Abstract
Mycobacterium tuberculosis (Mtb) infection induces pulmonary expression of the heme-degrading enzyme heme oxygenase-1 (HO-1). We have previously shown that pharmacological inhibition of HO-1 activity in experimental tuberculosis results in decreased bacterial loads and unexpectedly that this outcome depends on the presence of T lymphocytes. Here we extend these findings by demonstrating that IFNγ production by T lymphocytes and NOS2 expression underlie this T cell requirement and that HO-1 inhibition potentiates IFNγ-induced NOS2-dependent control of Mtb by macrophages in vitro. Among the products of heme degradation by HO-1 (biliverdin, carbon monoxide and iron), only iron supplementation reverted the HO-1 inhibition-induced enhancement of bacterial control and this reversal was associated with decreased NOS2 expression and NO production. Additionally, we found that HO-1 inhibition results in decreased labile iron levels in Mtb-infected macrophages in vitro and diminished iron accumulation in Mtb-infected lungs in vivo. Together these results suggest that the T lymphocyte dependence of the therapeutic outcome of HO-1 inhibition on Mtb infection reflects the role of the enzyme in generating iron that suppresses T cell-mediated IFNγ/NOS2-dependent bacterial control. In broader terms, our findings highlight the importance of the crosstalk between iron metabolism and adaptive immunity in determining the outcome of infection.
Introduction
Tuberculosis (TB), resulting from infection with the bacterium Mycobacterium tuberculosis (Mtb) is now the leading cause of mortality due to a single infectious agent 1. Although antibiotic therapy for Mtb has been available for almost 80 years 2, its effects on the global burden of TB have not kept pace with the results of interventions in the other major infectious diseases of mankind. The current treatment regimen for TB consists of 4 different antibiotics which typically are administered for 6 to 9 months and can be associated with adverse side effects. This scenario promotes noncompliance leading to both relapse and the development of drug resistance 3. Indeed, multi-drug resistance in TB infection is on the rise with over 390,000 cases reported in 2018 1. Thus, there is a need for more effective therapeutic approaches for TB treatment and in particular, those that can promote more rapid cure and act against drug resistant organisms.
In addition to the development of more efficient antibiotics, a second approach to achieving more rapid and effective cure is to target the Mtb-host interaction with host-directed therapies (HDT) which could be used as an adjunct to conventional drug treatment. By their nature, such therapeutic approaches do not target the pathogen directly, thereby circumventing the development of drug resistance. A number of different strategies involving different host targets affecting Mtb susceptibility have been proposed and several are currently being tested in clinical trials 4.
In a previous study, we described a novel HDT candidate based on the inhibition of heme oxygenase-1 (HO-1), a host enzyme critical for the recycling of iron 5, a metal important both as essential nutrient for Mtb growth as well as for many host-defense functions 6, 7. We showed that the treatment of Mtb-infected mice with a well characterized inhibitor of HO-1 enzymatic activity, tin protoporphyrin IX (SnPP), results in a reduction in pulmonary bacterial loads and when administered adjunctively with antibiotics, accelerates pathogen clearance. An unusual property of this experimental HDT, is that its efficacy depends on the adaptive immune system. Thus, SnPP fails to reduce bacterial loads when administered earlier than 3 weeks post infection, before the emergence of antigen-responsive T cells, or when given to mice lacking a T cell compartment 8.
The development of a Th1 immune response is critical for the control of Mtb infection and this effect is generally attributed to the production of IFNγ and TNF, and in murine infection, the induction of nitric oxide by this subset of T lymphocytes 9. While IFNγ itself has been tested clinically with mixed results as a treatment for multi-drug resistant Mtb 10, few if any of the other published HDT approaches directly target the Th1 response or its products. Nevertheless, in several cases candidate HDTs have been reported to enhance Th1 responses as an indirect outcome 4.
In the present study, we have examined the mechanisms underlying the unusual T cell dependence of the therapeutic effect of SnPP mediated HO-1 inhibition on experimental Mtb infection. We present evidence that SnPP treatment results in increased NOS2 expression and NO production, thereby enhancing IFNγ-mediated control of bacterial replication and implicate the inhibition of iron generation as the mechanism underlying this outcome. These findings suggest that in addition to affecting the availability of the metal as a nutrient for Mtb growth 11, the regulation of iron metabolism can influence microbicidal mechanisms induced by IFNγ activation in macrophages infected with the pathogen. In addition, they further support the process of iron homeostasis in Mtb-infected cells as an important target for the development of HDTs against tuberculosis.
Results
Characterization of HO-1 expressing cells in Mtb-infected mouse lungs
We have previously demonstrated that the pharmacological inhibition of host HO-1 activity in Mtb-infected mice with tin protoporphyrin IX (SnPP) results in a highly significant and reproducible reduction in pulmonary bacterial loads and that this outcome is dependent on the presence of an intact T cell compartment 8. As a first step in studying the role of T cell-immunity in the beneficial effects of HO-1 inhibition during experimental TB, we characterized the cellular source of HO-1 expression in the lungs of infected mice. Previous studies involving immunohistochemical staining had localized HO-1 to CD68+ myeloid cells in pulmonary human granulomas 12. We performed flow cytometry and western blotting to further characterize the myeloid cell populations involved in our mouse model.
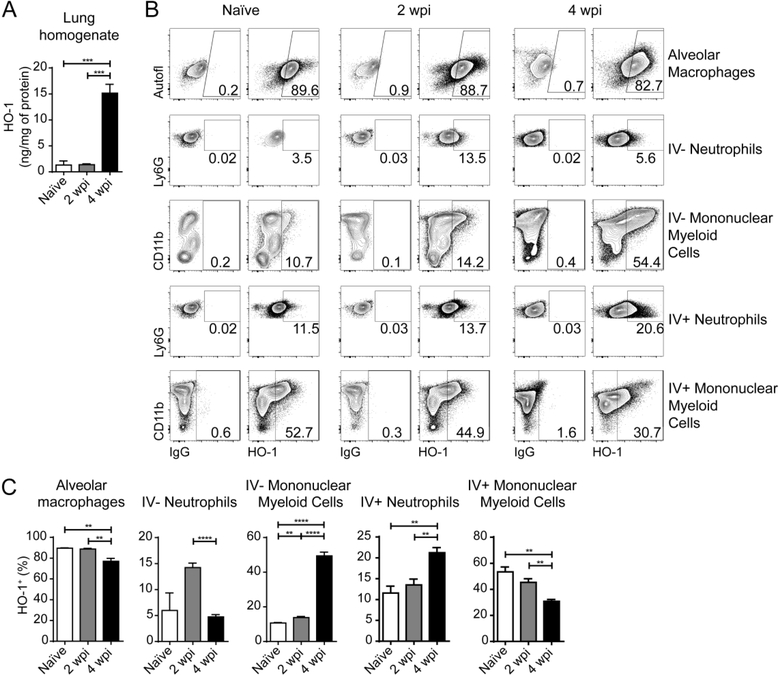
In agreement with our previous findings employing real-time PCR and western blotting measurement of the enzyme 8, we observed a sharp increase in the expression of HO-1 in lung homogenates by ELISA beginning at 4 weeks post-infection (wpi) (Fig. 1A). In order to differentiate HO-1-producing cells that are present in the circulation from those in the lung parenchyma and alveoli, we performed intravenous pan-leukocyte staining prior to euthanasia as previously described 13. We distinguished the different subsets based on their expression of specific surface markers as detailed in Figs. S1A, B and C.
Figure 1: HO-1 is upregulated mainly in parenchymal and alveolar mononuclear myeloid cells in the lungs of M. tuberculosis-infected mice.
(A) HO-1 concentration measured by ELISA in lung homogenates obtained from naïve, or M. tuberculosis-infected mice at 2 and 4 weeks post-infection (wpi) (n = 4 mice/group); (B) Representative dot plots showing HO-1 staining in the right hand columns and correspondent negative control on the left hand columns in alveolar macrophages, parenchymal / alveolar (IV−) neutrophils and mononuclear myeloid cells as well as intravascular (IV+) neutrophils, and mononuclear myeloid cells, from lungs of naïve or M. tuberculosis-infected mice at 2 and 4 wpi, gated as detailed in Fig. S1 (flow cytometry data concatenated from 4 samples) (C) Frequencies of HO-1+ alveolar macrophages, parenchymal / alveolar (IV−) neutrophils and mononuclear myeloid cells as well as intravascular (IV+) neutrophils, and mononuclear myeloid cells, measured by flow cytometry in lungs of naïve or M. tuberculosis-infected mice at 2 and 4 wpi, gated as detailed in Fig. S1. Data shown are representative of 3 independent experiments. Statistical analysis: unpaired Student’s t test. ** = p<0.01, *** = p<0.001, **** = p<0.0001, n.s = non-significant.
We found that alveolar macrophages express high levels of HO-1 even when obtained from naïve mice and that Mtb infection results in a small but significant decrease in the frequency of HO-1+ alveolar macrophages at 4 wpi (Fig. 1B and C). The frequency of HO-1+ IV− neutrophils underwent an increase at 2 wpi, this elevation returned to naïve levels by 4 wpi, while a small but significant increase of HO-1+ neutrophils occurred in the IV+ compartment at 4 wpi (Fig. 1B and C). In the remaining mononuclear myeloid cells, there was a reduction in the frequency of HO-1+ cells in the IV+ compartment, while the IV− mononuclear fraction displayed an enhanced frequency of HO-1+ cells following Mtb infection with a major increase occurring at 4 wpi (Fig. 1B and C).
