Abstract
Rationale:
Anandamide is an endocannabinoid that contributes to certain aspects of social behavior, like play and reward, by binding to cannabinoid receptor type 1 (CB1). Most interesting is the recent discovery that anandamide may be mobilized by oxytocin receptor activation under certain contexts, particularly in the nucleus accumbens.
Objectives:
Given the established role of oxytocin and the nucleus accumbens in the neurobiology of pair bonding, we investigated whether systemic administration of brain-permeable modulators of the endocannabinoid system could alter preferential partner contact in both male and female prairie voles.
Methods:
Specifically, we tested whether intraperitoneal administration of the neutral CB1 antagonist AM4113 (4.0–16.0 mg/kg) or the anandamide hydrolysis inhibitor URB597 (5.0–20.0 mg/kg) could prevent or facilitate partner preference formation, respectively. To further investigate the specificity of effects on partner preference, we repeated our URB597 dosing regimen on an additional group of females and tested their anxiety-related behavior in both an elevated-plus maze and a light/dark test.
Results:
AM4113 administration had no effect on partner preference. But while URB597 also had no effect on partner preference, low-dose females did increase absolute preferential contact with either the partner or the stranger; individual females spent significant contact time with either the partner or the stranger. None of our outcome measures in either anxiety test showed significant effects of treatment.
Conclusions:
Our results reveal that experimentally increasing anandamide levels in female prairie voles can increase social contact with both a familiar and novel male via unknown mechanisms that are likely separate from anxiety reduction.
Keywords: partner preference, anxiety, anandamide, prairie vole, pair bond
Introduction
Endocannabinoids are lipid metabolites that function within a relatively newly discovered physiological signaling system that is most widely studied for its role in mediating the effects of Cannabis sativa. The endocannabinoid system (ECS) includes an ever-growing list of endogenous neurotransmitters, receptors, and metabolic enzymes that operate within the same framework (Battista et al. 2012). Endocannabinoids are produced “on demand” from membrane phospholipid precursors and are rapidly degraded through local enzymatic activity, but not before acting as synaptic modulators (Battista et al. 2012). The signaling mechanism of endocannabinoids generally involves retrograde neurotransmission and the subsequent inhibition of neurotransmitter release; most of their receptors are found on the presynaptic terminals of neurons that produce glutamate and GABA (Castillo et al. 2012). The ECS regulates a wide variety of physiological processes, most relevantly the motivational salience of rewards (Vlachou and Panagis, 2014) and mediating the effects of glucocorticoids (Balsevich et al. 2017).
One of the many endocannabinoids is anandamide (AEA), a fatty acid neurotransmitter that has recently been studied for its role in mediating the effects of oxytocin. In C57BL/6J mice, researchers demonstrated that acute socialization increased AEA density in the nucleus accumbens and that oxytocin receptor antagonism inhibited this effect (Wei et al. 2015). These findings were reproduced with more direct approaches; both oxytocin receptor agonist and DREADD activation of oxytocinergic neurons within the paraventricular nucleus of the hypothalamus also mobilized AEA in the nucleus accumbens. Knockout of the enzyme responsible for the hydrolysis of anandamide, fatty acid amide hydrolase (FAAH), increased preference for the social context in a socially conditioned place preference paradigm, while antagonism of cannabinoid receptor type 1 (CB1) blocked this effect (Wei et al. 2015). Other studies have also functionally linked manipulation of the endocannabinoid system to sociality. Inhibition of FAAH increased social play in rats (Trezza et al. 2012, 2010) and CB1 antagonism impaired maternal behavior in mice (Schechter et al. 2012). These findings collectively warrant an investigation of endocannabinoid-centric mechanisms for sociality across other contexts.
One model species that has been predominantly used for research into social processes is the socially monogamous prairie vole. Unlike most rodents, prairie voles selectively form enduring social attachments that have been well-characterized on both a behavioral and physiological level (McGraw and Young 2010). In laboratory settings, the propensity of voles to form a pair bond has been measured using a partner preference test. This behavioral paradigm has helped elucidate the functional significance of several signaling systems in the regulation of social attachments, including oxytocin, dopamine, and opioids. To our knowledge, no study has ever explored the endocannabinoid system in prairie voles nor investigated the functional relationship between this system and social attachments. Our objective with this study was to merge these approaches.
With studies confirming a role for anandamide and CB1 in mediating social reward, we predicted that FAAH inhibition would increase social contact with a partner and that CB1 blockade would decrease social contact. We explored the effects of FAAH inhibition using URB597, a brain-permeable drug that is gaining prominence in the literature (Lodola et al. 2015; Piomelli et al. 2006). Traditional CB1 antagonists, however, have recently been scrutinized for exhibiting inverse agonist effects with high affinities for off-target signaling systems. Most notable is the ability of AM251 and rimonabant, the two most commonly used CB1 antagonists, to bind with mid-nanomolar affinities to mu opioid receptors (Seely et al. 2012), which have been shown to mediate pair-bond formation (Resendez et al. 2013). AM4113, on the other hand, is a neutral CB1 antagonist that does not exhibit inverse agonist capabilities or bind to opioid receptors. We tested whether CB1 antagonist could block partner preference formation by administering AM4113 systemically to both male and female prairie voles who were cohoused with a partner long enough to successfully form a social preference. We also tested whether FAAH inhibition could facilitate partner preference formation by administering URB597 to voles who underwent sub-optimal cohabitation times with a partner. Finally, we complemented any findings with additional tests of anxiety since stress differentially modulates partner preference formation in both male and female prairie voles (DeVries et al., 1996).
Materials and Methods
Subjects
Our experiments employed 224 prairie voles (132 females, 92 males) from our breeding colony located in the Department of Psychology at the University of California, Davis. We maintained the animals on a 14:10 h light cycle at approximately 21°C. The animals had access to food (Purina High Fiber Rabbit Chow, PMI Nutrition International, Brentwood, MO, USA) and water ad libitum. All animals were housed with their parents in large polycarbonate cages (44 × 22 × 16 cm) until weaning at postnatal day (P) 20. Subjects were then separated from their parents, given ear-clip markings for identification, and placed with a same-sex sibling in smaller cages (27 × 16 × 13 cm) until the time for behavioral testing. Our subjects were recruited into three experiments with varied testing regiments. Animals in Experiment 1 were paired with an opposite sex conspecific for testing and remained with this partner until sacrifice shortly after. Animals in experiments 2 and 3 were paired with an opposed-sex conspecific for testing and then were returned to their same-sex sibling until sacrifice. Please see the Behavioral Testing section for more specific details on the different conditions for each experiment.
Compliance with Ethical Standards
All procedures described were approved by the UC-Davis Animal Care and Use Committee.
Drug Preparation and Administration
AM4113 and URB597 were obtained from Tocris Bioscience (Ellisville, MO, USA) and dissolved in a vehicle of dimehthylsulfoxide (DMSO; Avantor, Allentwon, PA, USA), Tween-80 (Avantor, Allentown, PA, USA), and 0.9% saline in a 1:1:8 ratio. This vehicle was also used as the control group for all experiments. The drug pretreatment times, doses, and vehicle solution were selected using previously published research (Balla et al. 2018; Murillo-Rodríguez et al. 2016; Sink et al. 2008). Intraperitoneal administration was used for all treatments. For a timeline of our treatment procedures, please see Figure 1.
