Abstract
Background:
Drugs belonging to diverse therapeutic classes can prolong myocardial refractoriness or slow conduction. These drugs may be effective and well-tolerated, but the risk of sudden cardiac death from torsades de pointes (TdP) remains a major concern. The corrected QT interval has significant limitations when used for risk stratification. Measurement of GEH could help identify the substrate vulnerable to drug-induced ventricular arrhythmias.
Objectives:
To improve risk stratification for drug-induced TdP by measuring global electrical heterogeneity (GEH) on the ECG.
Methods:
We analyzed electrocardiographic data from a case-control study of patients with a history of drug-induced TdP, as well as age- and sex-matched controls. Vectorcardiograms were constructed from ECGs. GEH was measured via the spatial ventricular gradient (SVG) vector (magnitude, azimuth, and elevation). Log odds coefficients for TdP were estimated using multivariable logistic regression.
Results:
Among 17 cases and 17 controls (47% and 29% male, age 58.9 ± 12.5 and 61.0 ± 12.2 years), 34 ECGs were analyzed. SVG azimuth was significantly different between cases and controls (3.4 vs 22.0 deg, p = 0.02). After adjusting for gender and QTc interval, odds of TdP increased by a factor of 3.2 for each 1 standard deviation change in SVG azimuth from the control group mean (p = 0.04, 95% CI 1.07–9.14). QTc was not significant in the multivariable analysis (p = 0.20).
Conclusions:
SVG azimuth is correlated with a history of drug-induced TdP independent of the QTc. GEH measurement may help identify patients at high risk for drug induced arrhythmias.
Keywords: electrical heterogeneity, sudden cardiac death, electrocardiography, torsade de pointes, antiarrhythmic drugs
Introduction
Drugs belonging to diverse therapeutic classes can prolong myocardial refractoriness or slow conduction, either as therapeutic effects for antiarrhythmic drugs (AADs), or as unintended toxicities. One of the most severe cardiac toxicities related to drugs that affect myocardial repolarization is torsades de pointes (TdP) which carries a risk of sudden cardiac death. The risk of TdP is primarily assessed by the heart rate corrected QT-interval (QTc), but TdP can still occur even among patients with baseline normal QTc [1]. Additionally, it can be difficult to accurately measure the QT interval, and there is significant intraobserver variation in measurement that can impact use of QTc for risk stratification [2]. New ways to identify the vulnerable substrate for TdP beyond QTc are needed to facilitate safe use of both AADs and other non-cardiac medications with off-target electrophysiologic effects.
Normal myocardium is not electrically homogeneous; there exists normal spatial and temporal electrical heterogeneity which is required for normal cardiac function and which is responsible for the genesis of the QRST complex [3–5]. Although some degree of myocardial electrical heterogeneity likely protects from ventricular arrhythmias, excessive electrical heterogeneity, measured in a variety of ways, has been associated with an increased risk of ventricular arrhythmias in both experimental and clinical studies [6–8]. Disturbances in myocardial repolarization which are reflected in abnormal electrical heterogeneity are known causes for TdP [9]. A noninvasive measurement of myocardial electrical heterogeneity beyond the QTc therefore has the potential to improve electrophysiologic risk stratification and patient selection for various drugs such as AADs and antimicrobial and psychiatric agents.
Global electrical heterogeneity (GEH) is a set of noninvasive vectorcardiographic (VCG) measurements constructed from standard 12-lead digital ECG waveforms which quantifies abnormal myocardial electrical heterogeneity [10, 11]. GEH measurements have been correlated in humans with adverse cardiovascular outcomes including sudden cardiac death and adverse structural changes [12, 13]. Our group recently demonstrated that after AAD administration there are acute, drug specific GEH changes which are correlated with plasma drug levels [14]. In this study, we hypothesized that in patients with a history of drug induced TdP, GEH measurements will differ from those obtained in controls without such history.