Using an mCherry-expressing Mtb strain, we identified that at 4 weeks post-infection, a time point in which we found elevated HO-1 expression in lung homogenates (Fig. 1A), Mtb-infected cells are located predominantly in the IV− compartment, suggesting that they are either in the parenchyma or alveoli (Fig. 2A). We therefore, further analyzed the IV− myeloid cells and confirmed that alveolar macrophages are the major source of the low levels of HO-1 in the lungs of naïve and 2 week-infected mice, while mononuclear myeloid cells predominate at 4 wpi (Fig. 2B) indicating that they are the principal source of the HO-1 in the lung parenchymal and alveolar compartments at that time point. Additional characterization of the HO-1+ IV− mononuclear myeloid cells at 4 wpi revealed that more than 95% have a macrophage (CD11b+CD64+) phenotype, while around 70% are Ly6C+, indicating that the majority are of inflammatory monocyte origin (Fig. 2C). We then sorted the parenchymal and alveolar myeloid cells from Mtb-infected mouse lungs at 4 wpi, and further separated the CD11b+ mononuclear myeloid cells into Ly6C− and Ly6C+ subsets in order to compare their HO-1 production by western blot. The results confirmed the flow cytometry analysis demonstrating that CD11b+Ly6C+ cells are the major HO-1 expressing myeloid cell population (Fig. 2D).
Figure 2. The vast majority of Mtb-infected pulmonary parenchymal and alveolar mononuclear myeloid cells express HO-1.
(A) Representative dot plots showing mCherry M. tuberculosis staining in alveolar macrophages and parenchymal / alveolar (CD45 IV−) and intravascular (CD45 IV+) neutrophils (Neut) and mononuclear myeloid cells (MMC) from lungs of M. tuberculosis-infected mice at 4 weeks post infection (wpi), gated as detailed in Fig. S1 (flow cytometry data concatenated from 4 samples); (B) Percentage represented by each cell population among parenchymal / alveolar (IV−) HO-1+ cells isolated from naïve, or M. tuberculosis-infected mice at 2 and 4 wpi (n = 4 mice/group); (C) Representative dot plot showing phenotypic characterization by flow cytometry of parenchymal / alveolar (IV−) HO-1+ MMC (gate R1) isolated from lungs of mice at 4 wpi with M. tuberculosis (flow cytometry data concatenated from 4 samples); (D) HO-1 and beta actin detection by western blotting (bottom) in parenchymal / alveolar (IV−) cells sorted from lungs of mice at 4 wpi according to phenotypic characteristics detailed in Fig. S1 and Fig. 2C and quantification of HO-1 in each cell population normalized to beta actin (endogenous control) expression on the same samples (top) (n = 4 mice/group – pooled samples); (E) Percentage represented by Mtb-infected (black) or uninfected (gray) cells among HO-1+ AM and parenchymal / alveolar (IV−) Neut, CD11b+Ly6C− and CD11b+Ly6C+ MMC (n = 4 mice/group); (F) Frequency of HO-1+ cells among Mtb-infected AM and parenchymal / alveolar (IV−) Neut, CD11b+Ly6C− and CD11b+Ly6C+ MMC (n = 4 mice/group); (G) Representative dot plots showing HO-1 mCherry-Mtb and HO-1 staining in total AM and and parenchymal / alveolar (IV−) Neut, CD11b+Ly6C− and CD11b+Ly6C+ MMC populations (flow cytometry data concatenated from 4 samples). Data expressed as mean ± standard error of mean (B, E and F), dot plots from concatenated data (A, C and G) or pooled samples (D). Data shown are representative of 2 (A, E, F and G) or 3 independent experiments (B, C and D).
We next performed experiments in which mice were infected with an mCherry-expressing Mtb strain in order to determine whether HO-1 expression is limited to infected cells. When assayed 4 weeks later, we found that within the entire parenchymal and alveolar myeloid cell population there is a large proportion of HO-1+ cells that are uninfected. This was particularly true for alveolar macrophages where only around 2% of the HO-1+ cells are Mtb-infected (Fig. 2E). In contrast, in neutrophils as well as CD11b+Ly6C− and CD11b+Ly6C+ mononuclear myeloid cells, a larger frequency of HO-1+ cells are Mtb-infected (20.1, 23.2 and 22.4% respectively) (Fig. 2E). However, when we analyzed HO-1 expression only in Mtb-infected cells within the IV− parenchymal / alveolar compartment, we found that the overwhelming majority of Mtb+ alveolar macrophages, Ly6C+ and Ly6C− CD11b+ mononuclear myeloid cells are positive for HO-1 (91.3, 95.1 and 95.8% respectively), whereas only 26.2% of Mtb+ neutrophils express the enzyme (Fig. 2F). Together these results confirmed that mononuclear myeloid cells are the main source of HO-1 in the pulmonary parenchyma and alveoli of Mtb-acutely infected mice and that virtually all of these cells if infected with the bacillus express HO-1 (Fig. 2G).
IFNγ production by T cells is required for bacterial load reduction upon HO-1 pharmacological inhibition
HO-1 inhibition during murine TB is only effective in reducing pulmonary bacterial loads when treatment with tin protoporphyrin (SnPP) is initiated after the third week of infection, a time point at which expression of the enzyme is first detected in lung homogenates 8. Compared to SnPP, treatment with cobalt protoporphyrin (CoPP), a well characterized pharmacological HO-1 inducer results in a small, non-significant increase in pulmonary bacterial loads (Fig. S2A), suggesting that, although the inhibition of enzyme activity favors the control of bacterial replication, a further enhancement in HO-1 expression beyond that induced by M. tuberculosis infection itself does not result in enhanced susceptibility.
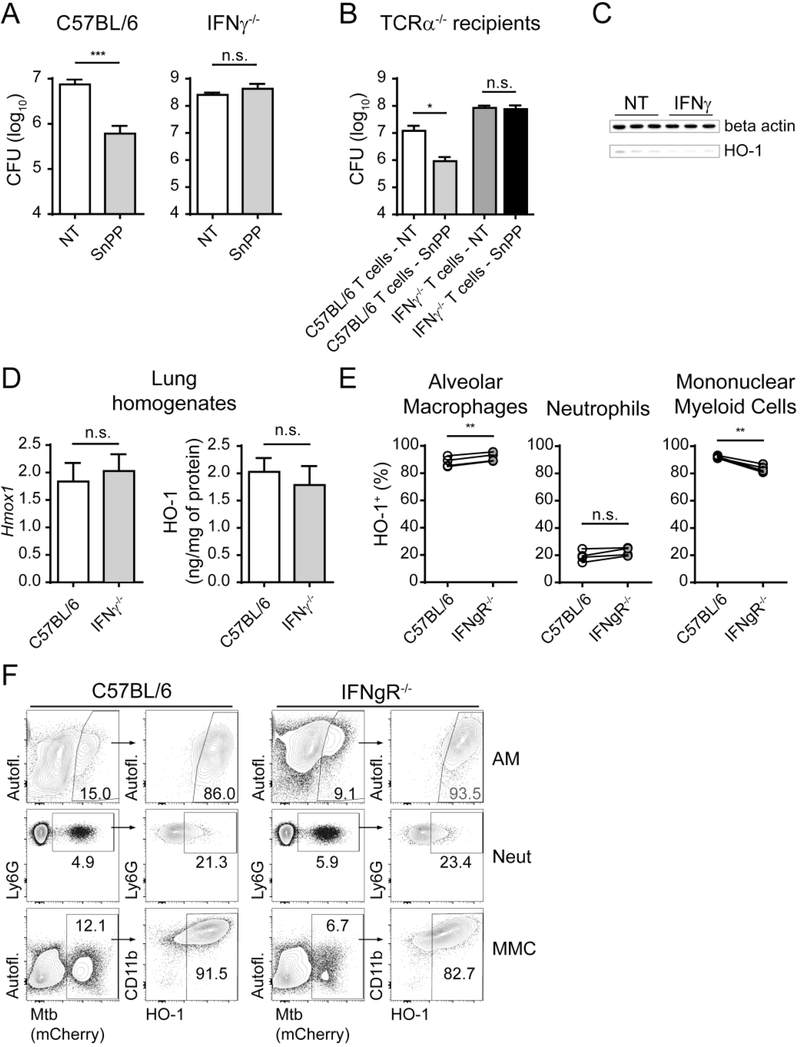
The time point at which HO-1 is first detected in whole M. tuberculosis-infected mouse lungs correlates kinetically with the development of the host Th1 response to Mtb as assessed by IFNγ and NOS2 mRNA expression and CD44+Tbet+ CD4+ T cell expansion in the lungs (Fig. S2B, C and D) as well as with the expression of HO-1 in the parenchymal and alveolar mononuclear myeloid cell compartments (Fig. 1). This association suggested that the ability to respond to SnPP treatment might depend on presence of Th1 products and in particular IFNγ. To test the latter hypothesis, C57BL/6 and IFNγ−/− mice were infected with Mtb and treated or not with SnPP for 15 days starting at 4 wpi. While treatment with the inhibitor resulted in a significant reduction in pulmonary bacterial loads in IFNγ sufficient C57BL/6 mice, no effect was observed in IFNγ−/− mice (Fig. 3A). To address the source of the required IFNγ, a similar SnPP treatment experiment was performed in T-cell deficient TCRα−/− mice that were adoptively transferred with total T cells from C57BL/6 or IFNγ−/− mice 1 week prior to infection. HO-1 pharmacological inhibition resulted in significant reductions in lung bacterial loads in mice adoptively transferred with C57BL/6 T cells, but not in animals receiving IFNγ−/− T cells (Fig. 3B), arguing that the production of IFNγ by T cells is essential for the anti-bacterial effects of SnPP treatment.
