Abstract
In this study, we sought to expand our previous research on associations between bioactivities in dust and associated organic contaminants. Dust samples were collected from central NC homes (n=188), solvent extracted, and split into two fractions, one for analysis using three different bioassays (nuclear receptor activation/inhibition and adipocyte development) and one for mass spectrometry (targeted measurement of 124 organic contaminants, including flame retardants, polychlorinated biphenyls, perfluoroalkyl substances, pesticides, phthalates, and polycyclic aromatic hydrocarbons). Approximately 80% of dust extracts exhibited significant adipogenic activity at concentrations that are comparable to estimated exposure for children and adults (e.g. ~20 μg/well dust) via either triglyceride accumulation (65%) and/or pre-adipocyte proliferation (50%). Approximately 76% of samples antagonized thyroid receptor beta (TRβ), and 21% activated peroxisome proliferator activated receptor gamma (PPARγ). Triglyceride accumulation was significantly correlated with TRβ antagonism. Sixty-five contaminants were detected in at least 75% of samples; of these, 26 were correlated with adipogenic activity and ten with TRβ antagonism. Regression models were used to evaluate associations of individual contaminants with adipogenic and TRβ bioactivities, and many individual contaminants were significantly associated. An exploratory g-computation model was used to evaluate the effect of mixtures. Contaminant mixtures were positively associated with triglyceride accumulation, and the magnitude of effect was larger than for any individually measured chemical. For each quartile increase in mixture exposure, triglyceride accumulation increased by 212% (RR=3.12 and 95% confidence interval: 1.58, 6.17). These results suggest that complex mixtures of chemicals present in house dust may induce adipogenic activity in vitro at environmental concentrations and warrants further research.
Key Terms: Endocrine Disrupting Chemicals, Adipogenesis, Obesogen, Obesity, Metabolic Disruption, House Dust
Introduction
Household dust is considered a reservoir for chemicals leaching from consumer products and building materials in the home, and is routinely reported to contain phthalates, flame retardants, pesticides, PAHs, phenols, and perfluoroalkyl substances (PFASs), that can span several orders of magnitude in concentration (Kademoglou et al., 2017; Mitro et al., 2016; Rasmussen et al., 2013; Rudel et al., 2003; Stapleton et al., 2008; Stapleton et al., 2005; Stapleton et al., 2006; Stapleton et al., 2009; Suzuki et al., 2007; Suzuki et al., 2013). Many of these chemicals have been described as endocrine disrupting chemicals (EDCs; able to disrupt normal hormonal action (Diamanti-Kandarakis et al., 2009; Gore et al., 2015)), and may contribute to adverse health risks for residents (Rudel et al., 2003; Stapleton et al., 2006; Stapleton et al., 2009). Residents are chronically exposed to indoor dust, via oral, dermal, and inhalation exposure routes (Fraser et al., 2012; Hoffman et al., 2015; Rudel et al., 2010; Watkins et al., 2011), and thus are exposed to EDCs present in the dust. Previous research has demonstrated significant activation or inhibition of nuclear hormone receptors at low concentrations of dust extracts (<100 μg/mL solvent), including the peroxisome proliferator activated receptor gamma (PPARγ), glucocorticoid receptor (GR), androgen receptor (AR), and thyroid receptor (TR), among others (Chou et al., 2015; Fang et al., 2015a; Fang et al., 2015b; Fang et al., 2015c; Kollitz et al., 2018; Suzuki et al., 2007). Children may be at greater risk of exposure to chemicals found in dust due to repetitive hand-to-mouth behaviors and greater time spent on dust-covered surfaces. In fact, the EPA estimates that children ingest 60–100 mg of dust per day from indoor environments (Agency, 2017), and studies have shown significant correlations between contaminant levels in dust and serum levels in children (Dixon et al., 2009; Stapleton et al., 2012; Watkins et al., 2011; Watkins et al., 2012), highlighting the importance of this exposure route. Humans are thus exposed to complex mixtures of EDCs throughout development, with exposure beginning during gestation (Dallaire et al., 2003; Houlihan et al., 2005) and continuing through lactation (Landrigan et al., 2002; Mogensen et al., 2015) and from the indoor environment throughout their lives (Agency, 2017; Fraser et al., 2012; Rudel et al., 2003; Watkins et al., 2013). While the vast majority of toxicological studies assess exposures to single contaminants, we know that humans are routinely exposed to hundreds or thousands of man-made chemicals from indoor and outdoor environments (Hoffman et al., 2018; Kassotis et al., 2016; Lyche et al., 2010; Phillips et al., 2018; Schilmann et al., 2010). As such, characterizing the specific contaminants present in household dust, and their associations with biological effects and subsequent potential health outcomes, is of profound importance.