Figure 1 –
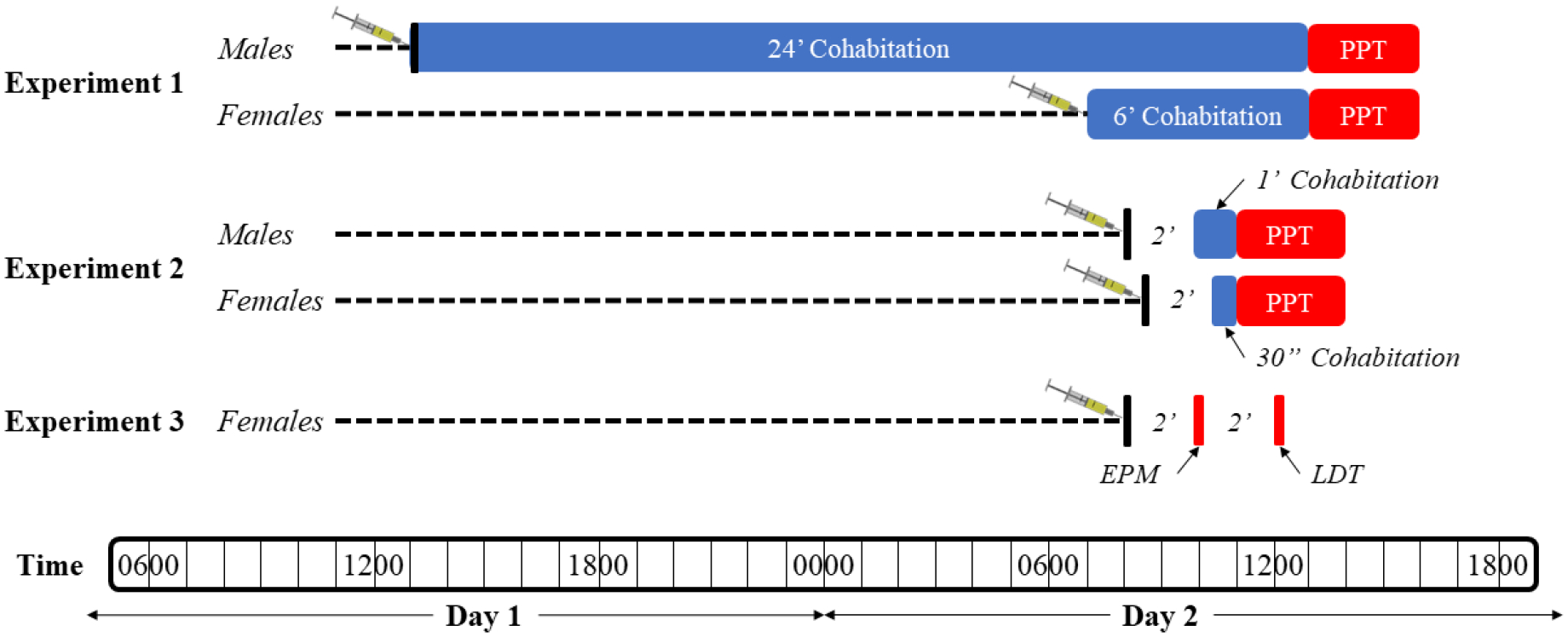
Timeline of experimental procedures. Subjects in Experiment 1 were treated with AM4113 immediately before entering a cohabitation period with a mate (“partner”). After the cohabitation, subjects were tested using the PPT. Subjects in experiments 2 and 3 were treated with URB597 2 hours before the cohabitation period and EPM, respectively. A 2-hour resting period was given between the EPM and LDT for the subjects of Experiment 3. Abbreviations: PPT = partner preference test; EPM = elevated-plus maze; LDT = light/dark test.
Behavioral Testing
As mentioned previously, our subjects were organized into three separate experiments with varied testing conditions (Figure 1). All tests were conducted by a male experimenter and subsequently recorded and manually scored by validated observers using Behavior Tracker 1.5 (www.behaviortracker.com). Our observers remained blind to subject group assignments and were trained to 95% or greater reliability on all behaviors before any data was collected.
Partner Preference Testing
Animals in Experiment 1 were treated with AM4113 as sexually mature adults and then immediately cohoused with opposite-sex conspecifics; male subjects were given 24 h cohabitations with a female while female subjects had 6 h cohabitations with males. These cohabitation times represent sex differences in the latency to form a partner preference with a potential mate (DeVries and Carter 1999). At the end of the cohabitation period, the partner was removed from the home cage and loosely tethered to a distinct chamber within the partner preference test (PPT) apparatus, which consisted of three identical polycarbonate cages (27 × 16 × 13 cm) connected by Plexiglas tubes (8.5 × 16 cm). In addition to the partner, a novel conspecific (“stranger”) selected to match the size and sex of the partner was also tethered within the apparatus. Once both the partner and stranger were securely confined to separate chambers, the test subject was introduced to the test and allowed to move freely throughout the apparatus for 3 h. Food and water was readily available in all chambers throughout the testing period.
Subjects from Experiment 2 also underwent a PPT but with URB597 treatment and shortened cohabitation periods (males 1 h, females 30 min). These periods were used because we have previously shown them to be insufficient to form a significant partner preference in an untreated animal, whereas even an hour was sufficient for females in our laboratory to form a preference (Bales and Carter, 2003; Bales et al., 2007; Bales, unpublished data). These subjects were also given a 2 h loading period prior to the cohabitation to ensure drug efficacy (Trezza and Vanderschuren 2008). The cohabitation times for Experiment 1 are designed to test for deficits in partner preference formation (Simmons et al. 2017) while those for Experiment 2 were used to test for facilitations (Bales and Carter 2003). The ethogram included time spent in social contact with the partner, time spent in social contact with the stranger, total social contact (time in contact with the partner and the stranger added together), the total time in each chamber, and the number of entries into each chamber.
Elevated-Plus Maze
Animals in Experiment 3 were treated with URB597 approximately 2 h before testing in the elevated-plus maze (EPM). The maze had four explorable arms with two opposing closed arms and two open arms. The closed arms had a dark floor and walls with an exposed ceiling while the open arms only had a clear Plexiglas floor. Each arm was 67 cm long and 5.5 cm wide, intersecting at a center Plexiglas square (5.5 cm × 5.5 cm). The entire apparatus was raised 1 m above the floor. All subjects were introduced to the maze at the center square and allowed to explore for 5 min. The test was paused whenever an animal jumped off the apparatus and resumed when it was reintroduced to the maze. However, the test was stopped and any collected data was removed from analysis if the subject jumped three times (as no animals actually jumped three times, none were actually removed). The ethogram was designed to measure anxiety-like behavior and included time spent in either the open or closed arms, the time in the center, the number of entries, and the time spent autogrooming.
Light/Dark Test
Animals in Experiment 3 were also examined using the light/dark test (LDT) approximately 2 h after completing the EPM. This timeline was chosen to give subjects time to recover from the EPM, but also to be well within the pharmacological profile of URB597, which suppresses FAAH activity for at least sixteen hours (Piomelli et al., 2006). The test apparatus consisted of two adjoining polycarbonate cages (27 × 16 × 13 cm) which were connected by a single Plexiglas tube (8.5 × 16 cm). One of the chambers was exposed to ambient light in the room while the second was encased in a cardboard box. All subjects were placed in the tube at the beginning of the test facing the darkened chamber and allowed to explore for 10 min. The ethogram was designed to measure anxiety-like behavior and included time spent in each chamber and the number of corresponding entries.