Methods
Data Source and Study Population
We analyzed existing data from a case-control study of patients who had experienced TdP in association with QT-prolonging drugs and a sample of age and sex matched controls. Data used for this research was provided by the Telemetric and Holter ECG Warehouse (THEW) of the University of Rochester, NY. These data may be obtained by registration at the University of Rochester’s Telemetric and Holter ECG Warehouse (http://thew-project.org) [15]. The study protocol has been previously described in detail [16, 17]. The case group of patients each had a documented history of previous TdP while undergoing drug therapy with QT prolonging potential: sotalol, sumatriptan, amiodarone, bisacodyl, cipramil, furosemide, clarithromycin, erythromycin. The control group consisted of age- and sex-matched patients, most of whom who were planned to start sotalol for the treatment of atrial fibrillation. All patients were enrolled after being admitted to the University Hospital of Munich, Germany, for an evaluation of the individual level of repolarization reserve by QT-interval measurement before and after sotalol infusion. All participants were genetically tested to exclude known mutations of the major long QT syndrome genes. All patients gave informed consent prior to enrollment. Of the sample of 40 patients described in the original paper, 34 were provided for analysis in the THEW dataset. This dataset has also been used for other ECG analyses [17]. This study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board.
Electrocardiographic Data
Continuous 1.5 or 3.6 minute surface 12-lead ECG recordings sampled at 1 kHz acquired at rest in the supine position at baseline were provided in the dataset. All of the ECGs were recorded with the subjects in sinus rhythm. None of the subjects were reported to be taking AADs when the baseline ECGs were acquired. We constructed median beat vectorcardiograms from the ECGs by transformation into the Frank X, Y, and Z leads using the Kors transformation matrix [18]. The X, Y, and Z leads were then baseline corrected so that the flattest part of the TP segment was the zero reference point for both area calculations and the origin of the VCG as this segment of the ECG is truly isolelectric. Fiducial points (QRSonset, QRSend, and Tend) were annotated using digital calipers by an investigator blinded to assignment to the case or control group. Further details of ECG processing, annotation, and median beat construction can be found in the supplemental methods. We followed previous conventions for orientation of axes with the positive X axis towards the left, the positive Y axis towards the feet, and the positive Z axis posterior. QT interval was corrected for heart rate using the Fridericia correction. The time of baseline ECG acquisition relative to the TdP event for cases was not recorded.
GEH Calculations
We assessed GEH measurements on a baseline ECG without any AAD effect and focused on the spatial ventricular gradient (SVG) which is a measurement of the electric dipole moment of the heart averaged over the cardiac cycle. The SVG is defined as the vector sum of the area QRS- and area T-vectors which are obtained by integrating the X- Y- and Z- components of the QRST complex (see Supplemental Figure 1). The SVG has magnitude and orientation in 3-dimensional space expressed as azimuth (angle in the XZ-transverse plane) and elevation (angle in the XY-frontal plane). We transformed the circular variable SVG azimuth into an adjusted azimuth (Az) defined as the absolute difference in azimuth from the control group mean:
| (1) |
Further details regarding definitions and calculations of other GEH measurements may be found in the Supplement. ECG and VCG processing and calculations were performed using Matlab R2018a (Mathworks, Natick, MA).
Statistical Analysis
The mean values of continuous GEH measurements and QTc for cases and controls were compared using two-sample t-tests, except for SVG azimuth (a circular variable), for which we used the two-sample Mardia-Watson-Wheeler test [19]. Categorical variables were compared using Fisher’s exact test. The mean 3-dimensional SVG vector for cases and controls was compared using the multivariate Modified Nel and Van der Merwe test [20]. Log odds coefficients for GEH measurements and QTc were estimated using multivariable logistic regression. Spearman correlation coefficient (using circular ranks) between SVG azimuth angle and QTc interval was calculated according to a previously published method [19]. All statistical analyses were performed with Stata 15 (StataCorp LP, College Station, TX). Two-sided P-values were computed without adjustment for multiple comparisons.
Results
Table 1 shows the demographic characteristics and comorbidities of the 34 study participants. Cases and controls were of similar age, 58.9 ± 12.5 and 61.0 ± 12.2 years (p = 0.63). There was a non-significant trend towards more males in cases compared to controls (47% and 29% male (p = 0.48)), respectively. Prevalence of coronary artery disease, history of myocardial infarction, and hypertension were similar among cases and controls (p > 0.5 for all). Atrial fibrillation trended towards being more prevalent among controls without reaching significance (p = 0.09). Supplemental Table 1 lists which drugs triggered TdP for each patient. Sotalol was the most common suspected cause of TdP, occurring in 7 out of 17 (41%) of cases. Two cases of amiodarone-induced TdP (a relatively rare cause of TdP [21, 22]) were reported.
