Abstract
Objectives:
To identify predictors of healthy arterial aging [long-term coronary artery calcification (CAC) of 0] among individuals with metabolic syndrome (MetS) or type 2 diabetes (T2D), which may improve primary prevention strategies.
Background:
Individuals with MetS or T2D have a heterogeneously increased risk of atherosclerotic cardiovascular disease (ASCVD) and not all have a high-intermediate risk.
Methods:
We included 574 participants from the Multi-Ethnic Study of Atherosclerosis with MetS or T2D who had CAC=0 at baseline and a repeat CAC scan 10 years later. Multivariable logistic regression assessed the association of traditional and novel ASCVD risk factors and the MetS severity score (based on the five MetS criteria) with healthy arterial aging.
Results:
The mean age of participants was 58.9 years, 67% were women, 422 participants had MetS, and 152 had T2D. The proportion with long-term CAC=0 was similar for MetS (42%) and T2D (44%). A younger age was the only individual low/normal traditional risk factor associated with an increased likelihood of long-term CAC=0 (OR=1.50, 95% CI: 1.22–1.85 per 10-years younger). The strongest associations of nontraditional risk factors were observed for an absence of thoracic calcification (OR=2.42, 95% CI: 1.24–4.72), absence of carotid plaque (OR=1.81, 95% CI: 1.25–2.61) and among persons with a high sensitivity troponin < 3 ng/mL (OR=1.55, 95% CI: 1.01–2.38). In addition, persons with the lowest quartile MetS severity score had a substantially higher odds of healthy long-term CAC=0 (OR=2.71, 95% CI: 1.27–5.76).
Conclusion:
More than 40% of adults with MetS or T2D and baseline CAC=0 had long-term absence of CAC, which was most strongly associated with an absence of extra-coronary atherosclerosis and a low MetS score. An optimal overall cardiovascular profile appears to be more important than an ideal value of any individual risk factor to maintain healthy arterial aging.
Keywords: coronary artery calcium, aging, diabetes mellitus, type 2, metabolic syndrome, prevention, healthy lifestyle, risk, cardiovascular diseases, multidetector computed tomography, atherosclerosis
Introduction
The prevalence of metabolic syndrome and type 2 diabetes disease have increased sharply over the past three decades(1,2) and now currently affect 115 million (35%)(3) and 30 million (9%)(4) individuals in the United States, respectively. While both of these conditions are often considered atherosclerotic cardiovascular disease (ASCVD) risk equivalents(5,6), there is significant heterogeneity in the risk for a future ASCVD event(7). In particular, approximately 40% of individuals with metabolic syndrome and/or type 2 diabetes do not have coronary artery calcium (CAC=0)(8–10). The absence of coronary artery calcium, as measured by non-contrast computed tomography, is associated with a very low risk for incident ASCVD(11), with a long-term event rate of <7/1,000 person-years of follow-up(8). These observations underline a need to further understand the predictors of healthy arterial aging (persistent long-term CAC=0) among individuals with metabolic syndrome and/or type 2 diabetes in order to develop a more precise approach for the primary prevention of ASCVD.
Coronary artery calcium (CAC), or the lack thereof, reflects the contribution of cumulative risk factor exposure, genetics, and susceptibility(12). While the risk factors associated with CAC progression are well-established(13), the predictors associated with persistent long-term CAC=0 remain largely unexplored. Furthermore, prospective studies of CAC conducted among individuals with metabolic syndrome or type 2 diabetes have been limited to relatively short follow-up times, and focused solely on CAC progression and traditional ASCVD risk factors(8,14). Understanding the risk predictors, or absence of risk factors, associated with persistent CAC=0 among individuals with metabolic syndrome or type 2 diabetes may provide insight into 1) the predictors of healthy arterial aging in this patient group that is otherwise considered at high risk for ASCVD and 2) which patients with metabolic syndrome or type 2 diabetes are most likely to have long-term healthy arterial aging.
Methods
Study Population
MESA is a community-based, multiethnic cohort study of 6,814 men and women free of clinical ASCVD 45 to 84 years old at baseline, which has been described in detail elsewhere(15). Participants with CAC > 0 at Visit 1 (n=3,398), participants without a repeat CAC scan at Visit 5 (n=1,566), and those who did not have metabolic syndrome or diabetes at baseline (n=1,276) were excluded resulting in a cohort of 574 individuals with CAC=0 and metabolic syndrome or type 2 diabetes at MESA Visit 1 (2000 to 2002) (Supplemental Figure 1). This group of 574 individuals was compared to participants with CAC=0 at baseline who had metabolic syndrome or type 2 diabetes that were not randomized to a follow-up CAC scan (n=559) (Supplemental Table 1). Type 2 diabetes was defined by a fasting blood glucose > 126 mg/mL (hemoglobin A1c was not measured at MESA Visit 1), reported utilization of insulin and/or oral hypoglycemic agents, or self-reported diabetes. Metabolic syndrome was defined using the National Cholesterol Education Program ATP III guidelines(16), characterized by any three out of the five following traits: 1) waist circumference > 102 cm in men or > 88 cm in women; 2) serum triglycerides ≥ 150 mg/mL or drug treatment for elevated triglycerides; 3) serum HDL-C < 40 mg/dL in men or < 50 mg/dL in women; 4) blood pressure ≥ 130/85 mmHg or drug treatment for elevated blood pressure; and 5) fasting blood glucose ≥ 100 mg/dL or drug treatment for elevated blood glucose.
All study participants provided written informed consent at each examination, and study protocols were approved by site-specific Institutional Review Boards at respective MESA-participating institutions.
Measurement of coronary and thoracic calcification
All MESA participants underwent a CAC scan at Visit 1, while half of MESA participants at Visit 5 were randomized to a CAC scan. Calcium scores were computed using the Agatston method and standardization of results among field centers was achieved using calcium phantoms scanned alongside participants(17,18). The phantom had 4 bars of known calcium density and was used to calibrate the level of brightness between study subjects and sites. The Chicago, Los Angeles, and New York field centers used electron beam computed tomography scanners and the Baltimore, Forsyth County, and St. Paul field centers used multidetector computed tomography scanners to acquire CAC scans.
Rescan agreement was robust for both electron beam computed scanners and multidetector computed tomography scanners with a Kappa statistic for interobserver and intraobserver agreement of 0.93 and 0.90, respectively(11,19). Using the Agatston method, the ascending aorta (aortic annulus to the lower edge of pulmonary artery) and descending aorta (lower edge of pulmonary artery to the cardiac apex) were evaluated for the presence (Agatston score ≥1) or absence (Agatston score =0) of thoracic aortic calcification(20).
