Abstract
Rationale:
Alcohol and nicotine co-dependence is common in humans and nicotine increases alcohol drinking in humans without alcohol use disorder (AUD). Nevertheless, there is little basic research on the interactions between the reinforcing effects of these two drugs.
Objectives:
The aim of this study was to investigate the effects of chronic nicotine injections on oral alcohol self-administration in alcohol-non-dependent rats.
Methods:
After stable alcohol self-administration was reached (baseline) and a period without alcohol access, adult male rats were treated with chronic nicotine or saline injections for 105 days during which time they were tested intermittently for alcohol self-administration. There were 3 experimental groups: 1) saline: rats treated with saline for 105 days; 2) early nicotine: rats treated with nicotine for 70 days, then with saline for 35 days; 3) late nicotine: rats treated with saline for 35 days, then with nicotine for 70 days.
Results:
Our results indicate that 1) chronic nicotine increases alcohol consumption regardless of whether exposure to alcohol was interrupted (early nicotine) or not (late nicotine) before the start of nicotine treatment, 2) the number of alcohol reinforcements correlates to blood-alcohol levels, 3) alcohol self-administration rapidly decreases when nicotine is no longer available (early nicotine).
Conclusions:
These discoveries may have clinical implications in social drinkers that use nicotine products, in that chronic nicotine can escalate alcohol drinking and cessation of nicotine exposure may decrease alcohol use.
Keywords: addiction, alcohol, blood-alcohol levels, nicotine, self-administration, tobacco
Introduction
Alcohol and nicotine dependence are two of the leading preventable causes of death worldwide (Miller and Gold 1998). Epidemiological studies indicate frequent comorbid alcohol and nicotine dependence (Bobo 1992; Miller and Gold 1998; Anthony and Wagner 2000) as well as worse health and treatment outcomes in patients that are dependent on both drugs in comparison to humans dependent on one drug or the other (Bobo 1992; Miller and Gold 1998; Littleton et al. 2007). One particular aspect of clinical interest is the ability of nicotine to increase alcohol drinking in humans without alcohol use disorder (AUD) (Barrett et al. 2006; Harrison and McKee 2008) to levels that could be considered a risk factor for the development of AUD. In this regard, smokers are in fact more likely to become alcohol dependent (DiFranza and Guerrera 1990; Grant et al. 2004) and to relapse to alcohol drinking (Gulliver et al. 2006) compared to non-smokers. Interestingly, in a human laboratory study conducted in smokers without AUD, pretreatment with a transdermal nicotine patch accelerated the time to peak ethanol concentration and increased the subjective reports of ethanol-induced euphoria and feeling drunk (Kouri et al. 2004).
Despite these clinical reports, only a few pre-clinical studies have examined the effects of nicotine on alcohol consumption. Those studies were performed in alcohol non-dependent rodents (except Leão et al. 2015, in which both alcohol non-dependent and dependent animals were employed) and reported conflicting results. Studies conducted using a two-bottle choice homecage drinking paradigm showed that nicotine increases alcohol consumption (Blomqvist et al. 1996) or that nicotine can increase or not alter alcohol consumption depending on the nicotine dose (Lê AD et al. 2000). In alcohol self-administration studies, some investigations reported that nicotine increases alcohol self-administration (López-Moreno et al 2004; Doyon et al. 2013; Leão et al. 2015), whereas others showed a decrease (Sharpe and Samson 2002) in alcohol self-administration, no effect (Nadal and Samson 1999; Clark et al. 2001), or both an increase or no change in consumption depending on the nicotine dose (Lê AD et al 2003). Interestingly, it was also shown that nicotine induces compulsive-like alcohol seeking in otherwise non-dependent animals (Leão et al. 2015) and increases alcohol self-administration when rats self-administered both alcohol (orally) and nicotine (i.v.) (Lê et al. 2014).
As discussed by Ostroumov et al. (2015), the reasons for these discrepancies are unclear, as the studies were conducted with comparable rat strains, ethanol and nicotine doses, session lengths, time between nicotine treatment and alcohol exposure, and other methodological variables. A caveat in the interpretation of the effects of nicotine on alcohol self-administration could be the estimation of alcohol consumption in terms of active lever presses. Only a few studies (Nadal and Samson 1998; Sharpe and Samson 2002; López-Moreno et al 2004; Doyon et al. 2013) showed alcohol consumption in g/kg, and none measured blood-alcohol levels (BALs). More research is necessary to understand the mechanisms underlying the facilitatory or inhibitory effect of nicotine on alcohol drinking and to investigate if these effects may change over time or fade when nicotine is no longer available. Within this scientific framework, the aim of our study was to evaluate the effects of a chronic nicotine treatment on alcohol self-administration in adult male Wistar rats when alcohol operant sessions were or were not interrupted prior to nicotine treatment. We also measured alcohol consumption after nicotine treatment was terminated, along with measurements of BALs, water and total liquid consumption, and anxiety-like behaviors. Our main hypotheses were that the chronic nicotine treatment would increase alcohol consumption and that this increase in alcohol drinking would persist (at least shortly) after cessation of nicotine treatment.
Material and Methods
Animals
Adult male Wistar rats (N=44) (Charles River, Raleigh, NC, USA) weighing ~300 g at arrival were pair-housed in a humidity- and temperature-controlled (22°C ±1) vivarium on an inverted 12hr light/dark cycle (lights off at 8 am). Rats were acclimated and handled for a week before the beginning of the experiments. Behavioral tests occurred approximately 8 hours into the 12-hour dark cycle. Animals had ad libitum access to food and water, except during the behavioral tests. All procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were in accordance with the National Institute of Health Guidelines.
Drugs
(−)-Nicotine hydrogen tartrate salt (Cat# GL9693, Glentham Life Sciences Ltd, Wiltshire, United Kingdom) was dissolved in a sterile 0.9% saline solution and administrated via subcutaneous (s.c.) injections at the dose of 0.8 mg/Kg (see Supplemental Information and Matta et al. 2006 for more details regarding nicotine dose calculation).
Experimental procedure
Alcohol self-administration (SA) Training:
After the first week of alcohol exposure (see Supplemental Information and Roltsch et al. 2014), the rats underwent daily 30-min alcohol operant sessions for 10 days, then 30-min sessions twice a week for 15 sessions, at which point stable levels of ethanol responses across 5 sessions were reached by each rat (baseline condition). Rats were trained to orally self-administer a 10% w/v alcohol solution and water on a Fixed Ratio (FR) 1 schedule of reinforcement (i.e., each operant response was reinforced with 0.1 ml of the solution) in a concurrent, two-lever, free-choice contingency that did not incorporate a sweet fading procedure as previously described (Roltsch et al. 2014). Self-administration sessions were conducted in standard operant conditioning chambers (Med Associates) in sound‐ and light‐attenuating cubicles.
