Abstract
Drugs of abuse and highly palatable foods (e.g. high fat or sweet foods) have powerful reinforcing effects, which can lead to compulsive and addictive drives to ingest these substances to the point of psychopathology and self-harm--specifically the development of Substance Use Disorder (SUD) and obesity. Both SUD and binge-like overeating can be defined as disorders in which the salience of the reward (food or drug) becomes exaggerated relative to, and at the expense of, other rewards that promote well-being. A major roadblock in the treatment of these disorders is high rates of relapse after periods of abstinence. It is common, although not universal, for cue-induced craving to increase over time with abstinence, often triggered by cues previously paired with the reinforcing substance. Accumulating evidence suggests that similar neural circuits and cellular mechanisms contribute to abstinence-induced and cue-triggered seeking of drugs and palatable food. Although much research has focused on the important role of corticolimbic circuitry in drug-seeking, our goal is to expand focus to the more recently explored hypothalamic–thalamic–striatal circuitry. Specifically, we review how connections, and neurotransmitters therein, among the lateral hypothalamus, paraventricular nucleus of the thalamus, and the nucleus accumbens contribute to abstinence-induced opioid- and (high fat or sweet) food-seeking. Given that biological sex and gonadal hormones have been implicated in addictive behavior across species, another layer to this review is to compare behaviors and neural circuit-based mechanisms of abstinence-induced opioid- or food-seeking between males and females when such data is available.
Keywords: incubation of craving, relapse, opioid, sucrose, food, paraventricular nucleus of the thalamus
1. Introduction
“Semper in absentis felicior aestus amantis” (Propertius, Elegies 2.33b.21)
[“Passion is always greater in absent lovers”] (Kline, A.S. transl., Liber Publications 2001)
The compulsive drive to self-administer drugs of abuse or palatable foods (e.g., fatty or sweet foods) to the point it harms one’s social, psychological, and physical health defines Substance Use Disorder (SUD) and binge eating disorders/obesity. The patterns of behavior and neurobiological mechanisms are similar between the two (Millan et al., 2017), and both are considered chronic, relapsing brain diseases (Volkow et al., 2013). The major impediment to the treatment of these disorders is high rates of relapse after periods of abstinence (O’Brien, 1997). There are numerous external and internal factors that lead to relapse in both humans and animal models. Broadly these factors lead to craving, which is defined as an overwhelmingly strong desire or need to use a drug or eat a palatable food. In humans, the experience of craving is a juggernaut that typically leads to relapse and yet has been nearly impossible to fully understand at a neurobiological level. Clinical and preclinical studies have shown that craving and relapse can be triggered by at least one of the following factors: acute exposure to the reinforcing stimulus (i.e., drug or food), cues or contexts associated with positive or negative reinforcers, or stress (for review, see Venniro et al., 2016). Importantly, stress and cues associated with negative affective states can be independent of the substance disorder or due to abstinence-induced withdrawal symptoms (Chartoff & Carlezon, 2014).
In this review we focus on the effect of abstinence on either opioid- or palatable food-seeking. It is critical to note that “abstinence” is a broad term in the context of this review. First, it can trigger a withdrawal state in the absence of the reinforcer, which is known to contribute to craving and—in animal models—reinstatement of reward-seeking (e.g. negative reinforcement; (Evans & Cahill, 2016; Koob, 2020). Second, abstinence can be as short as a day or as long as months or years. A particularly insidious feature of addictive-like behavior--whether the motivation is to ingest drug or food--is that craving can increase over time with abstinence. Preclinical scientists have dubbed this “incubation of drug craving” (Grimm, 2001), and defined it as a hypothetical motivational process in which there is a time-dependent increase in cue-induced reward-seeking. This has been shown to occur after withdrawal from both opioid and food self-administration in rats and humans (Grimm, 2020; Zhou et al., 2009). Of note, the data from humans include the common caveat that the timing and methods of manipulating abstinence periods are variable, thus adding to the complexity of trying to understand this phenomenon solely in humans.
A number of outstanding, in-depth reviews have covered extinction- and abstinence-based models of relapse to drug/food seeking (see Venniro et al., 2016; Nair et al., 2009; Grimm, 2020). Here we focus on abstinence-induced seeking of opioids and palatable foods, the mechanisms of which both overlap and are distinct from those determined for psychostimulants (Badiani et al. 2011). In that vein, we seize upon a slew of recent studies that have expanded focus outward from the traditional corticolimbic dopamine system to include hypothalamic–thalamic–striatal circuitry (Millan et al., 2017; Otis et al., 2019). Specifically, we describe how the lateral hypothalamus (LH), paraventricular nucleus of the thalamus (PVT), and the nucleus accumbens (NAc) interconnect and contribute to abstinence-induced opioid- and (high fat or sweet) food-seeking. As hypothalamic and midline thalamic regions are integral to the regulation of arousal, energy metabolism, reward and aversion, this broader neural circuit offers many mechanistic answers and avenues for future research.
Evidence suggests that gender and biological sex modulate compulsive, addictive-like behavior, including incubation of craving and relapse (Hallam et al., 2016; McHugh et al., 2018). Since relapse has been identified as one of the key blocks to successful treatment, understanding sex-dependent mechanisms contributing to relapse is an essential component of scientific inquiry. As such, another goal of this review is to describe, when known, studies that reveal sex-dependent differences or comparisons.
2. Clinical relevance of abstinence-induced reward-seeking
Drugs of abuse and highly palatable foods can establish Pavlovian association with external stimuli previously associated with their consumption (Volkow et al., 2013). In humans, drug or food cues elicit a subjective craving state (“urge” or “desire”) to consume or seek the reward. These craving states can lead to relapse, which for both drugs and food, is a major hurdle in the treatment of substance use disorder (O’Brien, 1997) and weight loss in obese people (Nair, 2009). This is particularly important because the subjective craving for both drugs and food increases during abstinence.
2.1. Abstinence-induced food-seeking in humans
Cues such as the sight, smell, and taste of food reliably signal food intake and act as conditioned stimuli that can potentially trigger food-seeking. These food cue responses could increase the probability of overeating (Wardle, 1990). In people who restrain their food intake, food predictive cue presentations can lead to a strong desire to eat and subsequent binging (Sobik et al., 2005). Moreover, people who were exposed to high-fat foods during dieting are more likely to relapse to their unhealthy eating habits (Gorin et al., 2004). Thus, both abstinence from eating and exposure to food cues increases food cravings and increases food-seeking and food intake. Additionally, food deprivation has been shown to increase the physiological responses specifically to food-related cues (Drobes et al., 2001).
