Abstract
Impella® devices are increasingly utilized for hemodynamic support in high risk percutaneous coronary intervention or cardiogenic shock despite a lack of randomized clinical trial data showing clinical benefit and newer observational data suggesting harm. In this retrospective analysis, our aim was to determine the most common adverse events associated with Impella® usage reported annually to the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database and to estimate via the National Inpatient Sample (NIS) database the number of percutaneous ventricular assist devices (pVAD) utilized and associated with inpatient mortality since introduction of the Impella®. Among the 885 complete reports submitted to the MAUDE database between 2008 and 2019 related to Impella® usage, there were 1206 complications coded; 88.2% of reports occurred between 2016 and 2019. Among patients with adverse events reported, bleeding (32.8%), device deployment or retrieval issues (18.2%), vascular complications (15.8%), and death (12.4%) were the most common, and 7.9% of all complications were attributable to operator decision-making or technique. Between 2007 and 2017 there was a >100-fold increase in pVAD use with an increase and plateau in in-hospital mortality to 31% between 2012—2016 based on NIS data. In conclusion, Impella® use has increased substantially over the last decade but remains associated with high inpatient mortality and serious complications based on data from the NIS and MAUDE databases. These findings emphasize the need for high quality randomized controlled trials to determine the clinical utility of Impella® in high risk percutaneous coronary intervention and cardiogenic shock.
Keywords: Impella®, mechanical circulatory support, high risk percutaneous coronary intervention, cardiogenic shock
Introduction
Mechanical circulatory support (MCS) is increasingly utilized to mitigate the adverse events associated with high risk percutaneous coronary intervention (PCI) and cardiogenic shock (CS).1 Intra-aortic balloon pumps (IABP) and Impella® devices are used most commonly despite a lack of evidence demonstrating improved outcomes.2–5 Recently, 2 large observational studies have further contested the benefit of improved hemodynamics achieved with Impella® support.6,7 These studies showed that among patients undergoing PCI with either Impella® or IABP support, Impella® use was associated with more adverse outcomes including death and major bleeding after adjusting for patient severity. As such, there remains considerable uncertainty regarding the benefits of MCS and incomplete understanding of the associated complications in contemporary clinical practice. Herein, we aimed to better define the complications related to Impella® usage in an analysis of the United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) post-marketing surveillance database which compiles voluntary reports of suspected device-associated deaths, serious injuries, and malfunctions. In addition, we utilized the nationally representative National Inpatient Sample (NIS) database to assess the temporal trends of percutaneous ventricular assist device (pVAD) utilization and in-hospital mortality.
Methods
The MAUDE database was queried for the time period January 1, 2008 through December 31, 2019 for reports pertaining to the brand name “Impella®” inclusive of the Impella® 2.5, CP, 5.0, 5.5, and RP. Complications were coded by independent physician review with multiple complications allowed per report. Reports that were incomplete, duplicate, or did not provide adverse event data were excluded. Complications were defined as follows:
Bleeding: Any blood loss resulting in transfusion occurring at the time of device placement, during patient support, or at the time of device retrieval.
Deployment or retrieval issue: Any event during device placement or retrieval resulting in a subsequent complication or placement of a new or alternative hemodynamic support device.
Vascular complication: Any device-related injury requiring vascular surgery intervention
Death: Any patient that expired after attempted or successful placement of a device
Hemolysis: Based on event description or abnormal lab value(s) consistent with hemolysis.
Limb ischemia: Any evidence of loss of ipsilateral limb perfusion.
Power failure: Any unintended loss of device function
Structural: Any cardiac chamber perforation resulting in pericardiocentesis or any valvular damage resulting in surgical repair or replacement
Defect/other: Any device or device component with compromised structural or functional integrity “out of the box” or any complication not listed.
Thrombosis: based on event description of thrombus in or on device resulting in malfunction.