Table 1:
Demographics
| Cases | Controls | ||||
|---|---|---|---|---|---|
| N/Mean | %/σ | N/Mean | %/σ | p | |
| Age (yrs) | 58.9 | 12.5 | 61.0 | 12.2 | 0.63 |
| Male | 8 | 47.1 | 5 | 29.4 | 0.48 |
| History of Coronary Artery Disease | 3 | 17.6 | 3 | 17.6 | 1.00 |
| History of Myocardial Infarction | 3 | 17.6 | 1 | 5.9 | 0.60 |
| History of Atrial Fibrillation | 11 | 64.7 | 16 | 94.1 | 0.09 |
| History of Hypertension | 7 | 41.2 | 9 | 52.9 | 0.73 |
Mean values and standard deviation (σ) or number and % of total for demographics of cases and controls. p-values computed using two-sample t-test or Fisher’s exact test.
Table 2 shows baseline ECG/GEH measurements of the study participants. The mean SVG vector had similar magnitude and elevation (angle in the XY-frontal plane) in cases and controls, but cases had more anterior azimuth than controls in the XZ-transverse plane (22.0 vs 3.4 deg (p = 0.02)), respectively. These differences are illustrated graphically in Figure 1. The absolute difference from the mean azimuth of the control group (adjusted azimuth) was also significantly different in cases and controls: 48.3 ± 35.1 vs 22.0 ± 16.9 deg (p = 0.01), respectively. Table 3 shows how the difference in SVG azimuth is reflected in the components of the population mean SVG vector. The Y-components are similar across both groups, but the X- and Z-components both are smaller in cases relative to controls (joint p = 0.004). Other GEH measurements were similar between cases and controls and are shown in in Supplemental Table 2. There was a trend towards QTc interval being slightly longer among cases compared to controls at 455 vs 438 ms without reaching statistical significance (p = 0.09). QRS duration was similar in cases and controls. Corresponding univariable odds ratios for risk of having a history of drug induced TdP are shown in Supplemental Table 3: adjusted azimuth (p = 0.02) was the only variable associated with TdP history.
Table 2:
ECG and GEH Measurements
| Cases | Controls | ||||
|---|---|---|---|---|---|
| Mean | σ | Mean | σ | p | |
| SVG Magnitude (mV · ms) | 40.65 | 23.93 | 46.67 | 24.18 | 0.47 |
| SVG Azimuth (deg) | 22.0 | 27.5 | 3.4 | 58.2 | 0.02 |
| Adjusted SVG Azimuth (deg) | 48.3 | 35.1 | 22.0 | 16.9 | 0.01 |
| SVG Elevation (deg) | 71.5 | 28.3 | 72.2 | 14.5 | 0.93 |
| QTc (ms) | 455 | 31 | 438 | 24 | 0.09 |
| QRS Duration (ms) | 98 | 12 | 95 | 12 | 0.50 |
Mean values and standard deviation (σ) of ECG and GEH measurements for cases and controls. p-values computed using two-sample t-test or Fisher’s exact test, except for SVG azimuth, which is computed using the two-sample Mardia-Watson-Wheeler test (due to the circular distribution of data). Adjusted SVG azimuth is SVG azimuth adjusted for the mean azimuth of the control group, that is, Adjusted Azimuth = |Azimuth − 22.0| deg. See Methods for details. Abbreviations: SVG=spatial ventricular gradient. QTc is computed via Fridericia’s formula.
Figure 1.