General Clinical Examination, ASCVD Risk Factors, and ASCVD events
Standardized survey methods were used to collect demographic and clinical information, including sex, race, education status, smoking status, and medication utilization history(15). Resting blood pressure was measured in triplicate on the right arm after five minutes in the seated position and the average of the second and third readings was used in analyses. Waist circumference was measured at the level of the umbilicus using a Gullick II 150 cm anthropometric steel measuring tape with standard 4-ounce tension (Sammons Preston, Chicago, IL)(21). Fasting blood glucose was measured using the Vitros 950 analyzer (Johnson & Johnson, Rochester, NY)(22). Fasting lipid values were measured using the cholesterol oxidase method (Roche Diagnostics)(22) and low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation(23). Using the five traditional metabolic syndrome components of 1) waist circumference, 2) systolic blood pressure, 3) HDL-C, 4) serum triglycerides and 5) fasting blood glucose, we calculated sex- and race-specific metabolic syndrome severity scores for each study participant(24–26). As previously described in greater detail (24), a confirmatory factor analysis was performed to establish the weighted contribution of each component to a latent metabolic syndrome factor on a sex- and race-specific basis. For each of the eight subgroups based on sex and race (Caucasian, African American, Hispanic, Chinese) factor loadings from the five metabolic syndrome components were determined and utilized to produce equations for calculating a metabolic syndrome severity score for each subgroup(24–26). The metabolic syndrome severity scores were initially derived from adults aged 20–64 years participating in the National Health and Nutrition Examination Survey(24) and have been since utilized in several cohorts, including the Atherosclerosis Risk and Communities (ARIC), Jackson Heart Study, Bogalusa Heart Study, and Princeton Lipid Research cohorts(27–29). The resulting score has a standard normal distribution that functions as a z-score. Serum creatinine was quantified using the Kinetic Jaffe method and was used to calculate estimated glomerular filtration rate (eGFR) via the CKD-EPI equation(30). Semiquantitative urine protein dipstick analysis was performed on collected urine samples. The presence of a carotid artery atherosclerotic plaque was defined by a distinct, focal wall thickening > 1.5 cm or focal thickening > 50% than the surrounding intima-media thickness and was measured using an M12L transducer (General Electric Medical Systems; common carotid artery frequency, 13 MHz)(31,32). Serum levels of high sensitivity C-reactive protein (hs-CRP) were measured using the BNIII nephelometer (Dade Behring, Deerfield, IL)(33). Cardiac biomarkers, N-terminal pro-brain natriuretic peptide (NT-proBNP) and high sensitivity cardiac troponin T (hs-cTnT), were quantified in EDTA plasma and were measured on the Cobas e601(Roche Diagnostics, Indianapolis, IN)(34). Lipoprotein a (Lp(a)) concentration was quantified using a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan)(35). Carotid plaque (n=545), NT-proBNP (n=474), and Lp(a) (n=303) were measured among a subset of all study participants (n=574).
ASCVD events were defined as incident coronary heart disease, incident stroke, or other incident ASCVD and adjudicated per a previously described MESA protocol(15). In order to examine differences in ASCVD event rates between participants with and without long-term persistent CAC=0, Visit 5 served as the baseline for follow-up time for all ASCVD event analyses.
Statistical Analysis
Study population characteristics are presented as means and standard deviations for continuous variables, while percentages are used for categorical variables. Normality of continuous variable distribution was assessed via the Kolmogorov-Smirnov test. The Student’s t-test and Wilcoxon signed-rank test were used to assess differences in normally and non-normally distributed continuous variables, respectively. Differences between categorical variables were evaluated through the Chi-square test.
We categorized continuous predictor variables as normal or elevated to assess their association with long term absence of CAC using the following interval values: waist circumference <102 cm for men and <88 cm for women, systolic and blood pressure <130 and diastolic blood pressure <80 mm Hg(36), fasting blood glucose <100 mg/dL, fasting serum triglycerides <150 mg/dL(37), and total cholesterol/HDL-C ratio <3.5(38), NT-proBNP < 125 pg/mL(39), hs-cTnT < 3 ng/mL(40), Lp(a) < 50 mg/dL for non-Hispanic White, Hispanic, and Chinese and < 30 mg/dL for non-Hispanic Black(35), eGFR ≥60 mL/min/1.73 m2, absence of urine protein, hsCRP < 2 mg/L(33), and absence of thoracic calcification. Lastly, individuals were categorized into quartiles based on their metabolic syndrome severity score consistent with prior publications(28,41), and the highest quartile was compared to the lowest quartile.
The association of ASCVD predictor variables with persistent CAC=0 was assessed through multivariable logistic regression adjusting for age, sex, race, education, antihypertensive medication, lipid-lowering medication, glucose-lowering medication, cigarette smoking, waist circumference, blood pressure, fasting blood glucose, fasting serum triglycerides, and total cholesterol/HDL-C ratio. The association between the metabolic syndrome severity score and persistent CAC=0 was assessed in the latter fully adjusted model and a model containing only age, sex, race, education, antihypertensive medication, lipid-lowering medication, glucose-lowering medication and cigarette smoking. The association between the Pooled Cohort Equations (PCE) ASCVD risk score and persistent CAC=0 was assessed in a bivariate logistic regression analysis using a cutoff of 7.5%. In order to investigate if there was a phenotype or specific group of risk predictors most strongly associated with persistent CAC=0 we examined the discriminative ability of groups of continuous ASCVD predictor variables using the concordance statistic via multivariable logistic regression modeling. The nonparametric approach developed by DeLong, DeLong, and Clarke-Pearson was used to compare ROC curves between models(42). ASCVD events were expressed as absolute numbers and proportions for each respective study sample. The total number of events was divided by person-years to calculate ASCVD rates (per 1,000-year follow-up). For the main study sample, 23 out of 574 persons experienced an event in between MESA Visit 1 and the follow-up CAC scans at Visit 5 and were thus excluded from the events analysis.
We performed three separate sensitivity analyses controlling for diabetes duration (insulin utilization at Visit 1), diabetes control (glycated hemoglobin measured at Visit 2), and changes in metabolic health over time to examine how these additional measures of risk may impact our results. Changes in metabolic health over time were assessed by averaging the metabolic syndrome severity score from MESA Visit 1 (2000–02), Visit 2 (2002–04), and Visit 3 (2004–05). We also conducted a sensitivity analysis calculating the ASCVD event rates for 1) participants with baseline CAC=0 and metabolic syndrome or diabetes, but without randomization to a follow-up CAC scan at Visit 5 (n=342) and 2) participants with baseline CAC=0, but without metabolic syndrome or type 2 diabetes who were and were not randomized to a follow-up CAC scan (n=1,988).
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). All hypothesis tests were two-sided. We used an alpha threshold of 0.05 for detecting differences in descriptive statistics and for detecting significant odds ratios in logistic regression models.
Results
The average age of the study population was 58.9 years (SD 9.1) and two-thirds of individuals were female (Table 1). There were 422 participants with metabolic syndrome and 152 with type 2 diabetes at baseline. Individuals with long-term absence of CAC were significantly younger, had lower fasting blood glucose, lower hs-cTnT, a higher eGFR, and were less likely to be prescribed a lipid lowering medication compared to CAC progressors. Those with persistent CAC=0 were also significantly less likely to have a carotid artery plaque or thoracic aortic calcification. Among participants with metabolic syndrome or type 2 diabetes and baseline CAC=0, individuals randomized to follow-up CAC scans were younger and had a lower prevalence of diabetes compared to those who were not randomized to follow-up CAC scans (Supplemental Table 1).
