Abstract
Objective.
To investigate the extent to which antibiotic exposure in the first two years of life is associated with the risk of immunological, metabolic, and neurobehavioral health conditions with childhood onset.
Patients and Methods.
In this population-based cohort study we identified all children born in Olmsted County, Minnesota, between January 1, 2003 and December 31, 2011 through the Rochester Epidemiology Project (REP) medical records-linkage system. Demographic characteristics, antibiotic prescriptions, and diagnostic codes through June 30, 2017 were retrieved using the REP infrastructure. Time-to-event analysis was conducted to assess the impact of antibiotic exposure on the risk of several adverse health conditions.
Results.
This study included 14,572 children (7,026 girls and 7,546 boys), of whom 70% received at least one antibiotic prescription during the first two years of life. Early antibiotic exposure was associated with an increased risk of childhood onset asthma, allergic rhinitis, atopic dermatitis, celiac disease, overweight, obesity, and attention deficit hyperactivity disorder (hazard ratios ranging from 1.20 to 2.89, all P<.05). The associations were influenced by the number, type, and timing of antibiotic exposure. Moreover, children exposed to antibiotics had a greater probability of experiencing combinations of conditions, particularly when given multiple prescriptions.
Conclusion.
Our study shows significant associations between early-life antibiotic exposure and several distinct health conditions with childhood onset. Additional research is warranted to establish practical guidelines to optimize the benefit and minimize the risk of antibiotics in children.
INTRODUCTION
Antibiotics prevent or cure serious infectious diseases and lessen their consequences. Their remarkable efficacy and presumed safety has led to widespread use often without clear-cut need.1 The evolution of drug-resistant bacteria exemplifies unintended consequences of antibiotic overuse.2 The increasing prevalence of health conditions with childhood-onset, including asthma, food allergies, obesity, and attention deficit hyperactivity disorder (ADHD), has triggered concern about antibiotic exposures during key developmental periods because of their impact on the microbiome.3
The bacterial communities colonizing the human body play essential roles in the development of host immunity, metabolism, and behavior.4–6 Their establishment parallels host growth, reflecting selection over an evolutionary time-scale.7 The intergenerational transfer of bacterial communities begins at birth8,9, and is fostered through social interaction, diet, and environmental factors.9 Cesarean-section delivery, formula-feeding, and pre- and postnatal antibiotic exposure negatively affect microbiome transmission and maturation and, therefore, may compromise childhood health.6,9 Increasing epidemiological evidence implicates factors disrupting the early-life microbiome in the development of multiple disorders.7,10–12 Although the causal role of the microbiome is not resolved in humans, murine studies support the hypothesis that microbiome perturbations during key developmental periods have long-lasting health consequences.13–15
Epidemiological studies investigating the relationship between early life antibiotic exposures and health have largely focused on a single disease. By contrast, we leveraged the unique infrastructure of the Rochester Epidemiology Project (REP)16, which has linked and archived the medical records of nearly all persons residing in Olmsted County for more than 50 years, and maintained an electronic index of demographic information, medical diagnoses, surgical interventions, and prescribed medications, to assess whether exposure to antibiotics in the first two years of life is associated with the incidence of distinct immunological, metabolic, and neurobehavioral diseases in children. Moreover, we investigated whether the risk of childhood health outcomes is influenced by the number, type and timing of antibiotic exposures.
METHODS
Study Population
Following approval by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center, all children (n = 18,160) who were born in Olmsted County, Minnesota, between January 1, 2003 and December 31, 2011, and whose mothers were residents at the time of delivery, were identified using the REP.16,17 Children who were part of a multiple birth, whose mother did not give research authorization, or who had <2 years of follow-up were excluded (Supplemental Figure 1). Children were followed through their entire medical records or the last available follow-up. Demographic characteristics, antibiotic prescriptions, diagnostic codes, and procedure codes for children and/or mothers were all identified using the REP infrastructure.
Childhood Health Outcomes
The diagnostic indices of the REP were searched electronically to identify the International Classification of Diseases, Ninth (ICD-9) and Tenth (ICD-10) Revision codes associated with any health care visit between January 1, 2003 and June 30, 2017. ICD-9 and −10 codes were pooled to define the diagnosis of asthma, allergic rhinitis, food allergy, atopic dermatitis, celiac disease, ADHD, autism, and learning disorders (Supplemental Table 1). Each included outcome appeared at least twice in the REP diagnostic index, at least 30 days apart, with the second diagnostic date used in the analysis to define disease onset. The 2000 CDC age/sex-specific child growth chart values were used for the diagnoses of overweight (body mass index (BMI) ≥ 85th percentile) and obesity (BMI ≥ 95th percentile).
Antibiotic Prescriptions
Outpatient antibiotic prescriptions issued from Mayo Clinic and Olmsted Medical Center were linked to specific individuals in the records-linkage system to define infant antibiotic exposure during the first 2 years of life. Electronic prescriptions were retrieved from the proprietary electronic systems of each institution and were converted into RxNorm codes retrospectively. The prescriptions were then grouped using the National Drug File-Reference Terminology (NDF-RT) classification system as described in Supplemental Table 2.
Infant and Maternal Confounders
Data on infant (sex, birth weight, ethnicity, cesarean section) and maternal (age, education, known smoking and antibiotic use during pregnancy) potential confounders were also identified by using the REP records-linkage system. Maternal exposure to antibiotics during pregnancy was assessed as described above for the children. Maternal smoking was defined at time of birth +/−2 years: mothers with missing documentation about their smoking status (n=3,336) were included in the “no smoking” category. By contrast, missing data for the variables birth weight (n = 4,500) and maternal education (n = 1,788) were imputed as described in the statistical analysis.