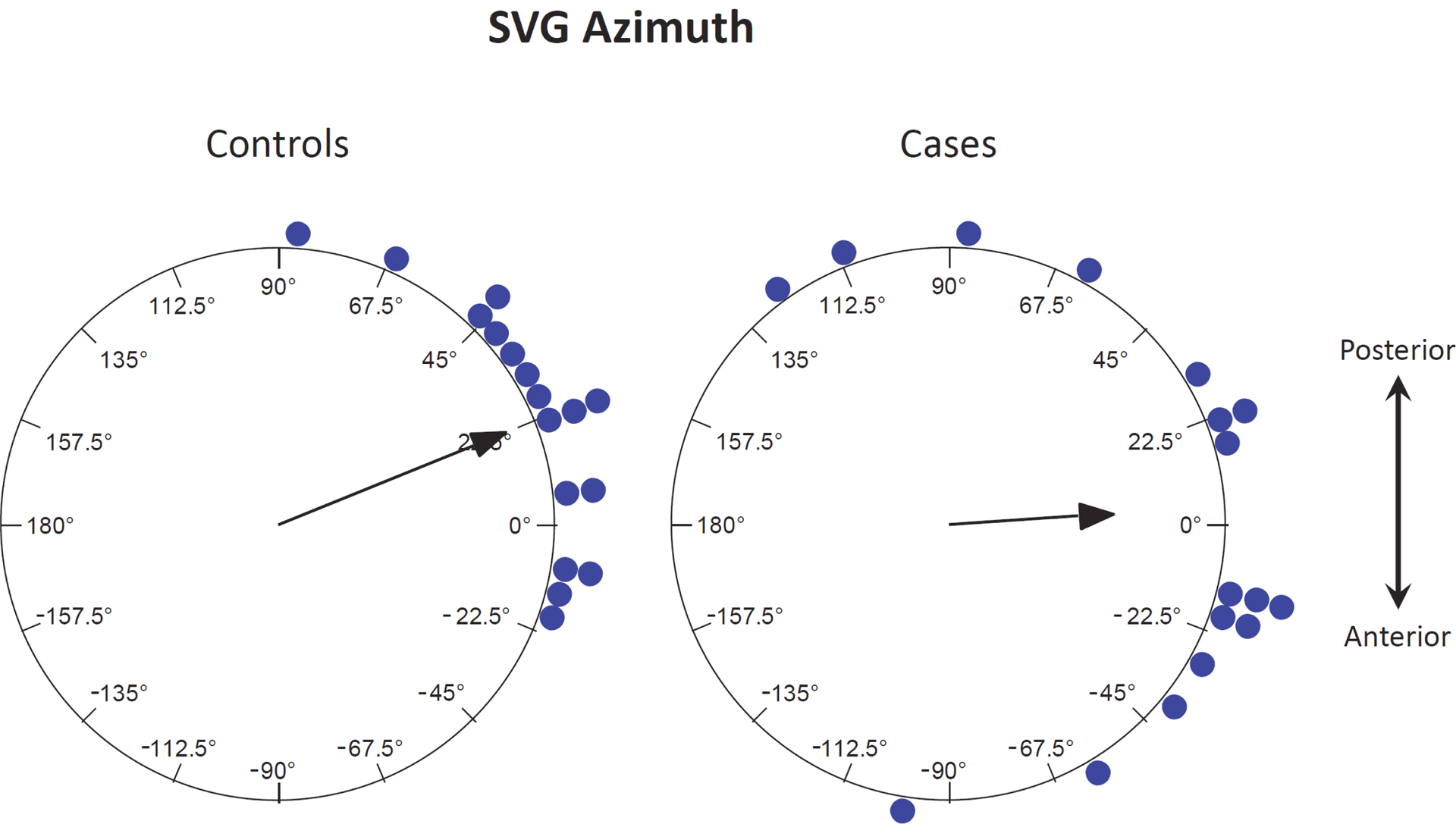
SVG azimuth in cases and controls — The arrow represents the mean direction in the transverse plane.
Table 3:
Mean SVG Vector
| Cases | Controls | P-Value | |
|---|---|---|---|
| SVG X (mV · ms) | 23.86 | 38.44 | 0.004 |
| SVG Y (mV · ms) | 16.25 | 15.02 | |
| SVG Z (mV · ms) | −3.73 | 10.02 |
Joint (multivariate) comparison of the population mean SVG vector for cases and controls. p-value computed using the multivariate Modified Nel and Van der Merwe test (see Methods for details).
The relationship between SVG azimuth and QTc is further illustrated in Figure 2. There was a wide spectrum of QTc values for cases, but there was a narrower QTc distribution in controls likely because the control group mostly excluded patients with prolonged QTc who would not have been eligible for sotalol therapy. Regardless of QTc value, however, cases had larger values of adjusted azimuth. There was no significant correlation between QTc and SVG azimuth (Spearman correlation coefficient 0.036, p = 0.56). Quantitative comparison can be made using Table 4, which shows the results of multivariable logistic regression for the odds of TdP as a function of adjusted SVG azimuth, including gender and QTc. Odds of TdP increased by a factor of 3.2 (95% CI 1.07–9.14, p = 0.04) per 1 standard deviation (30.2 deg) difference from the mean azimuth of the control group. QTc and gender were not significant in the multivariable analysis (p = 0.20 and p = 0.89, respectively).