Figure 3: IFNγ production by T cells is required for bacterial load reduction mediated by pharmacologic HO-1 inhibition.
(A) CFU loads in lung homogenates of C57BL/6 or IFNγ−/− Mtb-infected mice treated or not for 15 days with SnPP (n = 3 and 4 mice/group); (B) CFU loads in lung homogenates of TCRα−/− recipient mice treated or not with SnPP for 15 days (TCRα−/− recipient mice were adoptively transferred intravenously with 1 × 106 total T lymphocytes isolated from spleens of naïve C57BL/6 or IFNγ−/− mice 7 days prior to infection with Mtb) (n = 4 mice/group); (C) HO-1 and beta actin detection by western blot in cell lysates of BMDM cultures 24 hours post infection and stimulation or not with recombinant murine IFNγ (100 U/mL); (D) mRNA quantification by real time PCR (left) and protein quantification by ELISA (right) of HO-1 in lung homogenates obtained from C57BL/6 and IFNγ−/− mice at 4 weeks post-infection (wpi) with Mtb (n = 4 mice/group); (E) HO-1 expression measured by flow cytometry in mCherry Mtb+ donor alveolar macrophages (AM), parenchymal neutrophils (Neut) or parenchymal myeloid mononuclear cells (MMC) recovered from the lungs of infected receptor mice at 4 wpi and gated as detailed in Fig. S1 (C57BL/6J x B6.SJL F1 (CD45.1/2) mice were lethally irradiated (2 × 500 rad − 4 hours prior to cell transfer), injected with 1 × 107 total bone marrow cells containing equal amounts of cells from B6/SJL (CD45.1) and IFNgR−/− (CD45.2) mice and allotted 10 weeks for immune reconstitution prior to infection) (n = 4 mice/group); (F) Representative dot plots from data depicted in Fig. 3E, showing gating of Mtb+ cells in left hand panels and HO-1 expression in gated cells in right hand panels (flow cytometry data concatenated from 4 samples). Data expressed as mean ± standard error of mean (A, B and D), as individual samples (C), paired individual samples (E), or dot plots from concatenated data (F). Data shown are representative of 2 (B, C and E) or 3 independent experiments (A and D). Statistical analysis: unpaired (A, B and D) and paired (E) Student’s t test. * = p<0.05, ** = p<0.01, ***= p<0.001, n.s. = non-significant.
IFNγ and HO-1 expression are unlinked in Mtb-infected mice
The IFNγ-dependence of SnPP therapy could reflect a role for the enzyme in downregulating Th1 development and/or IFNγ production thereby suppressing host control of infection. This hypothesis would be consistent with previous data demonstrating a role for HO-1 in suppressing pro-inflammatory Th1 immune responses in experimental colitis and sickle cell alloimmunization 14, 15. Conversely, the IFNγ requirement could simply reflect a role for the cytokine in regulating HO-1 expression in Mtb-infected myeloid cells. In order to test the first hypothesis, C57BL/6 mice were treated or not with SnPP as described previously and pulmonary T cell responses assessed. No difference was found in the frequency and total numbers of CD4+ and CD8+ T lymphocytes or in the expression of the activation marker CD44 in parenchymal / alveolar compartments of lungs from SnPP treated versus non-treated mice (Fig. S3A, B and C). Moreover, the ex vivo production of IFNγ by pulmonary parenchymal / alveolar CD4+ T lymphocytes after Mtb antigen stimulation was indistinguishable between SnPP-treated and non-treated mice (Fig S3D and E), arguing that the effect of SnPP treatment on bacterial loads is not due to HO-1-mediated downmodulation of Th1 adaptative immune responses during Mtb infection. We did however observe a small but significant increase in the frequency and number of alveolar macrophages and a more pronounced decrease in the number of parenchymal / alveolar mononuclear myeloid cells in SnPP-treated Mtb-infected mice compared to non-treated animals (Fig. S3F), which could potentially contribute to or be an outcome of the reduced bacterial loads observed in these animals.
In order to test the second hypothesis of a possible role for IFNγ signaling in the induction of HO-1 expression, BMDM were infected with Mtb and treated or not with recombinant murine IFNγ. HO-1 expression by Mtb-infected macrophages was only minimally affected by IFNγ treatment (Fig. 3C). Moreover, no difference was found in the expression of either HO-1 mRNA or protein in lung homogenates of C57BL/6 and IFNγ−/− mice at 4 wpi (Fig. 3D). However, as described above, HO-1 is expressed differentially by the different pulmonary myeloid cell subsets, which also are not equally infected with Mtb (Fig. 2). We therefore assessed by flow cytometry if IFNγ signaling modulates HO-1 expression in isolated Mtb-infected parenchymal and alveolar pulmonary myeloid cell populations.
In contrast with wild-type C57BL/6 animals, IFNγ−/− mice develop an inflammatory infiltrate in the parenchyma and alveoli dominated by neutrophils (Fig. S4A and C), which also represent the most highly infected cells in the lungs of these animals (Fig. S4B and C). This major effect on neutrophil levels introduces a potential complication when comparing HO-1+ cells between the two mouse strains. We therefore employed a mixed bone marrow chimera approach in which CD45.1/2 wild type (WT) mice were irradiated and reconstituted simultaneously with equal numbers of CD45.1 WT and CD45.2 IFNgR−/− bone marrow cells. The Mtb-infected chimeric mice had similar levels of WT and IFNgR−/− cells (Fig. S4D) in the lungs and in particular neutrophils and mononuclear myeloid cells in the parenchymal / alveolar compartment (Fig. S4E and G), the latter representing the major Mtb-infected subset in both WT and IFNgR−/− cells (Fig. S4E and F), thus justifying the validity of the chimera approach.
When we compared HO-1 expression in WT and IFNgR−/− Mtb-infected parenchymal and alveolar pulmonary myeloid cells at 4 wpi, we found a slight increase in the frequency of HO-1+ alveolar macrophages in IFNgR−/− cells, similar frequencies of HO-1+ neutrophils and a small (less than 10%) but significant reduction in HO-1 expression in IFNgR−/− mononuclear myeloid cells (Fig. 3E and F). Therefore, the lack of response to SnPP in the absence of IFNγ cannot be accounted for by a wholesale impairment of HO-1 expression in infected cells.
The bacterial load reduction resulting from SnPP treatment is dependent on host expression of NOS2
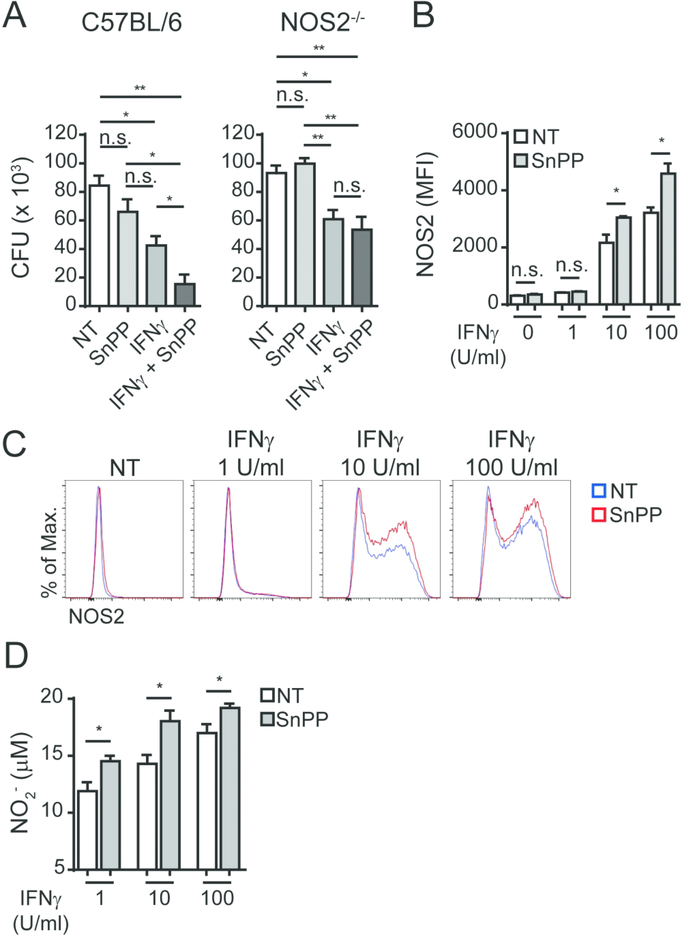
The expression of NOS2 is upregulated in Mtb-infected macrophages and is further enhanced in response to IFNγ activation (Fig. S5A). The resulting NO response play a critical role in the control of murine Mtb infection 16 although multiple mechanisms may be involved 17. As noted above, the upregulation of HO-1 protein expression in the lungs of Mtb-infected mice was found to be temporarily linked to the transcriptional expression of NOS2 in addition to IFNγ (Fig. S2B). Moreover, we found that a major increase in NOS2 protein expression occurs in alveolar macrophages and parenchymal / alveolar mononuclear myeloid cells 20 days post-infection with Mtb (Fig. S5B), a time point in which HO-1 expression also increases in the latter cell subset. To assess the involvement of NOS2 in the pharmacological effects of SnPP treatment, we infected C57BL/6 and NOS2−/− mice with Mtb and administered SnPP for 15 days starting at 4 wpi as described earlier. In contrast to the treated WT control animals, no bacterial load reduction was observed in NOS2−/− mice following SnPP administration (Fig. 4A), demonstrating a major requirement for NOS2 expression in the activity of the drug.