Statistical Analysis
We elected to prioritize comparisons across treatment groups and not between sexes because the experimental conditions varied by sex. In order not to bias results by litter, for experiments 1 and 2 we recruited only one animal per sex per litter. Experiment 3 only utilized female subjects because we wanted to follow-up on our sex-specific results from Experiment 2. We controlled for litter differences in Experiment 3 by representing all treatment groups in each litter we recruited. Litter was then added to these analyses as a blocking variable.
To analyze within-group partner preference, we created scores for each subject by subtracting the time in contact with the stranger from the time spent in contact with the partner (“preferential contact”). We then compared these preferences to zero.
For between-group analyses, we also analyzed the absolute values of these difference scores to explore whether preferences formed generally for one animal over the other (“absolute preferential contact”). Therefore, while a significant positive value of preferential contact indicated a preference for the partner over the stranger, a significant value of absolute preferential contact indicated a social preference for either the partner or the stranger. Chamber preference was the time spent in the chamber with the stranger subtracted from the time spent in the chamber with the partner. For EPM analyses, we calculated arm preference scores by subtracting time spent in the closed arms from the time spent in the open arms. Similar calculations were completed for LDT outcomes to create preference scores for the light chamber compared to the dark chamber.
We used R version 4.0.0 (R Core Team, 2019) to conduct all analyses. Within-group partner preferences (preferential contact) were analyzed using one-sample t-tests; p-values were adjusted for the false discovery rate using the procedure outline by Benjamini and Yekutieli (2001). One-tailed t-tests were used for Experiment 1 since the conditions were chosen specifically to create preferences for the partner. Two-tailed t-tests were used for Experiment 2 since no preference was expected to be formed at baseline.
Between-group differences for each dependent variable were analyzed using Dunnett tests. These tests are generally used as a post-hoc analysis to contrast treatment groups with a control after first finding a significant ANOVA F-test. However, we elected to use them in lieu of an ANOVA according to the recommendation by Hothorn (2016), who reported that using a conditional F-test before the Dunnett-test may increase type II error. Assumptions for t-tests were confirmed for each dependent variable using the Shapiro-Wilk and Levene’s tests. Alpha was set to 0.05.
Results
Experiment 1
The purpose of Experiment 1 was to determine whether administration of CB1 antagonist AM4113 could block the successful formation of a partner preference. One sample t-tests were conducted to determine whether the difference in time spent between the partner and the stranger (preferential contact) was significantly greater than 0. Preferential contact was not significantly greater than 0 for females treated with vehicle, or for females treated with any dose of AM4113 (Fig. 2). However, preferential contact was significant for males treated with drug vehicle and those treated with either 4.0 mg/kg or 8.0 mg/kg of AM4113 (Fig. 2). These results indicate that none of the female groups independently formed a partner preference, while all male groups—except those treated with 16.0 mg/kg AM4113—did successfully prefer their partner over the stranger. The lack of a partner preference in control females is contrary to expectation since their testing conditions (6 hr cohabitation) should have provided sufficient time for preferences to form (DeVries and Carter, 1999). It is worth noting that the only male group that failed to form a partner preference included those treated with the highest dose of AM4113. Please see Table 1 for all Experiment 1 test statistics.
Figure 2 –
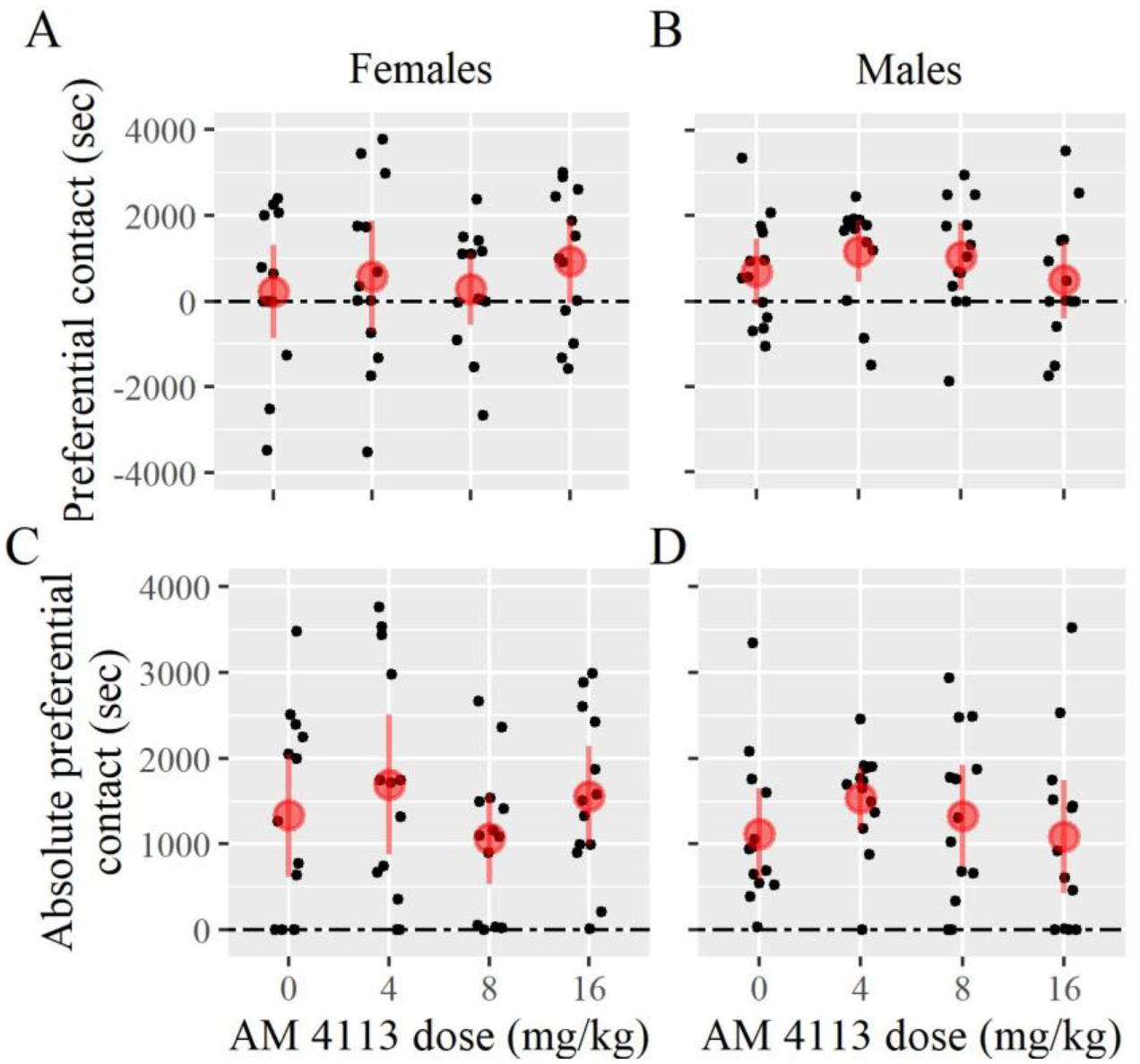
Partner preference test results from Experiment 1 using AM4113. Females from each treatment group spent similar time in preferential contact (calculated for each subject by subtracting the time in contact with the stranger from the time spent in contact with the partner) (A) or in absolute preferential contact (the absolute values of preferential social contact) (C). Males treated with either vehicle, low dose, or medium dose of AM4113 preferred their partner over the stranger (B) while absolute preferential contact was similar across the groups (D). Error bars are 95%-confidence intervals. Symbols: * p < 0.05.
