-test) and astrocytes (p = 0.33, t-test; microglia not included because of the transient nature of their expression) in the hippocampus. Thus, we made no distinction between these two groups in this study by considering them simply as D-serine treated animals.
The averaged neuronal density in the CA1 region, taken as a whole, was significantly reduced in epileptic animals relative to non-status controls (control: 3159 ± 185 versus epileptic: 1740 ± 76 cells per mm2, p < 0.0001, np 1-way ANOVA; Fig. 5D), and D-serine appeared ineffective in mitigating this loss (control: 3159 ± 185 versus D-serine: 1734 ± 55 cells per mm2, p < 0.0001; epileptic versus D-serine, p = 0.9, np 1-way ANOVA; Fig. 5D). However, averaged neuronal density was significantly reduced in all subdivisions of CA1 in epileptic animals compared with non-status controls and this overall pattern of neuronal loss and recovery with D-serine was consistent across regions C, B or A (p = 0.7, np 2-way ANOVA, Fig. 5E). Nonetheless, within each subdivision, there was a statistically significant difference between conditions (control, epileptic and D-serine treated; p < 0.0001, Fig. 5E).
A region-wise comparison within CA1 revealed no significant differences in neuronal densities in the control animals (C: 3413 ± 378, B: 3203 ± 333, A: 2861 ± 247, p = 0.57, 1-way ANOVA; Fig. 5F, left panel). However, in the epileptic animals (p < 0.01, 1-way ANOVA; Fig. 5F, middle panel) there were subdivision-specific differences between C and B (C: 1356 ± 52 versus B: 1835 ± 89, p < 0.001, pair-wise comparison, 1-way ANOVA), C and A (C: 1356 ± 52 versus A: 1871 ± 110, p < 0.001, pair-wise comparison, 1-way ANOVA), but not between B and A (B: 1835 ± 89 versus A: 1871 ± 110, p = 0.99, pair-wise comparison, 1-way ANOVA). These regional differences in neuronal densities were no longer manifest in D-serine-treated animals, despite the overall loss of neurons in the CA1 compared with controls, suggesting that it is efficacious in rescuing neurons in C (C: 1571 ± 83, B: 1878 ± 110, A: 1739 ± 72, p = 0.07, 1-way ANOVA; Fig. 5F, right panel). Taken together, this data suggests that region C is the most significantly affected of all subdivisions of the CA1 in terms of neuronal loss and D-serine is efficacious in rescuing this loss in the epileptic animals. To confirm this more directly, we compared neuronal densities in epileptic and D-serine-treated animals in each subdivision of the CA1 (Fig. 5G). Indeed, neuronal density was significantly higher in D-serine-treated animals compared with epileptic animals only in region C (control: 1356 ± 52 versus D-serine: 1571 ± 83, p < 0.05, t-test with Welch’s correction; Fig. 5G, left panel) and no other region (B, control: 1835 ± 89 versus D-serine: 1878 ± 110, A, control: 1871 ± 110 versus D-serine: 1739 ± 72, p = 0.55 and 0.64 for regions B and A respectively, t-test with Welch’s correction; Fig. 5G, middle and right panels).
Much to our surprise, we found that averaged neuronal density in the hilus actually went up under epileptic conditions (control: 131 ± 10 versus epileptic: 245 ±19 cells per mm2, p < 0.05, t-test, Fig. 5H left panel) and stayed up in animals treated with D-serine (control versus D-serine: 246 ± 23 cells per mm2, p < 0.01; D-serine versus epileptic, p = 0.1, t-test) in the MEA (p <0.01, 1-way ANOVA, Fig. 5H left panel). Next, we compared neuronal densities in control, epileptic and D-serine-treated animals across the DG (control: 1567 ± 97, epileptic: 1387 ± 27, D-serine: 1479 ± 124, p = 0.52, 1-way ANOVA; Fig. 5H, middle panel) and CA3 (control: 788 ± 57, epileptic: 945 ± 27, D-serine: 867 ± 45, p < 0.05, 1-way ANOVA; Fig. 5H, right panel) subfields of the hippocampus. We noted significant increases in neuronal density in the epileptic animals compared with controls in the hilus and CA3 regions, but not the DG, which seemed unaffected under all conditions (note however that the increase in neuronal density in CA3 was rendered not statistically significant when comparing between animals as opposed to sections, p = 0.15, One-Way nested ANOVA). Taken together, this data suggests that regions innervated by the PP are relatively spared in the epileptic animals and are uninfluenced by D-serine intervention in the MEA.
Region and condition-specific effects of D-serine intervention in MEA on astrocytic densities in the CA1 region.