Figure 4: NOS2 expression is required for the bacterial load reduction mediated by pharmacologic HO-1 inhibition in vivo.
(A) CFU loads in lung homogenates of C57BL/6 and NOS2−/− Mtb-infected mice treated or not for 15 days with SnPP (N = 3 mice/group); (B) HO-1 quantification by ELISA in lung homogenates obtained from C57BL/6 and NOS2−/− mice at 4 weeks post-infection (wpi) with Mtb (n = 4 and 3 mice/group); (C) HO-1 expression measured by flow cytometry in mCherry Mtb+ donor alveolar macrophages (AM), parenchymal neutrophils (Neut) or parenchymal myeloid mononuclear cells (MMC) recovered from the lungs of infected receptor mice at 4 wpi and gated as detailed in Fig. S1 (bone marrow chimeras were generated as described for Fig. 2F, but reconstituted with bone marrow from B6/SJL (CD45.1) and NOS2−/− (CD45.2) mice) (n = 3 mice/group); (D) Representative dot plots from data depicted in Fig. 4C, showing gating of Mtb+ cells in left hand panels and HO-1 expression in gated cells in right hand panels flow cytometry data concatenated from 3 samples). Data expressed as mean ± standard error of mean (A and B), paired individual samples (C) or dot plots from concatenated data (D). Data shown are representative of 2 (B, C and D) or 3 (A) independent experiments. Statistical analyses: unpaired (A and B) and paired (C) Student’s t test. ** = p<0.01, **** = p<0.0001, n.s. = non-significant.
The role of NOS2 in SnPP function is not due to an effect on HO-1 expression in Mtb-infected myeloid cells
The dependence of NOS2 expression for the HO-1 inhibition-induced reduction of bacterial burden in TB could reflect a role for NOS2 in the induction of HO-1 expression in Mtb-infected myeloid cells. In order to test this hypothesis, we first quantified HO-1 expression in lung homogenates of C57BL/6 and NOS2−/− mice and found no difference in enzyme levels between the two animal groups (Fig. 4B). In common with IFNγ−/− mice, infected NOS2−/− animals developed a neutrophil-dominated pulmonary parenchyma and alveolar response (Fig. S5C and D). To rule this out as a complicating factor we also measured HO-1 expression by flow cytometry using the same mixed bone marrow chimera approach described above in which in this instance, CD45.1/2 WT mice were irradiated and adoptively transferred with equal numbers of WT (CD45.1) and NOS2−/− (CD45.2) bone marrow cells. We found that the Mtb-infected chimeric mice displayed similar levels of each myeloid subset in parenchyma and alveoli (Fig. S5E and F) and that Mtb infection was comparable in WT and NOS−/− neutrophils and mononuclear myeloid cells from these compartments (Fig. S5G and H). In this normalized inflammatory niche, we analyzed parenchymal and alveolar myeloid cells and found no difference in HO-1 expression between WT and NOS2−/− Mtb-infected alveolar macrophages and neutrophils, while there was a slight but significant increase in the frequency of NOS2−/− HO-1+ mononuclear myeloid cells compared to the WT population (Fig. 4C and D). These data confirmed that the dependence on NOS2 for the bacterial load reduction triggered by SnPP treatment is not due to a role for NOS2 in the induction of HO-1 expression.
SnPP treatment results in increased NOS2 expression and NO production by infected macrophages in response to IFNγ activation
The requirement for IFNγ production and NOS2 expression for improved bacterial control following SnPP treatment could also be associated with a role for HO-1 in regulating NOS2 expression and NO production in response to IFNγ activation of Mtb-infected cells. We used an in vitro approach in which C57BL/6 or NOS−/− bone marrow derived macrophages (BMDM) were infected with Mtb in the presence or absence of SnPP and/or IFNγ to test this hypothesis. In C57BL/6 macrophages, SnPP-induced HO-1 inhibition alone induced a small, insignificant bacterial load reduction, while IFNγ treatment alone as expected induced a major decrease in pathogen levels. Importantly, simultaneous treatment with SnPP and IFNγ resulted in a highly significant further reduction in bacterial loads when compared to either SnPP or IFNγ treatment alone. In direct contrast to C57BL/6 cells, in cultures employing NOS2−/− macrophages, no difference in bacterial numbers was found between cells treated or not with SnPP, either in the presence or in the absence of IFNγ stimulation (Fig. 5A). We repeated this experiment using hemin as an inducer of HO-1 expression in C57BL/6 BMDM to test whether enhanced expression of the enzyme might impair bacterial replication control. We found that the bacterial loads were similar between non-treated and hemin-treated cells. Also, the reduction in the number of bacteria resulting from IFNγ treatment remained unchanged when hemin was included with the cytokine. As expected, the additional supplementation of SnPP to the IFNγ and hemin treated cultures, resulted in further reduction of bacterial loads (Fig. S6A). These data suggest that increasing HO-1 expression beyond that induced by M. tuberculosis infection does not impair bacterial replication control by macrophages in vitro.
Figure 5: The effect of HO-1 inhibition on intracellular bacterial levels in vitro is dependent on both IFNγ and NOS2 expression.
(A) CFU loads obtained from C57BL/6 or NOS2−/− BMDM 96 hours post infection and stimulation or not with IFNγ (250 U/mL), SnPP (1μM) or IFNγ (250 U/mL) plus SnPP (1μM) (triplicates); (B) NOS2 expression quantified in C57BL/6 BMDM by flow cytometry 24 hours post-infection and stimulation or not with IFNγ (1, 10 or 100 U/mL) in the presence or absence of SnPP (1 μM) (triplicates); (C) Representative histograms of data shown in Fig. 5B (flow cytometry data concatenated from triplicates); (D) Nitrite concentration quantified in supernatants of C57BL/6 BMDM 24 hours post-infection and stimulation with IFNγ (1, 10 or 100 U/mL) in the presence or absence of SnPP (1μM) (triplicates); Data expressed as mean ± standard error of mean (A, B and D) or histograms from concatenated data (C). Data shown are representative of 2 (A, B and C) or 3 (D) independent experiments performed in triplicates. * = p<0.05, ** = p<0.01, n.s. = non-significant.
We also assessed NOS2 expression by flow cytometry and quantified nitrite by Griess assay in the supernatants as a readout of NO production. We observed significantly increased expression of NOS2 in SnPP-treated macrophages activated with 10 and 100 U/ml of IFNγ (Fig. 5B and C) as well as higher NO production in macrophage cultures in the simultaneous presence of IFNγ and SnPP (Fig. 5D). These data argued that SnPP-mediated HO-1 inhibition enhances NOS2 expression and NO production in Mtb-infected IFNγ-activated macrophages, resulting in improved bacterial control.
The enhanced bacterial control resulting from pharmacological inhibition of HO-1 in IFNγ-activated macrophages is associated with a reduction in intracellular iron levels
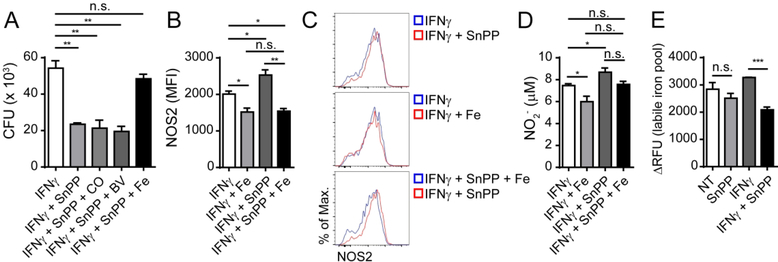
HO-1 catalyzes a critical step in heme degradation that results in the generation of equimolar amounts of carbon monoxide (CO), biliverdin and iron 18. These products can each have immunopharmacological effects in hematopoietic cells that can potentially affect host resistance to infection 19. In order to evaluate if the outcome of pharmacological HO-1 inhibition in IFNγ activated Mtb-infected macrophages stems from the reduced generation of any of these products, we treated these cells with either a CO donor (CORM2), biliverdin (biliverdin hydrochloride) or iron (FeSO4), to evaluate if any of these agents could reverse the effects of SnPP treatment. Treatment with the compounds alone or in combination with IFNγ in the absence of SnPP did not result in significant changes in bacterial levels in vitro (Fig. S6B). However, in macrophages treated with SnPP plus IFNγ, we found that the addition of iron reversed the bacterial load reduction resulting from HO-1 inhibition, while no effect was observed following the addition of either the CO donor or biliverdin (Fig. 6A). We also found that NOS2 expression in IFNγ-activated Mtb-infected macrophages, which increased with SnPP treatment, was lower in the presence of iron (Fig. 6B and C). A similar effect of iron supplementation was observed on the levels of NO produced by these cells, which were higher in the presence of SnPP, but returned to levels comparable to those expressed by IFNγ treated cells in the absence of the HO-1 inhibitor (Fig. 6D). As a control, we also treated IFNγ activated Mtb-infected macrophages with an iron chelating agent and as expected found an increase in NOS2 expression, both in the absence and presence of iron supplementation (Fig. S6C).