Table 1.
Partner Preference Test Statistics for Experiment 1
| Within-group T-Test | Between-groups Dunnett Test | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Outcome | Dose | M (SD) | T(df) = p | Contrast | Estimate (SE) | T (df) |
| Females | Absolute Preferential Contact | 0 | 1334 (1182) | - | - | - | - |
| 4 | 1692 (1353) | - | 4 – 0 | 358 (434) | 0.82 (48) = 0.747 | ||
| 8 | 1064 (871) | - | 8 – 0 | −270 (434) | −0.62 (48) = 0.869 | ||
| 16 | 1562 (960) | - | 16 – 0 | 228 (434) | 0.52 (48) = 0.915 | ||
| Chamber preference | 0 | 779 (3326) | - | - | - | - | |
| 4 | −239 (5040) | - | 4 – 0 | −1017 (1809) | −0.56 (48) = 0.898 | ||
| 8 | 433 (4212) | - | 8 – 0 | −345 (1809) | −0.19 (48) = 0.995 | ||
| 16 | 2766 (5558) | - | 16 – 0 | 1987 (1809) | 1.10 (48) = 0.560 | ||
| Preferential Contact | 0 | 219 (1809) | 0.44 (12) = 0.335 | - | - | - | |
| 4 | 565 (2142) | 0.95 (12) = 0.324 | 4 – 0 | 346 (690) | 0.50 (48) = 0.924 | ||
| 8 | 275 (1380) | 0.72 (12) = 0.324 | 8 – 0 | 56 (690) | 0.08 (48) = 1.000 | ||
| 16 | 929 (1622) | 2.06 (12) = 0.123 | 16 – 0 | 710 (690) | 1.03 (48) = 0.608 | ||
| Chamber Entries | 0 | 273 (142) | - | - | - | - | |
| 4 | 211 (104) | - | 4 – 0 | −62 (52) | −1.20 (48) = 0.490 | ||
| 8 | 254 (163) | - | 8 – 0 | −19 (52) | −0.38 (48) = 0.965 | ||
| 16 | 229 (108) | - | 16 – 0 | −45 (52) | −0.86 (48) = 0.721 | ||
| Males | Absolute Preferential Contact | 0 | 1121 (881) | - | - | - | - |
| 4 | 1533 (598) | - | 4 – 0 | 412 (354) | 1.16 (48) = 0.517 | ||
| 8 | 1332 (974) | - | 8 – 0 | 211 (354) | 0.60 (48) = 0.882 | ||
| 16 | 1092 (1087) | - | 16 – 0 | −29 (354) | −0.08 (48) = 1.000 | ||
| Chamber Preference | 0 | 1248 (3862) | - | - | - | - | |
| 4 | 2015 (3470) | - | 4 – 0 | 767 (1408) | 0.54 (48) = 0.906 | ||
| 8 | 2846 (2868) | - | 8 – 0 | 1598 (1408) | 1.13 (48) = 0.536 | ||
| 16 | 410 (4049) | - | 16 – 0 | −838 (1408) | −0.60 (48) = 0.882 | ||
| Preferential Contact | 0 | 686 (1276) | 1.94 (12) = 0.051 | - | - | - | |
| 4 | 1168 (1193) | 3.53 (12) = 0.008 | 4 – 0 | 482 (517) | 0.93 (48) = 0.674 | ||
| 8 | 1044 (1300) | 2.90 (12) = 0.013 | 8 – 0 | 358 (517) | 0.69 (48) = 0.829 | ||
| 16 | 496 (1486) | 1.20 (12) = 0.126 | 16 – 0 | −190 (517) | −0.37 (48) = 0.968 | ||
| Chamber Entries | 0 | 296 (148) | - | - | - | - | |
| 4 | 268 (104) | - | 4 – 0 | −28 (50) | −0.57 (48) = 0.894 | ||
| 8 | 247 (86) | - | 8 – 0 | −49 (50) | −0.98 (48) = 0.639 | ||
| 16 | 331 (154) | - | 16 – 0 | 35 (50) | 0.70 (48) = 0.822 | ||
Notes. Within-group analyses composed of one-sample t-tests analyzing whether group means were statistically greater than 0. P values for within-group analyses were adjusted for the false discovery rate using the procedure suggested by Benjamini & Yekutieli (2001). P values for between-groups analyses were adjusted using Dunnett test procedure.
Dunnett tests were used to analyze between-group differences in partner preference (preferential and absolute preferential), chamber preference, and activity levels (measured as chamber entries). No statistically significant differences in any of the dependent variables were found between control females and each treatment group, respectively (Fig. 2). There were also no statistically significant differences in any of the dependent variables analyzed for these comparisons in males (Fig. 2). These results suggest that AM4113 did not substantively alter preference behavior compared to vehicle control during the partner preference test for either sex.
Experiment 2
The objective of Experiment 2 was to test whether administration of FAAH inhibitor could facilitate the formation of partner preferences in suboptimal conditions (e.g. less cohabitation time). One sample t-tests were conducted to determine whether the difference in time spent between the partner and the stranger (preferential contact) was different than 0. These differences were not statistically different from 0 for any group, regardless of sex (Fig. 3). These results were expected for control groups as cohabitation times were shorter than what would generally lead to a partner preference (DeVries and Carter 1999). Thus, these results suggest that URB597 administration may not facilitate a selective preference for the partner over the stranger.