Figure 2.
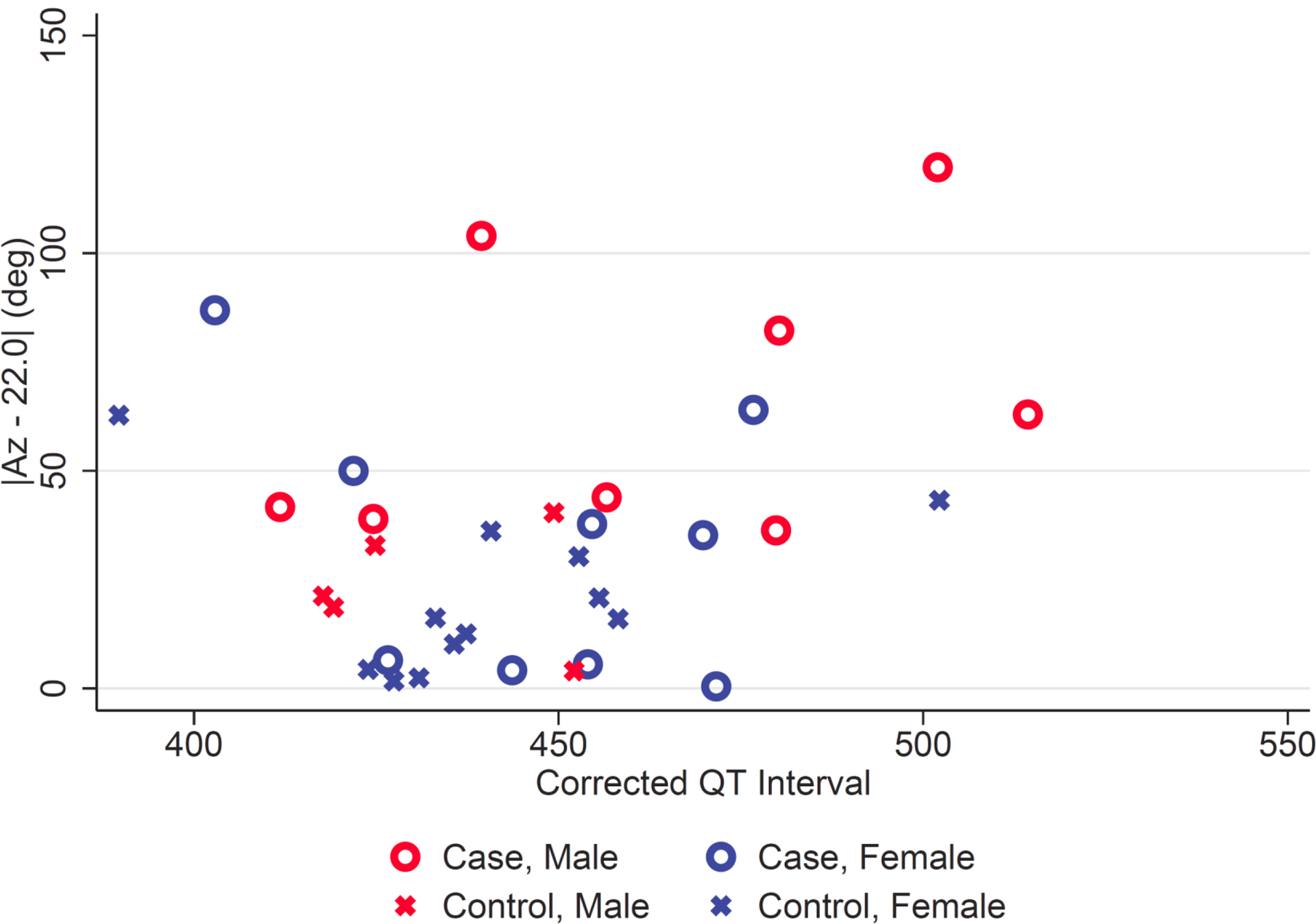
SVG azimuth vs QTc interval — Patients with (cases) and without (controls) a history of torsades de pointes. The SVG azimuth is plotted as the absolute difference from the control group mean (22 deg). See Methods for details.