Figure 6: The effects of HO-1 inhibition on IFNγ-mediated control of intracellular bacterial levels correlate with intracellular iron levels.
(A) CFU loads obtained from C57BL/6 BMDM 96 hours post infection and stimulation with IFNγ (250 U/ml), IFNγ (250 U/mL) plus SnPP (1μM), IFNγ (250 U/mL) plus SnPP (1μM) plus CORM2 (5μM) – IFNγ + SnPP + CO, IFNγ (250 U/mL) plus SnPP (1μM) plus biliverdin (5 μM) – IFNγ + SnPP + BV or IFNγ (250 U/mL) plus SnPP (1μM) plus FeSO4 (5 μM) – IFNγ + SnPP + Fe (triplicates); (B) NOS2 expression in C57BL/6 BMDM 24 hours post-infection and stimulation with IFNγ (100 U/mL), IFNγ (100 U/mL) plus SnPP (1μM) or IFNγ (100 U/mL) plus SnPP (1μM) plus FeSO4 (5 μM) – IFNγ + SnPP + Fe (triplicates); (C) Representative dot plots of data shown in Fig. 5B (flow cytometry data concatenated from triplicates); (D) Nitrite levels in supernatants of C57BL/6 BMDM infected and stimulated as in Fig. 5B (triplicates); (E) Labile iron pool readout measured as ΔRFU (relative fluorescence units) in BMDM lysates 24 hours post infection and stimulation or not with IFNγ (250 U/mL), SnPP (1μM) or IFNγ (250 U/mL) plus SnPP (1μM) (triplicates). The data are presented as the means ± standard error (A, B, D and E) or histograms from concatenated data (C). Data shown are representative of 2 (A, B, C, D and E) independent experiments. Statistical analysis: Student’s t test. * = p<0.05, ** = p<0.01, *** = p<0.001, n.s. = non-significant.
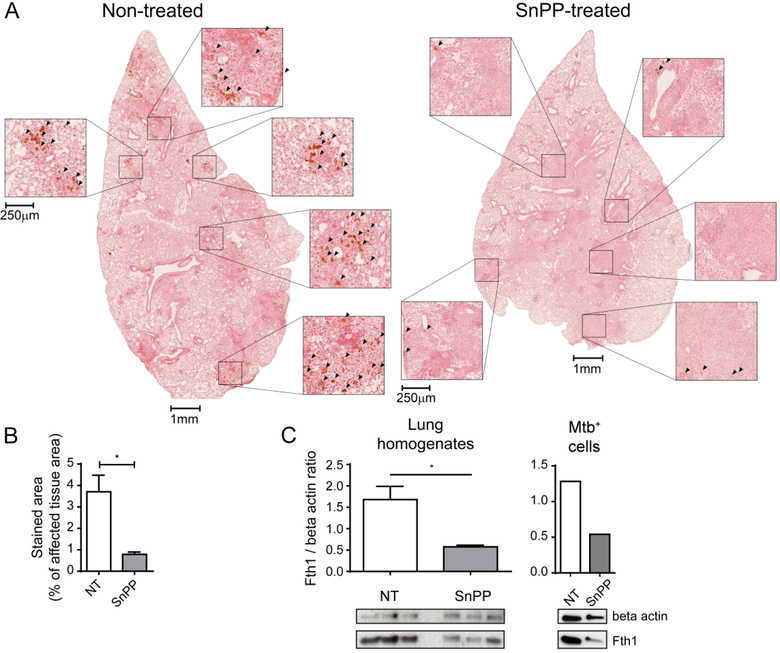
We next asked whether SnPP-induced HO-1 inhibition results in reduced intracellular iron in Mtb-infected cells using an assay for intracellular labile iron based on calcein-AM staining 20. We found that SnPP-induced HO-1 inhibition in IFNγ-activated Mtb-infected BMDM in vitro results in a significant decrease in intracellular iron levels (Fig. 6E). To assess whether SnPP treatment induces a similar reduction in iron levels in vivo, we performed Perls staining followed by diaminobenzidine (DAB) enhancement (Perls-DAB) to detect iron in lung sections from Mtb-infected mice treated or not with SnPP. By analyzing areas with infiltration of inflammatory cells, we found enhanced iron detection by Perls-DAB staining in lungs of non-treated mice compared to those of SnPP-treated animals (Fig. 7A and B). Furthermore, the levels of ferritin, an iron-chelating protein whose expression in induced in response to heightened cytosolic levels of the metal 5, 18, were substantially reduced in lung homogenates as well as in sorted Mtb-infected pulmonary myeloid cells from SnPP-treated mice in comparison with those of untreated animals (Fig. 7C). Based on these results, we propose a mechanism (Fig. 8) in which SnPP-mediated inhibition of HO-1 activity in Mtb-infected cells results in reduced intracellular iron levels that in addition to its predicted effects in restricting pathogen growth, enhances the control of bacterial replication mediated by IFNγ-dependent NO production.
Figure 7. Pharmacological HO-1 inhibition results in decreased iron accumulation in lungs of Mtb-infected mice.
(A) Photomicrographs of whole lung lobes (1 mm scale bars) and magnified fields (250 μm scale bars) showing Perls-DAB staining in histological sections from lungs of Mtb-infected C57BL/6 mice that were treated or not with SnPP for 21 days starting at 28 days post-infection (dpi) – arrowheads indicate areas with positive Perls-DAB staining; (B) Graphs showing percentage represented by Perls-DAB positively stained area in regions containing infiltration of inflammatory cells, in histological sections from lungs of Mtb-infected C57BL/6 mice treated or not SnPP for 21 days starting at 28 dpi (n = 4 and 3 mice/group); (C) Graphs showing quantification of ferritin heavy chain (Fth1) expression normalized to that of beta actin (endogenous control) (top) and images of western blot membrane showing Fth1 and beta actin expression (bottom) in lung homogenates (left) and mCherry Mtb+ pulmonary leukocytes isolated by sorting (right) from C57BL/6 Mtb-infected mice treated or not with SnPP for 21 days starting at 28 days post-infection (n = 3 mice/group on left panel and pooled samples from 3 mice/group in right panel). The data are presented as the means ± standard error (B and C), photomicrographs of individual samples (A) or individual samples (C). Data shown are representative of 2 independent experiments. Statistical analysis: Student’s t test. * = p<0.05.
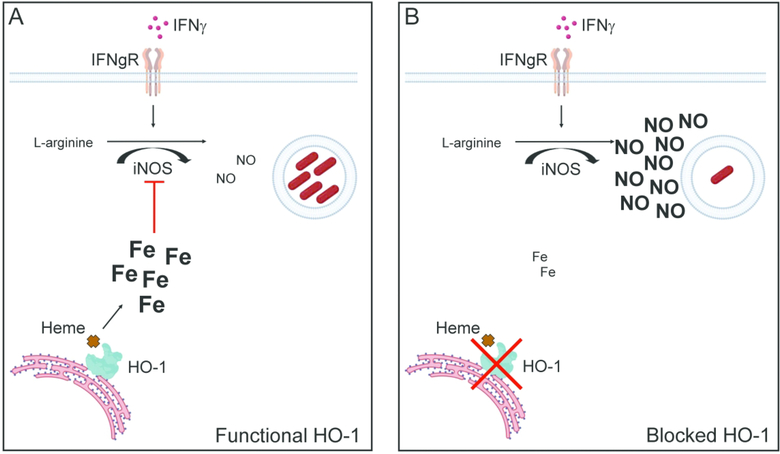
Figure 8: Proposed mechanisms for effect of HO-1 enzymatic inhibition on control of Mtb infection.
(A) M.tuberculosis infection induces HO-1 expression which catalyzes heme degradation and increases intracellular iron levels. This in turn suppresses NOS2-dependent NO production induced by IFNγ activation; (B) Inhibition of HO-1 with SnPP results in reduced free iron levels in M. tuberculosis-infected cells thereby enhancing IFNγ dependent NOS2-induced NO production and bacterial control.