Figure 3 –
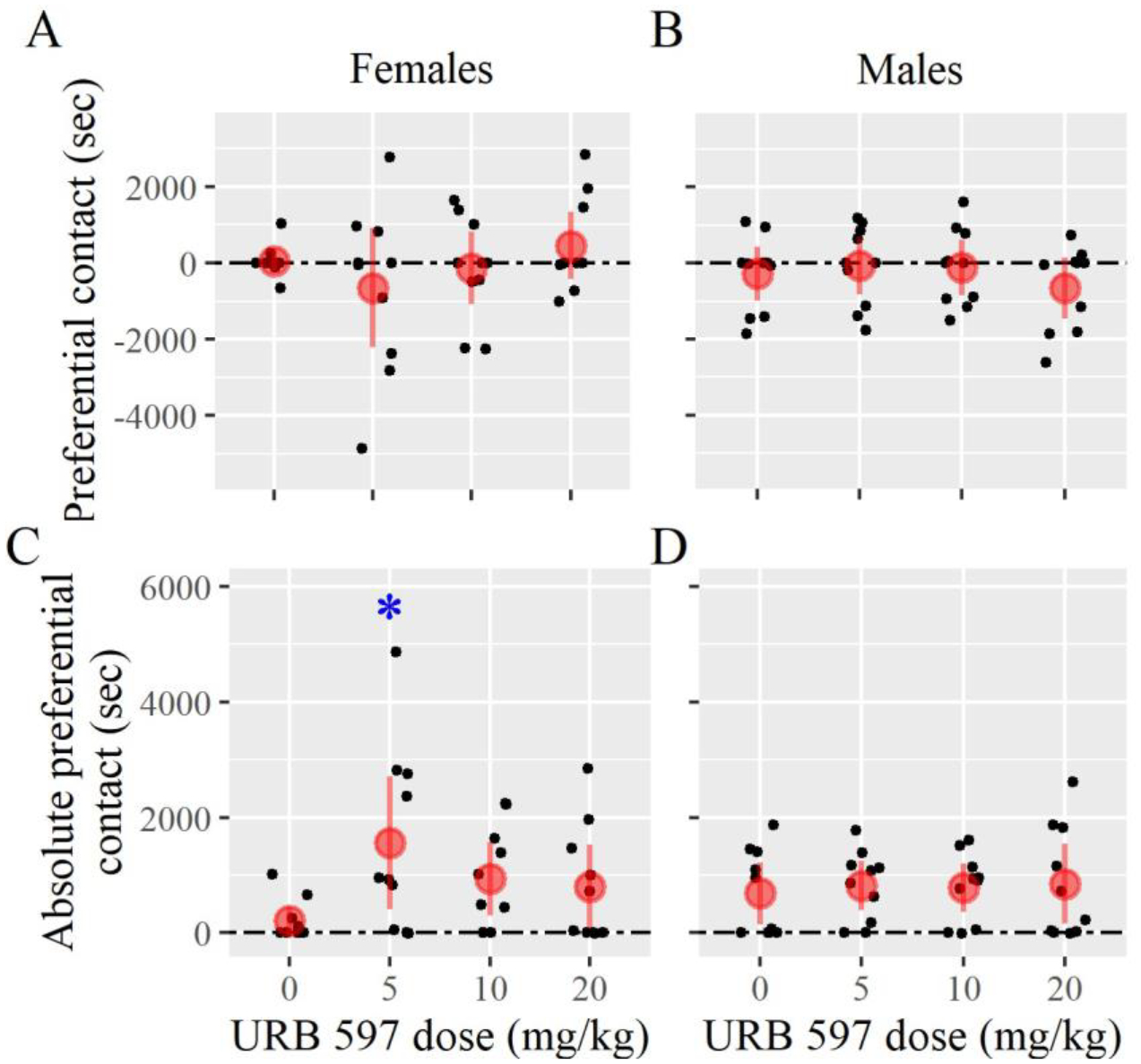
Partner preference test results from Experiment 2 using URB597. Females from each treatment group spent similar time in preferential contact (calculated for each subject by subtracting the time in contact with the stranger from the time spent in contact with the partner) (A) but low-dose females increased absolute preferential contact (the absolute values of preferential social contact) (C). Males from each treatment group spent similar time in preferential contact (B) or in absolute preferential contact (D). Error bars are 95%-confidence intervals. Symbols: * p < 0.05).
As with Experiment 1, we used Dunnett tests to analyze between-group differences in preferential and absolute preferential contact, chamber preference, and chamber entries. No statistically significant differences were found between each treatment group and control for preferential contact, chamber preference, or chamber entries, regardless of sex (Fig. 3). However, analyzing absolute preferential contact revealed a significant increase in females treated with 5.0 mg/kg of URB597 (Fig. 3). These results suggest that URB597 may have no impact on measured male behavior during the partner preference test. URB597 may also not facilitate female preferences for the male partner consistently, but may increase the degree of preference in side-to-side contact with either partner or stranger. In other words, these females are more likely to form a preference for one animal versus another, but this preference is not always for the partner. Please see Table 2 for all Experiment 2 test statistics.
Table 2.
Partner Preference Test Statistics for Experiment 2
| Between-groups Dunnett Test | |||||||
|---|---|---|---|---|---|---|---|
| Sex | Outcome | Dose | M (SD) | T(df) = p | Contrast | Estimate (SE) | T (df) = p |
| Females | Absolute Preferential Contact | 0 | 205 (355) | - | - | - | - |
| 5 | 1557 (1601) | - | 5 – 0 | 1352 (474) | 2.85 (36) = 0.020 | ||
| 10 | 943 (890) | - | 10 – 0 | 738 (474) | 1.56 (36) = 0.292 | ||
| 20 | 804 (1009) | - | 20 – 0 | 599 (474) | 1.26 (36) = 0.454 | ||
| Chamber Preference | 0 | 843 (2684) | - | - | - | - | |
| 5 | −948 (4394) | - | 5 – 0 | −1791 (1664) | −1.08 (36) = 0.577 | ||
| 10 | −875 (3961) | - | 10 – 0 | −1718 (1664) | −1.03 (36) = 0.607 | ||
| 20 | 1297 (3627) | - | 20 – 0 | 454 (1664) | 0.27 (36) = 0.986 | ||
| Preferential Contact | 0 | 53 (412) | 0.40 (12) = 0.750 | - | - | - | |
| 5 | −648 (2188) | −0.94 (12) = 0.748 | 5 – 0 | −700 (641) | −1.09 (36) = 0.567 | ||
| 10 | −138 (1327) | −0.33 (12) = 0.750 | 10 – 0 | −191 (641) | −0.30 (36) = 0.982 | ||
| 20 | 450 (1229) | 1.16 (12) = 0.748 | 20 – 0 | 397 (641) | 0.62 (36) = 0.870 | ||
| Chamber Entries | 0 | 204 (112) | - | - | - | - | |
| 5 | 182 (96) | - | 5 – 0 | −23 (39) | −0.57 (36) = 0.894 | ||
| 10 | 174 (59) | - | 10 – 0 | −30 (39) | −0.76 (36) = 0.790 | ||
| 20 | 148 (77) | - | 20 – 0 | −56 (39) | −1.41 (36) = 0.366 | ||
| Males | Absolute Preferential Contact | 0 | 688 (746) | - | - | - | - |
| 5 | 821 (606) | - | 5 – 0 | 133 (332) | 0.40 (36) = 0.958 | ||
| 10 | 786 (591) | - | 10 – 0 | 98 (332) | 0.30 (36) = 0.982 | ||
| 20 | 846 (965) | - | 20 – 0 | 159 (332) | 0.48 (36) = 0.933 | ||
| Chamber Preference | 0 | −1399 (3008) | - | - | - | - | |
| 5 | 756 (3767) | - | 5 – 0 | 2155 (1778) | 1.21 (36) = 0.487 | ||
| 10 | −808 (4153) | - | 10 – 0 | 591 (1778) | 0.33 (36) = 0.975 | ||
| 20 | −948 (4767) | - | 20 – 0 | 451 (1778) | 0.25 (36) = 0.989 | ||
| Preferential Contact | 0 | −276 (998) | −0.88 (12) = 0.808 | - | - | - | |
| 5 | −74 (1053) | −0.22 (12) = 0.829 | 5 – 0 | 202 (468) | 0.43 (36) = 0.949 | ||
| 10 | −114 (1011) | −0.36 (12) = 0.829 | 10 – 0 | 162 (468) | 0.35 (36) = 0.972 | ||
| 20 | −652 (1121) | −1.84 (12) = 0.397 | 20 – 0 | −375 (468) | −0.80 (36) = 0.763 | ||
| Chamber Entries | 0 | 205 (81) | - | - | - | - | |
| 5 | 250 (150) | - | 5 – 0 | 44 (63) | 0.70 (36) = 0.825 | ||
| 10 | 203 (150) | - | 10 – 0 | −2 (63) | −0.04 (36) = 1.000 | ||
| 20 | 250 (168) | - | 20 – 0 | 45 (63) | 0.70 (36) = 0.823 | ||
Notes. Within-group analyses composed of one-sample t-tests analyzing whether group means were statistically different than 0. P values for within-group analyses were adjusted for the false discovery rate using the procedure suggested by Benjamini & Yekutieli (2001). P values for between-groups analyses were adjusted using the Dunnett test procedure.