Table 4:
Multivariable Logistic Regression for SVG Azimuth and QTc.
| Variable | OR | p | 95% CI |
|---|---|---|---|
| QTc (ms) | 1.02 | 0.20 | 0.99–1.05 |
| Adjusted SVG Azimuth (deg) | 1.04 | 0.04 | 1.002–1.076 |
| Male Gender | 1.12 | 0.89 | 0.21—5.91 |
| QTc (per 1 SD) | 1.81 | 0.20 | 0.73–4.47 |
Abbreviations and definition of adjusted SVG azimuth as in Table 2. Standard deviations (SD) of adjusted SVG azimuth and QTc are 30.2 deg and 28.7 ms, respectively.
Discussion
Our results show that GEH measurements obtained from a standard 12-lead ECG are correlated with a history of drug induced TdP. We found that the odds of TdP were significantly higher in persons with an SVG vector that pointed in an abnormal direction when compared to age- and sex-matched controls. For every 1 standard deviation (30 deg) difference in SVG azimuth, odds of TdP having occurred increased by approximately 3.2, and the risk of TdP associated with SVG azimuth was independent of QTc. We conjecture that the correlation between incidence of TdP that we observed in persons with an abnormal SVG azimuth is caused by an abnormally heterogeneous electrical substrate, and that GEH measurements can identify patients at higher risk of drug-induced TdP.
Our results are consistent with previous work which has shown that the SVG azimuth is sensitive to drug induced electrophysiologic changes, and that it is associated with sudden death risk in the general population [12]. Temporal changes in SVG azimuth, especially rapid ones, have been associated with sudden death in human population studies [13]. Compared to ranolazine, the AADs dofetilide and quinidine, which are associated with higher risk of TdP, have been observed to cause larger posterior rotations of the SVG azimuth [14]. In the current study we found that large deviations in either direction of the SVG azimuth were associated with TdP. Differences among studies likely reflect different study populations and different mechanisms involved.
Abnormalities in ion channel function, caused by either hereditary or acquired changes in potassium, calcium, or sodium channel function, are associated with higher rates of sudden cardiac death from ventricular arrhythmia [23]. Many such ion channel perturbations, especially those which affect myocardial repolarization, can cause TdP which can degenerate into lethal ventricular fibrillation. The abnormal direction of the SVG vector that we observed in patients with a history of TdP likely reflects changes in myocardial ion channel function and/or expression in the myocardium which can predispose to TdP. The genetics associated with abnormal GEH involve genes which affect cardiac conduction system development and ion channel function [24]. The link between genetics and environment in how GEH is phenotypically expressed requires further investigation.
The QTc interval serves as a marker of abnormal repolarization by measuring the total time of myocardial depolarization and repolarization. When repolarization time is dramatically prolonged, such as in persons with genetic abnormalities in potassium channel function or after class III AAD exposure, the QTc can be a useful danger signal. However, other changes in ion channel expression and activity, which may not prolong the QT interval, are also known to be proarrhythmic and can dispose to TdP [25, 26]. Measurement of GEH using the SVG vector orientation in the transverse plane (azimuth) may help detect this risk even when QTc is normal or only mildly abnormal. If personal proarrhythmic risk beyond QTc can be noninvasively assessed with GEH, then it has the potential to improve our ability to safely select patients for AADs and other drugs that are associated with a risk of TdP. Such prospective studies are planned for the future.
Of related interest is how some drugs, such as amiodarone and ranolazine can significantly prolong the QT interval but do not tend to predispose to an especially elevated risk of TdP. This low rate of TdP is mediated in part by concomitant blockade of the late sodium current INa which counteracts proarrhythmic effects of potassium channel blockade. In our recent study, increasing plasma drug concentrations of ranolazine, which blocks the late sodium current, were associated with smaller changes in GEH measurements than drugs such as dofetilide and quinidine, which are known to cause TdP primarily via potassium channel (IKr) blockade [14]. Since mild to moderate baseline QTc prolongation, or an increase in QTc after drug initiation, can exclude patients from treatment with otherwise useful medications, GEH may in the future provide a more specific way to stratify patients according to risk of arrhythmia.