Just as in the MEA, we discovered a sparse distribution of astrocytes interspersed among neurons throughout the hippocampus in non-status control animals. This pattern was strikingly altered in the epileptics (schematic Figs. 6A, 4C) and curtailed to a significant extent by intervening in the MEA with D-serine (Fig. 4B, third horizontal panel from the top). This upregulation of astrocytic density in the CA1 region in epileptic animals can be easily gauged through visual inspection of the density grams, generated by plotting the precise location of the cells counted on a grid in various subdivisions of CA1. Note that the action-at-a-distance effect of D-serine intervention in the MEA is clearly restorative from the perspectives of astrocytes (Fig. 6B). The averaged astrocytic density in the CA1 region, taken as a whole, was significantly enhanced in epileptic animals relative to non-status controls (control: 372 ± 24 versus epileptic: 919 ± 36 cells per mm2, p < 0.0001, 1-way ANOVA; Fig. 6C), and D-serine mitigated this enhancement (control: 372 ± 24 versus D-serine: 787 ± 26 cells per mm2, p < 0.0001; epileptic versus D-serine, p < 0.01, 1-way ANOVA; Fig. 6C).
Epileptic animals saw significant increases in averaged astrocytic density in all subdivisions of CA1, which was opposite to that observed for neurons. However, astrocytic density was markedly enhanced in CA1c relative to other regions of CA1 in the epileptic animals compared with non-status controls and this overall pattern of increase in astrocyte counts and diminution with D-serine was different across regions A, B and C (p < 0.001, 2-way ANOVA, Fig. 6D). Regardless, within each subdivision, there was a statistically significant difference between conditions (p < 0.0001, Fig. 6D). A region-wise comparison within CA1 revealed no significant differences in astrocytic densities in control animals (C: 400 ± 51, B: 393 ± 33, A: 322 ± 40, p = 0.36, 1-way ANOVA; Fig. 6E, left panel). However, in epileptic animals (p < 0.0001, 1-way ANOVA; Fig. 6E, middle panel) there were subdivision-specific differences between C and B (C: 1090 ± 32 versus B: 858 ± 34, p < 0.0001, pair-wise comparison, 1-way ANOVA), C and A (C: 1090 ± 32 versus A: 809 ± 35, p < 0.0001, pair-wise comparison, 1-way ANOVA), but not between B and A (B: 858 ± 34 versus A: 809 ±35, p = 0.56, pair-wise comparison, 1-way ANOVA). These regional differences in astrocytic densities were no longer manifest in D-serine-treated animals, despite the overall increase in astrocytes in the CA1 compared with controls, suggesting that it is efficacious in reducing their numbers in C (C: 853 ± 48, B: 798 ± 43, A: 711 ± 41, p = 0.08, 1-way ANOVA; Fig. 6E, right panel). Taken together, this data suggests that region C is the most significantly affected of all subdivisions within the CA1 in terms of astrocytic enhancement and D-serine is efficacious in reducing it in the epileptic animals. To confirm this more directly, we compared astrocytic densities in epileptic and D-serine-treated animals in each subdivision of the CA1 (Fig. 6F). Indeed, astrocytic density was significantly lower in the D-serine-treated animals compared with epileptic animals only in region C (control: 1090 ± 32 versus D-serine: 853 ± 48, p < 0.05, t-test with Welch’s correction; Fig. 6F, left panel) and no other region (B, control: 858 ± 34 versus D-serine: 798 ± 43, A, control: 809 ± 35 versus D-serine: 711 ± 41, p = 0.28, 0.08 for regions B and A respectively, t-test with Welch’s correction; Fig. 6F, middle and right panels).
Similar to CA1, we found a statistically significant upregulation in averaged astrocytic density in the hilus under epileptic conditions (control: 357 ± 46 versus epileptic: 637 ± 25 cells per mm2, p < 0.0001, t-test, Fig. 6G) that stayed up with D-serine treatment (control: 357 ± 46 versus D-serine: 602 ± 18 cells per mm2, p < 0.0001; D-serine versus epileptic, p = 0.1, t-test) in the MEA (p < 0.0001, 1-way ANOVA, Fig. 6G). In addition to the CA1 and hilus, we also observed a diffuse upregulation of astrocytes in the CA2 and CA3 regions of the hippocampus in epileptic animals (not measured) despite a seemingly intact pyramidal cell layer. However, this upregulation appeared to be only partially mitigated by D-serine intervention in the MEA (Fig. 4B)
Microgliosis in CA1 is associated with neurodegeneration and neuroinflammation.