It is known that the primary cause of obesity is overeating. Human studies have shown that overweight individuals have stronger brain blood-oxygen-level-dependent (BOLD) imaging signals in response to food cues compared to healthy weight people (Frankort et al., 2012). These BOLD imaging signals have been shown to be strongest in the VTA, PFC, Amy, and the NAc (Martin et al., 2010). Additionally, clinical studies have shown that thalamic nuclei specifically activate in response to reward craving and reward cue presentation (George et al., 2001). However, the resolution in human imaging studies makes it difficult to distinguish the PVT from other thalamic nuclei. Nonetheless, thalamic nuclei closely interact with the mesocorticolimbic reward system and have been implicated in playing a key role in influencing food consumption and food-seeking in preclinical models (see Ferrario et al., 2016; Millan et al., 2017 for review).
There are clear biological sex differences in the regulation of food intake and body weight in humans (Woods et al., 2003). These differences have been reported to be driven by several factors such as: leptin and insulin sensitivity, gonadal hormones, sex chromosome-associated genes that influence energy homeostasis, fat distribution and appetite. Thus, understanding sex-specific mechanisms of the aforementioned factors is important to understand the development of obesity and overeating in both men and women. In women, caloric intake fluctuates across the menstrual cycle (Buffenstein et al., 1995). These changes in caloric intake are mediated by natural fluctuations of ovarian hormones throughout the cycle. For example, fluctuations in estradiol negatively predict shifts in food intake, progesterone shows a positive correlation, and the combination of both estradiol and progesterone mediates a periovulatory drop in eating (Roney and Simmons, 2017). A handful of studies have also shown that ovarian hormones play a key role in modulating the activity of the mesolimbic reward system nuclei (NAc, Amy and LH) in response to the presentation of food cues (Frank et al., 2010; Alonso-Alonso et al., 2011). Although obese or overweight men and women both exhibit increased responsivity to high-calorie food when compared to their lean counterparts, women tend to be more responsive. In addition, women show greater activation than men in cortical regions when food cues are presented (Killgore et al., 2010).
2.2. Abstinence-induced opioid-seeking in humans
Opioid use disorder (OUD) can be segmented into pathological use of illicit opioids (e.g., heroin) or prescription opioids (e.g., oxycodone). Patterns of OUDs have shifted recently, with an increase in concurrent prescription opioid- and heroin-use in people with OUD (Cicero et al., 2015). Although it has been difficult to obtain clear, direct evidence that opioid craving is heightened during periods of abstinence in humans, there are increasing studies examining stress, affect, impulsivity, and how triggers of these states change/increase over opioid abstinence periods. For example, it has been shown that although response inhibition is improved over time in heroin abstainers, that effect can be reduced by exposure to drug-related cues, which may increase the risk of relapse, and is a major impediment to treatment (Su et al., 2020). Increased opioid craving is positively correlated with stronger BOLD fMRI signals within mesocorticolimbic and other limbic regions of the brain, including cortex, dorsal and ventral striatum, thalamus, and hippocampus (Li et al., 2012). As discussed above, common triggers for craving and seeking include drug-paired cues, drug-paired contexts, and stress. Of note, the most effective trigger for opioid-seeking in dependent individuals is often exposure to the drug itself (McHugh et al., 2014).
Gender differences in OUD exist at multiple levels of the addiction cycle (McHugh et al., 2013). Not only do women report increased functional impairment and higher likelihood of misusing opioids to cope with negative affect and pain compared to men, women report significantly more craving (Back et al., 2011). These were not associated with medication dose or pretreatment sensitivity towards OUD. Such behavioral consequences span sensitivity to opioid-reward and negative affective state, and sensitivity to stress hormones in neural circuits that mediate opioid withdrawal-induced negative affective states (Chartoff and McHugh, 2016).
3. Preclinical models of abstinence-induced reward-seeking
In this section we will focus on abstinence-based relapse models. There are three phases to this model: training, abstinence and relapse testing. During training, animals self-administer either drugs or palatable food (high fat or sweet foods/sucrose) over several days. In each day’s self-administration session, responses on an active manipulandum are paired with a cue (auditory or visual stimulus), while simultaneously leading to reward (see Venniro et al., 2016 and Grimm, 2020 for reviews). Following training, rats undergo abstinence, which can be either forced or voluntary and importantly results in increased reward-seeking behaviors. The majority of studies utilize forced abstinence, in which animals are kept in their home cages and no longer have access to the reward. For relapse testing, rats are placed back in the operant chambers using conditions in which responding on the manipulandum results in cue presentation but no reward delivery (Counotte et al., 2014; Grimm, 2020). Depending on the length of abstinence, the original reinforcer, biological sex, and many other factors, cue-triggered responding (i.e., reward-seeking) is enhanced relative to early abstinence. This phenomenon of potentiated drug-seeking has been termed “incubation of craving” (Grimm et al., 2001; Grimm, 2020).
3.1. Abstinence-induced sucrose- and high-fat diet-seeking in rodent models
Incubation of food craving (e.g. sucrose, high-fat diets and saccharin) has been described in rodent models. The majority of studies examining incubation of food craving use a protocol slightly modified from that used with drugs of abuse. For example, studies by Grimm et al. use a palatable food self-administration training schedule similar to that used for drugs of abuse that incorporates a food-paired cue. Animals then undergo forced abstinence for a variable number of days. Unlike abstinence-induced drug-seeking tests; however, incubation of food craving is measured by ultimately allowing rats access to the active manipulandum (previously paired with palatable food) for several hours prior to re-introduction of cues. Broadly, the longer the “abstinence” period, the greater the response on the active manipulandum when cues are introduced. This is interpreted as incubation of craving (Grimm, 2020). Specifically, it has been found that after 15, 21 and 30 days of abstinence (Grimm, 2020; Li and Frantz, 2010; Counotte et al., 2014) from sucrose self-administration, rats have a time-dependent increase in cue-induced sucrose-seeking. Additionally, incubation of craving for high-fat/high-sugar diets has also been shown. For example, it was found that cues previously paired to high-fat food and standard chow pellets increase reward-seeking after 30 days of forced abstinence (Darling et al., 2016; McCue et al., 2019). Interestingly, no incubation of craving was found for chocolate pellets in rats (Noye Tuplin et al., 2018). Although saccharin does not have any caloric value, it is highly palatable (sweet) and saccharin-associated cues can trigger seeking behaviors after 30 days of abstinence in rats (Aoyama et al., 2014).