Stroke: Any new neurologic deficit in a patient with the device concurrently implanted
Unknown: No complication identified within a report
A report was coded as having a complication attributable to operator-related technique or decision-making if the device or its components were used without adherence to manufacturer recommendations (e.g., advancing device without a wire, using a non-compatible sheath, etc.). This definition does not encompass off-label use.
The NIS is an inpatient claims database provided by the Agency for Healthcare Quality and Research representative of the total U.S. population. We used the NIS to estimate annual pVAD volume based on ICD-9 codes (prior to Q4 2015) and ICD-10 codes thereafter (eTable 1). Although ICD codes for pVAD use include both Impella® and TandemHeart® devices, as of July 2020 only 1000 total TandemHeart® devices have been utilized worldwide making Impella® the most representative device by far.8 All volumes were converted to national averages based on the NIS survey weighting methodology.
Results
Between the years 2008 and 2019, 931 reports were submitted to the MAUDE database detailing complications related to Impella® use. A total of 46 reports were excluded that were either incomplete, duplicate, or did not include adverse event data. The remaining 885 reports were coded and included a total of 1206 complications (Figure 1A) including 2 complications in 35.4% of reports, and 3 complications in 6.1% of reports. Shock was the most common indication for Impella® use (42.2%) followed by PCI (23.2%), and post-cardiothoracic surgery (8.8%). An indication was not specified in 25.9% of reports. The relative frequency of coded complications is shown in Figure 1B. Bleeding was the most commonly coded complication (32.8%), followed by deployment or retrieval issue (18.2%) and vascular complications (15.8%). Patient death was included in 12.4% of the 885 coded reports. The majority (88.2%) of reports were submitted between 2016 and 2019. During this time reports of bleeding decreased from their highest in 2018 (44.0%) to their lowest in 2019 (27.9%) but the proportion of vascular complications remained high at approximately 19% and limb ischemia increased from a low of 2.4% in 2016 to a high of 14.9% in 2019. Reporting of patient death declined each year during this period however from a high of 25.9% in 2016 to a low of 6.0% in 2019. Although 7.9% of complications were operator related, this proportion decreased substantially over time from 33.3% of reports in 2008/9 to 3.8% of reports in 2019 (eFigure 1).
Figure 1:
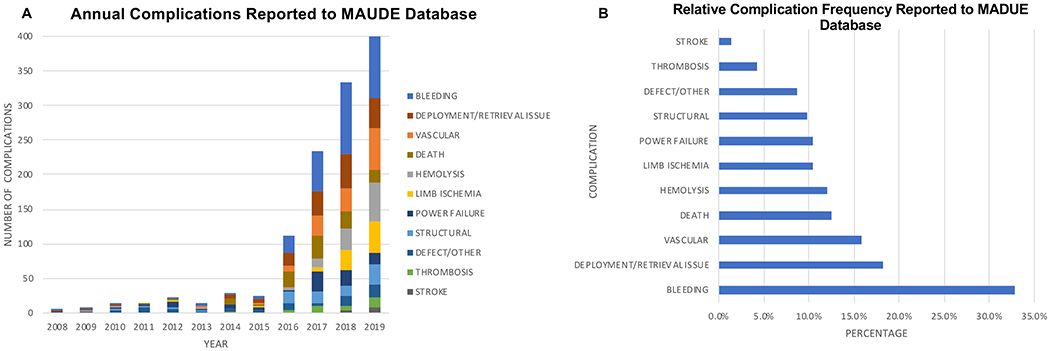
Complications Related to Impella® Usage Reported to the MAUDE Database.
A) Annual number of each complication with respective color is shown. B) Overall relative frequency of complications from all years is shown.
Based on NIS data estimates, pVAD use increased from 6,838 cases between 2007 and 2011 to 55,565 cases between 2012 and 2017 with 19,155 cases in 2017 alone (Figure 2). Since the introduction of the Impella® in 2008, unadjusted inpatient mortality rates among patients receiving the device increased from a low of 23.1% in 2009 to a plateau of ~31% between the years 2012 and 2016. In 2017, the last year for which data were available, in-hospital mortality decreased to 28.6%.