To determine if neuronal loss in CA1 is associated with neuroinflammation, we assayed for microglia (not distinguished from macrophages) with an antibody that specifically labeled these cells. The high-resolution confocal images clearly show proliferation and/or infiltration of microglia into CA1 and the adjoining CA3-CA2 regions of the hippocampus in epileptic animals (Fig. 7A). Microglia were not observed in the hippocampi of non-status control animals. The pattern of microglia staining coincided well with regions of cell loss within CA1 although lack of staining in CA1c in a few epileptic animals but not all (vertical panels 2 and 3, Fig. 7A), suggested a temporal order to their upregulation and diminution during phagocytosis, given the loss of neurons in all epileptic animals. D-serine intervention in the MEA did not seem to affect microgliosis in any region of the CA1 or the hilus, where there was a relatively modest elevation of microglia compared with the CA1, yet significant when compared with non-status controls. To ascertain whether the observed microgliosis is indeed correlated with degenerating neurons, we counter stained the sections with Fluro-Jade, a marker of dead or dying neurons. The high-resolution confocal images (Fig. 7B) clearly suggest an intermingling of the two signals, thereby confirming microglia-mediated phagocytic neurodegeration in CA1 in the epileptic animals. The averaged microglia density was significantly upregulated in all subdivisions of CA1 in the epileptics compared with non-status controls and this overall pattern was consistent across regions A, B and C (p = 0.5, 2-way ANOVA, Fig. 7C). Within each subdivision, there was a statistically significant difference between conditions (control, epileptic and D-serine treated; p < 0.0001, Fig. 7C) although differences between epileptic (C: 589 ± 65, B: 561 ± 54, A: 586 ± 32) and D-serine-treated animals were not significant in any subdivision of CA1 (C: 539 ± 72, B: 444 ± 38, A: 489 ± 42, p > 0.96, 1-way ANOVA pairwise comparison; Fig. 7C). We found a significant upregulation of microglial density in the hilus of epileptic animals that was unaffected by D-serine infusion into the MEA (p < 0.0001, 1-way ANOVA, Fig. 7C, right panel). Averaged microglia densities were significantly higher in the epileptics (185 ± 48 cells per mm2) or D-serine treated animals (206 ± 20 cells per mm2) compared to microglia-free controls (p < 0.001 for both, Fig. 7C) but not between epileptic and D-serine-treated animals (p = 0.75, Fig. 7C).
Discussion
This study examined neuronal, astroglial and microglial pathology in the CA1 subfield of the hippocampus in a rat model of TLE. Our results demonstrate and confirm significant neurodegeneration in this region and upregulation of astrocytic and microglial density in the epileptic animals, implicating neuroinflammatory processes in the pathogenesis. The loss of these neurons, which mediate memory-related processes including consolidation, may underlie TLE-related cognitive comorbidities. We found an unexpected gradation in the pathology, with the CA1c subdivision seeing the bulk of the disruption, followed by CA1b and CA1a. Intervention in the MEA with D-serine, an antagonist of t-NMDARs, prevented excitotoxicity-mediated death of LIII neurons, curtailed the deleterious effects of ensuing neuroinflammation, and prevented epileptogenesis in pilocarpine-treated post-status animals (Beesley, et al., 2020). The consequence of this intervention downstream in the hippocampus was seen mostly in the CA1c subdivision where neuron loss was curtailed and the upregulation in astrocytic density minimized. This suggests that the medial to lateral extents of the MEA are primarily mapped onto CA1c, extending a little perhaps into CA1b, while the LEA projects onto CA1b and mostly onto CA1a. The specificity of effects–disruption of stratum pyramidale in CA1c in the epileptic animals and its rescue through intervention in the MEA–reported, for the first-time to the best of our knowledge, highlights the importance of the less-studied TAP that connects the two regions and mediates the observed hippocampal pathogenesis. Changes in cell densities can be attributed in part to volumetric changes in the brain under epileptic conditions. Although this was neither noticeable nor measured, our cell density measurements may nonetheless be overestimated because we counted within fixed areas encompassing the CA1 in control and epileptic animals irrespective of possible reductions in brain volume.
The direct and indirect effects of TAP and PP
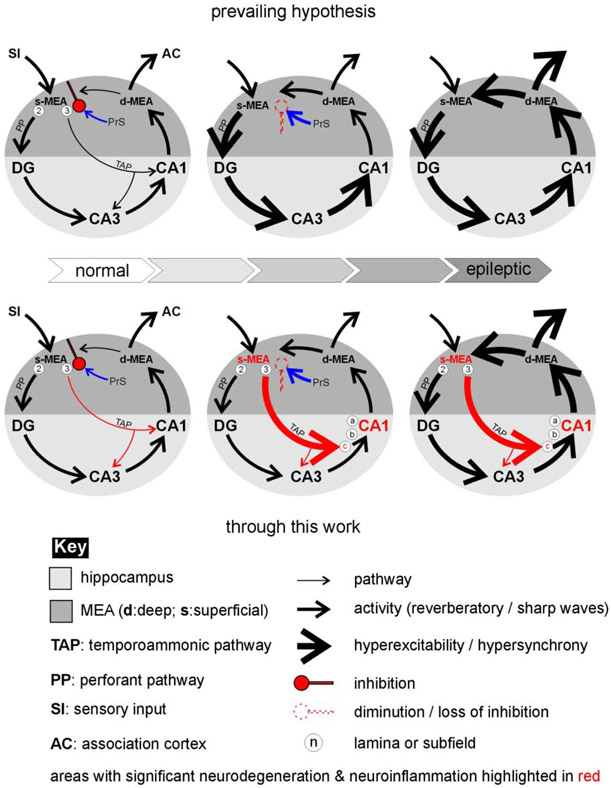
Other than intrinsic factors such as hyperexcitability of vulnerable populations of neurons, sprouting and neurogenesis that affect hippocampal pathology (Thom, 2014), the prevailing hypothesis is that TLE-related damage to CA1 is mediated indirectly through the driving of the trisynaptic circuit (MEA→DG→CA3→CA1) by the perforant pathway (Fig. 8, upper panel). However, much to our surprise, structures upstream of CA1 in this pathway appeared relatively unperturbed. In the hilus, the cell vulnerable region of the dentate gyrus, for example, we found an increase in neuronal density in the epileptic animals which was in sharp contrast with the CA1 subfield. This was also the case in the CA3 subfield. These are regions in which we had expected to see neurodegeneration and are unsure of precisely why the opposite effect is seen. Although this increase may be attributed to adult neurogenesis and/or abnormal positioning of granule cells (Parent, 2007; Scharfman and McCloskey, 2009), with the system reverting to a time point in early development when injured, the granule