Statistical Analysis
Descriptive statistics (percentages or medians and interquartile ranges (IQR)) were used to summarize characteristics of children and mothers. Comparisons between unexposed and exposed children were performed using chi-square and t-tests. The cumulative incidence of each outcome was estimated for up to 14 years using the Kaplan-Meier method, stratified by sex and antibiotic exposure. Children diagnosed with a given health outcome before the age of two were excluded from the analysis. Aalen-Johansen (a multistate generalization of cumulative incidence) methods were used to estimate the cumulative occurrence of multiple conditions over time. In this approach, values at each time point are shown for the percent of subjects who are in a given state, including at age 2 (baseline), and thus it includes information about all the subjects.
Cox proportional hazards models were used to compare the rate of developing each condition and the accumulation of multiple conditions between subjects with and without antibiotic exposure. Proportional hazard assumptions were checked and models were fit univariately and multivariably, adjusting for pre-specified infant and maternal confounders. Missing data were imputed five times using the “mice” package in R18; multivariable models were fit using each imputed dataset and pooled. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Participants
Our study included 14,572 children (7,026 girls and 7,546 boys) with a median follow-up of 8.8 years (interquartile range = 6.4 to 11.4 years), and a total follow-up of 128,425 person-years. The cohort was born to 10,335 unique mothers; 3,374 children (23%) were delivered by cesarean section. Characteristics of the children and mothers are summarized in Table 1. The numbers of children with diagnoses of the specified immunological, metabolic, and neurobehavioral conditions prior to the age of 2, and therefore excluded from the main analyses for that outcome, are reported in Supplemental Table 3.
Table 1.
Demographic and Clinical Characteristics of Children in the Study Cohort and their Mothers, according to Antibiotic Exposure in the First 2 Years of Life.
| Not exposed (n = 4352) | Exposed (n = 10220) | All (n = 14572) | |
|---|---|---|---|
| Children | |||
|
Sex – no. (%) Female Male |
2223 (51.1) 2129 (48.9) |
4803 (47.0) 5417 (53.0) |
7026 (48.2) 7546 (51.8) |
| Duration of follow-up – years a | |||
| Median (IQR) | 8.4 (6.1–10.4) | 9.1 (6.5–11.7) | 8.8 (6.4–11.4) |
| Birth weight - kg | |||
| Median (IQR) | 3.4 (3.1–3.7) | 3.4 (3.1–3.8) | 3.4 (3.1–3.8) |
| Ethnicity – no. (%) | |||
| White | 2951 (67.8) | 7397 (72.4) | 10348 (71.0) |
| Black | 365 (8.4) | 934 (9.1) | 1299 (8.9) |
| Asian | 338 (7.8) | 617 (6.0) | 955 (6.6) |
| Hawaiian/Pacific Islander | 19 (0.4) | 33 (0.3) | 52 (0.4) |
| American Indian | 18 (0.4) | 28 (0.3) | 46 (0.3) |
| Other/unknown | 661 (15.2) | 1211 (11.8) | 1872 (12.8) |
| Cesarean section – no. (%) | 940 (21.6) | 2434 (23.8) | 3374 (23.2) |
| Number of prescriptions – no. (%) | |||
| 1–2 | - | 4560 (44.6) | |
| 3–4 | - | 2434 (23.8) | |
| 5+ | - | 3226 (31.6) | |
| Categories – no. (%) | |||
| Penicillins | - | 9306 (63.9) | |
| Cephalosporins | - | 3401 (23.3) | |
| Sulfonamides | - | 777 (5.3) | |
| Macrolides | - | 3724 (25.6) | |
| Mothers | |||
| Age - years | |||
| Median (IQR) | 28.7 (24.7–32.5) | 29.1 (25.5–32.6) | 29.0 (25.3–32.6) |
| Education – no. (%) | |||
| High school or less | 789 (21.4) | 1614 (17.7) | 2403 (18.8) |
| Some college/4years | 1921 (52.1) | 4716 (51.8) | 6637 (51.9) |
| Post graduate | 978 (26.5) | 2766 (30.4) | 3744 (29.3) |
| Smoking – no. (%) | 457 (10.5) | 1151 (11.3) | 1608 (11.0) |
| Antibiotics during pregnancy – no. (%) | 1538 (35.3) | 4094 (40.1) | 5632 (38.7) |
Duration of follow-up includes also the first 2 years of life. IQR denotes interquartile range.
Early Life Antibiotic Exposure
Between birth and two years of age, 10,220 children (70%) were prescribed at least one antibiotic, with most receiving multiple antibiotics (Table 1). The most common pathologic conditions documented for unexposed and exposed children between birth and age two are summarized in Supplemental Table 4. Penicillins, cephalosporins, and macrolides (including lincomycins) were most often prescribed. Nitrofurantoin, quinolones, and other antibiotics (i.e. aminoglycosides, anti-tuberculars, chloramphenicol) together accounted for < 1% of prescriptions. The majority of prescriptions (99%) were for oral antibiotics.
Early Life Antibiotic Exposure and Childhood Health
To investigate the association of antibiotics with the incidence of several common childhood-onset health conditions, we first analyzed event rates of exposed and unexposed children. As expected, rates for many of these conditions differed significantly by sex, regardless of antibiotic exposure. In separate analyses, both girls and boys prescribed at least one course of antibiotics experienced a significantly greater cumulative incidence of asthma, allergic rhinitis, overweight and ADHD (Figure 1). Girls exposed to antibiotics were uniquely more prone to atopic dermatitis and celiac disease, whereas boys were uniquely more prone to obesity (Supplemental Figure 2).
Figure 1. Kaplan-Meier Curves of Time to Event for Health Conditions with Childhood Onset Stratified by Sex and Antibiotic Exposure in the First 24 Months of Life.