Other approaches to risk stratification for drug-induced arrhythmia have predominantly examined T-wave morphology. While it is known that females have longer average QTc intervals than males, and are more prone to TdP [27], this signal was not seen in our study, likely due to the small number of participants. Indices such as the interval from the T wave peak to the end of the T-wave [28], early and late T-wave repolarization durations [29], and microvolt T-wave alternans [30] have all been studied, but either have methodological issues related to measurement difficulty or limited clinical utility. GEH is supported by a sound physiologic and mathematical footing based on the dipole theory of the ECG and has experimental evidence showing it is associated with electrical heterogeneity and hard clinical outcomes such as sudden cardiac death.
Strengths of our study include age- and sex-matched populations with a documented history of an important but rare clinical outcome. Our analysis is also reproducible: ECG and clinical data and the annotated median beats used in this analysisis are available by request from THEW. QT is also difficult to measure accurately, and there can even be significant disagreement in QT measurements among experienced cardiologists. GEH measurements, which are primarily based on QRST areas, are insensitive to small errors in QT interval measurement. GEH measurements can be computed rapidly, used in real time, and are reproducible and stable over time [14, 31].
Limitations of our study include the small sample size because TdP is a rare event. While the study populations were matched by age and sex, the prevalence of atrial fibrillation was slightly but non-statistically higher in the control group. While atrial fibrillation itself should not confound our result, other unmeasured factors associated with the presence of atrial fibrillation, or other unaccounted factors that could affect GEH measurements cannot be excluded. Because almost all members of the control group were scheduled to start sotalol, this might also create some unknown bias in the control group. The time between the episode of TdP and the ECG used for this study is also unknown, but given the reproducibility of GEH measurements over time, [14, 31] we would expect the ECG used for this study (which would be free of QT prolonging medication effect) to approximate the ECG which would have been acquired prior to the drug that precipitated TdP. We also do not know if patients in the control group would potentially have experienced TdP in the future, and the risk of TdP may be dynamic where evaluation at a single time point may not be associated with long-term risk. The clinical relevance of our results remains unknown without a prospective study which will be needed before measurement of GEH can be adopted into clinical practice.
Conclusion
SVG azimuth is correlated with a history of drug-induced TdP independent of the QTc. GEH measurement may help identify patients at high risk for drug induced arrhythmias.
Supplementary Material
Funding
LGT: This work was partially supported by HL118277.
Abbreviations
- ECG
electrocardiogram
- AAD
anti-arrhythmic drug
- TdP
Torsades de Pointes
- QTc
corrected QT interval
- GEH
global electrical heterogeneity
- VCG
vectorcardiogram
- SVG
spatial ventricular gradient
- MI
myocardial infarction
- HTN
hypertension
- AFib
atrial fibrillation
- CAD
coronary artery disease
- Az
azimuth
Footnotes
Conflicts of Interest: None.
References
- [1].Pedersen HS, Elming H, Seibaek M, et al. Risk factors and predictors of Torsade de pointes ventricular tachycardia in patients with left ventricular systolic dysfunction receiving Dofetilide. Am J Cardiol 2007;100:876–880 [DOI] [PubMed] [Google Scholar]
- [2].Kautzner J QT interval measurements. Card Electrophysiol Rev 2002;6:273–277 [DOI] [PubMed] [Google Scholar]
- [3].Antzelevitch C, Dumaine R. Electrical Heterogeneity in the Heart: Physiological, Pharmacological and Clinical Implications. Compr Physiol 2011;Supplement 6: Handbook of Physiology, the Cardiovascular System, the Heart [Google Scholar]
- [4].Prenner SB, Shah SJ, Goldberger JJ, Sauer AJ. Repolarization Heterogeneity: Beyond the QT Interval. J Am Heart Assoc 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boukens BJ, Walton R, Meijborg VM, Coronel R. Transmural electrophysiological heterogeneity, the T-wave and ventricular arrhythmias. Prog Biophys Mol Biol 2016;122:202–214 [DOI] [PubMed] [Google Scholar]
- [6].Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation 1983;67:1356–1367 [DOI] [PubMed] [Google Scholar]
- [7].Vassallo JA, Cassidy DM, Kindwall KE, Marchlinski FE, Josephson ME. Nonuniform recovery of excitability in the left ventricle. Circulation 1988;78:1365–1372 [DOI] [PubMed] [Google Scholar]