Effects of nicotine injections on alcohol SA:
After stable alcohol consumption was reached (baseline), animals were kept in home cages for 30 days and handled twice per week. Afterwards, rats were injected daily with nicotine (0.8 mg/kg, s.c.) or 0.9% saline solution for 105 days and tested for alcohol SA twice per week for 30 operant sessions (we started the 1st post-baseline operant session on the 1st day of nicotine/saline injections and finished the 30th post-baseline session on the 105th injection day, see Figure 1). Rats were weighed and injected with nicotine/saline 15 min prior to 30-min operant alcohol SA sessions, similar to previous studies (see Ostroumov et al. 2015 for a review), in part because brain nicotine levels peak ~15 min after s.c. nicotine injection (Turner 1975) and plasma nicotine half-life is ~45 min (Matta et al. 2006) in rats. After baseline, rats were randomly assigned to one of three groups: 1) saline group (N=7), in which animals were injected with saline for 105 days (sessions 1-30); 2) early nicotine group (N=7), in which rats were injected with nicotine for 70 days (sessions 1-20) and then with saline for 35 days (sessions 21-30); 3) late nicotine group (N=7), in which rats were injected with saline for 35 days (sessions 1-10) and then with nicotine for 70 days (sessions 11-30) (see Figure 1). After the baseline period, we inserted one month of no alcohol SA for the following reasons: 1) to study the effects of nicotine on alcohol intake preceded by an interruption of alcohol drinking (early nicotine group, session 1-20); 2) to study if the effects of nicotine on alcohol self-administration could persist when nicotine is replaced by saline (early nicotine group, session 21-30); 3) to study the effects of nicotine on alcohol intake not preceded by interruption of alcohol drinking (late nicotine group, session 11-30). We acknowledge that alcohol SA was interrupted in the late nicotine group after baseline as with the early nicotine group; however, the 1st nicotine injection was administered at the 11th post-baseline session (i.e., the start of nicotine treatment was not immediately preceded by lack of alcohol access in this group). In order to verify that the number of lever presses to obtain ethanol was related to the amount of alcohol ingested, blood samples were collected to measure BALs from each rat at the end of both the last baseline session and the 29th (next-to-last) post-baseline session (see Supplemental Information and Avegno and Gilpin 2019 for more details regarding blood collection and BALs measurement). An additional 23 control rats were kept in home cages at all times and never exposed to alcohol, while still being handled and pharmacologically treated with nicotine/saline like the experimental groups (saline: N=8; early nicotine: N=7; late nicotine: N=8).
Fig. 1.
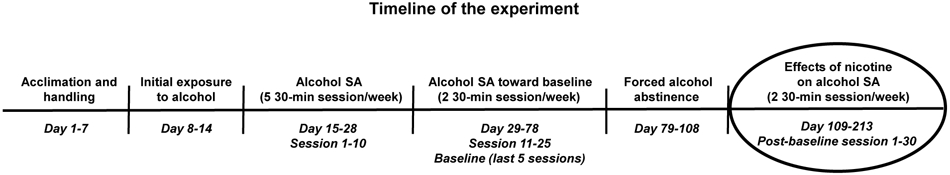
Schematic diagram of the timeline of the experiment. SA: self-administration.
The effects of alcohol x nicotine interaction on anxiety-like behaviors:
The day after the 25th post-baseline operant session, rats were tested for anxiety-like behaviors using a custom-built Elevated Plus Maze (EPM) made with black acrylic. The apparatus is elevated 60 cm from the floor and has 4 arms at right angles (each 10 cm wide x 50 cm long; two arms have 40-cm-high walls (closed arms), while two arms have no walls (open arms)). Rats were placed in the center of the apparatus and, during a 5-min test, were free to move in the different arms. The test was performed in a soundproof room, with a surgical light facing a wall and creating ~ 10-15 lux light intensity on the apparatus. The EPM is a well-established exploratory model of anxiety in rats, in which “anxious-like” animals spend less time in the open arms (Randall and Commissaris 2003). At the time of anxiety-like behavior testing (the day after the 25th post-baseline session, when animals were not tested for alcohol self-administration), nicotine had been replaced by saline for 2 weeks in the early nicotine group and administered for 7 weeks in the late nicotine group. Tests were run in a drug-free condition at the usual time of the operant sessions (the day of testing animals were not subjected to alcohol self-administration sessions, and daily nicotine/saline injections were given one hour after the EPM test). Control rats were subjected to the EPM test at the same time as the experimental groups. All tests were recorded using an Axis Camera Station to locally store the videos. The time spent (in sec) and the number of entries in the open vs closed arms (all four paws into an arm), were manually counted by a researcher that was blind to the study, while locomotor activity was measured utilizing ezTrack (Pennington et al. 2019), an open-source automated behavior tracking software.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 25 software. All variables are expressed as mean ± SEM. Significant main or interaction effects (p ≤ 0.05) were followed by Tukey post-hoc tests (which adjusts p-value significance criteria for multiple comparisons), except in analyses across the 30 post-baseline sessions, where Fisher’s LSD tests were performed (see Supplemental Information for details on the statistical analyses performed). Graphs were generated using GraphPad Prism 7.05 software.
Results
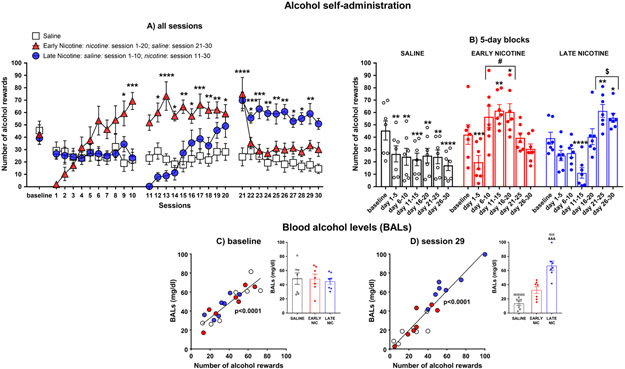
Figure 2. Effects of nicotine injections on alcohol SA and correlation between number of lever rewards and BALs
Fig. 2.
Mean (±SEM) number of alcohol rewards over all 30-min sessions (panel a), averaged by 5-day blocks (panel b), and linear regression between the number of alcohol rewards and the blood alcohol levels (BALs) measured after the last baseline session (panel c) and the 29th post-baseline session (panel d) in the saline, early nicotine, and late nicotine groups (N=7/group); *p≤0.05, **p≤0.01, ***p≤0.001, and ****p<0.0001 compared to baseline; #p≤0.05 compared to session 1-5 & 21-30, and the saline and late nicotine groups; $p≤0.05 compared to session 1-20 and the saline and early nicotine groups. Inset in panels c-d show BALs at the last baseline session (panel c) and the 29th post-baseline sessions (Inset panel d; ααp≤0.001, ααααp<0.0001 compared to baseline; &&&p≤0.001compared to the saline and early nicotine groups).