cell layer of the DG itself appeared relatively intact compared with any of the subdivisions in CA1. This observation is supported by the lack of microglial readout and the conspicuous absence of these phagocytic cells and Fluro-Jade staining within the DG in the epileptic animals and is consistent with observations reported in the literature that in the hippocampus, neuronal loss mainly included the hilus and pyramidal cell layers of areas CA1 and CA3–the DG and area CA2 cell layers were relatively resistant (Buckmaster and Dudek, 1997; Obenaus et al., 1993; Scharfman, 2019; Scharfman and Pedley, 2007). These observations, coupled with effects of D-serine intervention in the MEA on CA1 strongly implicate the more direct temporoammonic pathway in CA1 pathogenesis (Fig. 8, lower panel). While neuronal loss and microgliosis are only minimally observed in the DG and hilus, these regions, particularly the hilus, does see a conspicuous increase in astrocytic density (this could be due to proliferation and/or a change in reactive status) indicating inflammation. The PP does however seem to influence CA3 glial pathology, which although not quantified here, sees an increase in both astrocytic and microglial density and likely neurodegeneration (the Fluro-Jade stain extends into this subfield) under epileptic conditions (Liu et al., 2018). However, it is unclear whether this is mediated solely through PP connectivity or a bifurcating extension of the TAP to this region or both. Indeed, simultaneous recordings within the hippocampus and entorhinal cortex have demonstrated that epileptiform activity originated in the entorhinal cortex and propagated via the PP to the DG, CA3 and CA1 via Schaffer collaterals. However, after Schaffer collateral cut (used to mimic CA3 neuron damage known to occur in TLE (Ben-Ari, 1985) ictal discharges continued to occur in CA1 and subiculum and spread directly from the entorhinal cortex. Furthermore, the time delay for ictal discharge propagation from the entorhinal cortex to CA1 (~100 ms) was reduced by as much as a third, thereby “unmasking” the TAP as the principal pathway.
Fig 8: A hypothetical model for how the MEA might entrain the hippocampus in TLE.
Modifications made to the prevailing hypothesis through the current work on changes in activity patterns within the MEA-hippocampal network lead to the epileptic state highlighting the putative roles of the perforant (black) and temporoammonic (red) pathways in mediating these changes.
The selective vulnerability of CA1 neurons
An enduring question is why and/or how neurons in the CA1 region die in the epileptic animals. In animal models of TLE, the CA1 pyramidal neurons are more sensitive to Ca2+ induced excitotoxicity and subsequently to neurodegeneration compared with other regions of the hippocampus (CA4, CA3 and DG) (Santos, et al., 2019). This increased sensitivity is attributed, as least in part, to the prevalence of NMDA receptors (Martens et al., 1998) and the loss of parvalbumin (PV) and somatostatin (SOM) expressing GABAergic interneurons (Best et al., 1994; Liguz-Lecznar et al., 2016). Their loss, particularly in the CA1 stratum oriens, has been posited to influence epileptogenesis via disinhibition of the pyramidal cells (Lacaille et al., 1987; Spruston, 2008). The TAP may serve as an effective conduit for relaying the hyperexcitability of LIII neurons in MEA directly to pyramidal cells in CA1c and CA1b subfields of the hippocampus under epileptic conditions. Furthermore, these neurons receive many more synaptic inputs from other regions including CA3 (Schaffer collaterals) as well as from stellate cells in LII of the MEA through the PP, all which have the potential to enhance their excitability and vulnerability under epileptic conditions. This preponderance of synaptic inputs pyramidal neurons in CA1c receive from various sources compared for instance to those in the CA3 subfield, might well explain their selective vulnerabilities and define the extent to which these regions are disrupted in TLE. How does intervention within the MEA ameliorate neuron loss in CA1? We believe that TLE originates in LIII of the MEA from the hyperexcitability of glutamatergic neurons and the loss of GABAergic interneurons. The pathogenesis in CA1 likely occurs concomitantly because of events upstream wherein the hyperexcitability of MEA neurons is communicated to neurons in CA1c directly via the TAP leading eventually to the entrainment of the entire CA1 subfield of the hippocampus. Curtailing the hyperexcitability of neurons within MEA is therefore effective in dampening hyperexcitability of neurons in CA1c thereby mitigating excitotoxicity. GABAergic interneurons in superficial layers of MEA are ideally positioned to regulate spread of deep-to-superficial layer neuronal activity and/or implement a gating function that enables hippocampal activity returning to the entorhinal cortex to be channeled either to superficial layers for re-entry into the hippocampal-entorhinal circuit as reverberation or to the neocortex (Fig. 8). Under normal conditions, GABAergic interneurons within MEA target ~50% of deep-layer-mediated excitatory synaptic input destined for superficial layers II and III (van Haeften et al., 2003) and likely provide powerful inhibitory control over forward-projecting excitatory neurons in both layers, most notably LII stellate cells (de Curtis and Pare, 2004; Finch et al., 1988; Jones, 1993; Woodhall et al., 2005). Reverberation and propagation of neural activity in the entorhinal cortex is more prominent when inhibition is suppressed (Iijima et al., 1996) and reverberatory waves evoked by a single stimulus to entorhinal cortex stay confined to entorhinal and do not invade the hippocampus unless the strong local inhibition of neurons in superficial layers that project to the hippocampus is overcome either by GABAA receptor antagonists or by high frequency (≥ 1Hz) stimulation that also suppresses inhibition (Benardo, 1993; Deisz and Prince, 1989; Huguenard and Alger, 1986; McCarren and Alger, 1985; Thompson and Gahwiler, 1989). The loss of these GABAergic neurons in MEA in TLE facilitates hippocampal entrainment via the PP and/or TAP.