Discussion
HO-1 is a host enzyme that is strongly regulated by Mtb infection in humans and experimental models and its role in most studies has been linked to its antioxidant functions 21. Whether the function of HO-1 in Mtb infection is purely host protective is a subject of controversy. Thus, mice totally or conditionally genetically deficient in HO-1 are more susceptible to chronic Mtb infection 22, although in the case of the total HO-1 knockout animals this finding is complicated by baseline alterations in the myeloid lineage probably resulting from heme toxicity 23. On the other hand, in vitro studies employing HO-1 enzymatic inhibitors or knockdown of the HO-1 gene support a mycobacterium promoting role for the molecule. Thus, Mtb-infected human or as shown here (Fig. 6A) mouse macrophages show enhanced control of bacterial growth when treated with SnPP 12 as do human THP-1 cells knocked down for HO-1 when infected with Mycobacterium abcessus 24. In addition, as previously reported by us, SnPP-treated mice show highly significant and reproducible reductions in pulmonary bacterial loads, which in the first 3 weeks of administration are indistinguishable from those achieved with conventional antibiotic treatment 8. It is highly unlikely that these anti-bacterial outcomes of SnPP treatment represent a direct effect on the pathogen, since SnPP fails to restrict bacterial growth in the absence of host adaptive immunity in vivo (Figs 3 and 4) or in liquid bacterial culture 8, 12, 24. Instead, we speculate that the contrary outcomes of HO-1 suppression in these multiple studies reflect the difference between temporary versus chronic absence of the enzyme itself or in the case of SnPP treatment, its activity. In this regard, it has been demonstrated in silico that the HO-1 protein can serve as a scaffold for a variety of biologically important molecules 25 and consequently as suggested in a recent study 26 that the enzyme may have additional functions beyond heme degradation itself. Such secondary activities would be absent in HO-1 deficient mice and thus could provide an indirect explanation for the impaired resistance of these animals to mycobacterial infection seen in previous studies.
In the present report, we investigated the immune-dependence of SnPP treatment on Mtb infection and as a first step in doing so, characterized the source of the enzyme in our murine model. Myeloid cells and in particular splenic and liver macrophages have been traditionally considered to be the major site of HO-1 function because of the enzyme’s role in recycling iron from senescent red blood cells 27. Nevertheless, it is only recently that the HO-1-producing myeloid subsets in other tissue sites have been characterized 28, 29. In the current study we found that in mice, inflammatory monocyte derived macrophages are the major source of HO-1 in Mtb-infected lungs, with a minor contribution from alveolar macrophages, in the parenchymal and alveolar compartments. Importantly, we determined that while most of the HO-1 is derived from uninfected cells, essentially all Mtb-infected cells produce the enzyme. This observation which is consistent with in vitro findings from a previous study 12 indicates that in vivo HO-1 expression always accompanies Mtb intracellularly where it is appropriately situated to affect pathogen survival/growth.
We have previously shown that the activity of SnPP on Mtb infection is defective in TCRα−/− mice that lack functional conventional αβ T cells 8. In the present study we have extended these observations by demonstrating a key role for the major Th1 effectors IFNγ and NOS2 in this immune dependence consistent with the known kinetics of the Mtb-restricting Th1 response in infected mice and the corresponding delay in the responsiveness of these animals to SnPP treatment. Our data argue that the dependence of SnPP efficacy on IFNγ and NO is not due to a role for these effectors in either HO-1 induction or suppression of IFNγ production by HO-1. Instead, we favor the hypothesis that the induction of HO-1 expression in Mtb-infected cells suppresses IFNγ-dependent NOS2 and NO production, thus promoting bacterial survival and growth.
In contrast to our findings, a recent study by Singh et al. 30 employing IFNγ-treated RAW264.7 cells reported that SnPP treatment impairs rather than promotes the control of Mtb infection in vitro. The authors attributed this outcome to an effect of the CO generated as a result of HO-1 activity in promoting IFNγ-induced autophagic control of bacterial replication 30 but did not confirm the effects of SnPP on bacterial control or the role of this proposed HO-1 regulated autophagic pathway in vivo. At present the basis of the discordant in vitro effects of SnPP treatment on bacterial growth observed here and in the previous study by Singh et al. are unclear 407 but may relate to the different host cells (bone-marrow derived macrophages versus RAW tumor cells) employed.
A role for HO-1 in suppressing reactive nitrogen species production has also been proposed in human tuberculosis based on analysis of diseased versus non-diseased lung sections 28. However, in apparent contrast to our hypothesis, the authors of that study interpreted their findings as evidence for a protective role of HO-1 in preventing chronic tissue damage. Nevertheless, their observations do not exclude the existence of a beneficial effect of short-term suppression of the HO-1 activity in promoting bacterial control through the same mechanism of NO production enhancement. Such a host protective function might be of particular relevance in the context of adjunctive host-directed therapy where a HO-1 inhibitor such as SnPP would be administered in conjunction with conventional antibiotics.
The antioxidant and anti-inflammatory activities of HO-1 are usually attributed to its generation of biliverdin (and subsequently bilirubin) and carbon monoxide respectively from heme 21. Biliverdin and bilirubin scavenge superoxide (O2−) and peroxynitrite (ONOO−, a toxic metabolite resulting from the reaction of NO with O2−) 31, while CO triggers signaling pathways that culminate in decreased production of TNF in macrophages 32, a pro-inflammatory cytokine known to induce NOS2 expression 33. However, in the experiments presented here the addition of either biliverdin or CO to IFNγ-activated Mtb-infected macrophages failed to inhibit the enhanced NOS2-dependent reduction in bacterial loads resulting from SnPP treatment. Instead, it was only the addition of iron, the third enzymatic product of HO-1 activity, that reverted the effects of SnPP in the latter in vitro system and this outcome was associated with a corresponding reduction in NOS2 expression and NO production. Moreover, we observed that SnPP treatment results in reduction of intracellular iron levels in Mtb-infected macrophages in vitro and we provide correlative evidence supporting a role for this effect in vivo. HO-1 activity has been suggested to mediate intracellular iron accumulation in vivo in LPS-induced septic shock 34 and it was demonstrated that the iron overload found in experimental liver fibrosis could be reverted by pharmacological inhibition of the enzyme 35. These previously published observations support our own findings indicating that the enhanced HO-1 activity triggered by Mtb infection associates with iron overload in vivo and suggesting that this upregulation of intracellular iron levels to impaired IFNγ-induced NOS2-NO production thereby promoting pathogen survival. The latter conclusion is consistent with previous findings demonstrating that iron suppresses IFNγ and LPS-induced NO production by macrophages 36. Further experiments causally linking Mtb induced HO-1 activity, increased iron levels and decreased NO response are needed to firmly establish the existence of this regulatory pathway in vivo.
Fluctuations in iron levels have been long believed to influence the outcome of Mtb infection 37. This association in most cases has been linked with the well-known nutritional effects of iron on mycobacterial growth 6, 11, a mechanism that is not universally agreed upon (e.g. Harington-Kandt et al. 38). We have previously shown that excessive iron accumulation in Mtb-infected cells can induce necrotic cell death by ferroptosis, promoting tissue damage and favoring bacterial dissemination 39. Whether iron derived from heme degradation contributes to this process remains to be formally investigated along with the possible function of ferroptosis inhibition in the therapeutic effects of SnPP in vivo. Instead, the present study provides evidence for the role of iron resulting from HO-1 enzymatic activity in promoting survival of this pathogen through its activity in suppressing a major antimycobacterial effector, NO, by means of its known property as an inhibitor of NOS2 36, 40, 41. The precise mechanism by which iron regulates NOS2 synthesis has not been fully elucidated. It has been demonstrated that iron impairs the binding capacity of the NF-IL6 (C/EBPβ) transcription factor to the Nos2 gene promoter region thereby reducing IFNγ-induced NOS2 protein expression 42. A second mechanism that has been previously proposed involves the known property of iron as a cofactor for the activation of the enzyme prolyl hydroxylase (PHD), which in turn degrades HIF1α 43, 44, a major inducer of NOS2 expression 45. In this scenario, SnPP by inhibiting the generation of iron from heme would suppress PHD activity and consequently enhance HIF1α mediated NOS2 production and bacterial control. Alternatively, as observed in in vitro S. typhimurium infection, iron accumulation could also enhance the production of the anti-inflammatory cytokine IL-10, which can suppress NOS2 expression 46. Whether the reduction in intracellular iron levels triggered by SnPP treatment also contributes to its effects by decreasing the levels of the metal required as a nutrient for pathogen growth remains to be determined.
Importantly, our previous and ongoing studies indicate SnPP treatment as a successful and highly reproducible therapy for TB in experimental murine infection. In recent work (Adeleke et al. unpublished) we have been able to formulate the drug as a prolonged release intramuscular injection feasible for clinical use. Although never assessed for its effect on human TB, SnPP has been previously used clinically for the treatment of jaundice in infants 47, 48 and has been tested experimentally as a therapy for porphyria 49, 50. The most common side effect associated with its use is a transient phototoxicity 51. Although HO-1 plays an important role in iron metabolism, acute treatment with SnPP does not result in iron deficiency anemia 52, but this side effect has been observed during chronic administration of tin mesoporphyrin (another tin based porphyrin HO-1 inhibitor), but was reversed after cessation of treatment 53. In addition to repurposing SnPP, other HO-1 inhibitors exist or are in development (e.g. imidazole-dioxolane derivatives) that could be experimentally tested for their therapeutic effect on Mtb infection 54. As emphasized here, as well as in our previous study 8, the effects of HO-1 inhibition by SnPP are T cell-dependent and this may limit the efficacy of the drug in HIV-TB patients with lowered CD4+ T lymphocyte counts. On the other hand, the immune dependence of SnPP therapy raises the interesting counter question of whether an HO-1 inhibitor might enhance bacterial clearance when used as an adjunct to therapeutic vaccination 55, 56 of individuals with established infections. At a more general level, the results reported here underscore the importance of iron metabolism as a potential target for intervention in tuberculosis both at the levels of the pathogen and the host defense mechanisms to which it is susceptible.