Analysis of the number of alcohol rewards obtained by rats across all sessions yielded significant main effects of session [F(30,540)=6.964, p<0.0001] and group [F(2,18)=5.478, p=0.0139], and a significant session x group interaction [F(60,540)=10.92, p<0.0001]. As shown in Figure 2a, Fisher’s LSD post-hoc tests indicated that compared to baseline, alcohol self-administration in the saline group was reduced along all the post-baseline sessions (p≤0.05 for all comparisons). Compared to baseline, alcohol self-administration in the early nicotine group (see Figure 2a) was reduced in the first sessions of nicotine treatment (session 1-3, p≤0.001 for all comparisons), increased from session 9 to 20 of nicotine treatment (p≤0.05 for all comparisons except for session 11), and following replacement by saline (session 21, p<0.0001), decreased to baseline levels in the subsequent saline sessions (session 22-30). The same temporal pattern of nicotine effects on alcohol self-administration was observed in the late nicotine group (see Figure 2a). Compared to baseline, alcohol self-administration was reduced in some of the post-baseline sessions in which animals were treated with saline (session 3, 4, 7, and 10; p≤0.05 for all comparisons). Subsequently, nicotine initially decreased (session 11-14, p≤0.001 for all comparisons) but subsequently increased (session 21-29, p≤0.05 for all comparisons) alcohol self-administration compared to baseline. Since day by day differences within and between groups are kept when the results are averaged over 5-day increments, for simplicity of explanation, these differences are reported in the 5-day block analysis (see Figure 2b and Supplemental Information).
As shown in Figure 2c-d, the linear regression analysis between the number of alcohol rewards and the BALs indicated a significant correlation between the two variables in both the last baseline session [F(1,19)=71.82; p<0.0001] and the 29th post-baseline session [F(1,19)=141.1; p<0.0001]. Note that for each subgroup, the correlation was significant in both sessions (baseline: saline, p=0.0057; early nicotine, p=0.0038; late nicotine: p=0.0174; 29th post-baseline session: saline, p=0.0430; early nicotine, p=0.0375; late nicotine, p=0.0160).
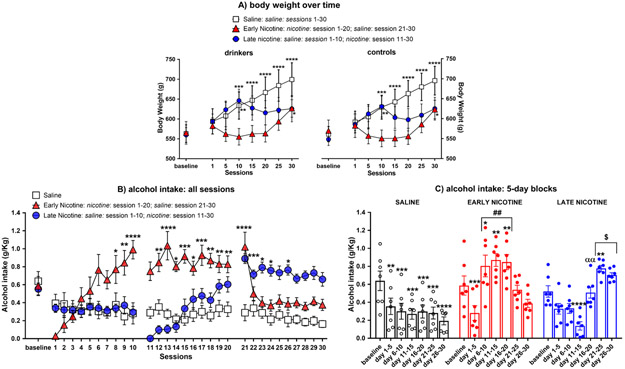
Figure 3. Weight of rats over time and effects of nicotine injections on alcohol intake (g/kg)
Fig. 3.
Mean (±SEM) body weight (panel a) over time in the drinker and control groups, and mean (±SEM) alcohol intake over all 30-min sessions (panel b) and averaged by 5-day blocks (panel c) in the saline, early nicotine, and late nicotine drinker groups (N=7/group); *p ≤ 0.05, **p ≤ 0.01, ***p≤0.001, and ****p<0.0001 compared to the post-baseline session 1 (panel a) or baseline (panel b-c); ##p≤0.01 compared to session 1-5 & 21-30, and the saline and late nicotine groups; $p≤0.05 compared to session 1-15 and the saline and early nicotine groups; ααp≤0.01 compared to session 21-25. For simplicity of explanation, the body weight of rats (panel a) is reported at the post-baseline session 1, 5, 10, 15, 20, 25, and 30 (or at the corresponding day for the control rats); however, rats were weighed before each injection, which occurred 15 min before alcohol sessions for the drinker groups in which alcohol intake was calculated daily for each rat (panel b-c; see text for more details).
For the statistical results of the body weight of rats over time, see Figure 3a and Supplemental Information. The analysis of alcohol intake (g/kg) across all sessions produced significant main effects of session [F (30,540)=6.824; p<0.0001] and group [F(2,18)=8.664; p=0.0023], and a significant session x group interaction [F(60,540) = 12.47; p<0.0001]. As shown in Figure 3b, Fisher’s LSD post-hoc indicated that compared to baseline, alcohol intake in the saline group was reduced across all post-baseline sessions (p≤0.05 for all comparisons), and it also decreased during the post-baseline sessions (session 1 vs session 30, p= 0.0006). Compared to baseline, alcohol intake in the early nicotine group was reduced in the first sessions of nicotine treatment (session 1-3, p≤0.0001), increased from session 8 to 20 of nicotine treatment (p≤0.05 for all comparisons except for session 11) and following replacement by saline (session 21, p<0.0001), decreased to or below baseline levels in subsequent saline sessions (session 22-30; session 23, 24, 26, 27, 28, and 30 under baseline levels, p≤0.05 for all comparisons; see Figure 3b). The same temporal pattern of nicotine effects on alcohol intake was observed in the late nicotine group (see Figure 3b). Compared to baseline, alcohol intake was reduced in all post-baseline sessions in which animals where treated with saline (session 1-10, p≤0.05 in all comparisons). Afterwards, nicotine initially decreased (session 11-15, p≤0.05 for all comparisons) but subsequently increased (session 21, 23, 24, and 26, p≤0.05 for all comparisons) alcohol intake in comparison to baseline. Since day by day differences within and between groups are kept when the results are averaged over 5-day increments, for simplicity of explanation, these differences are reported in the 5-day block analysis (see Figure 3c and Supplemental Information).
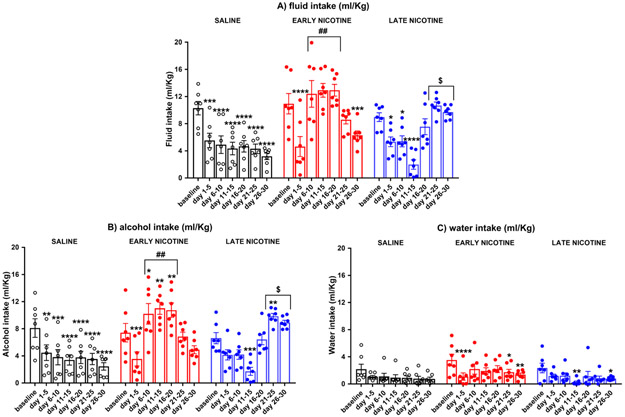
Figure 4. Effects of nicotine injections on alcohol, water, and total liquid consumption (ml/kg)
Fig. 4.
Mean (±SEM) ml/Kg total liquid consumption (panel a), and mean (±SEM) ml/Kg alcohol (panel b) and water (panel c) consumption averaged by 5-day blocks in the saline, early nicotine, and late nicotine groups (N=7/group); *p≤ 0.05, **p≤0.01, ***p≤0.001, and ****p<0.0001 compared to baseline; ##p≤0.01 compared to session 1-5 & 21-30, and the saline and late nicotine groups; $p≤0.05 compared to session 1-15 and the saline and early nicotine groups.