We did note that the finding on absolute preferential contact in females treated with 5.0 mg/kg appeared to be driven by one point in particular. A Dixon test indicated that this point was not actually a statistical outlier (Q = 0.43289, p = 0.13). Removal of that animal from the analysis resulted in a p-value of 0.06; however, the effect size was still very large (d = 1.1694). Based on these considerations, we believe the conclusions of a drug effect on absolute preferential contact to be robust.
Experiment 3
To follow-up on our findings from Experiment 2, we recruited a third group of females and tested whether the increase in stimulus animal preference experienced by those treated with URB597 could be attributed to reductions in anxiety. We used Dunnett tests to analyze whether chamber preferences (e.g. light versus dark chamber) or activity levels (e.g. arm or chamber entries) were different between controls and each treatment group. We found no evidence of an effect for URB597 treatment on any outcome measured during the EPM, including the arm preference and total arm entries (Fig. 4). We similarly found no evidence of a treatment effect for behaviors measured during the LDT, including preference for the light chamber versus the dark chamber, and total entries (Fig. 4). The results indicate that URB597 may not alter anxiety-related behavior in female prairie voles under these contexts. Please see Table 3 for all Experiment 3 test statistics.
Figure 4 –
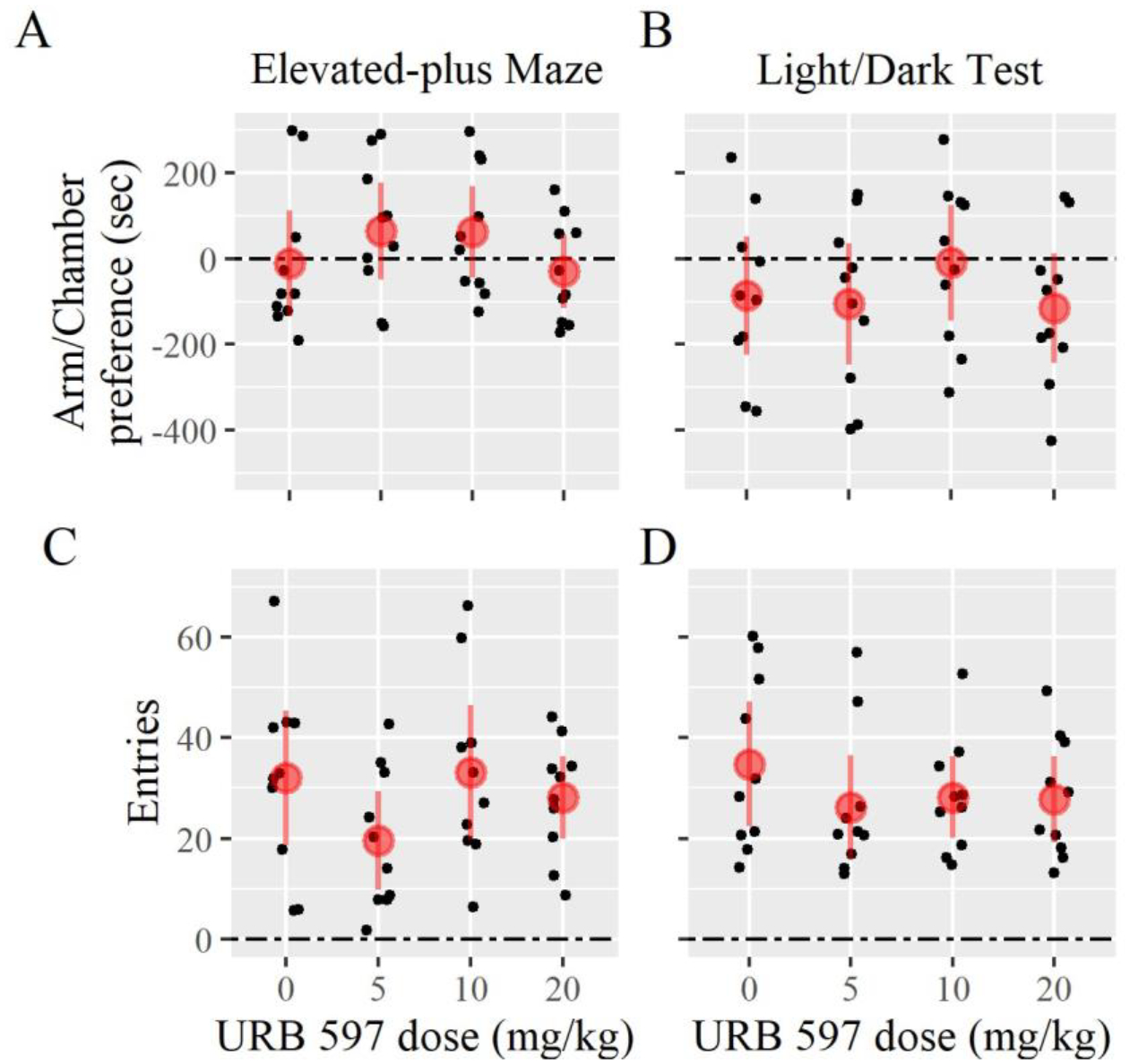
Anxiety-related paradigm results from Experiment 3 using URB597. Females tested using the elevated-plus maze spent similar amounts of time in either the open or closed arms (A) and entering the arms (C). Females tested using the light/dark test spent similar amounts of time in either the light or dark chambers (B) and in chamber entries (D). Error bars are 95%-confidence intervals.
Table 3.
Anxiety Test Statistics for Experiment 3
| Between-groups Dunnett Test | ||||||
|---|---|---|---|---|---|---|
| Test | Outcome | Dose | M (SD) | Contrast | Estimate (SE) | T (df) = p |
| Elevated Plus Maze | Arm Preference | 0 | −12 (172) | |||
| 5 | 64 (157) | 5 – 0 | 76 (67) | 1.13 (48) = 0.544 | ||
| 10 | 62 (150) | 10 – 0 | 74 (67) | 1.09 (48) = 0.567 | ||
| 20 | −29 (119) | 20 – 0 | −17 (67) | −0.26 (48) = 0.988 | ||
| Arm Entries | 0 | 32 (19) | ||||
| 5 | 20 (14) | 5 – 0 | −12 (7) | −1.74 (48) = 0.212 | ||
| 10 | 33 (19) | 10 – 0 | 1 (7) | 0.15 (48) = 0.997 | ||
| 20 | 28 (11) | 20 – 0 | −4 (7) | −0.55 (48) = 0.904 | ||
| Light/Dark Test | Chamber Preference | 0 | −86 (193) | |||
| 5 | −106 (198) | 5 – 0 | −20 (85) | −0.23 (48) = 0.991 | ||
| 10 | −9 (189) | 10 – 0 | 77 (85) | 0.91 (48) = 0.691 | ||
| 20 | −116 (179) | 20 – 0 | −30 (85) | −0.35 (48) = 0.972 | ||
| Chamber Entries | 0 | 35 (17) | ||||
| 5 | 26 (14) | 5 – 0 | −9 (6) | −1.40 (48) = 0.376 | ||
| 10 | 28 (11) | 10 – 0 | −7 (6) | −1.06 (48) = 0.589 | ||
| 20 | 28 (12) | 20 – 0 | −7 (6) | −1.12 (48) = 0.546 | ||
Note. P values were adjusted using the Dunnett test procedure.