Table 1.
Characteristics of 574 MESA Participants with Metabolic Syndrome and/or Type 2 Diabetes Mellitus
| Variable | All (n=574) | Long-Term CAC=0 (n=240) | Incident CAC (n=334) | P-Value § |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age, years | 58.9 (9.1) | 56.8 (8.9) | 60.3 (8.9) | < 0.0001 |
| Female, % | 66.6 | 68.8 | 65.0 | 0.34 |
| Race, % | ||||
| Caucasian | 29.5 | 29.2 | 29.6 | 0.98 |
| Chinese | 8.5 | 8.7 | 8.4 | |
| African American | 33.6 | 32.9 | 34.1 | |
| Hispanic | 28.4 | 29.2 | 27.9 | |
| Traditional CVD Risk Factors | ||||
| Systolic Blood Pressure, mmHg | 130.3 (20.3) | 129.1 (19.9) | 131.1 (20.6) | 0.23 |
| Diastolic Blood Pressure, mmHg | 73.1 (10.1) | 72.9 (10.5) | 73.2 (9.9) | 0.67 |
| Antihypertensive Medication, % | 44.1 | 40.4 | 46.7 | 0.13 |
| Total Cholesterol, mg/dL | 195.4 (36.7) | 192.7 (35.8) | 197.3 (37.3) | 0.15 |
| HDL Cholesterol, mg/dL | 44.5 (10.8) | 44.5 (10.9) | 44.5 (10.7) | 0.94 |
| Serum Triglycerides, mg/dL † | 162.0 (105.0, 212.0) | 159.0 (107.0, 205.0) | 162.0 (104.0,213.0) | 0.69 |
| Lipid-lowering Medication, % | 17.1 | 12.5 | 20.5 | 0.01 |
| Type 2 Diabetes Mellitus, % | 26.5 | 26.3 | 26.7 | 0.92 |
| Fasting Blood Glucose, mg/dL | 107.6 (37.9) | 102.7 (28.2) | 111.2 (43.2) | <0.001 |
| Glucose-lowering Medication, % | 17.3 | 15.4 | 18.6 | 0.33 |
| Waist Circumference, cm | 104.4 (12.4) | 103.9 (12.4) | 104.7 (12.4) | 0.48 |
| Never Smokers, % | 56.9 | 57.7 | 56.3 | 0.88 |
| Ten-Year ASCVD Risk | 8.5 (3.7, 15.4) | 6.9 (2.7, 12.6) | 9.5 (4.8, 16.6) | <0.001 |
| Cardiovascular Imaging | ||||
| CAC Score at Visit 5, † | 6.8 (0.0, 35.9) | 0.0 (0.0, 0.0) | 29.3 (12.2, 75.5) | <0.001 |
| Presence of Carotid Plaque, % ‡ | 43.3 | 33.8 | 50.3 | 0.0001 |
| Presence of Thoracic Calcification, % | 14.5 | 7.5 | 19.5 | <0.001 |
| Novel CVD Risk Factors | ||||
| hs-C-Reactive Protein, mg/L | 5.1 (6.4) | 4.9 (5.8) | 5.1 (6.8) | 0.79 |
| hs-Cardiac Troponin T, pg/dL † | 4.0 (2.9, 6.5) | 3.3 (2.9, 5.5) | 4.4 (2.9, 6.9) | <0.001 |
| NT-proBNP, pg/dL †‡ | 44.4 (19.9, 89.4) | 39.1 (19.3, 77.3) | 49.9 (20.6, 91.2) | 0.10 |
| Lipoprotein a, mg/dL‡ | 25.7 (30.2) | 23.1 (18.7) | 27.9 (31.3) | 0.12 |
| Metabolic Syndrome Severity Z-Score | 1.8 (1.1) | 1.7 (0.9) | 1.8 (1.2) | 0.13 |
| eGFR, mL/min/1.73m2 * | 81.2 (15.9) | 82.8 (15.7) | 80.1 (16.1) | 0.04 |
| Presence of Urine Protein, % | 9.9 | 9.2 | 10.6 | 0.60 |
Values are mean (SD) unless otherwise noted.
=median (Q1, Q3).
=measured in subset of population (carotid plaque, n=545; NT-proBNP, n=474; Lp(a), n=303).
= p-value for comparison between long-term CAC=0 and incident CAC.
ASCVD=atherosclerotic cardiovascular disease; CAC=coronary artery calcium; eGFR=estimated glomerular filtration rate; HDL=high-density lipoprotein; hs=high-sensitivity; mmHg=millimeters of mercury; MESA=multi-ethnic study of atherosclerosis; NT-proBNP = n-terminal pro-brain natriuretic peptide.
There was a similar proportion of individuals with persistent CAC=0 for participants with type 2 diabetes (41.5%) and metabolic syndrome (41.9%) (Central Illustration). Approximately one-third of individuals classified as having a high ASCVD risk (≥20%) by traditional scoring had a long-term absence of CAC, while nearly 50% of low risk individuals (<7.5%) had a long-term absence of CAC. Among participants who developed incident CAC, there was no significant difference in the median Agatston score at Visit 5 between groups classified as high or low risk by traditional ASCVD scoring (Figure 1).
Central Illustration. Proportion of participants with long-term CAC=0 versus incident CAC stratified by baseline risk.