The two-way ANOVA of the total liquid consumption showed a significant main effect of group [F(2,18)=9.573, p=0.0015] and session [F(6,108)=14.48, p≤0.0001], and a significant group x session [F(12,108)=17.31, p≤0.0001] interaction. Post-hoc comparisons indicated that in the saline group, compared to baseline, the total liquid consumption decreased in all the post-baseline sessions (p≤0.001 for all comparisons; see Figure 4a). The early nicotine group decreased liquid consumption in comparison to baseline over the first 5 sessions of nicotine treatment (sessions 1-5: p<0.0001) and the last 5 sessions of saline treatment (session 26-30: p=0.0003; see Figure 4a). Interestingly, the total liquid consumption from session 6 to session 20 of nicotine treatment was higher in comparison to: 1) the first 5 days of nicotine treatment (p<0.0001 for all comparisons); 2) the subsequent saline sessions (p≤0.01 for all comparisons); 3) the saline and late nicotine groups (p<0.0001 for all comparisons, see Figure 4a). The late nicotine group – in comparison to baseline – decreased liquid consumption during the saline post-baseline sessions (session 1-10, p≤0.05 for all comparisons) and the first 5 days of nicotine treatment (sessions 11-15, p<0.0001; see Figure 4a). The liquid consumption of the last 10 days of nicotine treatment (sessions 21-30) was higher in comparison to: 1) the saline sessions (p≤0.01 for all comparisons); 2) the first 5 days of nicotine treatment (sessions 11-15, p<0.0001 for all comparisons); 3) the saline and early nicotine groups (late nicotine vs saline, p<0.0001 for all comparisons; late nicotine vs early nicotine, p≤0.05 for all comparisons; see Figure 4a). There was also an increase in liquid consumption from session 11-15 to session 16-20 (p<0.0001), and from session 16-20 to 21-25 (p=0.0044) of nicotine treatment.
The three-way ANOVA of alcohol and water consumption (ml/Kg) yielded a significant main effect of lever [F(1,108)=90.387, p≤0.0001], session [F(6,108)=17.310, p≤0.0001], and group [F(2,18)=9.573, p≤0.0001]. There was also a significant session x group [F(12,108)=17.310, p≤0.0001], session x lever [F(6,108)=4.377, p≤0.001], and session x group x lever interaction [F(12,108)=13.106, p≤0.0001]. Tukey’s post-hoc tests indicated that all groups consumed more alcohol than water in all the 5-day blocks (p≤0.05 for all comparisons).
Regarding alcohol consumption, the saline group decreased alcohol intake in comparison to baseline in all the post-baseline 5-day blocks (p≤0.01 for all comparisons). The early nicotine group decreased alcohol consumption in comparison to baseline over the first 5 sessions of nicotine treatment (sessions 1-5: p=0.0007), while it increased during the remaining days of nicotine treatment (sessions 6-10: p= 0.0398; sessions 11-15: p= 0.0023; sessions 16-20: 0.0064; see Figure 4b). The amount of alcohol that rats consumed from session 6 to session 20 of nicotine treatment was also higher in comparison to: 1) the first 5 days of nicotine treatment (session 1-5, p<0.0001 for all comparisons); 2) the subsequent saline sessions (21-30; p≤0.01 for all comparisons); 3) the saline and late nicotine groups (p<0.0001 for all comparisons, see Figure 4b). Similarly, the late nicotine group – in comparison to baseline – decreased its alcohol intake during the first 5 days of nicotine treatment (sessions 11-15, p≤0.001), increasing it during later nicotine treatment (sessions 21-25, p= 0.009; see Figure 4c). Moreover, the amount of alcohol ingested in the last 10 days of nicotine treatment (sessions 21-30) was higher in comparison to: 1) the saline sessions (sessions 1-10; p<0.0001 for all comparisons); 2) the first 5 days of nicotine treatment (sessions 11-15, p<0.0001 for all comparisons); 3) the saline and early nicotine groups (late nicotine vs saline, p<0.0001 for all comparisons; late nicotine vs early nicotine, p≤0.05 for all comparisons; see Figure 4b). There was also an increase in alcohol consumption from session 11-15 to session 16-20 (p<0.0001), and from session 16-20 to session 21-25 (p=0.0045) of nicotine treatment.
Regarding water consumption, both the early and the late nicotine group decreased - compared to baseline - water consumption in the first 5-day block of nicotine exposure (early nicotine: session 1-5, p≤0.00001; late nicotine: session 11-15: p=0.0020; see Figure 4c). The early nicotine group also decreased (compared to baseline) water intake in session 21-30 (p≤0.05 for all comparisons), while the late nicotine group did so in session 26-30 (p=0.0484; see Figure 4c). Comparison between groups indicated a significant difference between the early and late nicotine group in the 11-15 day block (p=0.0475).
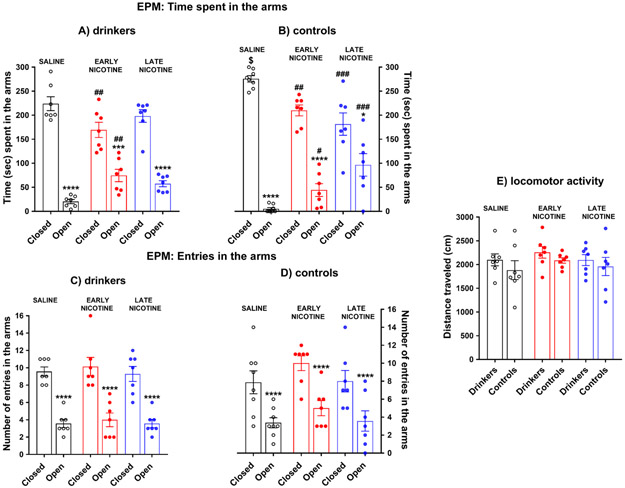
Figure 5. The effects of alcohol x nicotine interaction on anxiety-like behaviors
Fig. 5.