CA1 pathology from the neuroinflammatory perspective
The loss of neurons in CA1c and CA1b is inversely correlated with increases in astrocytic and microglial densities raising the question of their role in neurodegeneration under epileptic conditions. We noted a sparse distribution of astrocytes, known for their normal homeostatic functions, throughout the entire hippocampus in control animals. However, this pattern was dramatically altered in the epileptic animals with the emergence of hotspots in the CA1c, CA3 (stratum radiatum) and the hilus in which there was marked increase in astrocytic density due to proliferation and/or infiltration and/or change in reactive status. Intervention within the MEA disrupted this upregulation, particularly in CA1 relative to the hilus, suggesting that astrocytes are particularly sensitive changes in the status of neurons. Indeed, these cells are implicated in mobilizing the neuroinflammatory response to injury through the release of pro- and anti-inflammatory cytokines. It is interesting to note in this context that while astrogliosis is negatively correlated with neuron loss in the CA1 subfield, it is positively correlated neuronal proliferation in the hilus under epileptic conditions. This pattern of change is quite different for microglia which are significantly upregulated in CA1 and CA3, but not the hilus or DG in epileptic animals. Infiltration and/or proliferation of these phagocytic cells are likely triggered by dead or dying neurons and the upregulation of astrocytes. Unlike astrocytes though, microglia were unresponsive to intervention in the MEA although their expression in the various subdivisions of CA1 appeared transitory in nature as their numbers seemed to wax and wane with the clearing of degenerating neurons. This impression was further strengthened through staining with Fluoro-Jade, a marker of neurodegeneration. It is worth mentioning in this context, that the lack of microglia in the hilus does not necessarily mean that neurons are somehow spared in this region under epileptic conditions given the possibility that pathology in the hilus might follow a different temporal sequence of events after initial precipitating injury compared with either CA1 or CA3. Nonetheless, the manifestation of microglia is a telltale sign of neurodegeneration in any region of the hippocampus or MEA.
Can the observed astrogliosis and microgliosis cause neuron loss independently of hyperexcitability mediated excitotoxicity? We have observed that hyperexcitability alone is insufficient to account for the massive loss of neurons and that it takes only a few neurons to perish, a trigger of sorts, to get the vicious cycle of neurodegeneration and neuroinflammation started. Indeed, in a collection of studies, the neuron-centric hypothesis of epileptogenesis was brought into question and astrocytes were placed at the forefront of this degenerative process (Fellin et al., 2006; Fellin et al., 2004; Gomez-Gonzalo et al., 2010; Tian et al., 2005). For example, it was demonstrated that widespread neuronal synchrony was possible through astrocytic glutamate release via the activation of extrasynaptic NMDA receptors within the CA1 (Fellin, et al., 2004). Astrocytic glutamate, as well as GABA (Le Meur et al., 2012), ATP (Zhang et al., 2003) and D-serine (Mothet et al., 2005) is released principally via Ca2+-mediated exocytosis and likely mediated via G-protein coupled receptor activation. This release of gliotransmitters is thought to mediate neuronal excitation (or inhibition) (Fellin, et al., 2004; Kang et al., 1998; Parri et al., 2001) together suggesting multiple ways through which astrocytic glutamate can be released to excite neurons and potentially lead to ictogenesis. Astrogliosis has also been shown to induce neuronal hyperexcitability (Ortinski et al., 2010), through the down regulation of glutamate synthetase, and is believed to contribute to the development of epilepsy (Seifert et al., 2010). Other groups have studied the effects of reactive astrogliosis on the induction of seizures in mice (Robel et al., 2015). The β1- integrin (Itgβ1) gene was conditionally knocked out in radial glia, resulting in chronic astrogliosis throughout the brain, without detectable abnormalities or inflammation (Robel et al., 2009). These mice developed marked astrogliosis and spontaneous recurrent seizures beginning as early as 4 weeks after birth, yet a similar β1-integrin deletion in the neuron did not induce hyperexcitability, ruling out the possibility that neurons were initiating seizure development. These astrocytes showed reduced Kir4.1- potassium currents, which are associated with stress-induced seizures (Djukic et al., 2007). Neuronal death in CA1 has been directly linked to increased expression of tumor necrosis factor-alpha (TNF-α), astrocytes (Liddelow et al., 2017), and nitric oxide (NO) using TgCRND8, a transgenic mouse model (Ugolini et al., 2018). Astrocytes have been shown to respond to neuronal damage/injury by convert from resting, or naïve, to a reactive state and that there are two forms of reactive astrocytes (A1 and A2), that had pro-inflammatory and anti-inflammatory actions, like