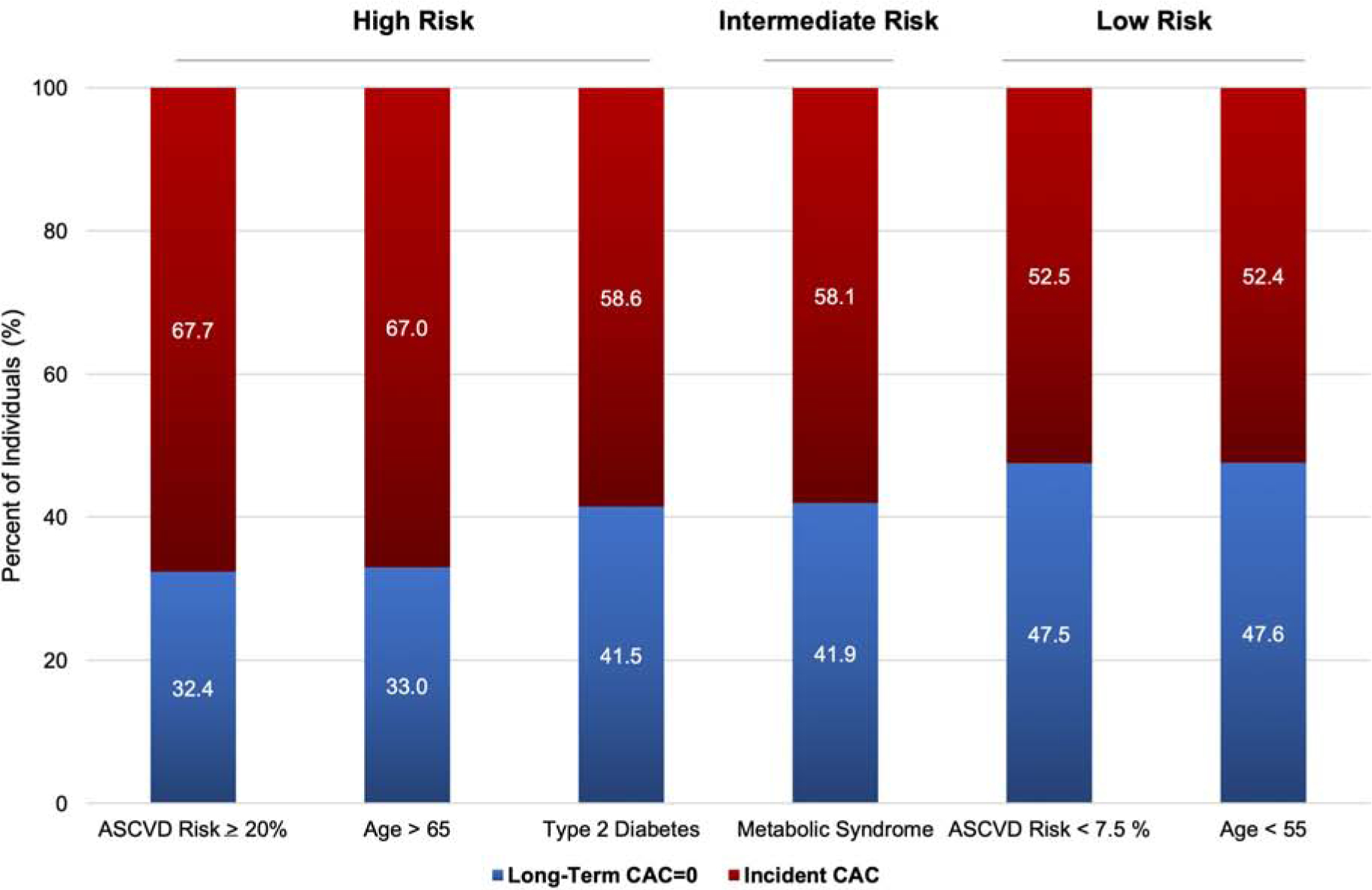
Similar proportion of participants with long-term CAC=0 among persons with metabolic syndrome and/or type 2 diabetes
Figure 1. Median incident coronary artery calcification score at Visit 5 stratified by baseline risk.
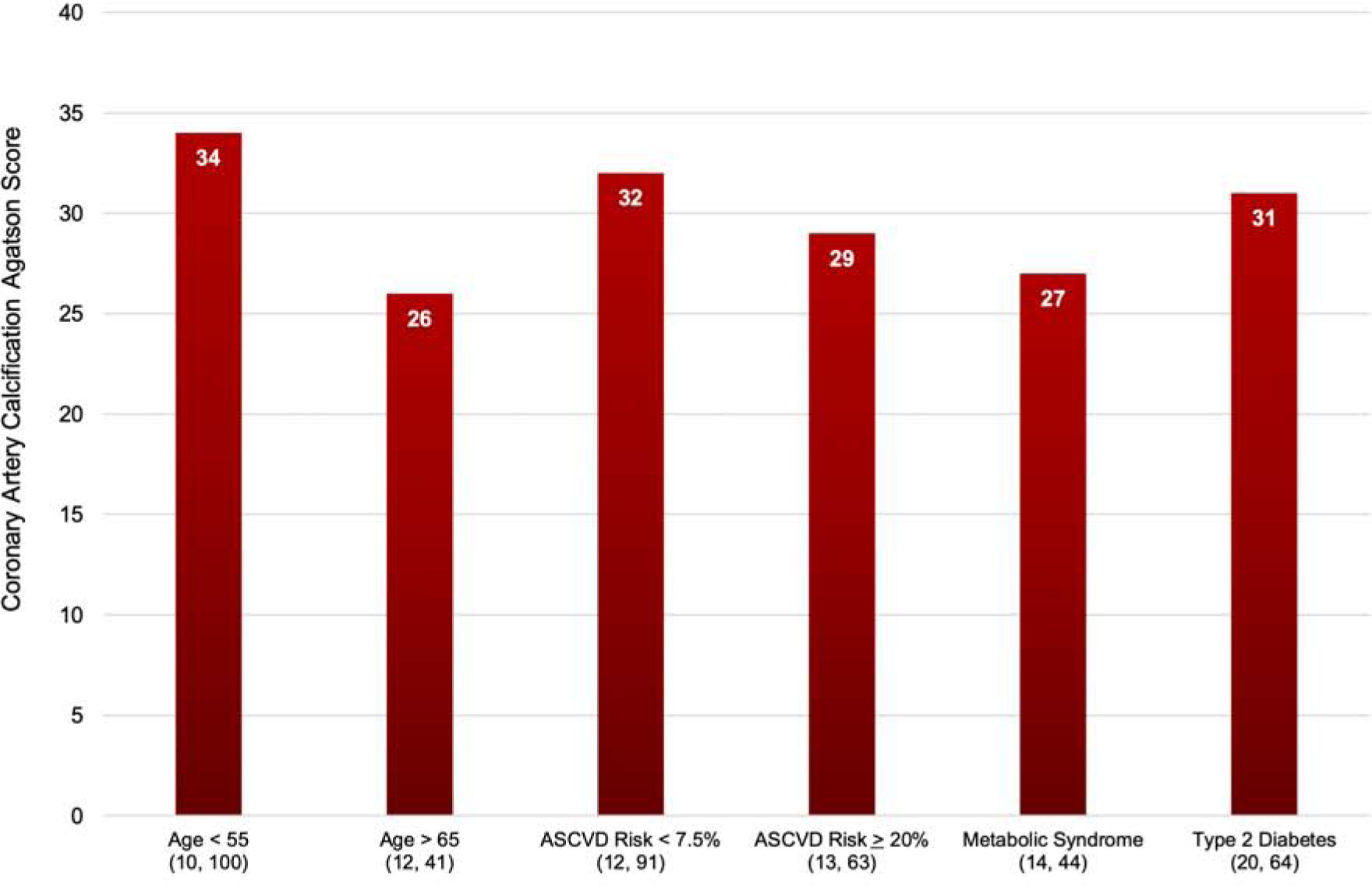
The 25th and 75th percentiles are displayed in parentheses under their representative risk group
After adjusting for traditional ASCVD risk factors, participants had 50% higher odds of persistent long-term CAC=0 per every 10-years younger age (OR=1.50, 95% CI=1.22–1.85; p-value<0.01), but no significant associations with other individual traditional ASCVD risk factors were found (Table 2). In bivariate analysis, individuals with a low 10-year ASCVD risk of <7.5% had 51% higher odds of maintaining CAC=0 compared to those with an intermediate or higher risk (OR=1.51, 95% CI=1.08, 2.11; p-value=0.02).