Mean (±SEM) time spent (panel a-b) and mean (±SEM) number of entries (panel c-d) in the open and closed arms of the elevated plus maze (EPM), along with the mean (±SEM) distance traveled (panel e) during the 5-min test in drinker (N=7/group; animals subjected to alcohol operant sessions and treated with nicotine/saline, panels a-c-e) and control rats (N=7-8/group; animas only treated with nicotine/saline and never exposed to alcohol, panels b-d-e); *p ≤ 0.05, ***p≤0.001, and ****p<0.0001 compared to the closed arms; #p≤0.05, ##p≤0.01, and ###p≤0.001 compared to the saline group; $p≤0.05 compared to drinkers. At the time of testing, nicotine had been replaced by saline 2 weeks prior in the early nicotine groups and administered for 7 weeks in the late nicotine groups. EPM tests were run the day after the 25th post-baseline sessions (when there was not any alcohol self-administration session for the drinker group) at the usual time of the operant sessions, with the animals treated with nicotine/saline an hour following the test (see text for more details)
The 3-way ANOVA of the time spent in the EPM arms yielded significant main effects of arms [F(1,37)=231.680; p<0.0001] and group [F(1,37)=10.735; p=0.002], but not treatment [F(2,37)=2.272; p=0.117]. There were also arms x treatment [F(2,37)=13.921; p<0.0001] and arms x group x treatment [F(2,37)=3.865, p=0.030] significant interactions, but not arms x group [F(1,37)=1.685; p=0.202] nor group x treatment [F(2,37)=1.162, p=0.324] interactions. Tukey’s post-hoc tests indicated that all groups of animals spent less time in the open arms than in the closed arms (see Figure 5a-b; drinkers: saline and late nicotine, p<0.0001; early nicotine, p= 0.0008; controls: saline and early nicotine, p<0.0001; late nicotine, p= 0.0320). As shown in Figure 5a, in the drinker group (animals subjected to alcohol self-administration sessions and treated with nicotine/saline), the early nicotine subgroup spent more time in the open arms (and less in the closed arms) than the saline subgroup (open arms: p=0.0078; closed arms: p=0.0074). On the other hand, in the control group (animals only injected with nicotine/saline), both the early and late nicotine subgroups spent more time in the open arms (and less in the closed arms) in comparison to the saline subgroup (see Figure 5b; open arms: early nicotine vs saline, p=0.0539; late nicotine vs saline, p=0.0002; closed arms: early nicotine vs saline, p=0.0087; late nicotine vs saline, p=0.0002). Interestingly, comparisons between groups showed that in animals treated with saline, control rats spent more time in the closed arms compared to drinker animals (p=0.039; see Figure 5a-b).
The 3-way ANOVA of entries in the EPM arms indicated a significant main effect of arms [F(1,37)=113.047, p≤0.0001] but not group [F(1,37)=0.543, p=0.466] or treatment [F(2,37)=2.233, p=0.121], and neither a group x treatment [F(2,37)=0.629, p=0.538], arms x group [F(1,37)=1.727, p=0.0197], arms x treatment [F(2,37)=0.085, p=0.919], nor arms x group x treatment [F(2,37)=0.011, p=0.989] significant interaction effect. As follow-up of the main factor ‘arms’, Tukey post-hoc tests indicated that all groups of rats entered fewer times into the open arms than the closed arms (p<0.0001; see Figure 5c-d).
The 2-way ANOVA of locomotor activity did not yield any significant effect: group [F(1,36) =2.213, p=0.1455]; treatment [F(2,36)=0.8917; p=0.4188]; or group x treatment [F(2,36)=0.04177; p=0.9591] effects (see Figure 5e).
Discussion
In this study, we show that in alcohol-non-dependent adult male Wistar rats, 1) chronic nicotine treatment – after an initial decrease – increases alcohol self-administration and alcohol consumption regardless of whether exposure to alcohol was interrupted or not before the nicotine treatment, 2) the number of alcohol reinforcements correlates to BALs in two phases of the experiment, 3) despite the fact that our animals were chronically treated with nicotine, the increase in alcohol drinking rapidly decreases when nicotine is no longer available, 4) nicotine selectively increases alcohol consumption, as it did not increase water consumption, 5) exposure to only alcohol or nicotine or the interaction between the two produces different “anxiety-like phenotypes”.
Exposure to alcohol started at the beginning of adulthood, exposure to nicotine at ~ 5 months of age (corresponding to the human age of ~ 18 years), and the experiment ended at ~ 9 months of age (corresponding to the human age of ~ 30 years; Sengupta 2013). Animals were exposed to alcohol and nicotine at ages corresponding to life epochs during which humans are particularly sensitive to the effects of these drugs (Jordan and Andersen 2016), and in which the prevalence of alcohol and tobacco use and co-abuse is very high (Anthony and Wagner 2000; Barrett et al. 2006). We did not measure plasma-nicotine levels, however Javadi-Paydar et al. (2019) showed that a nicotine injection at the same dose used by us (0.8 mg/Kg, s.c.) induced nicotine-plasma levels in adult male Sprague–Dawley rats similar to those reported in human smokers (10 to 50 ng/mL; see Matta et al. 2007).
The major finding of this study is that nicotine increased alcohol consumption when alcohol operant sessions were both interrupted (early nicotine group) or not interrupted (late nicotine group) prior to the nicotine treatment, producing blood-alcohol levels that correlated to the number of alcohol rewards (see Figure 2, 3b-c, 4b). Overall, we observed that in both the early and late nicotine groups, the number of alcohol rewards and alcohol consumption decreased in the first 3-5 sessions of nicotine treatment, and progressively increased in the following sessions to reach - compared to baseline - higher levels starting from the 8-11th day of nicotine treatment (see Figures 2a-b, 3b-c, 4b). Alcohol intake in the late nicotine group was only higher in comparison to baseline from session 21-26; however, alcohol consumption from session 21-30 was higher than in the post-baseline saline sessions (session 1-10, Figures 2a-b, 3b-c, 4b).
It has been reported (Parnell et al. 2006) that nicotine decreases blood alcohol concentrations in adult rats when single high doses of alcohol (4 g/Kg) and nicotine (2-8 mg/Kg) are administered via intragastric (IG) intubation. However, it seems that our nicotine-treated groups did not develop tolerance to alcohol effects; in comparing BALs from the baseline sessions (in absence of nicotine) and day 29 of the experimental phase, late nicotine rats that achieved higher alcohol rewards also exhibited higher corresponding BALs (see Insets of Figure 2 c-d).
To the best of our knowledge, this is the first study to evaluate the effects of nicotine on alcohol drinking in rats over 10 weeks. Previous studies evaluated alcohol drinking in rats treated with nicotine for relatively short periods of 5-10 days (Lê AD et al. 2000, 2003; López-Moreno et al 2004) or longer periods of 3-4 weeks (Blomqvist et al. 1996; Nadal and Samson 1999; Clark et al. 2001; Sharpe and Samson 2002). Leão et al. (2015) measured alcohol drinking in rats treated with nicotine over 7 weeks, however the last 3 weeks served to evaluate alcohol drinking in a progressive-ratio schedule of reinforcement and aversion-resistant alcohol responding. In another study, rats were treated with nicotine for 16 weeks, but that study focused on relapse and animals were subjected to cycles of alcohol exposure followed by periods without alcohol, with nicotine administration interrupted by weekends (Alen et al. 2009). In our study, rats increased alcohol intake from the 8-11th day of nicotine treatment. We continued the nicotine treatment until session 20 to evaluate if nicotine-induced increases in alcohol drinking would stabilize, increase, or fade over time. The nicotine-induced reduction of alcohol intake during the first days of nicotine treatment has been reported in other studies with the same nicotine dose as ours (Lê AD et al. 2000, 2003) and may be due to an initial aversive and hypo-locomotive effect induced by the drug. This hypothesis is supported by a report (López-Moreno et al 2004) in which 5 days of nicotine treatment (0.8 mg/kg) reduced locomotor activity and induced conditioned place aversion (CPA) for a compartment paired with nicotine injections (López-Moreno et al 2004).