To date, only one study has directly compared sex differences in incubation of sucrose craving (Madangopal et al., 2019). Although both males and females showed incubation of sucrose craving, this study did not find overt sex differences in sucrose-seeking after abstinence (1, 21, 60, 120 and 200 days). Other studies have examined the role of ovarian hormones on stress-induced food-seeking but no effects of ovarian hormones were observed (Calu et al., 2014; Pickens et al., 2011). Nevertheless, these findings do not exclude the possibility of ovarian hormones and the estrous cycle having a role in incubation of craving as it has been observed in humans and for other types of rewards (e.g. cocaine: Nicolas et al., 2019). Additionally, ovarian hormones and the estrous cycle have been previously shown to play a role in modulating the motivational responses to food cues (in the absence of reward delivery) in female rats (Alonso-Caraballo and Ferrario, 2019).
Importantly, either ovarian or testicular hormones can underlie sex-specific effects on reward-seeking. For example, abstinence from a junk-food diet enhanced NAcC glutamatergic transmission in males but not females (Alonso-Caraballo et al., 2020). Additionally, a role for testicular hormones in modulating the mesocorticolimbic reward system has also been described (Tobiansky et al., 2018). Overall, females show greater impulsive choice for food reward compared to males, and testicular hormones act to reduce impulsive choice in a food-seeking paradigm in males (Hernandez et al., 2020). As such, research studies examining the role of both ovarian and testicular gonadal hormones in reward-seeking (in general and during abstinence) is essential.
3.2. Abstinence-induced opioid-seeking in rodent models
Incubation of opioid craving has been described in animal models (Zhou et al., 2009), using procedures that incorporate forced or voluntary abstinence from drugs (Reiner et al., 2019). At the most basic level, forced abstinence involves removing the ability of the subject to obtain drug. The temporal effect of abstinence length on opioid-seeking behavior is consistent with an inverted U-shaped curve; there is typically a peak in opioid-seeking observed between 6–25 days post-abstinence, depending on conditions including the opioid itself, and whether context, cue, or stress is used to trigger reinstatement (Shalev et al., 2001).
In addition, there have been several recent studies examining incubation of opioid craving and relapse after voluntary abstinence in both males and females, including (Reiner et al., 2020; Venniro et al., 2019), and no sex differences have been observed in behavior. Interestingly, Venniro et al. (2019) showed that incubation of craving was observed in both sexes only after forced, but not voluntary, abstinence using their procedures (Venniro et al., 2017). Together, these findings suggest that the behavioral expression of incubation of opioid craving are similar in males and females, although it remains to be determined if the neurobiological mechanisms are the same. This is critical information for understanding the behavior itself and for considering translational studies aimed at mitigating incubation of craving. Interestingly, a recent study demonstrated that exogenously administered estradiol to freely cycling female rats nominally improved extinction of heroin-seeking, whereas a combination of estradiol and progesterone had a stronger impact (Vazquez et al., 2020). Although these findings point towards an important role of circulating gonadal hormones in regulating opioid-seeking, the study was under-powered to determine if estrous cycle stage was associated with levels of heroin seeking. As noted with Hernandez et al., 2020)above, it is also likely that male gonadal hormones play a role in incubation of craving. Regardless, these findings are consistent with a role for gonadal hormones in opioid seeking and further study is warranted.
4. Neural circuit-based mechanisms for abstinence-induced reward-seeking
There are multiple regions in the brain that mediate different aspects of reward-processing, motivation state, salience, and reward-seeking, with the mesocorticostriatal pathway being one of the most studied. Recent studies however have brought into sharp focus the effects of subcortical nuclei, such as the PVT and LH, and its interaction with the NAc. The LH in particular has emerged as a key brain region in mediating reward-seeking. The LH is the origin of brain-wide orexinergic projections (Peyron et al., 1998), which are involved in both arousal states and reward-related behavior. Accumulating evidence implicates LH orexin projections in mediating relapse to both drugs of abuse and natural rewards.
The PVT is considered part of hypothalamic–thalamic–striatal circuitry that integrates information related to motivation, reward, and energy balance control (Millan, 2017; Kelley et al., 2005). The major projections of the PVT are to the NAc, amygdala (Amy), and bed nucleus of the stria terminalis (BNST). These regions have been extensively studied in relation to motivated behavior, reward, aversion, and fear/anxiety (Kirouac, 2015). As such, the PVT is a lynchpin for coordinating responses to positive and negative affective states that lead to drug or food seeking and relapse to unregulated intake. The vast majority of PVT neurons project to the NAc (Dong et al., 2017), forming excitatory synaptic contact with NAc medium spiny neurons (MSNs). There is a distinct topography in which the anterior PVT (aPVT) projects preferentially to the dorsal NAc shell (NAcSh), whereas the posterior PVT (pPVT) projects preferentially to the ventromedial NAcSh and NAc core (NAcC) (Dong et al., 2017). This anatomical distinction is important, although not fully understood, because the dorsal and ventral subregions of the NAcSh can have opposing effects on motivated behavior (Al-Hasani et al., 2015; Marchant et al., 2009; Millan et al., 2017). The pPVT also projects strongly to the central nucleus of the amygdala (CeA), where it is understood to regulate the expression of fear responses (Do-Monte et al., 2015; Penzo et al., 2015). In this section we will focus on aspects of the hypothalamic–thalamic–striatal circuitry neuronal projections that mediate reward-seeking (see Fig.1).
Figure 1. Hypothalamic–thalamic–corticostriatal circuitry involved in food- and opioid-seeking.