those of microglia (Liddelow, et al., 2017). A1 astrocytes form as a pathological response to neuroinflammation, requiring microglia to do so and present in an array of neurodegenerative diseases including Alzheimer’s, Huntington’s, Parkinson’s and multiple sclerosis, and are neurotoxic. A2 astrocytes, on the other hand, form in response to ischemic damage, such as in stroke, and promote neuronal survival (Faulkner et al., 2004; Liddelow, et al., 2017; Okada et al., 2006; Zamanian et al., 2012). Yet, these astrocytic changes have not been fully explored in the context of epilepsy. These and other studies nonetheless highlight the importance of astrocytes and microglia in the non-neuronally initiated pathogenesis in the hippocampus and MEA and the worthiness of D-serine like intervention in the MEA that has the potential to prevent/reduce neuronal damage, particularly in the CA1 region, and attenuate and/or reduce mediators of inflammation. Furthermore, knowing the pathogenesis in interlinked structure of the temporal lobe can inform us about underlying mechanisms and points of intervention as a means of exploring therapeutic avenues for TLE.
Highlights:
TLE related pathology in MEA influences neuron loss and gliosis in the hippocampus.
The CA1c region is the most susceptible of the three subdivisions of CA1.
Intervention in MEA with D-serine mitigated hippocampal pathology.
Entrainment of the hippocampus in TLE is mediated via the temporoammonic pathway.
This work is of relevance to TLE-related neurodegeneration and neuroinflammation.
Acknowledgments
Grants: This work was supported in part by grants from the CRC and CoM at Florida State University, Epilepsy Foundation, and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen P, Bliss TV, Skrede KK (1971), Lamellar organization of hippocampal pathways. Exp Brain Res 13:222–238. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA (2005), Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci 25:9567–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA (2016), Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351:aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley S, Sullenberger T, Crotty K, Ailani R, D'Orio C, Evans K, Ogunkunle EO, Roper MG, et al. (2020), D-serine mitigates cell loss associated with temporal lobe epilepsy. Nat Commun 11:4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley S, Sullenberger T, Kumar SS (2020), The GluN3 subunit regulates ion selectivity within native N-methyl-Daspartate receptors. IBRO Reports 9:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley S, Sullenberger T, Pilli J, Abbasi S, Gunjan A, Kumar SS (2019), Colocalization of distinct NMDA receptor subtypes at excitatory synapses in the entorhinal cortex. J Neurophysiol 121:238–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr J, Heinemann U (1996), Low Mg2+ induced epileptiform activity in the subiculum before and after disconnection from rat hippocampal and entorhinal cortex slices. Neurosci Lett 205:25–28. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y (1985), Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14:375–403. [DOI] [PubMed] [Google Scholar]
- Benardo LS (1993), GABAA receptor-mediated mechanisms contribute to frequency-dependent depression of IPSPs in the hippocampus. Brain Res 607:81–88. [DOI] [PubMed] [Google Scholar]
- Best N, Mitchell J, Wheal HV (1994), Ultrastructure of parvalbumin-immunoreactive neurons in the CA1 area of the rat hippocampus following a kainic acid injection. Acta Neuropathol 87:187–195. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Thom M, Wiestler OD (2002), Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 12:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS (2004), Laboratory animal models of temporal lobe epilepsy. Comp Med 54:473–485. [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE (1997), Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 385:385–404. [PubMed] [Google Scholar]
- Covolan L, Mello LE (2000), Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res 39:133–152. [DOI] [PubMed] [Google Scholar]
- Damisah EC, Hill RA, Rai A, Chen F, Rothlin CV, Ghosh S, Grutzendler J (2020), Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv 6:eaba3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Pare D (2004), The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol 74:101–110. [DOI] [PubMed] [Google Scholar]
- Deisz RA, Prince DA (1989), Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol 412:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow MJ, Eid T, Du F, Schwarcz R, Lothman EW, Steward O (2001), Disruption of inhibition in area CA1 of the hippocampus in a rat model of temporal lobe epilepsy. J Neurophysiol 86:2231–2245. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD (2007), Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27:11354–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento AL, Dos Santos NF, Campos Pelagio F, Aparecida Teixeira S, de Moraes Ferrari EA, Langone F (2012), Neuronal degeneration and gliosis time-course in the mouse hippocampal formation after pilocarpine-induced status epilepticus. Brain Res 1470:98–110. [DOI] [PubMed] [Google Scholar]
- Drexel M, Preidt AP, Sperk G (2012), Sequel of spontaneous seizures after kainic acid-induced status epilepticus and associated neuropathological changes in the subiculum and entorhinal cortex. Neuropharmacology 63:806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Kohler C, Schwarcz R (1995), Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci 15:6301–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA (2007), A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron 56:866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004), Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG (2006), Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci 26:9312–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G (2004), Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43:729–743. [DOI] [PubMed] [Google Scholar]