We also observed that saline-treated animals decreased the number of reinforcements and alcohol intake in comparison to their baseline levels (see Figures 2a-b, 3b-c, 4b).We speculate that, in non-dependent animals trained to self-administer alcohol twice per week, the decrease in alcohol consumption may be due to the 30-day interval between the baseline and the post-baseline sessions and/or to the injection procedure itself. Other studies reported no change in alcohol intake after interruption of alcohol exposure (Blomqvist et al. 1996; López-Moreno et al 2004). However, in these studies, rats were exposed to alcohol daily and alcohol exposure was interrupted for only one week (Blomqvist et al. 1996; López-Moreno et al 2004). It has been previously shown that the injection procedure itself affects physiological and affective measures of stress and anxiety (Cloutier et al. 2015; Stuart and Robinson 2015). Here, rats were injected with saline for 3 days prior to the first post-baseline session to habituate them to the injection procedure. Finally, the transient reduction in alcohol intake might be due to an aversion towards the operant chamber related to the tail bleeding that occurred at the end of the baseline phase, although it seems unlikely that this would account for the decrease of alcohol intake near the end of the experiment.
Another important finding of our study is that even though nicotine was administered chronically for 70 days, when the treatment was interrupted in the early nicotine group, alcohol consumption remained high only on the first post-nicotine day and later decreased to baseline levels (see Figures 2a-b, 3b-c, 4b).
Prior work examining post-nicotine effects on alcohol drinking have reported either an increase in alcohol intake for 4 weeks after termination of 24 days of nicotine treatment (0.35 mg/Kg; Blomqvist et al. 1996), no change in alcohol drinking for 2 weeks after termination of 26 days of nicotine treatment (9 days 0.35 mg/Kg + 17 days 0.6 mg/Kg; Nadal and Samson 1999), or a decrease in alcohol intake for one week after termination of 4 weeks of nicotine treatment (0.35 mg/Kg for 3 weeks, then 0.7 mg/Kg for a week; Sharpe and Samson 2002). Similar to our results, another study showed that, following 10 days of nicotine treatment (0.2, 0.4, and 0.8 mg/Kg), alcohol drinking remained higher during only the first day after termination of nicotine treatment (Lê et al 2003). Although in our study alcohol drinking returned to baseline levels during the 2nd session after nicotine treatment ended (in the early nicotine group), we treated animals with saline for 5 more weeks to evaluate long-term alcohol drinking levels after termination of nicotine treatment. Internal states induced by both drugs and vehicles can function as interoceptive cues able to promote drug-taking in animals and humans (see LeCocq et al. 2020 for a review). Because the interoceptive state induced by both drugs and vehicles can function as a cue (Besheer et al. 2004; Thompson et al. 2019), and because in our study and previous work (Blomqvist et al. 1996; Lê AD et al. 2000; López-Moreno et al 2004; Doyon et al. 2013; Leão et al. 2015) only animals treated with nicotine increased alcohol intake, the increased alcohol consumption in our animals is likely to be due at least in part or perhaps largely to the physiological effects of nicotine. That said, in the early nicotine group, alcohol intake remained high on the first day (but not beyond) that nicotine injection was replaced by saline injection, suggesting that the injection itself may also contribute to the increased alcohol intake.
A caveat in interpreting our results could be that nicotine increased alcohol consumption by a non-selective increase in fluid intake or locomotor activity. Our results, however, indicate that nicotine only decreased water consumption during the first days of treatment, and it did not increase, compared to baseline, the total liquid consumption (see Figure 4). It has been already shown that nicotine, while increasing alcohol intake, did not influence intake of water (Blomqvist et al. 1996; Olausson et al. 2001), saccharin (Doyon et al. 2013), or a flavored solution isocaloric to 10% ethanol solution (Potthoff et al. 1983). A lack of association between nicotine-induced increases in alcohol drinking and nicotine-induced alterations in locomotor activity has also been reported previously (Blomqvist et al. 1996; Olausson et al. 2001). Overall, the effect of nicotine on locomotion in rats exposed to alcohol seems to be time- (in terms of days of treatment) and dose-dependent, with a high dose (0.8 mg/Kg) reducing locomotor activity over 5 days (Olausson et al. 2001) and a medium dose (0.35 mg/Kg) increasing it over 10-15 days of treatment (Blomqvist et al. 1996; Nadal and Samson 1999). To our knowledge, no study investigated the effects of nicotine on locomotor activity over 70 days of chronic treatment.
As shown in Figure 3a, nicotine blocked the body weight gain observed over time in the saline groups. The body weight of the late nicotine groups increased until animals were treated with saline (session 1-10), whereas in the early nicotine groups, it increased when nicotine treatment was interrupted (session 21-30). It seems that the changes in body weight we observed are not due to the amount of alcohol consumed; in fact, there was no difference between the body weight of control and drinker rats, and the body weight of the drinker nicotine groups was stable when animals were treated with nicotine and consumed more alcohol, whereas it increased when animals were treated with saline and consumed less alcohol. Our results are corroborated by the literature in both humans (Albanes et al. 1987; O’Hara et al. 1998) and rats (Bishop et al. 2002), in which it was shown that chronic nicotine decreases food consumption and body weight, while nicotine cessation increases both.