Table 2.
Association between traditional ASCVD risk factors and long-term CAC=0 among persons with metabolic syndrome and/or type 2 diabetes
| Variable | All | Metabolic Syndrome Alone | Type 2 Diabetes | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Demographic | |||||||
| Female Sex | 1.25 (0.83, 1.89) | 0.28 | 1.29 (0.80, 2.09) | 0.29 | 1.22 (0.52, 2.84) | 0.65 | |
| Age, per 10 years younger | 1.50 (1.22, 1.85) | <0.01 | 1.70 (1.33, 2.17) | <0.0001 | 1.11 (0.72, 1.70) | 0.64 | |
| Traditional ASCVD Risk Factors* | |||||||
| Never cigarette smoker | 1.07 (0.75, 1.55) | 0.70 | 0.96 (0.63, 1.47) | 0.85 | 1.67 (0.76, 3.68) | 0.20 | |
| Waist circumference | 1.10 (0.66, 1.82) | 0.73 | 1.02 (0.54, 1.92) | 0.94 | 1.45 (0.54, 3.89) | 0.46 | |
| Blood pressure | 1.14 (0.71, 1.83) | 0.58 | 1.15 (0.67, 1.98) | 0.61 | 0.81 (0.27, 2.43) | 0.70 | |
| Fasting Blood Glucose | 1.27 (0.84, 1.91) | 0.26 | 1.18 (0.75, 1.87) | 0.47 | 5.22 (0.89, 30.59) | 0.07 | |
| Triglycerides | 0.98 (0.63, 1.53) | 0.92 | 1.04 (0.60, 1.81) | 0.89 | 0.83 (0.34, 2.01) | 0.68 | |
| Total cholesterol / HDL-cholesterol | 1.27 (0.73, 2.21) | 0.40 | 1.34 (0.65, 2.79) | 0.43 | 1.02 (0.40, 2.63) | 0.96 | |
| ASCVD risk < 7.5% † | 1.51 (1.08, 2.11) | 0.02 | 1.68 (1.13, 2.48) | <0.01 | 1.11 (0.53, 2.31) | 0.78 | |
Adjusted for: age, sex, race, education, antihypertensive medication, lipid-lowering medication, glucose-lowering medication, cigarette smoking, waist circumference, blood pressure, fasting blood glucose, fasting serum triglycerides, and total cholesterol/HDL-C ratio.
waist circumference < 40 inches in males, < 35 inches in females; blood pressure < 130 / < 80 mmHg; fasting blood glucose < 100 mg/dL; triglycerides < 150 mg/dL;
total cholesterol / HDL-cholesterol ratio < 3.5.
= bivariate analysis.
ASCVD=atherosclerotic cardiovascular disease; CAC=coronary artery calcium; HDL=high-density lipoprotein.
Participants with the lowest quartile metabolic syndrome severity score had nearly 3-fold higher odds of persistent CAC=0 compared to individuals in the upper quartile (OR=2.71, 95% CI=1.27–5.76, p-value<0.01) (Table 3). This association was especially strong among individuals with type 2 diabetes, as persons in the lowest quartile had nearly 6-fold higher odds of maintaining an absence of CAC versus those in the highest quartile (OR=5.96, 95% CI=1.27–28.07, p=0.02). Odds ratios and significance for the metabolic syndrome severity score were consistent when comparing the fully adjusted model to a model without traditional biologic ASCVD risk factors (OR=2.29, 95% CI=1.33, 5.26, p-value<0.01). In addition, individuals with a serum hs-cTnT concentration < 3 mg/dL had 55% higher odds of long-term absence of CAC compared to those with a hs-cTnT concentration ≥3 mg/dL (p-value=0.04). Persons without thoracic calcification had more than double the odds of maintaining long-term absence of CAC in the overall sample (OR=2.42, 95% CI=1.24–4.72; p-value<0.01). Similarly, absence of carotid plaque was associated with 81% higher odds of persistent CAC=0 among persons with metabolic syndrome or diabetes (OR=1.81, 95% CI=1.25–2.61, p-value<0.01). Parameter estimates and significance values of ASCVD risk factors with long-term CAC=0 remained consistent after additionally controlling for diabetes duration, diabetes control, and after further adjustment of metabolic health over time, leveraging an averaged metabolic syndrome severity score through MESA Visit 3.
Table 3.
Association between novel ASCVD risk factors and long-term CAC=0 among persons with metabolic syndrome and/or type 2 diabetes
| Variable | All | Metabolic Syndrome Alone | Type 2 Diabetes | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Novel ASCVD Risk Factors* | ||||||
| hs-CRP | 1.23 (0.80, 1.89) | 0.34 | 1.14 (0.69, 1.88) | 0.62 | 1.43 (0.59, 3.47) | 0.43 |
| hs-cardiac troponin T | 1.55 (1.01, 2.38) | 0.04 | 1.37 (0.84, 2.24) | 0.21 | 3.35 (1.22, 9.15) | 0.02 |
| NT-proBNP | 1.20 (0.66, 2.18) | 0.55 | 0.92 (0.46, 1.82) | 0.80 | 3.06 (0.70, 13.47) | 0.14 |
| Lipoprotein (a) | 1.85 (0.92, 3.71) | 0.08 | 1.73 (0.79, 3.80) | 0.17 | 3.22 (0.58, 17.76) | 0.18 |
| eGFR | 1.67 (0.70, 3.97) | 0.25 | 1.40 (0.53, 3.65) | 0.50 | 4.63 (0.45, 47.67) | 0.20 |
| Absence of Urine Protein | 1.05 (0.53, 2.09) | 0.88 | 0.75 (0.26, 2.18) | 0.59 | 1.51 (0.56, 4.03) | 0.41 |
| Absence of Thoracic Calcification | 2.42 (1.24, 4.72) | <0.01 | 2.72 (1.23, 6.02) | 0.01 | 2.42 (0.59, 9.81) | 0.22 |
| Absence of Carotid Plaque | 1.81 (1.25, 2.61) | <0.01 | 1.61 (1.04, 2.49) | 0.03 | 2.86 (1.37, 5.98) | <0.01 |
| Metabolic Syndrome Severity Score† | 2.71 (1.27, 5.76) | <0.01 | 2.25 (0.87, 5.87) | 0.09 | 5.96 (1.27, 28.07) | 0.02 |
Adjusted for: age, sex, race, education, antihypertensive medication, lipid-lowering medication, glucose-lowering medication, cigarette smoking, waist circumference, blood pressure, fasting blood glucose, fasting serum triglycerides, and total cholesterol/HDL-C ratio.