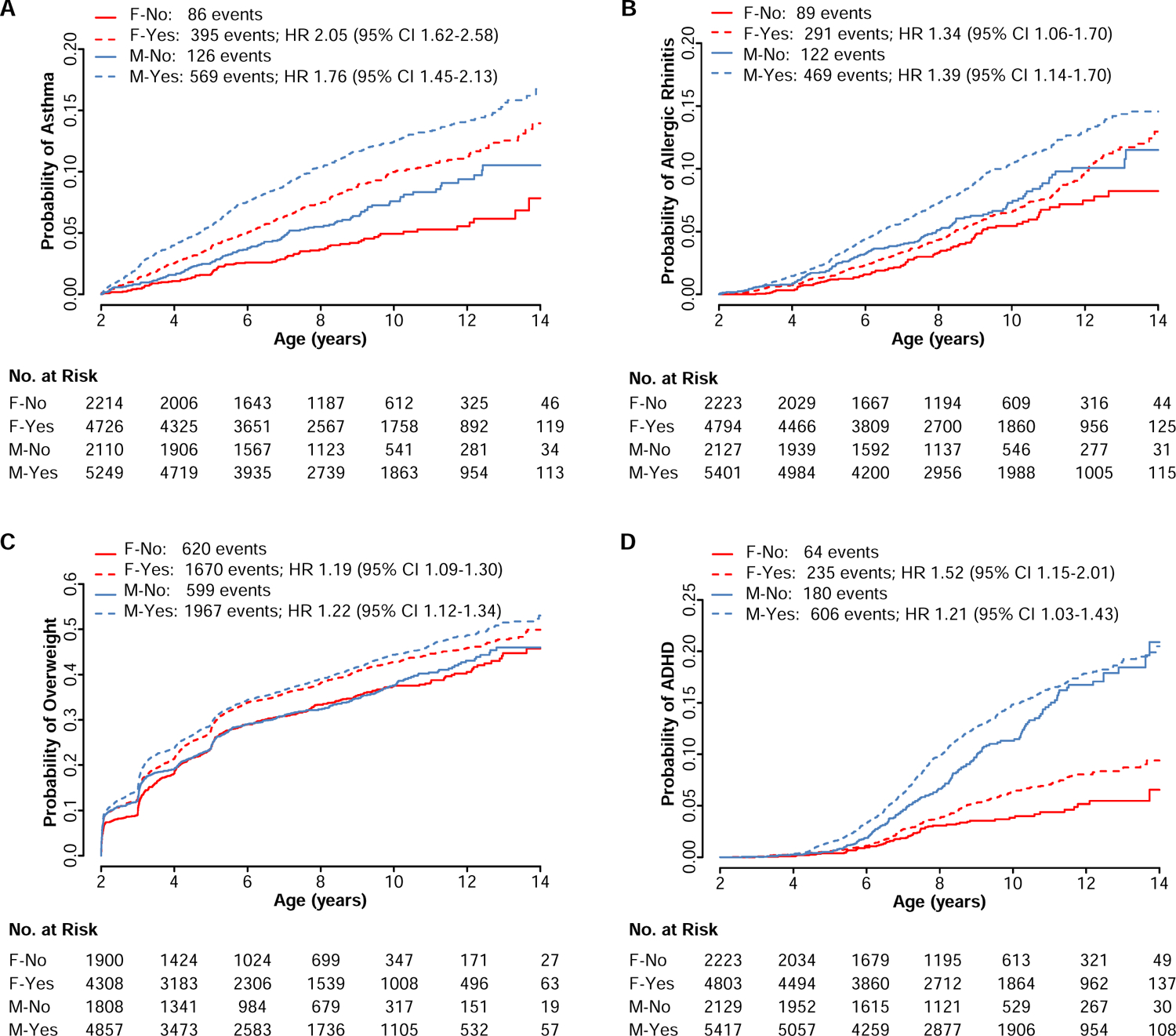
The numbers (No.) of females unexposed (F-No), females exposed (F-Yes), males unexposed (M-No), and males exposed (M-Yes) to antibiotics and at risk for (A) asthma, (B) allergic rhinitis, (C) overweight, and (D) attention-deficit hyperactivity disorder (ADHD) are reported below the x-axis for the specified ages. The total numbers of events for each group are reported in the legend for each condition.
The Influence of the Number, Type, and Timing of Antibiotic Exposures
We next examined the extent to which the number of antibiotic prescriptions in the first two years of life was associated with the risk of childhood health outcomes. Among children who received one or two prescriptions, only girls were at significantly higher risk to develop asthma and celiac disease compared to those unexposed (Table 2). By contrast, receiving three to four prescriptions was associated with a higher incidence of asthma, atopic dermatitis, and overweight in both sexes, ADHD and celiac disease in girls, and obesity in boys. Both girls and boys who received five or more prescriptions had significantly higher risk to develop asthma, allergic rhinitis, overweight, obesity, and ADHD, and girls also were at higher risk of celiac disease.
Table 2.
Associations between the Number of Antibiotic Prescriptions and Risk of Several Common Health Conditions with Childhood Onset.