The results of the EPM test indicate that animals never exposed to any drug (controls saline) spent more time in the closed arms in comparison to animals exposed to alcohol during the operant sessions (drinkers saline, see Figure 5a-b). This result suggests that alcohol self-administration can reduce anxiety-like behavior, in agreement with previous rat (Henniger et al. 2002; Morales-Mulia 2019) and human literature (see Turner et al. 2018 for a review). Our results also suggest that nicotine may have an anxiolytic-like effect that persists when the treatment is interrupted. In fact, rats never exposed to alcohol but previously or currently treated with nicotine (controls early and late nicotine groups, respectively) spent more time in the open arms (and less in the closed arms) in comparison to animals never exposed to any drug (controls saline; see Figure 5b). Control saline animals spent most of their time in the closed arms, perhaps due to the injection itself, which has been shown affect physiological and affective measures of stress and anxiety (Stuart and Robinson 2015). Therefore, observed alcohol and nicotine effects on anxiety-like behavior may be overestimated, although all experimental and control groups were chronically exposed to the same injection regimen. Several studies have investigated the effects of nicotine on anxiety-like behaviors in rats using the EPM test – overall, those past studies agree with our findings in which chronic nicotine and protracted nicotine abstinence are both associated with reduced anxiety-like behavior. It was shown that nicotine has an anxiogenic effect after one day (0.1 mg/kg, Irvine et al. 2001; 0.6 and 0.8 mg/kg, Zarrindast et al. 2012), two days (0.1, 0.5, and 1 mg/kg, Elliott et al. 2004) or 4 days (0.45 mg/Kg, Irvine et al 2001b) of treatment, while anxiolytic effects were reported after 1 week (0.1 mg/Kg, Irvine et al. 2001) and 4 weeks of nicotine treatment (0.45 mg/Kg, Irvine et al. 2001b). Concerning anxiety-like behaviors during nicotine withdrawal, prior work in rats showed an increase in anxiety-like behavior 24 hours after termination of one week of nicotine exposure (0.1 mg/Kg, Irvine et al. 2001), but not 24 hours or 72 hours after termination of 4 weeks of nicotine exposure (0.45 mg/Kg, Irvine et al. 2001b), nor 2 or 3 months after termination of a 3-week nicotine treatment (0.36 mg/kg, Morud et al. 2018). Human studies indicate that nicotine reduces anxiety in smokers (Kutlu et al. 2015), whereas increased anxiety is observed during acute withdrawal (24 hours, Hogle et al. 2010) but not over a protracted abstinence of 1 month (Gross et al. 1989) or 6 months (McDermott et al. 2013). The differences between rats and humans regarding the anxiety status 24 hours into nicotine withdrawal may be explained by species differences in nicotine metabolism (see Matta et al. 2007) and/or to the fact the 24 hours may have a different value in the 2 species due to the different life span (see Sengupta 2013). Finally, we showed that in alcohol self-administering animals (drinkers), rats spent more time in the open arms (and less time in the closed arms) only when previously treated with nicotine (early nicotine), compared to rats never treated with nicotine (saline, see Figure 5a). To the best of our knowledge no study (either in animals or humans) has investigated anxiety-like behavior or anxiety in subjects previously exposed to alcohol and nicotine but currently in nicotine abstinence (as our early nicotine group). Olausson et al. (2001) showed that rats exposed to alcohol and nicotine for 2 weeks spent more time in the open arms compared to control animals, albeit in this study animals were treated with nicotine (0.35 mg/kg) before testing while our test was conducted in a drug-free state. Interestingly, a human study indicated an additive effect of nicotine and alcohol on decreasing anxiety in a population of drinkers who smoke (Novak et al. 2003).
This study develops a model of nicotine-induced increases in alcohol drinking to be tested in future studies using both sexes, in which we plan to test nicotine x alcohol effects on the brain and neural control of nicotine-induced increases in alcohol drinking. Both human (DiFranza and Guerrera 1990; Bobo 1992; Anthony and Wagner 2000; Grant et al. 2004; Barrett et al. 2006; Harrison and McKee 2008) and rat experiments (Potthoff et al. 1983; Hauser et al. 2014) have demonstrated that the ability of nicotine to increase alcohol drinking is not exclusive to male subjects. Additionally, the ventral tegmental area (Doyon et al 2013; see also Britt and Bonci 2013 & Doyon et al. 2018 for reviews) and basolateral amygdala (Leão et al. 2015) seem to be two brain areas in which nicotine interacts with alcohol to promote alcohol drinking in non-dependent animals.
Human studies have shown alcohol and nicotine co-dependence (Bobo 1992; Miller and Gold 1998; Anthony and Wagner 2000) and an exacerbation on alcohol drinking by nicotine in humans without AUD (Barrett et al. 2006; Harrison and McKee 2008). In spite of this, there is little basic research on the interactions between the reinforcing effects of these two drugs. Our results demonstrate that in alcohol-non-dependent rats, chronic nicotine robustly escalates alcohol consumption regardless of whether exposure to alcohol was interrupted or not before the nicotine treatment. Though we chronically treated animals with nicotine, alcohol drinking rapidly decreased when nicotine was no longer available. If we extrapolate these results to a human population of smokers and social drinkers, we may argue that nicotine may be able to escalate alcohol consumption regardless of whether these subjects are drinking often or not, and that this increased alcohol ingestion may be reduced by smoking cessation.
Supplementary Material
Acknowledgments:
Funding for this award was provided by National Institute of Health (NIH) Award R01 AA023305 (NWG) and by Merit Review Award #I01 BX003451 (NWG) from the United States (U.S.) Department of Veterans Affairs, Biomedical Laboratory Research and Development Service. All the authors declare no competing interests.
References
- Albanes D, Jones DY, Micozzi MS, Mattson ME (1987) Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health 77(4):439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alén F, Gómez R, González-Cuevas G, Navarro M, López-Moreno JA (2009) Nicotine causes opposite effects on alcohol intake: Evidence in an animal experimental model of abstinence and relapse from alcohol. Nicotine Tob Res 11(11):1304–1311. doi: 10.1093/ntr/ntp139. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Echeagaray-Wagner F (2000) Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health 24(4):201–208. [PMC free article] [PubMed] [Google Scholar]
- Avegno EM, Gilpin NW (2019) Inducing Alcohol Dependence in Rats Using Chronic Intermittent Exposure to Alcohol Vapor. Bio Protoc 9(9):e3222. doi: 10.21769/BioProtoc.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO (2006) Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend 81(2):197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA (2004). Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 172(1):108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bishop C, Parker GC, Coscina DV (2002) Nicotine and its withdrawal alter feeding induced by paraventricular hypothalamic injections of neuropeptide Y in Sprague-Dawley rats. Psychopharmacology (Berl) 162(3):265–272. doi: 10.1007/s00213-002-1101-7. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Söderpalm B (1996) Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol 314(3):257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Bobo JK. Nicotine dependence and alcoholism epidemiology and treatment (1992) J Psychoactive Drugs 24(2):123–129. doi: 10.1080/02791072.1992.10471633. [DOI] [PubMed] [Google Scholar]
- Britt JP, Bonci A (2013) Alcohol and tobacco: how smoking may promote excessive drinking. Neuron 79(3):406–407. doi: 10.1016/j.neuron.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, Little HJ (2001) Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology 41(1):108–117. doi: 10.1016/s0028-3908(01)00037-5. [DOI] [PubMed] [Google Scholar]
- Commissaris RL (1993) Techniques in the Behavioral and Neural Sciences Chapter 17: Conflict behaviors as animal models for the study of anxiety. Edited by van Haaren Frans, 1993, Volume 10, Pages 443–474 . doi:org/ 10.1016/B978-0-444-81444-9.50022-5. [DOI] [Google Scholar]