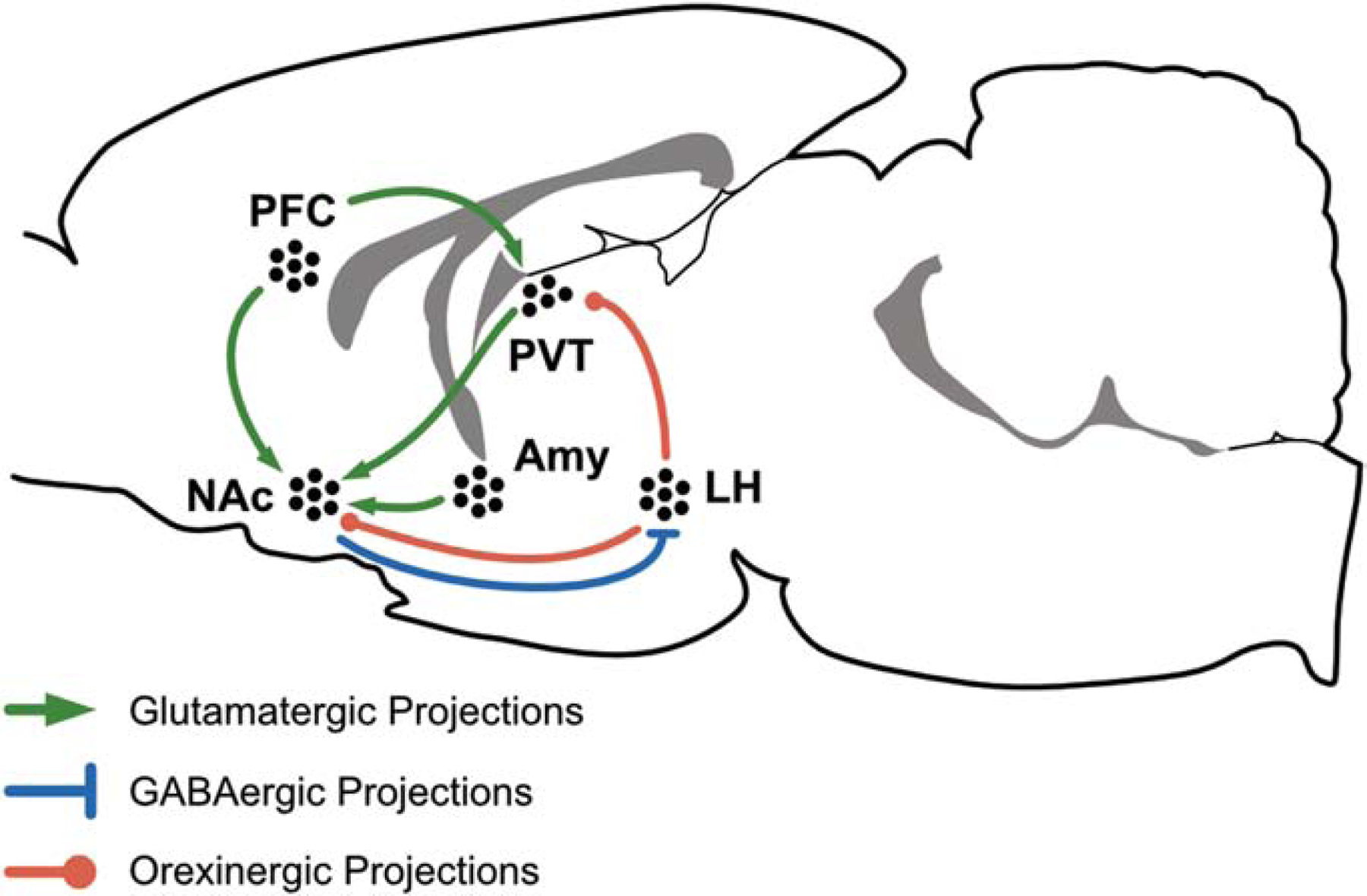
Schematic representation of long-range projections across cortical, hypothalamic, thalamic and limbic structures. Abbreviations: Amy, Amygdala; LH, Lateral Hypothalamus; NAc, Nucleus Accumbens; PVT, Paraventricular Nucleus of the Thalamus; PFC, Prefrontal Cortex. Tables 1 and 2 for roles of specific neural projections in either palatable food (Table 1) or opioid (Table 2) seeking.
An important consideration for the study of PVT projections and their functions is the high degree of collateralization in PVT projections. As an example, it was found through retrograde labeling that 50% of PVT neurons projecting to the dorsolateral BNST and the CeA also projected to the NAcSh (Dong, 2017). This extensive collateralization of PVT axons suggests this region coordinates a complex network of cortical and extended amygdala regions important for motivated behavior, reward-seeking, and emotional valence. To date, relatively little is known about how PVT collateralization translates to behavior, resulting in a gap in knowledge.
4.1. Neural circuit-based mechanisms for abstinence-induced food-seeking
Food cues elicit strong activation responses in the brain’s reward system areas. In this section, we will discuss potential neural circuit-based mechanisms underlying incubation of food craving. Our main focus will be on circuits composed of connections among the PVT, LH, Amy, and NAc, as well as projection-specific manipulations and their behavioral outcomes (Fig. 1 and Table 1). Both the PVT and the NAc are strategically positioned as major interfaces between cortical, hypothalamic and mesolimbic structures. In addition, both PVT and NAc play key roles in the control of feeding behaviors, motivation, reward and learning (for an extensive review on the PVT see Millan et al., 2017).
TABLE 1:
Projection-specific role in food-seeking behaviors.
| Projection | Role | References |
|---|---|---|
| PVT to NAc | Food-predictive signals, hunger-induced food-seeking and cue-induced food-seeking | Ong et al., 2017; Labouébe et al., 2016; Meffre et al., 2019; Do-Monte et al., 2017 |
| mPFC PFC to PVT | Food-primed-induced reinstatement Incentive salience attribution | Sun and Rebec, 2005 Campus et al., 2019 |
| Amy to NAc PVT to Amy | Food consumption and cue-induced food-seeking | Stuber et al., 2011; Do-Monte et al., 2017 |
| PFC to PVT to NAc | Cue-reward associations and cue-induced anticipation of food. | Otis et al., 2017;2019 |
| LH to PVT to NAc | Reward consumption and promotion of feeding | Otis et al., 2019 |
Abbreviations: Amy, Amygdala; BLA; Basolateral Amygdala; LH, Lateral Hypothalamus; NAc, Nucleus Accumbens; PVT, Paraventricular Nucleus of the Thalamus; PFC, Prefrontal Cortex; vmPFC, Ventro-medial PFC.