| Condition | No. | Females HR (95% CI), | P-value | Males HR (95% CI), | P-value |
|---|---|---|---|---|---|
| Asthma | 1–2 | 1.57 (1.20–2.05) | .001 | 1.28 (1.01–1.61) | .037 |
| 3–4 | 1.85 (1.37–2.50) | <.001 | 1.92 (1.52–2.44) | <.001 | |
| 5+ | 3.00 (2.31–3.90) | <.001 | 2.24 (1.81–2.78) | <.001 | |
| Allergic rhinitis | 1–2 | 0.94 (0.70–1.26) | .680 | 1.04 (0.82–1.33) | .749 |
| 3–4 | 1.17 (0.84–1.62) | .348 | 1.24 (0.95–1.61) | .110 | |
| 5+ | 2.12 (1.62–2.77) | <.001 | 1.92 (1.54–2.39) | <.001 | |
| Food allergy | 1–2 | 1.27 (0.76–2.12) | .371 | 1.31 (0.85–2.02) | .224 |
| 3–4 | 0.83 (0.41–1.68) | .596 | 1.49 (0.93–2.39) | .097 | |
| 5+ | 1.66 (0.97–2.86) | .064 | 1.42 (0.91–2.21) | .119 | |
| Atopic dermatitis | 1–2 | 1.41 (0.90–2.23) | .136 | 1.29 (0.83–2.00) | .251 |
| 3–4 | 1.87 (1.13–3.09) | .015 | 1.71 (1.08–2.70) | .023 | |
| 5+ | 1.33 (0.79–2.23) | .276 | 1.35 (0.86–2.12) | .188 | |
| Celiac disease | 1–2 | 8.12 (1.03–64.10) | .047 | 0.87 (0.22–3.47) | .840 |
| 3–4 | 8.87 (1.04–75.95) | .046 | 1.02 (0.23–4.56) | .980 | |
| 5+ | 12.32 (1.56–97.32) | .017 | 2.42 (0.76–7.74) | .136 | |
| Overweight | 1–2 | 1.06 (0.95–1.18) | .276 | 1.08 (0.97–1.20) | .163 |
| 3–4 | 1.14 (1.01–1.30) | .041 | 1.23 (1.09–1.39) | .001 | |
| 5+ | 1.45 (1.30–1.63) | <.001 | 1.40 (1.26–1.56) | <.001 | |
| Obesity | 1–2 | 1.00 (0.86–1.17) | .962 | 1.05 (0.90–1.21) | .543 |
| 3–4 | 1.11 (0.92–1.33) | .270 | 1.22 (1.04–1.43) | .016 | |
| 5+ | 1.37 (1.16–1.60) | <.001 | 1.45 (1.25–1.67) | <.001 | |
| ADHD | 1–2 | 1.31 (0.95–1.80) | .097 | 1.06 (0.87–1.29) | .575 |
| 3–4 | 1.60 (1.12–2.29) | .009 | 1.13 (0.91–1.41) | .264 | |
| 5+ | 1.78 (1.28–2.46) | .001 | 1.45 (1.20–1.75) | <.001 | |
| Autism | 1–2 | 0.79 (0.29–2.18) | .647 | 1.00 (0.59–1.70) | .990 |
| 3–4 | 0.67 (0.18–2.52) | .551 | 1.27 (0.72–2.23) | .405 | |
| 5+ | 1.04 (0.36–3.00) | .943 | 1.57 (0.96–2.57) | .075 | |
| Learning disability | 1–2 | 1.07 (0.76–1.51) | .702 | 1.16 (0.92–1.47) | .209 |
| 3–4 | 1.23 (0.83–1.84) | .305 | 1.17 (0.90–1.52) | .251 | |
| 5+ | 1.38 (0.96–1.99) | .080 | 1.21 (0.95–1.53) | .125 | |
Notably, exposure to cephalosporins was associated with increased risk for the greatest number of conditions and, uniquely, autism and food allergies (Table 3). Penicillins were associated with increased risk for asthma and overweight in both sexes, celiac disease and ADHD in girls, and obesity in boys, whereas they were associated with reduced risk for autism in girls. Sulfonamides only increased the risk for overweight in boys. Macrolides were associated with an increased risk for asthma and overweight in both sexes, and allergic rhinitis and obesity in boys, but were associated with lower risk of atopic dermatitis in girls and learning disability in boys.
Table 3.
Association between the Type of Antibiotics Prescribed and Risk of Several Common Childhood Health Conditions.
| Condition | Class | Females HR (95% CI) | P-value | Males HR (95% CI) | P-value |
|---|---|---|---|---|---|
| Asthma | Penicillins | 1.50 (1.21–1.87) | <.001 | 1.35 (1.12–1.62) | .001 |
| Cephalosporins | 1.39 (1.13–1.71) | .002 | 1.29 (1.09–1.53) | .004 | |
| Sulfonamides | 1.27 (0.93–1.74) | .130 | 0.92 (0.66–1.27) | .602 | |
| Macrolides | 1.33 (1.08–1.62) | .006 | 1.34 (1.14–1.58) | .001 | |
| Allergic rhinitis | Penicillins | 1.15 (0.91–1.46) | .247 | 1.16 (0.95–1.41) | .148 |
| Cephalosporins | 1.83 (1.46–2.30) | <.001 | 1.29 (1.07–1.55) | .008 | |
| Sulfonamides | 1.16 (0.81–1.66) | .405 | 1.10 (0.80–1.52) | .557 | |
| Macrolides | 1.11 (0.88–1.40) | .359 | 1.39 (1.16–1.66) | <.001 | |
| Food allergy | Penicillins | 0.74 (0.47–1.15) | .181 | 1.06 (0.73–1.53) | .774 |
| Cephalosporins | 2.73 (1.74–4.28) | <.001 | 1.96 (1.39–2.76) | <.001 | |
| Sulfonamides | 1.27 (0.65–2.49) | .490 | 0.67 (0.31–1.43) | .299 | |
| Macrolides | 1.00 (0.62–1.59) | .985 | 0.99 (0.69–1.41) | .944 | |
| Atopic dermatitis | Penicillins | 1.27 (0.87–1.87) | .220 | 1.40 (0.96–2.03) | .079 |
| Cephalosporins | 1.69 (1.14–2.52) | .010 | 1.29 (0.90–1.84) | .166 | |
| Sulfonamides | 0.98 (0.49–1.96) | .953 | 1.37 (0.75–2.51) | .301 | |
| Macrolides | 0.59 (0.37–0.92) | .021 | 0.83 (0.57–1.19) | .304 | |
| Celiac disease | Penicillins | 6.74 (1.56–29.23) | .011 | 1.61 (0.51–5.12) | .417 |
| Cephalosporins | 0.49 (0.16–1.50) | .213 | 1.53 (0.59–3.95) | .383 | |
| Sulfonamides | 0.57 (0.07–4.28) | .582 | 1.42 (0.32–6.34) | .648 | |