Discussion
The present study provides evidence that supports a role for AEA in modulating female social behavior. We show that lower doses of FAAH inhibitor administered systemically in females can facilitate the formation of a preference for a specific male, although not necessarily with the familiar partner. We pursued this finding by confirming that URB597 was not acting through changes in anxiety-like behavior. Finally, our male subjects demonstrated no effect of treatment in any of our outcome measures across all our behavioral paradigms and experiments.
In Experiment 1, we designed our cohabitation periods to reflect the well-established sex difference in time it takes for males and females to form a partner preference. Previous research from our lab (Bales et al., 2007, 2013; Hostetler et al., 2011) and others (DeVries and Carter, 1999) supports the use of 24 h cohabitations for males and 6 h for females to form a preference for a familiar partner. Our vehicle-treated males in this experiment did successfully prefer their partner but our control females did not. We suspect two potential explanations for these findings. First, the stress of receiving the intraperitoneal injection may have interfered with the initial interactions between the subject and mate during the cohabitation. We treated our female subjects in Experiment 1 immediately before the cohabitation. Previous research suggests that stress affects vole social preferences in sexually dimorphic ways (DeVries et al. 1996). The formation of partner preferences in males is facilitated by swim stress or injections of corticosterone while adrenalectomies inhibit them; this may partially explain why our control males were not inhibited by the injection procedure. Conversely, females do not form partner preferences when exposed to swim stress or corticosterone treatment beforehand; adrenalectomy also facilitates the formation of partner preferences in females. It is worth mentioning that intracranial injections of oxytocin do not seem to impair partner preferences in females despite the differences in handling intensity between the two administration techniques (Liu and Wang 2003). While the process of intracranial administration is arguably more stressful to the rodent, oxytocin has been shown to play a role in fear extinction (Triana-Del Río et al. 2019) and social reward (Dölen et al. 2013), which may mitigate the stress of the administration procedure.
Second, there may be some component of the vehicle control that is impairing social behavior. Saline is often used as a solvent for drugs in prairie vole studies, but the specific use of DMSO and Tween-80 for intraperitoneal administration is uncharted territory in this species. A study using male CD2F1 mice found that intraperitoneal administration of Tween-80 decreased locomotor activity at concentrations above 16%, while DMSO had a similar effect above 32% (Castro et al. 1995); the concentrations in our experiments were 10% for both Tween-80 and DMSO. This may also explain why only control males and not females successfully formed a partner preference, as the longer cohabitations for males could have buffered any potential effects of the vehicle.
Regardless of the reason that control females did not form a preference in Expt 1, we still believe that the data are interpretable, given that these same conditions were experienced by all groups.
However, further testing, with a longer cohabitation time, would be necessary before concluding that CB1 receptor inhibition does not impair partner preference in females.
Experiment 1 also revealed that the only male group which was unsuccessful in forming a partner preference included animals treated with the highest dose of CB1 inhibitor. However, it is difficult to say that AM4113 uniquely impaired partner preference in that group, since the magnitude of preferential contact for the partner across groups was not significantly different from control. Repeating this experiment with a larger sample size and alternate doses should help clarify these results.
Experimental manipulations that increase absolute preferential social contact (a preference for either a familiar or novel mate, as found here) are uncommon in the prairie vole literature, especially with suboptimal cohabitation times. Prairie voles are most likely to form a preference to the animal with whom they were cohoused. However, it is important to note that in females treated with URB597, while many formed a social preference (i.e. they chose a male, they did not spend equal time in contact with both), they did not consistently prefer the partner over the stranger. There are a number of behavioral mechanisms that could be responsible for this effect. We investigated and eliminated one possibility, changes in anxiety, which fits with the data from mice suggesting that AEA’s effects on social behavior are not secondary to anxiety (Wei et al., 2015). A second possibility might be an impairment in social memory or social reward – in other words, maybe the female did not appropriately form memories or reward-related associations with her familiar partner. These processes are oxytocin-dependent in female voles (Lieberwirth and Wang, 2016), and interference with oxytocin’s ability to mobilize AEA could have resulted in alterations in memory or reward. This hypothesis is consistent with results from social discrimination tests in rats (Scheyer et al., 2020). Further research would be necessary to test these possibilities.
It is also possible that this variability in social choice may be explained by the nature of the drug itself. As mentioned previously, AEA is produced “on demand” and URB597 increases AEA levels by inhibiting its degradative enzyme, FAAH, rather than directly activating cannabinoid receptors. Thus, the impact of the drug is contingent on the natural release of AEA that likely varies from subject to subject depending on the nature of the social interactions on an individual level with either the partner or stranger during the early stages of the partner preference paradigm. If this were the case, more direct approaches like microinjections of CB1 agonist into targeted regions of the brain would likely produce effects that contrast those in this study. Given our findings and the interaction between the oxytocin and endocannabinoid systems, targeted central manipulations are warranted. Finally, we do not know whether the preference demonstrated while still under the effects of URB597 would be long-lasting or exist only during acute treatment, suggesting that additional retests of animals would be valuable.
While we found interesting effects of endocannabinoid effects on female pair bonding behavior in prairie voles, these effects were smaller than we expected; we also found no effects on male pair bonding behavior. Removal of one data point (which while a visual outlier, was not a statistical outlier) from the analysis of female absolute preferential contact, resulted in loss of the significant effect. It is worth noting that with the sample size used in this study, removal of any data point is a substantial loss that would affect power.
However, consideration of the negative findings in this paper may be important to our understanding of the relationship between CB1 receptors and pair bonding. Previous studies have shown that oxytocin receptor agonism mobilizes AEA and therefore CB1 activity in the nucleus accumbens (Wei et al., 2015). One possibility is that any reduction in the effects of oxytocin subsequent to CB1 manipulation may be compensated for by arginine vasopressin, another neuropeptide critical to pair bonding, and which is particularly important for pair bonding in males (Walum and Young, 2018). Following that logic, any increase in oxytocin effects with CB1 manipulation may encounter a ceiling effect. Alternatively, the effects of oxytocin on pair bonding specifically, as opposed to more general types of social behavior tested in previous studies, may not be dependent on CB1 activation. Species differences in oxytocin receptor distribution may be relevant, as prairie voles and other pair bonding species differ from non-pair-bonding species such as mice (Freeman and Young, 2016). Another possibility is that we might see stronger effects with chronic rather than acute exposures. Finally, our results in females should be replicated to confirm whether or not the effect is consistent.