- Finch DM, Tan AM, Isokawa-Akesson M (1988), Feedforward inhibition of the rat entorhinal cortex and subicular complex. J Neurosci 8:2213–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa DG (1996), The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res 725:11–22. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, et al. (2010), An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol 8:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Villarreal DM, Morales IS, Derrick BE (2016), Long-term Potentiation at Temporoammonic Path-CA1 Synapses in Freely Moving Rats. Front Neural Circuits 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Ishizuka N (2004), Organization of connectivity of the rat presubiculum: I. Efferent projections to the medial entorhinal cortex. J Comp Neurol 473:463–484. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Alger BE (1986), Whole-cell voltage-clamp study of the fading of GABAactivated currents in acutely dissociated hippocampal neurons. J Neurophysiol 56:1–18. [DOI] [PubMed] [Google Scholar]
- Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G (1996), Entorhinal-hippocampal interactions revealed by real-time imaging. Science 272:1176–1179. [DOI] [PubMed] [Google Scholar]
- Jones RS (1993), Entorhinal-hippocampal connections: a speculative view of their function. Trends Neurosci 16:58–64. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Hasselmo ME (2004), Theta rhythmic stimulation of stratum lacunosum-moleculare in rat hippocampus contributes to associative LTP at a phase offset in stratum radiatum. J Neurophysiol 92:1615–1624. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M (1998), Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1:683–692. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S (2014), Island cells control temporal association memory. Science 343:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodam A, Ourdev D, Maulik M, Hariharakrishnan J, Banerjee M, Wang Y, Kar S (2019), A role for astrocyte-derived amyloid beta peptides in the degeneration of neurons in an animal model of temporal lobe epilepsy. Brain Pathol 29:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS (2016) Functional detection of novel triheteromeric NMDA receptors In: Ionotropic Glutamate Receptor Technologies, vol. (Popescu GK, ed), pp. 71–80. New York: Springer [Google Scholar]
- Kumar SS, Buckmaster PS (2006), Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci 26:4613–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Jin X, Buckmaster PS, Huguenard JR (2007), Recurrent circuits in layer II of medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci 27:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA (1987), Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci 7:1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent F, Brotons-Mas JR, Cid E, Lopez-Pigozzi D, Valero M, Gal B, de la Prida LM (2015), Proximodistal structure of theta coordination in the dorsal hippocampus of epileptic rats. J Neurosci 35:4760–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur K, Mendizabal-Zubiaga J, Grandes P, Audinat E (2012), GABA release by hippocampal astrocytes. Front Comput Neurosci 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, et al. (2017), Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Urban-Ciecko J, Kossut M (2016), Somatostatin and Somatostatin-Containing Neurons in Shaping Neuronal Activity and Plasticity. Front Neural Circuits 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JT, Wu SX, Zhang H, Kuang F (2018), Inhibition of MyD88 Signaling Skews Microglia/Macrophage Polarization and Attenuates Neuronal Apoptosis in the Hippocampus After Status Epilepticus in Mice. Neurotherapeutics 15:1093–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R (1934), Studies on the structure of the cerebral cortex ii continuation of the studies of the ammonic system. Journal f Psychologie und Neurologie:113–177. [Google Scholar]
- Martens U, Capito B, Wree A (1998), Septotemporal distribution of [3H]MK-801, [3H]AMPA and [3H]Kainate binding sites in the rat hippocampus. Anat Embryol (Berl) 198:195–204. [DOI] [PubMed] [Google Scholar]
- Masurkar AV, Srinivas KV, Brann DH, Warren R, Lowes DC, Siegelbaum SA (2017), Medial and Lateral Entorhinal Cortex Differentially Excite Deep versus Superficial CA1 Pyramidal Neurons. Cell Rep 18:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK (1995), The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain 118 ( Pt 1):105–118. [DOI] [PubMed] [Google Scholar]
- McCarren M, Alger BE (1985), Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol 53:557–571. [DOI] [PubMed] [Google Scholar]
- Morin-Brureau M, Milior G, Royer J, Chali F, LeDuigou C, Savary E, Blugeon C, Jourdren L, et al. (2018), Microglial phenotypes in the human epileptic temporal lobe. Brain 141:3343–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G (2005), Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A 102:5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S (2008), Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319:1260–1264. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR (1993), Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 13:4470–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Zhu G, Yoshida S, Hirose S, Kaneko S (2004), Protein kinase associated with gating and closing transmission mechanisms in temporoammonic pathway. Neuropharmacology 47:485–504. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, et al. (2006), Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA (2010), Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM (2007), Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res 163:529–540. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V (2001), Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4:803–812. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. Academic Press. [DOI] [PubMed] [Google Scholar]