| Macrolides | 1.26 (0.53–3.03) | .600 | 1.08 (0.42–2.79) | .869 | |
| Overweight | Penicillins | 1.11 (1.02–1.22) | .021 | 1.13 (1.03–1.24) | .007 |
| Cephalosporins | 1.10 (1.00–1.22) | .057 | 1.11 (1.01–1.22) | .027 | |
| Sulphonamides | 1.11 (0.94–1.31) | .232 | 1.20 (1.01–1.41) | .033 | |
| Macrolides | 1.16 (1.05–1.28) | .002 | 1.11 (1.01–1.21) | .027 | |
| Obesity | Penicillins | 1.05 (0.92–1.19) | .497 | 1.15 (1.02–1.31) | .023 |
| Cephalosporins | 1.17 (1.02–1.35) | .029 | 1.08 (0.96–1.22) | .217 | |
| Sulfonamides | 1.15 (0.92–1.45) | .221 | 1.11 (0.89–1.39) | .338 | |
| Macrolides | 1.08 (0.94–1.24) | .263 | 1.15 (1.02–1.29) | .022 | |
| ADHD | Penicillins | 1.50 (1.14–1.96) | .003 | 1.08 (0.91–1.27) | .382 |
| Cephalosporins | 1.21 (0.92–1.59) | .175 | 1.18 (1.00–1.39) | .053 | |
| Sulfonamides | 0.65 (0.38–1.10) | .110 | 1.19 (0.89–1.58) | .233 | |
| Macrolides | 1.03 (0.79–1.34) | .846 | 1.09 (0.93–1.28) | .283 | |
| Autism | Penicillins | 0.39 (0.16–0.95) | .038 | 0.96 (0.62–1.47) | .836 |
| Cephalosporins | 2.77 (1.09–7.02) | .032 | 1.89 (1.25–2.84) | .002 | |
| Sulfonamides | 1.08 (0.24–4.81) | .915 | 1.13 (0.54–2.35) | .752 | |
| Macrolides | 1.21 (0.47–3.14) | .695 | 0.83 (0.55–1.28) | .406 | |
| Learning disability | Penicillins | 1.18 (0.88–1.58) | .279 | 1.06 (0.87–1.29) | .562 |
| Cephalosporins | 1.36 (0.99–1.85) | .056 | 1.48 (1.21–1.80) | <.001 | |
| Sulfonamides | 0.96 (0.56–1.64) | .874 | 1.03 (0.71–1.51) | .866 | |
| Macrolides | 0.93 (0.68–1.28) | .666 | 0.79 (0.65–0.98) | .028 | |
Timing of antibiotic exposure was associated with overlapping and differential risks. Antibiotics prescribed before 6 months of age were significantly associated with risk of atopic dermatitis, overweight, and ADHD in both sexes, asthma, allergic rhinitis, and obesity in girls, and food allergy in boys (Supplemental Table 5). Antibiotics prescribed between 6- and 12-months were most strongly associated with the incidence of asthma, allergic rhinitis, overweight and obesity in both sexes. Antibiotics prescribed from 12- to 24-months were associated with significantly increased risk of asthma and overweight in both sexes, ADHD in girls, and allergic rhinitis in boys.
The Influence of Infant and Maternal Factors on Risk
We next studied child and maternal factors that might confound the risk estimations. Male sex was associated with significantly increased risk for asthma, allergic rhinitis, food allergy, overweight, obesity, ADHD, autism, and learning disability (Supplemental Table 6). Delivery by caesarian section was associated with increased risk for allergic rhinitis, atopic dermatitis, overweight, obesity, and ADHD. Higher birthweight was associated with increased risk of overweight, obesity, and celiac disease, but reduced risk of asthma and learning disability. Ethnicity differentially affected the risk of several conditions.
Mothers were prescribed antibiotics during 5,632 (39%) of the observed pregnancies (Table 1). Maternal exposure to antibiotics was associated with increased risk for asthma, allergic rhinitis, overweight, obesity, ADHD, and learning disability in their children (Supplemental Table 6). Maternal smoking was associated with increased risk for asthma, overweight, obesity, ADHD, and learning disability. Older maternal age was associated with increased risk for allergic rhinitis, food allergy, and autism, but lowered risk for asthma, overweight, obesity, and ADHD. Maternal post-high school education appeared to mitigate the risk for childhood asthma, overweight, obesity, ADHD, and learning disability, but was associated with a higher risk of food allergy in the children.
Given the strong influence of child and maternal confounders, we conducted multivariate analysis to better characterize the risks associated with antibiotics. After adjustment for male sex, ethnicity, delivery by cesarean section, and maternal age, education, smoking and antibiotic exposure during pregnancy, antibiotic-associated risks remained significant for childhood onset of asthma, allergic rhinitis, atopic dermatitis, celiac disease, overweight, obesity, ADHD, and learning disability (Figure 2, panel A). Multivariate analyses stratified by sex are presented in Supplemental Table 7.
Figure 2. Associations between Antibiotic Exposure in the First Two Years of Life and Risk of Several Common Health Conditions with Childhood Onset.