Methods
Experimental animals and in vivo bacterial infections
C57BL6J, B6.SJL, C57BL/6J x B6.SJL F1, IFNγ−/−, TCRα−/−, IFNgR−/− and NOS2−/− mice were obtained through a National Institute of Allergy and Infectious Diseases (NIAID) supply contract with Taconic Farms (Germantown, NY), or purchased from The Jackson Laboratory (Ben Harbor, ME). All animals were housed at BSL-2 and BSL-3 animal facilities at the NIAID, National Institutes of Health (NIH) and all the experimental protocols were approved by the NIAID Animal Care and Use Committee (ACUC). Mice were aerosol-infected with approximately 100 CFU of M. tuberculosis (Mtb) strain H37Rv or a H37Rv strain transformed with an mCherry reporter plasmid driven by the pMSP12 promoter 57 in whole body exposure/inhalation system (Glas Col, Terre Haute, IN). Bacterial loads were quantified in organ homogenates through a limiting dilution assay utilizing 7H11 agar medium (Sigma-Aldrich, Saint Louis, MO) enriched with OADC (BD Biosciences, San Jose, CA).
Bone marrow derived macrophage (BMDM) cultures and in vitro infection
For BMDM generation, marrow was obtained from femurs and tibia and cultured in Petri dishes (100 × 15 mm) containing 10 ml of DMEM/F-12 (Gibco, ThermoFisher Scientific, Waltham, MA) differentiation medium as described previously 39. The cells were next seeded in flat bottom 96 well plates in RPMI medium (Gibco, ThermoFisher Scientific, Waltham, MA) at a concentration of 8 × 104 cells/well, allowed to adhere overnight and exposed to Mtb strain H37Rv at a multiplicity of infection of 3 (MOI:3) for 4 hours after which non-internalized bacteria were removed by washing and different stimuli were added. For bacterial load quantification, BMDM were lysed for 10 minutes in PBS containing 0.05% (w/v) of saponin and a limiting dilution colony count performed with the cell lysates as described above.
SnPP, CORM2, biliverdin and iron sulfate treatment
For all in vivo tin protoporphyrin IX (SnPP) (Frontier Scientific, Logan, UT) treatments, mice were intraperitoneal injected daily (5 mg/kg/mouse in 200ul of PBS) starting 28 days post-infection. In vitro, SnPP was added to culture medium for at a concentration of 1μM. In in vitro experiments, biliverdin hydrochloride (Frontier Scientific, Logan, UT), Tricarbonyldichlororuthenium II (CORM2) or FeSO4 (both from Sigma-Aldrich, Saint Louis, MO), were added to cell culture medium all at 5μM.
Pulmonary cell isolation
Mice were euthanized by cervical dislocation following anesthesia by isoflurane inhalation. Lungs were perfused with PBS prior to excision. Lungs and spleens were incubated for 40 minutes at 37°C in digestion medium (RMPI (Gibco, ThermoFisher Scientific, Waltham, MA) + collagenase IV (100 U/ml) + DNAse I (50 U/ml) (both from Sigma-Aldrich, Saint Louis, MO), after which they were mashed through 100 μm nylon strainers. Viable cell concentrations were determined by microscopic counting on Neubauer chambers in the presence of trypan blue.
Flow cytometry
For ex vivo flow cytometric analyzes, mice were intravenously injected with a solution containing 1μg of anti-mouse CD45 antibody and 10 U of heparin, 3 minutes prior to euthanasia. Single cell suspensions from harvested organs or from BMDM cultures were labeled with a fixable viability dye (ThermoFisher Scientific, Waltham, MA) and then with fluorochrome-conjugated antibodies specific for cell surface markers. For intracellular staining, cells were further permeabilized by utilizing a Cytofix/Cytoperm™ kit (BD Biosciences, San Jose, CA) or with an eBioscience FoxP3/Transcription Factor Staining Buffer Set™ (ThermoFisher Scientific, Waltham, MA) prior to addition of specific antibodies. The antibodies used are depicted in Table S1. LSRFortessa and FACSymphony flow cytometers (BD Biosciences, San Jose, CA) were used in cell acquisition. Data was analyzed utilizing FlowJo software (FlowJo LLC, Ashland, OR).
Quantification of mRNA expression by real time PCR
Perfused lungs from naïve and Mtb-infected mice were disrupted in 2 ml tubes containing 2.7 mm glass beads and Trizol reagent (ThermoFisher Scientific, Waltham, MA) using a Precellys Evolution™ tissue homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France). mRNA was extracted from samples utilizing Qiagen RNeasy mini kits (Qiagen, Hilden, Germany) and Direct-zol™ RNA mini-prep kits (Zymo Research, Irvine, CA). 1 μg of RNA was then reverse transcribed into cDNA using superscript II reverse transcriptase and random primers (ThermoFisher Scientific, Waltham, MA). SYBR Green, 7900HT Fast Real-Time PCR and Quant-Studio 7 Real-Time PCR Systems (Applied Biosystems, ThermoFisher Scientific, Waltham, MA) were employed for real time PCR reactions. Relative expression of genes of interest in Mtb-infected mouse lungs was calculated using the ΔΔ cycle threshold method. mRNA expression in each sample was normalized to that of β-actin and further analyzed in relation to those of uninfected naïve mice lungs. Murine primers used: Actb F: 5’ AGC TGC GTT TTA CAC CCT TT 3’; Actb R: 5’ AAG CCA TGC CAA TGT TGT CT 3’; Hmox1 F: 5’ GCC ACC AAG GAG GTA CAC AT 3’; Hmox1 R: 5’ GCT TGT TGC GCT CTA TCT CC 3’.
Immunoassays
Perfused lungs from naïve and Mtb-infected mice were disrupted in 2 ml tubes containing 2.7 mm glass beads and PBS with cOmplete ULTRA™ protease inhibitor cocktail (Roche, Basel, Switzerland) and 2mM of PMSF (Sigma-Aldrich, Saint Louis, MO), using a Precellys Evolution™ tissue homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France). Cell cultures were lysed in Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA) containing cOmplete ULTRA™ protease inhibitor cocktail (Roche, Basel Switzerland) and 2mM of PMSF (Sigma-Aldrich, Saint Louis, MO). For western blot, samples were denatured for 5 min at 95°C in reducing buffer (ThermoFisher Scientific, Waltham, MA), separated in mini-protean TGX gels (Bio-Rad, Hercules, CA) and transferred to PVDF membranes prior to staining with specific antibodies and development with luminol. Protein expression in samples was calculated utilizing ImageJ software in relation to β-actin expression. The concentration of HO-1 in samples was measured by ELISA using mouse HO-1 ELISA set (Enzo Life Sciences, Farmingdale, NY). Antibody specifications are provided in Table S1.
Labile iron concentration measurement and Perls staining of iron in tissues
For the quantification of intracellular labile iron concentration, we used a modified Calcein-AM staining protocol as described previously in which intracellular labile iron concentration is determined by the difference between Calcein-AM fluorescence values of a sample incubated in the presence and absence of an iron chelating agent 20. Briefly, samples of BMDM lysate (lysed for 10 minutes in PBS containing 0.05% of saponin) were incubated for 15 minutes at room temperature with 250μM of deferoxamine mesylate (DFO) or PBS, followed by staining with Calcein-AM (ThermoFisher Scientific, Waltham, MA) at 125nM for 30 minutes at 37°C, in opaque 96 well plates (Corning, Corning, NY). Relative fluorescence units (RFU) were measured and labile iron levels are represented as ΔRFU (DFO-treated sample RFU – PBS treated sample RFU). Perls staining followed by DAB enhancement was performed by Histoserv, Inc. (Germantown, MD, USA) in histological sections from Mtb-infected mouse lungs previously fixed in PBS buffer containing 10% formalin, as described elsewhere 58. Images were acquired in an Aperio Digital Pathology Slide Scanner (Leica, Buffalo Grove, IL, USA) and analyzed using ImageScope (Leica, Buffalo Grove, IL, USA) and ImageJ 59 softwares.
Statistical Analyses
Differences between groups were statistically evaluated by paired and unpaired Student’s t test using Prism software (GraphPad) and considered significant when p≤0.05.
Supplementary Material
Acknowledgments
The authors would like to thank Sandra Oland and the staff from the NIAID animal facilities for technical assistance and Dr. Dragana Jankovic, Dr. Daniel Barber and Dr. Katrin Mayer-Barber for scientific discussion during manuscript preparation. This work was supported by the Intramural Research Program of the NIAID, NIH.
This work was financially supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
Disclosure
The authors declare no conflict of interest.
Supplementary Material
Supplementary Material is linked to the online version of the paper at http://www.nature.com/mi.