=each novel risk factor was evaluated independently and added to model 1: hs-CRP < 2 mg/L; hs-cardiac troponin T < 3 ng/mL; NT-proBNP < 125 pg/mL; Lp(a) < 50 mg/dL in whites, Chinese, and Hispanic, Lp(a) < 30 mg/dL in blacks; eGFR > 60 mL/min/1.73m2.
=quartile 1 (lowest) versus quartile 4 (reference).
ASCVD=atherosclerotic cardiovascular disease; CAC=coronary artery calcium; eGFR=estimated glomerular filtration rate; hs-CRP=high sensitivity C-reactive protein; NT-proBNP=n-terminal prohormone brain natriuretic peptide.
The addition of traditional ASCVD risk factors to demographic information yielded a higher C-statistic of 0.671 compared to a model that included the PCEs risk score, C-statistic of 0.599 (Table 4). The addition of the metabolic syndrome severity score had a similar improvement in the predictive ability for long-term absence of CAC (C-statistic=0.673), while the addition of novel serum biomarkers (C-statistic=0.686) and absence of extra-coronary atherosclerosis (C-statistic=0.698) had higher predictive values.
Table 4.
AUC analysis for prediction of long-term CAC=0 among individuals with metabolic syndrome or type 2 diabetes.
| C-Statistic (95% CI) | Model P-value | Change in C-Statistic* | AUC Contrast P-value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age, Sex, Race | 0.625 (0.577, 0.673) | <0.001 | - | - |
| Traditional Risk Factors/Risk Scoring | ||||
| Individual Risk Factors † | 0.671 (0.625, 0.718) | <0.0001 | +0.046 | <0.01 |
| Pooled Cohort Equations ASCVD Risk | 0.599 (0.551, 0.648) | <0.01 | −0.026 | 0.17 |
| Novel Risk Factors/Risk Scoring | ||||
| Individual Risk Factors + Metabolic Syndrome Severity Score | 0.673 (0.627, 0.719) | <0.001 | +0.048 | <0.01 |
| Individual Risk Factors + hs-cTnT, NT-proBNP, Lp(a), hs-CRP, eGFR, Absence of Urine Protein | 0.686 (0.625, 0.747) | 0.05 | +0.061 | 0.12 |
| Imaging | ||||
| Individual Risk Factors + Absence of Extra-Coronary Atherosclerosis ‡ | 0.698 (0.650, 0.740) | <0.0001 | +0.073 | <0.01 |
| Demographics, Traditional Risk Factors, Novel Risk Factors, and Imaging § | 0.709 (0.650, 0.768) | <0.01 | +0.084 | 0.03 |
All models, except pooled cohort equations model, include age, sex, and race as covariates.
=ROC curves were compared to the reference model including age, sex, and race.
= systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, fasting blood glucose, waist circumference, triglycerides, antihypertensive medication, glucose-lowering medication,
lipid-lowering medication.
= carotid plaque and thoracic calcification.
= all individual risk factors listed above, except the pooled cohort equations ASCVD risk and the metabolic syndrome severity score.
ASCVD=atherosclerotic cardiovascular disease; AUC=area-under-the-curve; eGFR=estimated glomerular filtration rate; hs-CRP=high sensitivity C-reactive protein; hs-cTnT=high-sensitivity cardiac troponin T; Lp(a)=lipoprotein a;
NT-proBNP=n-terminal prohormone brain natriuretic peptide.
There were 28 (5.1%) ASCVD events over a median follow-up time of 4.8 years. The overall event rate for persons with metabolic syndrome or type 2 diabetes with baseline CAC=0 was similar for those underwent follow-up CAC scans (10.9 events per 1,000 person-years) versus those who did not undergo a repeat CAC scan (11.7 events per 1,000 person-years), while individuals without metabolic syndrome or type 2 diabetes who were and were not randomized to a follow-up CAC scan had an ASCVD event rate of 5.9 per 1,000 person-years (Supplemental Table 2). Compared to incident CAC, long-term CAC=0 conferred a 62% and 66% lower risk of an ASCVD event among persons with metabolic syndrome (4.9 events per 1,000 person-years) and type 2 diabetes (7.1 events per 1,000 person-years), respectively. (Supplemental Figure 2).
Discussion
This is one of the first population-based studies to assess the predictors of healthy arterial aging, defined here by persistent CAC=0, in persons with metabolic syndrome or type 2 diabetes. We found that among the approximately 40% of MESA participants with metabolic syndrome or type 2 diabetes who have baseline CAC=0, that 42% had long-term absence of CAC over a 10-year follow-up period. Aside from a lower age, the absence of any specific individual traditional ASCVD risk factor was not associated with an increased likelihood of healthy arterial aging, while a low metabolic syndrome severity score and absence of thoracic aortic calcification were associated with an approximately 2.5-fold greater likelihood of healthy arterial aging. Overall, our results suggest that future studies are required to determine the risk/benefit of primary prevention pharmacotherapy among younger persons with diabetes who have a low metabolic severity score, as they are most likely to maintain healthy arterial aging.
Although the absence of individual traditional ASCVD risk factors were not associated with long-term CAC=0, a normal hs-cTnT (< 3 mg/dL) emerged as a novel predictor for this protective phenotype. Elevated hs-cTnT is a marker of subclinical myocardial damage that is associated with myocardial fibrosis and alterations in left ventricular structure among adults without ASCVD(43). Additionally, hs-cTnT is also associated with higher pulse wave velocity in persons with type 2 diabetes(44), suggesting that elevated hs-cTnT may reflect both subclinical myocardial injury and systemic arterial stiffness in persons with metabolic disease. Accordingly, further studies evaluating whether long-term normal hs-cTnT values improve the prediction of healthy arterial aging are warranted.
An absence of carotid plaque was associated with an 81% higher likelihood of persistent CAC=0 among individuals with metabolic syndrome or type 2 diabetes. This association was more prominent among individuals with diabetes, as an absence of carotid plaque conferred a nearly three-fold higher odds of persistent CAC=0 among in this subgroup. Our findings build on previous studies that have demonstrated similar associations between carotid and coronary atherosclerosis, as the presence of a carotid plaque identified through ultrasound is associated with a 37% higher risk of incident CAC among individuals without clinical ASCVD(45). However, less than 15% of patients harboring carotid artery plaque(s) go on to develop CAC(45), suggesting differences in the pathobiology of atherosclerosis between the two sights. Thus, the fairly modest associations between the two imaging modalities underline that assessment and treatment of additional risk factors outside of carotid atherosclerosis may be required to successfully prevent incident CAC.