- DiFranza JR, Guerrera MP (1990) Alcoholism and smoking. J Stud Alcohol 51(2):130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, Dani JA (2013) Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 79(3):530–540. doi: 10.1016/j.neuron.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE (2004) Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav 77(1):21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- LeCocq MR, Randall PA, Besheer J, Chaudhri N (2020) Considering Drug-Associated Contexts in Substance Use Disorders and Treatment Development. Neurotherapeutics 17(1):43–54. doi: 10.1007/s13311-019-00824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA (2004) Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Gross J, Stitzer ML (1989) Nicotine replacement: ten-week effects on tobacco withdrawal symptoms. Psychopharmacology (Berl) 98(3):334–341. doi: 10.1007/BF00451684. [DOI] [PubMed] [Google Scholar]
- Gulliver SB, Kamholz BW, Helstrom AW (2006) Smoking cessation and alcohol abstinence: what do the data tell us?. Alcohol Res Health 29(3):208–212. [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, McKee SA (2008) Young adult non-daily smokers: patterns of alcohol and cigarette use. Addict Behav 33(5):668–674. doi: 10.1016/j.addbeh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Hölter SM (2002) Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology 26(6):729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ (2010) Nicotine withdrawal increases threat-induced anxiety but not fear: neuroadaptation in human addiction. Biol Psychiatry 68(8):719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE (2001a) Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav 68(2):319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, Marshall M, File SE (2001b) Different treatment regimens and the development of tolerance to nicotine’s anxiogenic effects. Pharmacol Biochem Behav 68(4):769–776. doi: 10.1016/s0091-3057(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE (2004) Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend 75(1):55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Parikh V, Gould TJ (2015) Nicotine Addiction and Psychiatric Disorders. Int Rev Neurobiol 124:171–208. doi: 10.1016/bs.irn.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, & Andersen SL (2017). Sensitive periods of substance abuse: Early risk for the transition to dependence. Developmental cognitive neuroscience, 25, 29–44. 10.1016/j.dcn.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res 24(2):155–163. doi: 10.1111/j.1530-0277.2000.tb04585. [DOI] [PubMed] [Google Scholar]
- Lê AD, Wang A, Harding S, Juzytsch W, Shaham Y (2003) Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 168(1-2):216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Lê AD, Funk D, Lo S, Coen K (2014) Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology (Berl) 231(20):4019–4029. doi: 10.1007/s00213-014-3541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Cruz FC, Vendruscolo LF, et al. (2015) Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking [published correction appears in J Neurosci 2015 Aug 5;35(31):11169]. J Neurosci 35(15):6241–6253. doi: 10.1523/JNEUROSCI.3302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! shouldn't we do something about it? (2007) Alcohol Alcohol 42(3):167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- López-Moreno JA, Trigo-Díaz JM, Rodríguez de Fonseca F, et al. (2004) Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology 47(7):1036–1044. doi: 10.1016/j.neuropharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190(3):269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P (2013) Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study. Br J Psychiatry January;202(1):62–7. doi: 10.1192/bjp.bp.112.114389. [DOI] [PubMed] [Google Scholar]
- Miller NS, Gold MS (1998) Comorbid cigarette and alcohol addiction: epidemiology and treatment. J Addict Dis 17(1):55–66. doi: 10.1300/J069v17n01_06. [DOI] [PubMed] [Google Scholar]
- Morales-Mulia M (2019) Intra-accumbal orexin-1 receptor inhibition prevents the anxiolytic-like effect of ethanol and leads to increases in orexin-A content and receptor expression. Pharmacol Biochem Behav. 2019;185:172761. doi: 10.1016/j.pbb.2019.172761. [DOI] [PubMed] [Google Scholar]
- Morud J, Strandberg J, Andrén A, Ericson M, Söderpalm B, Adermark L (2018) Progressive modulation of accumbal neurotransmission and anxiety-like behavior following protracted nicotine withdrawal. Neuropharmacology 128:86–95. doi: 10.1016/j.neuropharm.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Nadal R, Samson HH (1999) Operant ethanol self-administration after nicotine treatment and withdrawal. Alcohol 7(2):139–147. doi: 10.1016/s0741-8329(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Novak A, Burgess ES, Clark M, Zvolensky MJ, Brown RA (2003) Anxiety sensitivity, self-reported motives for alcohol and nicotine use, and level of consumption. J Anxiety Disord 17(2):165–180. doi: 10.1016/s0887-6185(02)00175-5. [DOI] [PubMed] [Google Scholar]
- O’Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R (1998) Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol 148(9):821–830. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Löf E, Engel JA, Söderpalm B (2001) Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol 417(1-2):117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Dani JA, Doyon WM (2015) Cigarettes and alcohol: The influence of nicotine on operant alcohol self-administration and the mesolimbic dopamine system. Biochem Pharmacol 97(4):550–557. doi: 10.1016/j.bcp.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, Dani JA (2018) Convergent Neuronal Plasticity and Metaplasticity Mechanisms of Stress, Nicotine, and Alcohol. Annu Rev Pharmacol Toxicol 58:547–566. doi: 10.1146/annurev-pharmtox-010617-052735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30(8):1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Pennington ZT, Dong Z, Feng Y, et al. (2019) ezTrack: An open-source video analysis pipeline for the investigation of animal behavior. Sci Rep 9(1):19979 Published 2019 December 27. doi: 10.1038/s41598-019-56408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff AD, Ellison G, Nelson L (1983) Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav 18(4):489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- Roltsch EA, Baynes BB, Mayeux JP, Whitaker AM, Baiamonte BA, Gilpin NW (2014) Predator odor stress alters corticotropin-releasing factor-1 receptor (CRF1R)-dependent behaviors in rats. Neuropharmacology 79:83–89. doi: 10.1016/j.neuropharm.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P (2013). The Laboratory Rat: Relating Its Age With Human’s. International journal of preventive medicine, 4(6), 624–630. [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH (2002) Repeated nicotine injections decrease operant ethanol self-administration. Alcohol 28(1):1–7. doi: 10.1016/s0741-8329(02)00238-0. [DOI] [PubMed] [Google Scholar]
- Stuart SA, Robinson ES (2015) Reducing the stress of drug administration: implications for the 3Rs. Sci Rep 5:14288. doi: 10.1038/srep14288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL (2000) Animal models in alcohol research. Alcohol Res Health. 2000;24(2):77–84. [PMC free article] [PubMed] [Google Scholar]
- Thompson BM, Barrett ST, Bevins RA (2019) Exploring the interoceptive stimulus effects of nicotine and varenicline. Pharmacol Biochem Behav 181:9–16. doi: 10.1016/j.pbb.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DM (1975) Influence of route of administration on metabolism of [14C] nicotine in four species. Xenobiotica 5(9):553–561. doi: 10.3109/00498257509056125. [DOI] [PubMed] [Google Scholar]
- Turner S, Mota N, Bolton J, Sareen J (2018) Self-medication with alcohol or drugs for mood and anxiety disorders: A narrative review of the epidemiological literature. Depress Anxiety 35(9):851–860. doi: 10.1002/da.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12(3-4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Aghamohammadi-Sereshki A, Rezayof A, Rostami P (2012) Nicotine-induced anxiogenic-like behaviours of rats in the elevated plus-maze: possible role of NMDA receptors of the central amygdala. J Psychopharmacol 26(4):555–563. doi: 10.1177/0269881111412094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.