While the role of the PVT on abstinence-induced food-seeking or incubation of craving has not yet been meticulously examined, there are several studies that clearly describe the role of the PVT in modulating food cue motivated behaviors. For example, ibotenic acid lesions of the PVT increase motivational responses towards food-predictive cues in goal-tracker rats (Haight et al., 2015). These results suggest that the PVT plays a critical role in attenuating the attribution of incentive salience to reward-predictive cues. In contrast, PVT pharmacological inactivation with muscimol (GABAA agonist; Do-Monte et al., 2017) increases cue-induced sucrose-seeking (only when expected reward is omitted; a frustrative condition). In addition, specific intra-PVT activation of glucagon-like peptide receptor leads to decreased cue-induced sucrose-seeking (Ong et al., 2017), whereas specific stimulation of glucose transporter 2 (GLUT2) in the PVTGLUT2-NAc increases motivation to obtain sucrose in an operant conditioning task (Labouébe et al., 2016). Furthermore, photostimulation of aPVT projections to NAcSh decreases sucrose-seeking (Do-Monte et al., 2017), whereas aPVT-NAcSh photoinhibition increases sucrose-seeking (when expected reward was omitted). Additionally, facilitation of pPVT orexin-A transmission facilitated neuronal responses in the NAcC and photostimulation of pPVT to NAcC pathway led to increased cue-induced sucrose-seeking (Meffre et al., 2019). Both photostimulation and photoinhibition of aPVT projections to the central nucleus of the amygdala (CeA) inhibits sucrose-seeking in rats (Do-Monte et al., 2017). Moreover, PFC-PVT-NAc connections are involved in formation of cue-reward associations (Otis et al., 2017; 2019), whereas LH-PVT-NAc connections are involved in reward consumption and initiation of feeding behaviors (Otis et al., 2019). It has also been found that photoinhibition of BLA to NAc projections decreases sucrose-seeking (Stuber et al., 2011). When taken together, the wide range of methods, anatomical subregions, behavioral outcomes, and complexity of the systems makes it impossible to ascribe a single function to the PVT in the context of palatable food-seeking. However, the combination of findings described suggests the PVT integrates stimulus valence, affective and arousal states, and metabolic balance to stimulate or suppress food-seeking. This has important ramifications for treatment of compulsive food-related disorders.
Unfortunately, little is known about the role of the estrous cycle or male/female gonadal hormones in the modulation of these pathways. However, sex-specific effects of abstinence from a junk-food diet has been found in obesity-susceptible rats. In this case, NAcC glutamatergic transmission was enhanced in males but not females (Alonso-Caraballo et al., 2020). Given that gonadal hormones play an important role in food intake and motivational responses to food cues, it will be important to extend this line of research to studies of incubation of craving for food reinforcers.
4.2. Neural circuit-based mechanisms for abstinence-induced opioid-seeking
Chemogenetic and optogenetic studies of the PVT-NAc and NAc-LH pathways play key roles in mediating various aspects of addictive-like behaviors such as withdrawal-associated aversion, retrieval, and relapse to opioid-seeking behavior (Keyes et al., 2020; Zhu et al., 2016). Glutamatergic PVT neurons project onto both dopaminergic D1 and D2 receptor-expressing MSNs in the NAcSh. However, repeated opioid exposure increases synaptic potentiation specifically at PVT-D2-MSNs synapses (Zhu et al., 2016). D2-MSNs that project onto neighboring D1-MSNs enhances the feed-forward inhibition of these D1-MSNs that in turn precipitates opioid-relapse. Direct activation of these D1 NAcSh–LH neurons prevents relapse (Keyes et al., 2020). The role of the PVT in addiction is an area of active exploration. The PVT has connections—some being reciprocal—with multiple reward/aversion-related regions of the brain, and numerous studies implicate these pathways in addictive-like behavior (Zhou & Zhu, 2019). LH orexinergic neurons play a major role in motivated behavior, including cocaine- and opioid-seeking in rodent models of addictive-like behavior (James et al., 2017). Studies pairing opioid self-administration and reinstatement models, as well as behavioral economics with systemic inhibition of orexin receptors have shown that the orexinergic system regulates motivation towards drug-seeking (James et al., 2017). However, the projection-specific role of orexinergic neurons in mediating motivation towards opioid-seeking remains to be explored. Studies from natural reward and LH–PVT projections (Meffre et al., 2019) lend credence to this. In addition, projections to the NAc from the ventro-medial prefrontal cortex (vmPFC) mediate opioid-seeking (Bossert et al., 2012). Context-induced heroin-reinstatement is mediated through interaction of vmPFC glutamatergic projections and postsynaptic dopaminergic D1 signaling.
5. Conclusions
This review summarizes a relatively nascent collection of studies suggesting a role for hypothalamic–thalamic–striatal circuitry in both opioid- and palatable food-seeking during abstinence—a strong preclinical model of relapse. Specifically, connections among the LH, PVT, NAc, and AMY serve to integrate internal and external signals related to affective state, arousal, metabolic balance, and motivation to drive relapse-related behaviors. The similarities between opioid- and food- seeking behavior and neural mechanisms are striking and often contrary to literature on psychostimulants (Badiani et al., 2011).
Few studies have as yet directly compared behaviors and neural mechanisms of incubation of craving in both males and females. On balance, the studies that have been reported find similar behavioral responses: both males and females show an increase in reward-seeking with abstinence. This is critical information, as the development of effective treatments depends on how both males and females would respond. It also highlights the importance of digging deeper to parse out the molecular, cellular, and circuit-based mechanisms for behavior. As discussed, gonadal hormones regulate reward-seeking and reward-based behavior, and since estrogen and testosterone are obviously different hormones, it is likely that different mechanisms underlie behavioral responses. This is a rich area for discovery.
Through this review, our goal was to describe what is known thus far and then identify some key gaps in knowledge that we and others can address in future preclinical and clinical studies. For example, one gap is identifying the neural circuits and modulators that connect affective state to motivated behavior. Specifically, does cue-triggered reward-seeking during abstinence arise through positive or negative mood states, and what brain region(s) with hypothalamic–thalamic–striatal circuitry is necessary? Another gap in knowledge is detailed understanding of how neurotransmitters (e.g. glutamate, GABA) and neuromodulators (e.g. dopamine, orexin, dynorphin) interact within this circuitry to affect specific aspects of motivated behavior related to opioid- and palatable food-seeking. There are numerous other questions, but a final gap in knowledge raised in this review is a general lack of understanding of the impact of biological sex on the functioning of this fundamental circuit.