References
- 1.WHO. Global Tuberculosis Report 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 2.Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N. Eng. J. Med. 2012; 367(10): 931–936. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Guidelines for treatment of tuberculosis. 4th edn. WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- 4.Young C, Walzl G, Du Plessis N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020; 13(2): 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antiox. Redox Signal. 2014; 20(11): 1754–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells RM, Jones CM, Xi Z, Speer A, Danilchanka O, Doornbos KS et al. Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Path. 2013; 9(1): e1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015; 15(8): 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa DL, Namasivayam S, Amaral EP, Arora K, Chao A, Mittereder LR et al. Pharmacological Inhibition of Host Heme Oxygenase-1 Suppresses Mycobacterium tuberculosis Infection In Vivo by a Mechanism Dependent on T Lymphocytes. mBio 2016; 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst JD. The immunological life cycle of tuberculosis. Nat. Rev. Immunol. 2012; 12(8): 581–591. [DOI] [PubMed] [Google Scholar]
- 10.Dawson R, Condos R, Tse D, Huie ML, Ress S, Tseng CH et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PloS One 2009; 4(9): e6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE 3rd. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Nat. Acad. Sci. U.S.A. 2000; 97(3): 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharn CR, Collins AC, Nair VR, Stamm CE, Marciano DK, Graviss EA et al. Heme Oxygenase-1 Regulates Inflammation and Mycobacterial Survival in Human Macrophages during Mycobacterium tuberculosis Infection. J. Immunol. 2016; 196(11): 4641–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 2014; 192(7): 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takagi T, Naito Y, Mizushima K, Hirai Y, Harusato A, Okayama T et al. Heme oxygenase-1 prevents murine intestinal inflammation. J. Clin. Biochem. Nutr. 2018; 63(3): 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong H, Bao W, Friedman D, Yazdanbakhsh K. Hemin controls T cell polarization in sickle cell alloimmunization. J. Immunol. 2014; 193(1): 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Nat. Acad. Sci. U.S.A. 1997; 94(10): 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra BB, Lovewell RR, Olive AJ, Zhang G, Wang W, Eugenin E et al. Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat. Microbiol. 2017; 2: 17072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010; 50: 323–354. [DOI] [PubMed] [Google Scholar]
- 19.Singh N, Ahmad Z, Baid N, Kumar A. Host heme oxygenase-1: Friend or foe in tackling 670 pathogens? IUBMB Life 2018; 70(9): 869–880. [DOI] [PubMed] [Google Scholar]
- 20.Picard V, Epsztejn S, Santambrogio P, Cabantchik ZI, Beaumont C. Role of ferritin in the control of the labile iron pool in murine erythroleukemia cells. J. Biol. Chem. 1998; 273(25): 15382–15386. [DOI] [PubMed] [Google Scholar]
- 21.Soares MP, Bach FH. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol. Med. 2009; 15(2): 50–58. [DOI] [PubMed] [Google Scholar]
- 22.Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect. Immun. 2013; 81(7): 2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood 2010; 116(26): 6054–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdalla MY, Ahmad IM, Switzer B, Britigan BE. Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells. Redox. Biol. 2015; 4: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paez AV, Pallavicini C, Schuster F, Valacco MP, Giudice J, Ortiz EG et al. Heme oxygenase-1 in the forefront of a multi-molecular network that governs cell-cell contacts and filopodia-induced zippering in prostate cancer. Cell Death Dis. 2016; 7(12): e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pek RH, Yuan X, Rietzschel N, Zhang J, Jackson L, Nishibori E et al. Hemozoin produced by mammals confers heme tolerance. eLife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soares MP, Hamza I. Macrophages and Iron Metabolism. Immunity 2016; 44(3): 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinta KC, Rahman MA, Saini V, Glasgow JN, Reddy VP, Lever JM et al. Microanatomic Distribution of Myeloid Heme Oxygenase-1 Protects against Free Radical-Mediated Immunopathology in Human Tuberculosis. Cell Rep. 2018; 25(7): 1938–1952 e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luz NF, DeSouza-Vieira T, De Castro W, Vivarini AC, Pereira L, Franca RR et al. Lutzomyia longipalpis Saliva Induces Heme Oxygenase-1 Expression at Bite Sites. Front. Immunol. 2018; 9: 2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh N, Kansal P, Ahmad Z, Baid N, Kushwaha H, Khatri N et al. Antimycobacterial effect of IFNG (interferon gamma)-induced autophagy depends on HMOX1 (heme oxygenase 1)-mediated increase in intracellular calcium levels and modulation of PPP3/calcineurin-TFEB (transcription factor EB) axis. Autophagy 2018; 14(6): 972–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen T, Daiber A. Direct Antioxidant Properties of Bilirubin and Biliverdin. Is there a Role for Biliverdin Reductase? Front. Pharmacol. 2012; 3: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000; 6(4): 422–428. [DOI] [PubMed] [Google Scholar]
- 33.Lin JY, Seguin R, Keller K, Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect. Immun. 1994; 62(5): 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Huttemann M et al. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab. Invest. 2008; 88(1): 70–77. [DOI] [PubMed] [Google Scholar]
- 35.Wang QM, Du JL, Duan ZJ, Guo SB, Sun XY, Liu Z. Inhibiting heme oxygenase-1 attenuates rat liver fibrosis by removing iron accumulation. World J. Gastroenterol. 2013; 19(19): 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 1994; 180(3): 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J. Infect. Dis. 2007; 195(12): 1745–1753. [DOI] [PubMed] [Google Scholar]
- 38.Harrington-Kandt R, Stylianou E, Eddowes LA, Lim PJ, Stockdale L, Pinpathomrat N et al. Hepcidin deficiency and iron deficiency do not alter tuberculosis susceptibility in a murine M.tb infection model. PloS One 2018; 13(1): e0191038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaral EP, Costa DL, Namasivayam S, Riteau N, Kamenyeva O, Mittereder L et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 2019; 216(3): 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komarov AM, Mattson DL, Mak IT, Weglicki WB. Iron attenuates nitric oxide level and iNOS expression in endotoxin-treated mice. FEBS Lett. 1998; 424(3): 253–256. [DOI] [PubMed] [Google Scholar]
- 41.Agoro R, Taleb M, Quesniaux VFJ, Mura C. Cell iron status influences macrophage polarization. PloS One 2018; 13(5): e0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dlaska M, Weiss G. Central role of transcription factor NF-IL6 for cytokine and iron-mediated regulation of murine inducible nitric oxide synthase expression. J. Immunol. 1999; 162(10): 6171–6177. [PubMed] [Google Scholar]
- 43.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 2008; 134(3): 756–767. [DOI] [PubMed] [Google Scholar]
- 44.Phelan JJ, Basdeo SA, Tazoll SC, McGivern S, Saborido JR, Keane J. Modulating Iron for Metabolic Support of TB Host Defense. Front. Immunol. 2018; 9: 2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int. Immunol. 2013; 25(2): 67–75. [DOI] [PubMed] [Google Scholar]
- 46.Fritsche G, Nairz M, Werner ER, Barton HC, Weiss G. Nramp1-functionality increases iNOS expression via repression of IL-10 formation. Eur. J. Immunol. 2008; 38(11): 3060–3067. [DOI] [PubMed] [Google Scholar]
- 47.Drummond GS, Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc. Nat. Acad. Sci. U.S.A. 1981; 78(10): 6466–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kappas A, Drummond GS, Manola T, Petmezaki S, Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics 1988; 81(4): 485–497. [PubMed] [Google Scholar]
- 49.Dover SB, Graham A, Fitzsimons E, Moore MR, McColl KE. Haem-arginate plus tin-protoporphyrin for acute hepatic porphyria. Lancet 1991; 338(8761): 263. [DOI] [PubMed] [Google Scholar]
- 50.Dover SB, Moore MR, Fitzsimmons EJ, Graham A, McColl KE. Tin protoporphyrin prolongs the biochemical remission produced by heme arginate in acute hepatic porphyria. Gastroenterology 1993; 105(2): 500–506. [DOI] [PubMed] [Google Scholar]
- 51.Schulz S, Wong RJ, Vreman HJ, Stevenson DK. Metalloporphyrins - an update. Front. Pharmacol. 2012; 3: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berglund L, Galbraith RA, Emtestam L, Drummond GS, Angelin B, Kappas A. Heme oxygenase inhibitors transiently increase serum ferritin concentrations without altering other acute-phase reactants in man. Pharmacology 1999; 59(1): 51–56. [DOI] [PubMed] [Google Scholar]
- 53.Kappas A, Drummond GS, Galbraith RA. Prolonged clinical use of a heme oxygenase inhibitor: hematological evidence for an inducible but reversible iron-deficiency state. Pediatrics 1993; 91(3): 537–539. [PubMed] [Google Scholar]
- 54.Pittala V, Salerno L, Romeo G, Modica MN, Siracusa MA. A focus on heme oxygenase-1 (HO-1) inhibitors. Curr. Med. Chem. 2013; 20(30): 3711–3732. [DOI] [PubMed] [Google Scholar]
- 55.Cardona PJ. The Progress of Therapeutic Vaccination with Regard to Tuberculosis. Front. Microbiol. 2016; 7: 1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis--a systematic review. Vaccine 2014; 32(26): 3162–3168. [DOI] [PubMed] [Google Scholar]
- 57.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat. Immunol. 2004; 5(8): 828–835. [DOI] [PubMed] [Google Scholar]
- 58.Philippot Q, Deslee G, Adair-Kirk TL, Woods JC, Byers D, Conradi S et al. Increased iron sequestration in alveolar macrophages in chronic obstructive pulmonary disease. PloS One 2014; 9(5): e96285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012; 9(7): 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.