Figure 2:
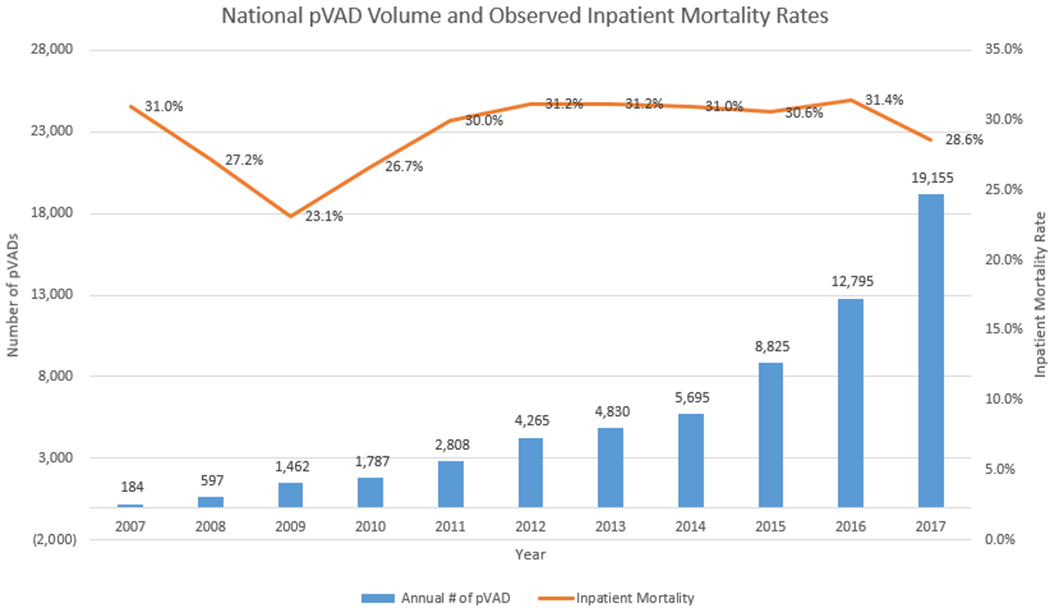
National pVAD Volume and Observed Inpatient Mortality Rates.
Blue bars represent annual number of pVADs and orange line represents annual inpatient mortality. pVAD: percutaneous ventricular assist device
Discussion
Impella® devices are increasingly utilized for hemodynamic support in high risk PCI and CS but with limited randomized clinical trial data showing improved clinical outcomes and more recent observational data suggesting a net clinical harm compared with IABP.6,7 In our analysis of the MAUDE database, we describe the frequency of complications among patients with a voluntarily reported adverse event related to Impella® use. The most commonly reported complications included bleeding, issues with device deployment or retrieval, vascular complications, and in-hospital death. Between initial device approval in 2008 and 2017, reports of death and bleeding declined but vascular complications and limb ischemia reports increased, which may be due to increased utilization of larger bore Impella® devices or use in more challenging patients. Complications that were attributable to operator decision-making or technical skill decreased over the course of MAUDE reporting but still accounted for 3.8% of all reported complications in 2019 highlighting the need for appropriate operator training and experience in the placement and later management of these large bore devices.
Although reports of Impella®-related death to the MAUDE database decreased over time, a different picture emerges when examining in-hospital outcomes using the NIS database. Following introduction of the Impella® in 2008, inpatient mortality rates increased in those who received a pVAD, reaching a plateau between 2012 and 2016. The decrease in mortality in the year 2017 may represent increased device utilization in less critically-ill patients as described by Amin et al6. Overall, the NIS estimates a lower observed inpatient mortality rate when compared to the 45.0% mortality risk estimated by Dhruva et al.7
The MAUDE database is a passive reporting system with potential inclusion of incomplete, inaccurate or biased device reporting. Importantly, the number of complications coded from the database likely underestimate the true number events due to underreporting to the FDA MAUDE system.9 Moreover, proportions of adverse events related to Impella® use reported to the MAUDE database do not represent true frequencies of events due to an unknown denominator. The NIS has a number of limitations including potential for miscoding, minimal clinical data, and the mortality data not being risk adjusted. Rates of adverse outcomes associated with pVAD use reflect a range of devices most notably TandemHeart® in addition to Impella®, although Impella® is by far the most common of these devices in current clinical practice.