Human research suggests that marijuana, an activator of CB1 receptors, has effects on social salience that can vary in direction depending on many factors including context and dose (de Wit and Sayette, 2018; Wei et al. 2015, 2017). The psychological and neurobiological mechanisms subserving these effects are not well understood. A reduction in social anxiety is sometimes credited, and there is evidence that cannabinoids reduce the amygdala’s responsiveness to social threat (Gorka et al., 2015). Another possible cause might be emotional disinhibition (Salzman et al., 1978). Our study confirms that endocannabinoid manipulations may impact social behaviors in prairie voles as well. The vole model provides a utility to questions of social attachments and pair-bonding behavior that few other models replicate. Our study provides the foundational insight that informs future vehicle considerations and approaches when addressing these questions.
Acknowledgments and conflicts of interest:
The authors acknowledge funding from the University of California, Davis and NIH grant MH108319, and report no conflicts of interest. The authors would like to thank Drs. Cindy Clayton and Rhonda Oates-O’Brien, Jessica Bond, and the husbandry staff for research support.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References Cited
- Bales KL, Carter CS, (2003) Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav Neurosci 117: 854–859. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP (2013) Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry 74: 180–188. doi: 10.1016/j.biopsych.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS (2007) Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav 52: 274–279. doi: 10.1016/j.yhbeh.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Dong B, Shilpa BM, Vemuri K, Makriyannis A, Pandey SC, Sershen H, Suckow RF, Vinod KY (2018) Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology 131: 200–208. 10.1016/j.neuropharm.2017.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsevich G, Petrie GN, Hill MN (2017) Endocannabinoids: Effectors of glucocorticoid signaling. Front Neuroendocrin. 10.1016/j.yfrne.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Battista N, Di Tommaso M, Bari M, Maccarrone M (2012) The endocannabinoid system: an overview. Front Behav Neurosci 6:9 10.3389/fnbeh.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D.(2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29, 1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y (2012) Endocannabinoid signaling and synaptic function. Neuron 76, 70–81. 10.1016/j.neuron.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR (1995) Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacol Biochem Behav 50: 521–526. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Carter CS (1999) Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Can J Zool 77: 885–889. 10.1139/z99-054 [DOI] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS (1996) The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci 93: 11980–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Sayette M (2018) Considering the context: social factors in responses to drugs in humans. Psychopharmacology 235: 935–945. doi: 10.1007/s00213-018-4854-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501: 179–184. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S (2011) An {R} Companion to Applied Regression. Sage, Thousand Oaks, CA. [Google Scholar]
- Freeman SM, Young LJ (2016) Comparative perspectives on oxytocin and vasopressin research in rodents and primates: Translational implications. J Neuroendocrinol 28: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, de Wit H, Phan KL (2015) Cannabinoid modulation of amygdala subregion functional connectivity to social signals of threat. Intl J Neuropsychopharm 18: pyu104. doi: 10.1093/ijnp/pyu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121: 537–544. [DOI] [PubMed] [Google Scholar]
- Lodola A, Castelli R, Mor M, Rivara S (2015) Fatty acid amide hydrolase inhibitors: a patent review (2009 – 2014). Exp Opin Ther Pat 25: 1247–1266. 10.1517/13543776.2015.1067683 [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Harkey SL, Krzywosinski TB, Aragona BJ, Bales KL (2011) Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole. Behav Pharmacol 22:703–710. DOI: 10.1097/FBP.0b013e32834affd2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn LA (2016) The two-step approach—a significant ANOVA F-test before Dunnett’s comparisons against a control—is not recommended, Comm Stat - Theory Meth, 45:11, 3332–3343, doi: 10.1080/03610926.2014.902225 [DOI] [Google Scholar]
- Lieberwirth C, Wang Z (2016) The neurobiology of pair bond formation, bond disruption, and social buffering. Curr Opin Neurobiol 40: 8–13. doi: 10.1016/j.conb.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Young LJ (2010) The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci 33: 103–109. 10.1016/j.tins.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Rodríguez E, Machado S, Rocha NB, Budde H, Yuan T-F, Arias-Carrión O (2016) Revealing the role of the endocannabinoid system modulators, SR141716A, URB597 and VDM-11, in sleep homeostasis. Neuroscience 339: 433–449. 10.1016/j.neuroscience.2016.10.011 [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D (2006) Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev 12: 21–38. 10.1111/j.1527-3458.2006.00021.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, Aragona BJ (2013) mu-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci 33: 9140–9. 10.1523/JNEUROSCI.4123-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman C, van der Kolk B, Shader RI (1976) Marijuana and hostility in a small-group setting. Am J Psychiatry 133: 1029–1033. doi: 10.1176/ajp.133.9.1029 [DOI] [PubMed] [Google Scholar]
- Schechter M, Pinhasov A, Weller A, Fride E (2012) Blocking the postpartum mouse dam’s CB1 receptors impairs maternal behavior as well as offspring development and their adult social-emotional behavior. Behav Brain Res 226: 481–92. 10.1016/j.bbr.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Scheyer AF, Borsoi M, Pelissier-Alicot A-L, Manzoni OJJ (2020) Perinatal THC exposure via lactation induces lasting alterations to social behavior and prefrontal cortex function in rats at adulthood. Neuropsychopharmacology 0: 1–8. doi: 10.1038/s41386-020-0716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Brents LK, Franks LN, Rajasekaran M, Zimmerman SM, Fantegrossi WE, Prather PL (2012) AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: Implications for opioid/cannabinoid interaction studies. Neuropharmacology 63: 905–915. 10.1016/j.neuropharm.2012.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons TC, Balland JF, Dhauna J, Yang SY, Traina JL, Vazquez J, Bales KL (2017) Early intranasal vasopressin administration impairs partner preference in adult male prairie voles (Microtus ochrogaster). Front Endocrinol 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD (2008) Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology (Berl) 196: 565–574. 10.1007/s00213-007-0988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ (2010) The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci 31: 463–9. 10.1016/j.tips.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, Petrosino S, Van Kerkhof LW, Pasterkamp RJ, Zhou Y, Campolongo P, Cuomo V, Di Marzo V, Vanderschuren LJ (2012) Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J Neurosci 32: 14899–908. 10.1523/JNEUROSCI.0114-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ (2008) Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl) 197: 217–227. 10.1007/s00213-007-1025-3 [DOI] [PubMed] [Google Scholar]
- Triana-Del Río R, van den Burg E, Stoop R, Hegoburu C (2019) Acute and long-lasting effects of oxytocin in cortico-limbic circuits: consequences for fear recall and extinction. Psychopharmacology (Berl) 236: 339–354. 10.1007/s00213-018-5030-5 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Panagis G (2014). Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des 20: 2072–88. [DOI] [PubMed] [Google Scholar]
- Walum H, Young LJ (2018). The neural mechanisms and circuitry of the pair bond. Nat Neurosci 19: 643–654. doi: 10.1038/s41583-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Lee D, Cox CD, Karsten CA, Penagarikano O, Geschwind DH, Gall CM, Piomelli D (2015) Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci 112: 14084–9. 10.1073/pnas.1509795112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Allsop S, Tye K, Piomelli D (2017) Endocannabinoid signaling in the control of social behavior. Trends Neurosci 40: 385–396. 10.1016/j.tins.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