TABLE 2:
Projection-specific role in opioid-seeking behaviors.
| Projection | Role | References |
|---|---|---|
| PFC to NAc | Expression of withdrawal-associated aversion. | Zhu et al., 2016 |
| PVT to NAc to LH | Relapse to context-induced opioid-seeking. | Keyes et al., 2020 |
| vmPFC to NAc | Context-induced opioid relapse. | Bossert et al., 2012 |
| LH orexinergic projections | Motivation for opioid-seeking | James et al., 2017 |
Abbreviations: Amy, Amygdala; BLA; Basolateral Amygdala; LH, Lateral Hypothalamus; NAc, Nucleus Accumbens; PVT, Paraventricular Nucleus of the Thalamus; PFC, Prefrontal Cortex; vmPFC, Ventro-medial PFC.
Highlights.
Abstinence-induced craving and reward-seeking are phenomena described in humans and animals, respectively, and they are thought to underlie relapse.
We speculate that hypothalamic-thalamic-striatal circuitry is a key modulator of abstinence-induce reward-seeking.
Biological sex and gonadal hormones affect incubation of craving depending on the reinforcer and the method of abstinence, but underlying mechanisms are not fully understood.
Acknowledgments:
We thank Ms. Jameson Morris-Kliment, MA (Arthur Vining Davis Professor of Greek and Latin; Chair, Classics Department, The Roxbury Latin School, Boston, MA) for insightful discussions on the classical relevance of abstinence and incubation of craving; culminating in a quote and its English translation from the Roman poet Sextus Propertius.
Funding: This work was supported by the National Institutes of Health [R01DA045000] and [K00DA053527].
Abbreviations:
- Amy
amygdala
- BLA
basolateral amygdala
- BOLD
blood-oxygen-level-dependent imaging
- CeA
central amygdala
- GLUT2
glucose transporter 2
- LH
lateral hypothalamus
- MSNs
medium spiny neurons
- NAc
nucleus accumbens
- NacC
NAc core
- NAcSh
NAc shell
- PVT
paraventricular nucleus of the thalamus
- aPVT
anterior PVT
- pPVT
posterior PVT
- PFC
prefrontal cortex
- OUD
opioid use disorder
- SUD
substance use disorder
- vmPFC
ventro medial PFC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: The authors have no declarations of interest.
References
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park S II, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, & Bruchas MR (2015). Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron, 87(5), 1063–1077. 10.1016/j.neuron.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, Rifkin-Graboi A, Yannakoulia M, Rojas R, Pascual-Leone A, & Mantzoros CS (2011). Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: Relation to fasting and feeding. Am J Clin Nut, 94(2), 377–384. 10.3945/ajcn.110.010736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caraballo Y, & Ferrario CR (2019). Effects of the estrous cycle and ovarian hormones on cue-triggered motivation and intrinsic excitability of medium spiny neurons in the Nucleus Accumbens core of female rats. Hormones and Behavior, 116, 104583–104583. 10.1016/j.yhbeh.2019.104583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caraballo Y, Fetterly TL, Jorgensen ET, Nieto AM, Brown TE, & Ferrario CR (2020). Sex specific effects of “junk-food” diet on calcium permeable AMPA receptors and silent synapses in the nucleus accumbens core. Neuropsychopharmacology, 1–10. 10.1038/s41386-020-0781-1 [DOI] [PMC free article] [PubMed]
- Aoyama K, Barnes J, & Grimm JW (2014). Incubation of saccharin craving and within-session changes in responding for a cue previously associated with saccharin. Appetite, 72, 114–122. 10.1016/j.appet.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, & Brady KT (2011). Characteristics and correlates of men and women with prescription opioid dependence. Addictive Behaviors, 36(8), 829–834. 10.1016/j.addbeh.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, & Shaham Y (2011). Opiate versus psychostimulant addiction: the differences do matter. Nat Rev, Neurosci, 12(11), 685–700. 10.1038/nrn3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FRM, Marchant NJ, Wang H-L, Morales M, & Shaham Y (2012). Role of Projections from Ventral Medial Prefrontal Cortex to Nucleus Accumbens Shell in Context-Induced Reinstatement of Heroin Seeking. J Neurosci, 32(14), 4982–4991. 10.1523/JNEUROSCI.0005-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, & Prentice AM (1995). Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiology and Behavior, 58(6), 1067–1077. 10.1016/0031-9384(95)02003-9 [DOI] [PubMed] [Google Scholar]
- Calu DJ, Chen YW, Kawa AB, Nair SG, & Shaham Y (2014). The use of the reinstatement model to study relapse to palatable food seeking during dieting In Neuropharmacology (Vol. 76, Issue PART B, pp. 395–406). Elsevier; 10.1016/j.neuropharm.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campus P, Covelo IR, Kim Y, Parsegian A, Kuhn BN, Lopez SA, … Flagel SB (2019). The paraventricular thalamus is a critical mediator of top-down control of cue-motivated behavior in rats. ELife, 8 10.7554/eLife.49041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, & Carlezon WA (2014). Drug withdrawal conceptualized as a stressor. Behavioural Pharmacology, 25(5–6), 473–492. 10.1097/FBP.0000000000000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, & McHugh RK (2016). Translational Studies of Sex Differences in Sensitivity to Opioid Addiction. Neuropsychopharmacology, 41(1), 383–384. 10.1038/npp.2015.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, & Harney J (2015). Shifting Patterns of Prescription Opioid and Heroin Abuse in the United States. NEJM, 373(18), 1789–1790. 10.1056/NEJMc1505541 [DOI] [PubMed] [Google Scholar]
- Counotte DS, Schiefer C, Shaham Y, & O’Donnell P (2014). Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology, 231(8), 1675–1684. 10.1007/s00213-013-3294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling RA, Dingess PM, Schlidt KC, Smith EM, & Brown TE (2016). Incubation of food craving is independent of macronutrient composition. Scientific Reports, 6 10.1038/srep30900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte Fabricio H., Minier-Toribio Angélica, Quiñones-Laracuente Kelvin, Medina-Colón Estefanía M., & Quirk Gregory J. (2017). Thalamic Regulation of Sucrose Seeking during Unexpected Reward Omission. Neuron, 94(2), 388–400. 10.1016/j.neuron.2017.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte Fabricio H., Quiñones-Laracuente Kelvin, & Quirk Gregory J. (2015). A temporal shift in the circuits mediating retrieval of fear memory. Nature, 519(7544), 460–463. 10.1038/nature14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Li S, & Kirouac GJ (2017). Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Br Struct Funct, 222(9), 3927–3943. 10.1007/s00429-017-1445-8 [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Miller EJ, Hillman CH, Bradley MM, Cuthbert BN, & Lang PJ (2001). Food deprivation and emotional reactions to food cues: Implications for eating disorders. Biol Psychol, 57(1–3), 153–177. 10.1016/S0301-0511(01)00093-X [DOI] [PubMed] [Google Scholar]