In conclusion, based on analysis of the MAUDE and NIS databases, Impella® use has increased substantially since its introduction but remains associated with serious complications and high inpatient mortality. These findings emphasize the need for high quality randomized controlled trials to determine the clinical utility of Impella® in high risk PCI and CS.10,11
Supplementary Material
eTable 1: ICD-9 and 10 codes used to estimate annual percutaneous ventricular assist device volume from National Inpatient Sample database.
ICD-9 codes were used prior to the fourth quarter of 2015 and corresponding ICD-10 codes were used after the transition date.
eFigure 1: Annual Operator Related Complications Reported to the MAUDE Database. Blue line represents percentage of MAUDE reports with complications attributable to operator decisionmaker or technique.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S. Trends in the use of percutaneous ventricular assist devices: Analysis of National Inpatient Sample data, 2007 through 2012. JAMA Intern Med 2015;175:941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill WW, Kleiman NS, Moses J, Henriques JPS, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Džavík V, Popma J, Douglas PS, Ohman M. A prospective, randomized clinical trial of hemodynamic support with impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: The PROTECT II study. Circulation 2012;126:1717–1727. [DOI] [PubMed] [Google Scholar]
- 4.Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke JT, Møller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Möbius-Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schafer A, Westermann D. Impella support for acute myocardial infarction complicated by cardiogenic shock: Matched-pair iabp-shock II trial 30-day mortality analysis. Circulation 2019;139:1249–1258. [DOI] [PubMed] [Google Scholar]
- 5.Ouweneel DM, Eriksen E, Seyfarth M, Henriques JPS. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump for Treating Cardiogenic Shock: Meta-Analysis. J Am Coll Cardiol 2017;69:358–360. Available at: 10.1016/j.jacc.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, McNeely C, Al-Badarin F, House JA, Kulkarni H, Rao S V. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support.; 2020. [DOI] [PubMed]
- 7.Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, Berkowitz A, Masoudi FA, Messenger JC, Parzynski CS, Ngufor C, Girotra S, Amin AP, Shah ND, Desai NR. Association of Use of an Intravascular Microaxial Feft Ventricular Assist Device vs Intra-aortic Balloon Pump with In-Hospital Mortality and Major Bleeding among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA - J Am Med Assoc 2020;323:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush M. CardiacAssist, Inc . Announces 1000th TandemHeart System Procedure. Advent Life Sci 2020. [Google Scholar]
- 9.Anon. MAUDE - Manufacturer and User Facility Device Experience. 2016. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM. Accessed January 2, 2020.
- 10.Anon. Danish Cardiogenic Shock Trial: DanGer Shock. Available at: https://clinicaltrials.gov/ct2/show/NCT03947619. Accessed March 3, 2020.
- 11.Anon. Primary Unloading and Delayed Reperfusion in ST-Elevation Myocardial Infarction: The STEMI-DTU Trial. Available at: https://clinicaltrials.gov/ct2/show/NCT03947619. Accessed March 3, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1: ICD-9 and 10 codes used to estimate annual percutaneous ventricular assist device volume from National Inpatient Sample database.
ICD-9 codes were used prior to the fourth quarter of 2015 and corresponding ICD-10 codes were used after the transition date.
eFigure 1: Annual Operator Related Complications Reported to the MAUDE Database. Blue line represents percentage of MAUDE reports with complications attributable to operator decisionmaker or technique.