- [8].Thomsen MB, Verduyn SC, Stengl M, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation 2004;110:2453–2459 [DOI] [PubMed] [Google Scholar]
- [9].Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature 2006;440:463–469 [DOI] [PubMed] [Google Scholar]
- [10].Burger HC. A theoretical elucidation of the notion ventricular gradient. Am Heart J 1957;53:240–246 [DOI] [PubMed] [Google Scholar]
- [11].Waks JW, Tereshchenko LG. Global electrical heterogeneity: A review of the spatial ventricular gradient. J Electrocardiol 2016;49:824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Waks JW, Sitlani CM, Soliman EZ, et al. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Biering-Sørensen T, Kabir M, Waks JW, et al. Global ECG Measures and Cardiac Structure and Function: The ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol 2018;11:e005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stabenau HF, Shen C, Tereshchenko LG, Waks JW. Changes in global electrical heterogeneity associated with dofetilide, quinidine, ranolazine, and verapamil. Heart Rhythm 2020;17:460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cardiac patients with and without a history of drug-induced torsades de pointes. Telemetric and Holter ECG Warehouse, University of Rochester Medical Center, 2012. http://thew-project.org/Database/E-OTH-12-0068-010.html. [Google Scholar]
- [16].Kaab S, Hinterseer M, Nabauer M, Steinbeck G. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome–a case-control pilot study using i.v. sotalol. Eur Heart J 2003;24:649–657 [DOI] [PubMed] [Google Scholar]
- [17].Couderc JP, Kaab S, Hinterseer M, et al. Baseline values and sotalol-induced changes of ventricular repolarization duration, heterogeneity, and instability in patients with a history of drug-induced torsades de pointes. J Clin Pharmacol 2009;49:6–16 [DOI] [PubMed] [Google Scholar]
- [18].Kors JA, van Herpen G, Sittig AC, van Bemmel JH. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J 1990;11:1083–1092 [DOI] [PubMed] [Google Scholar]
- [19].Mardia KV, Jupp PE. Directional Statistics. Wiley, 2000 [Google Scholar]
- [20].Krishnamoorthy K, Yu J. Modified nel and van der merwe test for the multivariate behrensfisher problem. Statistics & Probability Letters 2004;66:161–169 [Google Scholar]
- [21].Connolly SJ. Evidence-based analysis of amiodarone efficacy and safety. Circulation 1999;100:2025–2034 [DOI] [PubMed] [Google Scholar]
- [22].Shenthar J, Rachaiah JM, Pillai V, Chakali SS, Balasubramanian V, Chollenhalli Nanjappa M. Incidence of drug-induced torsades de pointes with intravenous amiodarone. Indian Heart J 2017;69:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Skinner JR, Winbo A, Abrams D, Vohra J, Wilde AA. Channelopathies That Lead to Sudden Cardiac Death: Clinical and Genetic Aspects. Heart Lung Circ 2019;28:22–30 [DOI] [PubMed] [Google Scholar]
- [24].Tereshchenko LG, Sotoodehnia N, Sitlani CM, et al. Genome-Wide Associations of Global Electrical Heterogeneity ECG Phenotype: The ARIC (Atherosclerosis Risk in Communities) Study and CHS (Cardiovascular Health Study). J Am Heart Assoc 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prenner SB, Shah SJ, Goldberger JJ, Sauer AJ. Repolarization Heterogeneity: Beyond the QT Interval. J Am Heart Assoc 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Antzelevitch C Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 2007;293:H2024–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sur S, Han L, Tereshchenko LG. Comparison of sum absolute qrst integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PloS one 2013;8:e57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Malik M, Huikuri HV, Lombardi F, Schmidt G, Verrier RL, Zabel M. Is the Tpeak-Tend interval as a measure of repolarization heterogeneity dead or just seriously wounded? Heart Rhythm 2019;16:952–953 [DOI] [PubMed] [Google Scholar]
- [29].Johannesen L, Vicente J, Hosseini M, Strauss DG. Automated Algorithm for J-Tpeak and Tpeak-Tend Assessment of Drug-Induced Proarrhythmia Risk. PLoS ONE 2016;11:e0166925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Verrier RL, Klingenheben T, Malik M, et al. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility–consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol 2011;58:1309–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Perez-Alday EA, Hamilton C, Li-Pershing A, et al. The Reproducibility of Global Electrical Heterogeneity ECG Measurements. Comput Cardiol (2010) 2018;45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS ONE 2013;8:e57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tereshchenko LG, Cheng A, Fetics BJ, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol 2011;44:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