- Evans CJ, & Cahill CM (2016). Neurobiology of opioid dependence in creating addiction vulnerability. F1000Research, 5, F1000 Faculty Rev-1748. 10.12688/f1000research.8369.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, & O’Connor EC (2016). Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci, 36(45), 11469–11481. 10.1523/JNEUROSCI.2338-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TC, Kim GL, Krzemien A, & Van Vugt DA (2010). Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Br Res, 1363, 81–92. 10.1016/j.brainres.2010.09.071 [DOI] [PubMed] [Google Scholar]
- Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, & Jansen A (2012). Reward activity in satiated overweight women is decreased during unbiased viewing but increased when imagining taste: an event-related fMRI study. Int J Obes (Lond), 36(5), 627–637. 10.1038/ijo.2011.213 [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, & Vincent DJ (2001). Activation of Prefrontal Cortex and Anterior Thalamus in Alcoholic Subjects on Exposure to Alcohol-Specific Cues. Arch Gen Psych, 58(4), 345 10.1001/archpsyc.58.4.345 [DOI] [PubMed] [Google Scholar]
- Gorin AA, Phelan S, Wing RR, & Hill JO (2004). Promoting long-term weight control: Does dieting consistency matter? Intl J Obesity, 28(2), 278–281. 10.1038/sj.ijo.0802550 [DOI] [PubMed] [Google Scholar]
- Grimm JW (2020). Incubation of food craving in rats: A review. J Exp Anal Behav, 113(1), 37–47. 10.1002/jeab.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, & Shaham Y (2001). Incubation of cocaine craving after withdrawal. Nature, 412(6843), 141–142. 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight Joshua L., Fraser Kurt M., Akil Huda, & Flagel Shelly B. (2015). Lesions of the paraventricular nucleus of the thalamus differentially affect sign-and goal-tracking conditioned responses. Eur J Neurosci, 10.1111/ejn.13031 [DOI] [PMC free article] [PubMed]
- Hallam J, Boswell RG, DeVito EE, & Kober H (2016). Gender-related Differences in Food Craving and Obesity. Yale J Biol Med, 89(2), 161–173. [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Orsini C, Wheeler AR, Eyck TWT, Betzhold SM, Labiste CC, … Bizon JL (2020). Testicular hormones mediate robust sex differences in impulsive choice in rats. ELife, 9, 1–21. 10.7554/ELIFE.58604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, & Aston-Jones G (2017). A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? In Curr Topics Behav Neurosci (Vol. 33, pp. 247–281). Springer Verlag; 10.1007/7854_2016_57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, & Will MJ (2005). Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Phys Behav, 86(5), 773–795. 10.1016/j.physbeh.2005.08.066 [DOI] [PubMed] [Google Scholar]
- Keyes PC, Adams EL, Chen Z, Bi L, Nachtrab G, Wang VJ, Tessier-Lavigne M, Zhu Y, & Chen X (2020). Orchestrating Opiate-Associated Memories in Thalamic Circuits. Neuron, 107, 1–11. 10.1016/j.neuron.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, & Yurgelun-Todd DA (2010). Sex differences in cerebral responses to images of high versus low-calorie food. NeuroReport, 21(5), 354–358. 10.1097/WNR.0b013e32833774f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ (2015). Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neuroscience & Biobehavioral Reviews, 56, 315–329. 10.1016/j.neubiorev.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Koob GF (2020). Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psych. 10.1016/j.biopsych.2019.05.023 [DOI] [PubMed]
- Labouèbe G, Boutrel B, Tarussio D, & Thorens B (2016). Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci, 19(8), 999–1002. 10.1038/nn.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, & Frantz KJ (2010). Time-dependent increases in cue-induced reinstatement of sucrose seeking after sucrose self-administration in adolescence. Behav Br Res, 213(1), 109–112. 10.1016/j.bbr.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, Wu N, Chang H, Zheng Y, Qin W, Zhao L, Yuan K, Liu J, Wang W, & Tian J (2012). Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: An event-related fMRI study. Br Res, 1469, 63–72. 10.1016/j.brainres.2012.06.024 [DOI] [PubMed] [Google Scholar]
- Madangopal R, Tunstall BJ, Komer LE, Weber SJ, Hoots JK, Lennon VA, Bossert JM, Epstein DH, Shaham Y, & Hope BT (2019). Discriminative stimuli are sufficient for incubation of cocaine craving. ELife, 8 10.7554/eLife.44427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, & McNally GP (2009). Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci, 29(5), 1331–1342. 10.1523/JNEUROSCI.5194-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, & Savage CR (2010). Neural Mechanisms Associated With Food Motivation in Obese and Healthy Weight Adults. Obesity, 18(2), 254–260. 10.1038/oby.2009.220 [DOI] [PubMed] [Google Scholar]
- McCue DL, Kasper JM, Ara A, & Hommel JD (2019). Incubation of feeding behavior is regulated by neuromedin U receptor 2 in the paraventricular nucleus of the hypothalamus. Behav Br Res, 359, 763–770. 10.1016/j.bbr.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, DeVito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, Connery HS, & Weiss RD (2013). Gender differences in a clinical trial for prescription opioid dependence. J Sub Ab Treat, 45(1), 38–43. 10.1016/j.jsat.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Park S, & Weiss RD (2014). Cue-induced craving in dependence upon prescription opioids and heroin. A J Addictions, 23(5), 453–458. 10.1111/j.1521-0391.2014.12129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Sugarman DE, & Greenfield SF (2018). Sex and gender differences in substance use disorders. Clinical Psychology Review, 66, 12–23. 10.1016/j.cpr.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre J, Sicre M, Diarra M, Marchessaux F, Paleressompoulle D, & Ambroggi F (2019). Orexin in the Posterior Paraventricular Thalamus Mediates Hunger-Related Signals in the Nucleus Accumbens Core. Current Biology, 29(19), 3298–3306.e4. 10.1016/j.cub.2019.07.069 [DOI] [PubMed] [Google Scholar]