- Pilli J, Kumar SS (2012), Triheteromeric N-methyl-D-aspartate receptors differentiate synaptic inputs onto pyramidal neurons in somatosensory cortex: involvement of the GluN3A subunit. Neuroscience 222:75–88. [DOI] [PubMed] [Google Scholar]
- Racine RJ (1972), Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM (2002), Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature 416:736–740. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM (2004), Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431:699–703. [DOI] [PubMed] [Google Scholar]
- Robel S, Buckingham SC, Boni JL, Campbell SL, Danbolt NC, Riedemann T, Sutor B, Sontheimer H (2015), Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 35:3330–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Mori T, Zoubaa S, Schlegel J, Sirko S, Faissner A, Goebbels S, Dimou L, et al. (2009), Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia 57:1630–1647. [DOI] [PubMed] [Google Scholar]
- Santos VR, Melo IS, Pacheco ALD, Castro OW (2019), Life and death in the hippocampus: What's bad? Epilepsy Behav:106595. [DOI] [PubMed] [Google Scholar]
- Scharfman HE (2019), The Dentate Gyrus and Temporal Lobe Epilepsy: An "Exciting" Era. Epilepsy Curr 19:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, McCloskey DP (2009), Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res 85:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pedley TA (2007) The Neurobiology of Disease. New York, NY: Academic Press. [Google Scholar]
- Seifert G, Carmignoto G, Steinhauser C (2010), Astrocyte dysfunction in epilepsy. Brain Res Rev 63:212–221. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Wang L, Ribak CE (2008), Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia 49 Suppl 2:33–41. [DOI] [PubMed] [Google Scholar]
- Spruston N (2008), Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9:206–221. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA (1976), Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol 169:347–370. [DOI] [PubMed] [Google Scholar]
- Surmeli G, Marcu DC, McClure C, Garden DLF, Pastoll H, Nolan MF (2015), Molecularly Defined Circuitry Reveals Input-Output Segregation in Deep Layers of the Medial Entorhinal Cortex. Neuron 88:1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y (1995), Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol 353:379–390. [DOI] [PubMed] [Google Scholar]
- Tang Q, Ebbesen CL, Sanguinetti-Scheck JI, Preston-Ferrer P, Gundlfinger A, Winterer J, Beed P, Ray S, et al. (2015), Anatomical Organization and Spatiotemporal Firing Patterns of Layer 3 Neurons in the Rat Medial Entorhinal Cortex. J Neurosci 35:12346–12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M (2014), Review: Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol 40:520–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH (1989), Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol 61:501–511. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, et al. (2005), An astrocytic basis of epilepsy. Nat Med 11:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA (1989), Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse 3:154–171. [DOI] [PubMed] [Google Scholar]
- Ugolini F, Lana D, Nardiello P, Nosi D, Pantano D, Casamenti F, Giovannini MG (2018), Different Patterns of Neurodegeneration and Glia Activation in CA1 and CA3 Hippocampal Regions of TgCRND8 Mice. Front Aging Neurosci 10:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya D, Kodali M, Gitai D, Castro OW, Zanirati G, Upadhya R, Attaluri S, Mitra E, et al. (2019), A Model of Chronic Temporal Lobe Epilepsy Presenting Constantly Rhythmic and Robust Spontaneous Seizures, Co-morbidities and Hippocampal Neuropathology. Aging Dis 10:915–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, Kesner RP (2008), Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res 189:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP (2003), Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus 13:943–952. [DOI] [PubMed] [Google Scholar]
- Varga C, Lee SY, Soltesz I (2010), Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci 13:822–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP (2007), The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res 163:43–61. [DOI] [PubMed] [Google Scholar]
- Woodhall GL, Bailey SJ, Thompson SE, Evans DI, Jones RS (2005), Fundamental differences in spontaneous synaptic inhibition between deep and superficial layers of the rat entorhinal cortex. Hippocampus 15:232–245. [DOI] [PubMed] [Google Scholar]
- Wozny C, Gabriel S, Jandova K, Schulze K, Heinemann U, Behr J (2005), Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol Dis 19:451–460. [DOI] [PubMed] [Google Scholar]
- Wyatt-Johnson SK, Herr SA, Brewster AL (2017), Status Epilepticus Triggers Time-Dependent Alterations in Microglia Abundance and Morphological Phenotypes in the Hippocampus. Front Neurol 8:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012), Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, et al. (2003), ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40:971–982. [DOI] [PubMed] [Google Scholar]