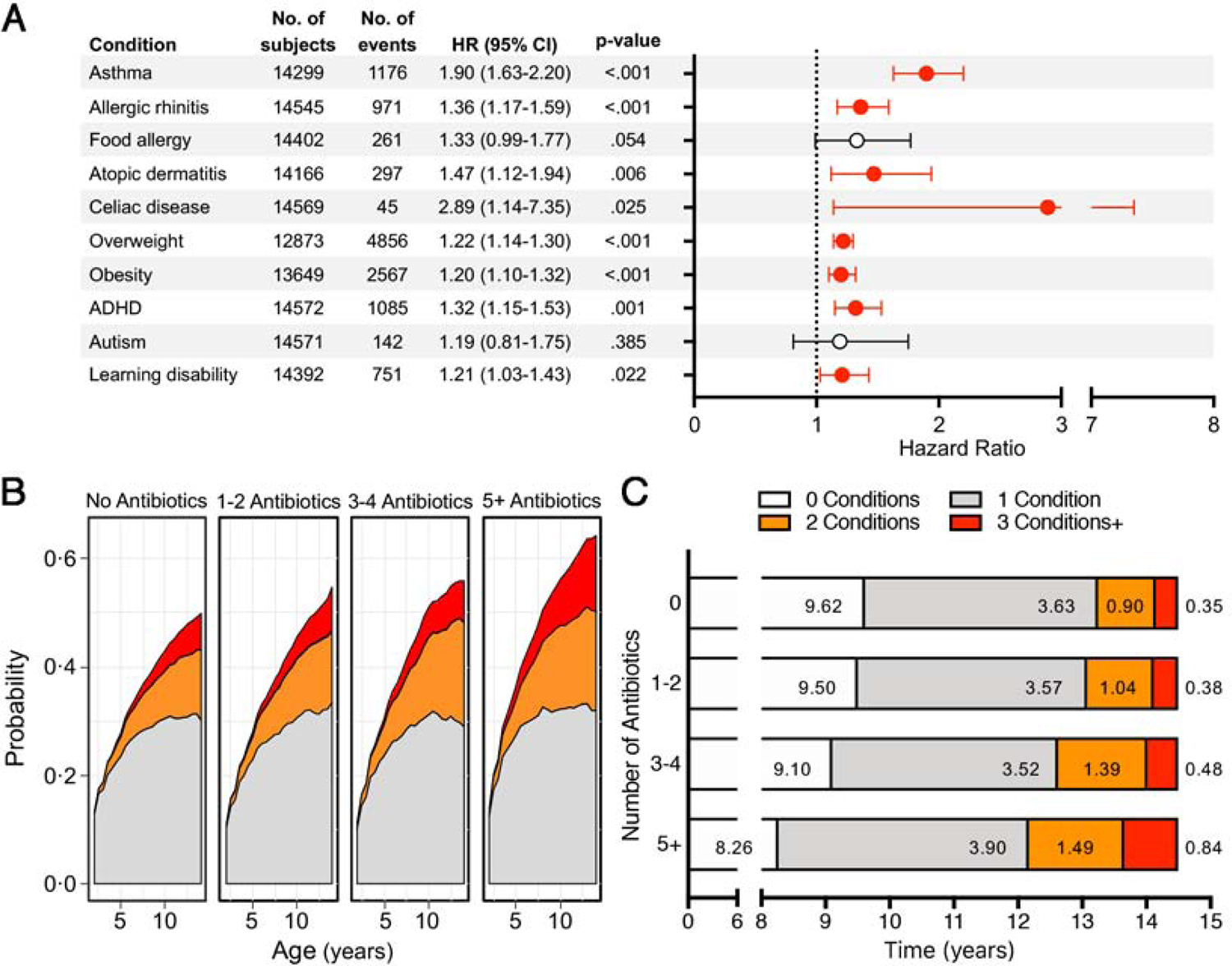
(A) Hazard ratios (HR) and 95% confidence intervals (95% CI) for antibiotic exposure and risk of health conditions with childhood onset, after adjustment for child confounders (male sex, birth weight, ethnicity, and cesarean section) and maternal confounders (age, education, smoking, and antibiotic use during pregnancy). HRs and CIs highlighted in red are statistically significant. Attention-deficit hyperactivity disorder is abbreviated as ADHD. (B) Cumulative probability of developing 1, 2, and 3 or more health conditions in children unexposed or exposed to antibiotics stratified by number of prescriptions. (C) Number of years spent with 0, 1, 2, or 3 or more health conditions during the first 14.5 years of life stratified by number of prescriptions.
Antibiotics and Risk for Multiple Childhood Health Conditions
We next evaluated whether children exposed to antibiotics during the first two years of life were more likely to develop multiple health conditions. Exposed children had a greater probability of experiencing more than one condition and, on average, spending greater time having two or more conditions, especially if having received multiple prescriptions (Figure 2, panels B and C). The most common disease dyads among children exposed to antibiotics included those of obesity with asthma, ADHD, and learning disability, and asthma with allergic rhinitis. Similar dyads were observed in unexposed children (Supplemental Figure 3 and Supplemental Table 8). Obesity, asthma, and allergic rhinitis was the most common disease triad in both exposed and unexposed children, whereas combinations of asthma, obesity, and either atopic dermatitis or ADHD were more frequently observed in children exposed to antibiotics (Supplemental Table 8).
DISCUSSION
Our study highlights the prevalent use of antibiotics in infants and reveals concerning associations between exposure to antibiotics and distinct immunological, metabolic, and neurobehavioral health conditions and the occurrence of combinations of these conditions during childhood. The health risks associated with antibiotic exposure in the first two years of life relate to the number, type, and timing of prescriptions. Notably, the association between antibiotic exposures and adverse health outcomes, including asthma, allergic rhinitis, atopic dermatitis, celiac disease, overweight, obesity, ADHD, and learning disability persisted after adjusting for recognized and important child and maternal confounders.