- Millan EZ, Ong ZY, & McNally GP (2017). Paraventricular thalamus: Gateway to feeding, appetitive motivation, and drug addiction In Progress in Brain Research (Vol. 235, pp. 113–137). Elsevier B.V. 10.1016/bs.pbr.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, & Shaham Y (2009). The neuropharmacology of relapse to food seeking: Methodology, main findings, and comparison with relapse to drug seeking In Progress in Neurobiology (Vol. 89, Issue 1, pp. 18–45). 10.1016/j.pneurobio.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, McCarthy MM, Shaham Y, & Ikemoto S (2019). Incubation of Cocaine Craving After Intermittent-Access Self-administration: Sex Differences and Estrous Cycle. Biological Psychiatry, 85(11), 915–924. 10.1016/j.biopsych.2019.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noye Tuplin EW, Lightfoot SHM, & Holahan MR (2018). Comparison of the time-dependent changes in immediate early gene labeling and spine density following abstinence from contingent or non-contingent chocolate pellet delivery. Frontiers in Behavioral Neuroscience, 12, 144–144. 10.3389/fnbeh.2018.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien Charles P. (1997). A range of research-based pharmacotherapies for addiction. Science, 278(5335), 66–70. 10.1126/science.278.5335.66 [DOI] [PubMed] [Google Scholar]
- Ong Zhi Yi, Liu Jing Jing, Pang Zhiping P., & Grill Harvey J. (2017). Paraventricular thalamic control of food intake and reward: Role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology, 42(12), 2387–2397. 10.1038/npp.2017.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Namboodiri VMK, Matan AM, Voets ES, Mohorn EP, Kosyk O, McHenry JA, Robinson JE, Resendez SL, Rossi MA, & Stuber GD (2017). Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature, 543(7643), 103–107. 10.1038/nature21376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Zhu M, Namboodiri VMK, Cook CA, Kosyk O, Matan AM, Ying R, Hashikawa Y, Hashikawa K, Trujillo-Pisanty I, Guo J, Ung RL, Rodriguez-Romaguera J, Anton ES, & Stuber GD (2019). Paraventricular Thalamus Projection Neurons Integrate Cortical and Hypothalamic Signals for Cue-Reward Processing. Neuron, 103(3), 423–431.e4. 10.1016/j.neuron.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, & Li B (2015). The paraventricular thalamus controls a central amygdala fear circuit. Nature, 519(7544), 455–459. 10.1038/nature13978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van Den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, & Kilduff TS (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 10.1523/jneurosci.18-23-09996.1998 [DOI] [PMC free article] [PubMed]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, & Shaham Y (2011). Neurobiology of the incubation of drug craving. Trends Neurosci, 34(8), 411–420. 10.1016/j.tins.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, & Shaham Y (2019). Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology, 44(3), 465–477. 10.1038/s41386-018-0234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Lofaro OM, Applebey SV, Korah H, Venniro M, Cifani C, … Shaham Y (2020). Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice-induced voluntary abstinence. J Neuroscience, 40(12), 2485–2497. 10.1523/JNEUROSCI.2693-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney JR, & Simmons ZL (2017). Ovarian hormone fluctuations predict within-cycle shifts in women’s food intake. Hormones and Behavior, 90, 8–14. 10.1016/j.yhbeh.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, & Shaham Y (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl), 156(1), 98–107. [DOI] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, & Craighead L (2005). Cue-elicited craving for food: A fresh approach to the study of binge eating. Appetite, 44(3), 253–261. 10.1016/j.appet.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, Van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, & Bonci A (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475(7356), 377–382. 10.1038/nature10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Li S, Yang L, & Zheng M (2020). Reduced response inhibition after exposure to drug-related cues in male heroin abstainers. Psychopharmacology, 237(4), 1055–1062. 10.1007/s00213-019-05434-6 [DOI] [PubMed] [Google Scholar]
- Sun Wen Lin, & Rebec George V. (2005). The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology, 177(3), 315–323. 10.1007/s00213-004-1956-x [DOI] [PubMed] [Google Scholar]
- Tobiansky DJ, Wallin-Miller KG, Floresco SB, Wood RI, & Soma KK (2018). Androgen Regulation of the Mesocorticolimbic System and Executive Function. Frontiers in Endocrinology, 9(JUN), 1 10.3389/fendo.2018.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez M, Frazier JH, Reichel CM, & Peters J (2020). Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learning & memory (Cold Spring Harbor, N.Y.), 27(1), 6–11. 10.1101/lm.050187.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, & Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence In Prog Br Res (2016/01/30, Vol. 224, pp. 25–52). 10.1016/bs.pbr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Zhang M, & Shaham Y (2019). Operant Social Reward Decreases Incubation of Heroin Craving in Male and Female Rats. Biol Psych, 86(11), 848–856. 10.1016/j.biopsych.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, & Caprioli D (2017). Incubation of Methamphetamine but not Heroin Craving after Voluntary Abstinence in Male and Female Rats.Neuropsychopharmacology,42(5),1126–1135. 10.1038/npp.2016.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, & Baler RD (2013). Obesity and addiction: Neurobiological overlaps. Obesity Reviews, 14(1), 2–18. 10.1111/j.1467-789X.2012.01031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J (1990). Conditioning processes and cue exposure in the modification of excessive eating. Addictive Behaviors, 15(4), 387–393. 10.1016/0306-4603(90)90047-2 [DOI] [PubMed] [Google Scholar]
- Woods SC, Gotoh K, & Clegg DJ (2003). Gender Differences in the Control of Energy Homeostasis. Experimental Biology and Medicine, 228(10), 1175–1180. 10.1177/153537020322801012 [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, & Kalivas PW (2009). Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology (Berl), 203(4), 677–684. 10.1007/s00213-008-1414-2 [DOI] [PubMed] [Google Scholar]
- Zhou K, & Zhu Y (2019). The paraventricular thalamic nucleus: A key hub of neural circuits underlying drug addiction Pharmacological Research. Academic Press; 10.1016/j.phrs.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Zhu Yingjie, Wienecke Carl F. R., Nachtrab Gregory, & Chen Xiaoke. (2016). A thalamic input to the nucleus accumbens mediates opiate dependence. Nature, 530(7589), 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]