Overall, the absence of individual traditional and novel risk factors was not strongly associated with persistent CAC=0, and these findings may have been sensitive to how continuous variables were categorized or dichotomized. In contrast to individual ASCVD risk factors, we observed strong and consistent associations of persistent CAC=0 with composite risk scores, including the PCEs and metabolic syndrome severity scores. The metabolic syndrome severity score was more robustly associated with CAC=0 compared to 10-year ASCVD risk, as individuals in the lowest quartile of metabolic syndrome severity score had over two-fold higher odds of maintaining CAC=0 compared to those in the highest quartile. This latter association was nearly six-fold among those with diabetes. However, differences in the strength of association of the PCE and metabolic syndrome severity score with persistent CAC=0 may have also been due to the methods of categorizing independent variables. The metabolic syndrome severity score odds ratio compared extremes in the distribution (1st quartile versus 4th quartile) while the PCE odds ratio compared those below 7.5% versus those with 7.5% or higher 10-year ASCVD predicted risk. The metabolic syndrome severity score and PCEs collectively incorporate and define risk factor burden, which may explain why these scores perform significantly better than any individual risk factor alone. Thus, given the heterogeneity and inconsistent associations of individual risk factors with CAC, the metabolic syndrome severity score represents a powerful supplemental tool for the prediction of healthy arterial aging in individuals with metabolic syndrome or type 2 diabetes.
While type 2 diabetes is often considered an ASCVD risk equivalent(46), our results fit into a broader context of research that demonstrates considerable heterogeneity in risk among individuals with diabetes and metabolic syndrome(47). We observed that >40% of individuals with type 2 diabetes or metabolic syndrome have a persistent absence of CAC over a 10-year period.
Furthermore, our results underline the “power of zero”(48) and the concept that individual traditional ASCVD risk markers have a limited utility for de-risking individuals compared to atherosclerosis imaging modalities, especially CAC imaging. An absence of extra-coronary atherosclerosis, carotid or thoracic, were the strongest individual negative risk markers and similar in strength compared to a combination of risk factors as integrated by the metabolic syndrome score. We observed no significant association of hsCRP, Lp(a), nor NT-proBNP with persistent CAC=0. These findings support data that demonstrate little reduction in the post-test versus pre-test ASCVD risk for a low/normal finding of traditional and novel risk factors, including hsCRP, Lp(a), and NT-proBNP(49,50). On the other hand, a finding of CAC=0 has up to an 80% reduction in post-test compared to pre-test risk for coronary heart disease(50). Thus, intrinsic differences between risk enhancers and risk reducers may be a further explanation for our observation that individual ASCVD risk factors were not significantly associated with healthy arterial aging.
Strengths of this study include the longitudinal measurement of CAC in a diverse population over a 10-year follow up period. Consistent with previous studies of healthy arterial aging(51), two-thirds of our study sample were women and the participants were ethnically diverse, giving our results wider generalizability. Similarly, 62% of individuals with CAC=0 in MESA are women, and women are more likely to have and absence of CAC compared to men of the same age(52). In addition, we examined both traditional and novel ASCVD risk factors, underlining potential new risk stratification tools that may help improve prediction of healthy arterial aging, including hs-cTnT and the metabolic syndrome severity score.
Limitations of our analysis include the heterogeneity within metabolic disease as well as different definitions of the metabolic syndrome. However, all definitions are based on similar risk factors and we used the most widely recognized metabolic syndrome definition(16) to overcome this limitation and related ambiguity. Overall, our study also had a relatively small sample size and there were only 152 participants with diabetes at baseline. Accordingly, differences in risk markers for long-term CAC=0 between participants with metabolic syndrome or diabetes should be interpreted with caution. A larger sample size would help to determine whether the observed differences in individual risk factors for the prediction of healthy arterial aging between those with metabolic syndrome and type 2 diabetes may have been due to either low statistical power or true biological heterogeneity. Furthermore, there were 559 individuals with metabolic syndrome or type 2 diabetes and CAC=0 at baseline whom did not have a CAC measurement 10 years later, and thus were not included in the current analysis. Aside from a slightly older mean age and higher prevalence of diabetes, this group had a similar cardiovascular risk profile and ASCVD event rates were similar when comparing persons who underwent follow-up CAC scans compared to those who were not randomized to a follow-up CAC scan. However, long-term ASCVD event rates were lower for participants without metabolic syndrome or type 2 diabetes. Similar findings have previously been reported by Valenti et al who found that among individuals with baseline CAC=0, the 5-year cumulative mortality was similar for persons with and without diabetes, but at 15 years follow-up the cumulative mortality was significantly higher for persons with diabetes (11.7%) compared to persons without diabetes (4.5%) (p<0.05)(53). In our cohort, the difference in ASCVD rates between years 10–15 of follow-up after a baseline CAC score of 0 may be at least partially explained by the higher 10-year ASCVD risk of 12.4% among participants with metabolic syndrome or type 2 diabetes compared to 5.9% for participants without metabolic syndrome or type 2 diabetes.
In conclusion, among individuals with metabolic syndrome or type 2 diabetes and baseline CAC=0, approximately 40% maintained long-term absence of CAC over at least 10 years. Younger individuals, those with a lower metabolic syndrome severity score, and those with an absence of extra-coronary atherosclerosis were the most likely to maintain a healthy arterial aging phenotype of long-term CAC=0. These results emphasize the significant ASCVD heterogeneity within these two groups of participants and highlight the significant heterogeneity in arterial aging among patients with metabolic syndrome and diabetes. Our findings suggest that an optimal overall cardiovascular profile may be more important than an ideal value of any individual risk factor for the maintenance of healthy arterial aging.
Clinical Perspectives
Competency in Medical Knowledge:
Among the approximately 40% of MESA participants with metabolic syndrome or type 2 diabetes who have baseline CAC=0, 42% had long-term absence of CAC over a 10-year follow-up period. Younger age, a lower metabolic syndrome severity score, and absence of extra-coronary atherosclerosis serve as key predictors for the long-term absence of CAC among persons with metabolic syndrome or type 2 diabetes.
Translational Outlook:
Future studies are required to determine the risk/benefit of primary prevention pharmacotherapy among younger persons with diabetes who have a low metabolic severity score, as they are most likely to maintain healthy arterial aging.
Supplementary Material
Supplemental Figure 1. Flow diagram of study participant inclusion
Supplemental Figure 2. Incident rate of ASCVD for persons with metabolic syndrome and/or type 2 diabetes, stratified by CAC status.
Acknowledgements:
This research was supported by R01 HL071739 and MESA was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01 HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1 TR 001079, and UL1-RR-025005 from National Center for Research Resources. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Sources of Funding: Alexander C. Razavi is currently funded through a fellowship training grant supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under grant number F30HL147486.
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcification
- hs-cTnT
high-sensitivity cardiac troponin T
- eGFR
estimated glomerular filtration rate
- HDL-C
high-density lipoprotein cholesterol
- hs-CRP
high sensitivity C-reactive protein
- Lp(a)
lipoprotein a
- MESA
Multi-Ethnic Study of Atherosclerosis
- NT-proBNP
N-terminal pro-brain natriuretic peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no financial disclosures to report.