Early life host-microbiome interactions contribute to the proper development of the immune system4. Antibiotics markedly impact microbial composition; even transient perturbations during critical developmental periods may compromise both immune tolerance and inflammatory responses.4 Children with immature intestinal microbiota19 and low abundance of specific bacterial taxa20 may have increased asthma susceptibility. We found that children exposed in the first two years of life to the most commonly prescribed antibiotic classes (penicillins, cephalosporins, and macrolides) were more likely to develop asthma and allergic rhinitis, with strong dose-response relationships. The risk of atopic dermatitis also was increased, particularly in children receiving antibiotics early, in multiple doses, or, specifically, cephalosporins.
Antibiotic exposure also was associated with food allergy risk, with increased risk in both sexes with cephalosporins, and with exposure to any antibiotic prior to 6 months. A recent study also found associations of early exposure to antibiotics with food allergies, with effects specific to cow’s milk and eggs. 11 In that study, the risk was influenced by anti-acid medications, a potential confounder that we did not include. A Finnish study also found an association of allergy to cow’s milk with antibiotic exposures in the child before disease onset, and with use of antibiotics in their mother during and before pregnancy.21
Although the risk of celiac disease in our study was increased in girls who received one or more antibiotic prescriptions and, specifically, penicillins, the number of affected persons was small. Controlling for several confounders did not eliminate significance; however, the wide confidence interval in our study is consistent with the mixed results of prior studies.22,23 Overall, our findings extend the associations between early antibiotic exposure and later development of asthma, allergic diseases, and autoimmune conditions described previously.11,24–26 We hypothesize that antibiotics play a causal role in the pathogenesis of childhood immune disorders through disruption of the microbiome during critical developmental periods.4,7
Beside shaping host immunity, the microbiome also affects body composition and systemic metabolism.6 The growth promoting effects of low-dose antibiotics in livestock have been long-appreciated in animal husbandry. Murine studies have shown that early life antibiotics increase adiposity, even with recovery of the initial intestinal dysbiosis, particularly in the context of high fat feeding.13,14 Most27–30, but not all studies10,31, have found an association between early-life antibiotic exposure and childhood obesity. Our observation that multiple antibiotic prescriptions significantly increased risk of overweight and obesity agrees with prior reports.27,29 However, it may be that infections, rather than antibiotics exposures, are actually heightening the risk of childhood obesity.31 Our analysis did not include the underlying infections for which antibiotics were prescribed and, therefore, confounding by indication may have occurred. This limitation is common to all of the associations we found; however, animal models eliminate this variable.13,14 The specificity of associations with particular antibiotics, especially cephalosporins, but not others, is consistent with those antibiotics playing a pathogenetic role.
The microbiome may affect neural development5; therefore, practices compromising the establishment of microbial communities may affect the risk of neurobehavioral disorders.32 We found a significant association between antibiotic prescriptions in the first two years of life and with current ADHD,33 but their significance remains uncertain.12,34 In addition, we observed a significantly increased risk of autism and learning disabilities, only after exposure to cephalosporins. In fact, cephalosporin exposure was associated with increased risk for several conditions, suggesting the importance of distinguishing amongst antibiotics. If confirmed, differential selection of the developing microbiota by cephalosporins could provide an underlying mechanism. In contrast, the association of penicillins with lower risk that we observed in girls also may indicate a variation in developmental effects due to the differential selection. These selective effects of antibiotics should be considered in future studies. As a precedent, marked antibiotic-specific differential predisposition to C. difficile infections has been reported.35 Two recent studies did not observe an association between antibiotic exposure and autism36,37, but they did not discern between antibiotic classes. By contrast, a recent Danish nationwide study found an association between any treated infection and several childhood and adolescent neurobehavioral conditions. For autism, the risk was significantly increased only in children who required hospitalization for infection34, a circumstance that we did not investigate.
Strengths of this study include the well-characterized population-based cohort, the long follow-up duration, and the capture and confirmation of antibiotic prescriptions and of a broad set of medical diagnoses through the records-linkage system, which eliminates recall bias. However, our results need to be interpreted in the context of the study’s limitations. We could not disentangle the effects of antibiotics from those of the underlying conditions, which may have resulted in confounding by indication (e.g., antibiotics were prescribed for respiratory infection, which conveyed the risk for subsequent asthma) or reverse causation (e.g., respiratory infections are an early manifestation of undiagnosed asthma and may be treated with antibiotics). However, excluding participants with illnesses diagnosed before age 2, including several health outcomes with presumably distinct pathogenic mechanisms, and estimating the cumulative occurrence of multiple conditions, make our findings robust. We could not verify compliance with antibiotic prescriptions on record nor account for prescriptions issued outside of the records-linkage system, which may have affected our estimates of absolute exposure. Despite controlling for several major child and maternal confounders, we did not account for breast feeding, lifestyle behaviors (e.g., diet, physical activity, sleep), other medications, or familial factors (e.g., siblings).
CONCLUSIONS
In our study of a population-based cohort of children, antibiotic exposure in the first two years of life was associated with increased risk for several immunological, metabolic, and neurobehavioral childhood-onset health conditions, even after adjusting for several established child and maternal confounders. Our findings are consistent with the hypothesis that early-life microbiome composition is a critical health determinant, and that perturbations during key developmental periods can have long-term consequences. Although our findings reflect associations, not causation, they generate testable hypotheses related to the influence of antibiotic dose, class, and timing on childhood health. When antibiotics were first developed and deployed, the overwhelming consideration was control of pathogens. We now realize that their widespread application has considerable collateral effect on the microbiome, which may be of special importance in developing children. Antibiotic prescribing patterns in childhood are extremely variable38–40. With further study, practical clinical guidelines can be established to optimize benefit and minimize risk of antibiotics in children.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the generous support of the Pritzker Foundation (NKL), Leonard and Mary Lou Hoeft Fund in Healthy Aging and Independent Living Research (NKL), and the Rochester Epidemiology Project (AG034676 and AG052425; WAR). This work was also supported in part by U01 AI22285, by the C & D fund, and the Transatlantic Network of the Fondation Leducq (MB).