References
- 1.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014;312(12):1218–26. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore JX, Chaudhary N, Akinyemiju T Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis 2017;14:160287 Doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevention C for DC and. National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States Background 2018. Doi: 10.2196/jmir.9515. [DOI] [Google Scholar]
- 5.Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112(20):3066–72. Doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339(4):229–34. Doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S, Orimoloye OA, Nass CM, Blumenthal RS, Martin SS. Cardiovascular Risk Heterogeneity in Adults with Diabetes: Selective Use of Coronary Artery Calcium in Statin Use Decision-making. J Gen Intern Med 2019;34(11):2643–7. Doi: 10.1007/s11606-019-05266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the multi-ethnic study of atherosclerosis. JAMA Cardiol 2017;2(12):1332–40. Doi: 10.1001/jamacardio.2017.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoni AG, Wong ND, Shea S, et al. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2007;30(11):2951–6. Doi: 10.2337/dc07-1042. [DOI] [PubMed] [Google Scholar]
- 10.Wong ND, Sciammarella MG, Polk D, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol 2003;41(9):1547–53. Doi: 10.1016/S0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 11.Detrano R, Guerci AD, Carr JJ, et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med 2008;358(13):1336–45. Doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018:434–47. Doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gassett AJ, Sheppard L, McClelland RL, et al. Risk Factors for Long-Term Coronary Artery Calcium Progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2015;4(8):e001726 Doi: 10.1161/JAHA.114.001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong ND, Nelson JC, Granston T, et al. Metabolic Syndrome, Diabetes, and Incidence and Progression of Coronary Calcium: The Multiethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging 2012;5(4):358 Doi: 10.1016/J.JCMG.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol 2002;156(9):871–81. Doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.High Blood Cholesterol Evaluation Treatment Detection National Cholesterol Education Program Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. n.d.
- 17.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA Distribution of Coronary Artery Calcium by Race, Gender, and Age. Circulation 2006;113(1):30–7. Doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 19.Budoff MJ, McClelland RL, Chung H, et al. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: The multi-ethnic study of atherosclerosis. Am J Roentgenol 2009;192(3):613–7. Doi: 10.2214/AJR.08.1242. [DOI] [PubMed] [Google Scholar]
- 20.Takasu J, Katz R, Nasir K, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2008;155(4):765–71. Doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostchega Y, Seu R, Sarafrazi N, Zhang G, Hughes JP, Miller I Waist Circumference Measurement Methodology Study: National Health and Nutrition Examination Survey, 2016. Vital Health Stat 2 2019;(182):1–20. [PubMed] [Google Scholar]
- 22.Levin G, Kestenbaum B, Ida Chen Y Der, et al. Glucose, insulin, and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2010;172(10):1144–54. Doi: 10.1093/aje/kwq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502. [PubMed] [Google Scholar]
- 24.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014;63(2):218–25. Doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AM, Gurka MJ, DeBoer MD A metabolic syndrome severity score to estimate risk in adolescents and adults: current evidence and future potential. Expert Rev Cardiovasc Ther 2016;14(4):411–3. Doi: 10.1586/14779072.2016.1143360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low S, Khoo KCJ, Wang J, et al. Development of a metabolic syndrome severity score and its association with incident diabetes in an Asian population—results from a longitudinal cohort in Singapore. Endocrine 2019;65(1):1–8. Doi: 10.1007/s12020-019-01970-5. [DOI] [PubMed] [Google Scholar]
- 27.DeBoer MD, Gurka MJ, Morrison JA, Woo JG Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes 2016;40(9):1353–9. Doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurka MJ, Golden SH, Musani SK, et al. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia 2017;60(7):1261–70. Doi: 10.1007/s00125-017-4267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LX, Filipp SL, Urbina EM, Gurka MJ, DeBoer MD Longitudinal Associations of Metabolic Syndrome Severity between Childhood and Young Adulthood: The Bogalusa Heart Study. Metab Syndr Relat Disord 2018;16(5):208–14. Doi: 10.1089/met.2017.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens L Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am J Kidney Dis 2010;55(4):622 Doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JH, Korcarz CE, Hurst RT, et al. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008:93–111. Doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Tattersall MC, Gassett A, Korcarz CE, et al. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke 2014;45(11):3257–62. Doi: 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cainzos-Achirica M, Miedema MD, McEvoy JW, et al. The prognostic value of high sensitivity C-reactive protein in a multi-ethnic population after > 10 years of follow-up: The Multi-Ethnic Study of Atherosclerosis (MESA). Int J Cardiol 2018;264:158–64. Doi: 10.1016/j.ijcard.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seliger SL, Hong SN, Christenson RH, et al. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135(16):1494–505. Doi: 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan W, Cao J, Steffen BT, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35(4):996–1001. Doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018;138(17):e426–83. Doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 37.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Coll Cardiol 2019;74(10):e177–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafsson F, Steensgaard-Hansen F, Badskjaer J, Poulsen AH, Corell P, Hildebrandt P Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure. J Card Fail 2005;11(5):S15–20. [DOI] [PubMed] [Google Scholar]
- 40.Seliger SL, Hong SN, Christenson RH, et al. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure. Circulation 2017;135(16):1494–505. Doi: 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deboer MD, Filipp SL, Musani SK, Sims M, Okusa MD, Gurka M Metabolic syndrome severity and risk of CKD and Worsened GFR: The jackson heart study. Kidney Blood Press Res 2018;43(2):555–67. Doi: 10.1159/000488829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLong ER, DeLong DM, Clarke-Pearson DL Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–45. [PubMed] [Google Scholar]
- 43.Seliger SL, Hong SN, Christenson RH, et al. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135(16):1494–505. Doi: 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yiu KH, Zhao CT, Chen Y, et al. Association of subclinical myocardial injury with arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2013;12(1). Doi: 10.1186/1475-2840-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polak JF, Tracy R, Harrington A, Zavodni AEH, O’Leary DH Carotid artery plaque and progression of coronary artery calcium: The multi-ethnic study of atherosclerosis. J Am Soc Echocardiogr 2013;26(5):548–55. Doi: 10.1016/j.echo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction. N Engl J Med 1998;339(4):229–34. Doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 47.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6(5):361–9. Doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 48.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133(9):849–58. Doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133(9):849–58. Doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mortensen MB, Fuster V, Muntendam P, et al. Negative Risk Markers for Cardiovascular Events in the Elderly. J Am Coll Cardiol 2019;74(1):1–11. Doi: 10.1016/j.jacc.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 51.Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of Long-Term Healthy Arterial Aging. JACC Cardiovasc Imaging 2015;8(12):1393–400. Doi: 10.1016/j.jcmg.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 52.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113(1):30–7. Doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 53.Valenti V, Hartaigh B, Cho I, et al. Absence of coronary artery calcium identifies asymptomatic diabetic individuals at low near-term but not long-term risk of mortality: A 15-year follow-up study of 9715 patients. Circ Cardiovasc Imaging 2016. Doi: 10.1161/CIRCIMAGING.115.003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow diagram of study participant inclusion
Supplemental Figure 2. Incident rate of ASCVD for persons with metabolic syndrome and/or type 2 diabetes, stratified by CAC status.