ROLE OF THE FUNDING SOURCE
The funding sources for this study did not have a role in in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Financial support: Pritzker Foundation (NKL), Leonard and Mary Lou Hoeft Fund in Healthy Aging and Independent Living Research (NKL), and the Rochester Epidemiology Project (AG034676 and AG052425; WAR). This work was also supported in part by U01 AI22285, by the C & D fund, and the Transatlantic Network of the Fondation Leducq (MB).
ABBREVIATIONS
- ADHD
attention deficit hyperactivity disorder
- ICD
International Classification of Disease
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure: The authors do not have conflicts of interest to declare.
REFERENCES
- 1.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. Jama. 2016;315(17):1864–1873. [DOI] [PubMed] [Google Scholar]
- 2.Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial Resistance. Jama. 2016;316(11):1193–1204. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science (New York, NY). 2016;352(6285):544–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science (New York, NY). 2016;352(6285):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. Journal of psychiatric research. 2015;63:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nature medicine. 2016;22(10):1079–1089. [DOI] [PubMed] [Google Scholar]
- 7.Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic diseases. Nature reviews Immunology. 2017;17(8):461–463. [DOI] [PubMed] [Google Scholar]
- 8.Moeller AH, Suzuki TA, Phifer-Rixey M, Nachman MW. Transmission modes of the mammalian gut microbiota. Science (New York, NY). 2018;362(6413):453–457. [DOI] [PubMed] [Google Scholar]
- 9.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Science translational medicine. 2016;8(343):343ra382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad MB, Moossavi S, Owora A, Sepehri S. Early-Life Antibiotic Exposure, Gut Microbiota Development, and Predisposition to Obesity. Nestle Nutrition Institute workshop series. 2017;88:67–79. [DOI] [PubMed] [Google Scholar]
- 11.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood. JAMA pediatrics. 2018;172(6):e180315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axelsson PB, Clausen TD, Petersen AH, et al. Investigating the effects of cesarean delivery and antibiotic use in early childhood on risk of later attention deficit hyperactivity disorder. Journal of child psychology and psychiatry, and allied disciplines. 2019;60(2):151–159. [DOI] [PubMed] [Google Scholar]
- 13.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nature microbiology. 2016;1(11):16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology. 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. 2011. 2011;45(3):67. [Google Scholar]
- 19.Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nature communications. 2018;9(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science translational medicine. 2015;7(307):307ra152. [DOI] [PubMed] [Google Scholar]
- 21.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Mother’s and offspring’s use of antibiotics and infant allergy to cow’s milk. Epidemiology (Cambridge, Mass). 2013;24(2):303–309. [DOI] [PubMed] [Google Scholar]
- 22.Kemppainen KM, Vehik K, Lynch KF, et al. Association Between Early-Life Antibiotic Use and the Risk of Islet or Celiac Disease Autoimmunity. JAMA pediatrics. 2017;171(12):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canova C, Zabeo V, Pitter G, et al. Association of maternal education, early infections, and antibiotic use with celiac disease: a population-based birth cohort study in northeastern Italy. American journal of epidemiology. 2014;180(1):76–85. [DOI] [PubMed] [Google Scholar]
- 24.Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007;131(6):1753–1759. [DOI] [PubMed] [Google Scholar]
- 25.Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2013;24(8):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch AG, Pollak J, Glass TA, et al. Early-life antibiotic use and subsequent diagnosis of food allergy and allergic diseases. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2017;47(2):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA pediatrics. 2014;168(11):1063–1069. [DOI] [PubMed] [Google Scholar]
- 28.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. International journal of obesity (2005). 2014;38(10):1290–1298. [DOI] [PubMed] [Google Scholar]
- 29.Scott FI, Horton DB, Mamtani R, et al. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology. 2016;151(1):120–129.e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark CM, Susi A, Emerick J, Nylund CM. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68(1):62–69. [DOI] [PubMed] [Google Scholar]
- 31.Li DK, Chen H, Ferber J, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. The lancet Diabetes & endocrinology. 2017;5(1):18–25. [DOI] [PubMed] [Google Scholar]
- 32.Vuong HE, Yano JM, Fung TC, Hsiao EY. The Microbiome and Host Behavior. Annual review of neuroscience. 2017;40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aarts E, Ederveen THA, Naaijen J, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PloS one. 2017;12(9):e0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohler-Forsberg O, Petersen L, Gasse C, et al. A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA psychiatry. 2018. [DOI] [PMC free article] [PubMed]
- 35.Leffler DA, Lamont JT. Clostridium difficile infection. The New England journal of medicine. 2015;372(16):1539–1548. [DOI] [PubMed] [Google Scholar]
- 36.Hamad AF, Alessi-Severini S, Mahmud SM, Brownell M, Kuo IF. Early childhood antibiotics use and autism spectrum disorders: a population-based cohort study. International journal of epidemiology. 2018;47(5):1497–1506. [DOI] [PubMed] [Google Scholar]
- 37.Axelsson PB, Clausen TD, Petersen AH, et al. Relation Between Infant Microbiota and Autism?: Results from a National Cohort Sibling Design Study. Epidemiology (Cambridge, Mass). 2019;30(1):52–60. [DOI] [PubMed] [Google Scholar]
- 38.Gerber JS, Prasad PA, Russell Localio A, et al. Variation in Antibiotic Prescribing Across a Pediatric Primary Care Network. Journal of the Pediatric Infectious Diseases Society. 2015;4(4):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 40.Stam J, van Stuijvenberg M, Gruber C, et al. Antibiotic use in infants in the first year of life in five European countries. Acta paediatrica (Oslo, Norway : 1992). 2012;101(9):929–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