We previously demonstrated that house dust extracts promoted robust adipocyte differentiation and precursor cell proliferation in 3T3-L1 cells (Kassotis et al., 2019), where approximately 90% of dust extracts promoted adipocyte development via either triglyceride accumulation (a marker for differentiation) or via pre-adipocyte proliferation. The extent of triglyceride accumulation was significantly and positively correlated with the concentration of 12 brominated and organophosphate flame retardants (BFRs and OPFRs, respectively) in the dust extracts (Kassotis et al., 2019). We further evaluated associations between the extent of dust extract-induced effects in vitro and the health of adult residents. Positive associations between house dust-induced triglyceride accumulation and the body mass index (BMI) of residents living in these homes was observed (Kassotis et al., 2019). In addition, we previously found that antagonism of TRβ measured in house dust extracts was significantly correlated with participants serum thyroid hormone levels, potentially suggesting that exposures to mixtures in dust mixtures were interfering with hormone regulation in the general population [20]. Furthermore, we found that antagonism of TR was one of the most active/potent adipogenic pathways modulating triglyceride accumulation in our cell model (Kassotis et al., 2017b). In our cell culture models, co-treatment with T3 significantly reduced dust-induced triglyceride accumulation, and siRNA knock-down of TR inhibited dust-induced adipocyte development (Kassotis et al., 2019); in sum, these results provide evidence for a contributory causative role of TR antagonism in these effects.
These studies provide evidence that complex mixtures of chemicals present in house dust may be affecting multiple pathways that influence metabolism, hormone regulation and overall health within the general population. While we previously demonstrated a role for TR antagonism in dust extract-induced adipogenic effects, and positive correlations with twelve BFRs and OPFRs, we suspect that these are not the causative chemicals promoting adipogenesis. While correlated, many of these flame retardants were independently inactive in our cell model (Kassotis et al., 2017a; Kassotis et al., 2017b), and could not account for the magnitude of activity exhibited by the dust extracts. It is likely that mixture effects or other co-occurring contaminants present in the dust that we were not yet measuring were instead responsible for observed activities.
As such, the goals of this study were to more broadly assess the chemical space present in the indoor environment and its potential association (both on an individual chemical and mixture basis) with adipogenic (triglyceride accumulation and/or pre-adipocyte proliferation) and nuclear receptor bioactivities in a large set of residential house dust samples. Murine 3T3-L1 cells were used to screen and evaluate indoor house dust extracts for triglyceride accumulation and pre-adipocyte proliferation. As noted above, both TRβ antagonism and PPARγ activation have been reported in household dust extracts, and both are highly efficacious pathways for adipocyte differentiation and triglyceride accumulation (Kassotis et al., 2017b; Shao et al., 2016) (Li et al., 2011). As such, we measured these bioactivities using reporter gene assays (these assays measure total receptor activation or inhibition for the mixtures of chemicals extracted from the household dust samples) for a set of dust extracts from the Toddlers Exposure to SVOCs in Indoor Environments (TESIE) cohort (Cooper et al., 2019; Hammel et al., 2019; Hoffman et al., 2018; Phillips et al., 2018), and to assess the potential and relative roles of these underlying mechanisms in promoting adipogenic outcomes. In this study we measured concentrations of 124 chemicals, including BFRs, OPFRs, polychlorinated biphenyls (PCBs), PFAS, pesticides, phthalates, and polycyclic aromatic hydrocarbons (PAHs), vastly expanding our previous work to a wider chemical space. We hypothesized that house dust extracts would promote triglyceride accumulation and pre-adipocyte proliferation at low concentrations, and that this would be mediated in part by inhibition of TRβ, rather than PPARγ, via a complex mixture of bioactive chemicals. As such, we sought to characterize associations between individual contaminants and bioassay outcomes and to utilize a g-computation approach to measuring the total mixture effect of all contaminants.
Materials and Methods
Chemicals.
Chemicals for use in bioassays were purchased as follows: rosiglitazone (Sigma cat # R2408, ≥ 98%), 1–850 (Millipore cat # 609315, ≥ 98%), and triiodothyronine (T3; VWR cat # 80057–656, ≥ 98%). Stock solutions were prepared in 100% cell-culture grade DMSO (Sigma cat # D2650) and stored at − 20 °C between uses. Analytical and internal standards were all purchased from Wellington Laboratories Inc., and Cambridge Isotope Laboratories.
Participant enrollment and clinical assessments.
Extensive study characteristics and recruitment procedures for the Toddlers Exposure to SVOCs in Indoor Environments (TESIE), utilized herein, have been described in detail previously (Hoffman et al., 2018). Briefly, 190 families with children were between 3 and 6 years of age, were enrolled between August 2014 and April 2016. Mothers (or another legal guardian) provided informed consent for children’s participation. Demographic characteristics of the cohort reflected those of the general Durham, NC community (41.4% non-Hispanic white, 36.9% non-Hispanic black, and 20.2% Hispanic white; 55.7% male; and 44.3% college graduate). Home visits were conducted for all participants, and environmental samples were collected from each household. All study protocols were reviewed and approved by the Duke University Health System Institutional Review Board.
Dust sample collection and processing.
House dust samples (n=188 unique samples, n=194 with replicates) were collected and processed as described previously (Hoffman et al., 2017; Phillips et al., 2018). The entire exposed floor area of the main living area for each household was vacuumed with a Eureka Mighty Mite vacuum fitted with a cellulose thimble within the hose attachment during collection (area in which the participant reported spending the most time awake), and dust was collected in a cellulose thimble (Stapleton et al., 2012; Stapleton et al., 2014), wrapped in foil, and stored at −20 °C. Between home visits, the hose attachment of the vacuum was cleaned with soap and water and rinsed with solvent to prevent cross-contamination. Samples of varying weights were first sieved to <500 μm, a set of isotopically labeled standards were added to each sample for target analyte quantification (Table S1), dust samples were then extracted with 50:50 dichloromethane:hexane (used to extract a wide range of chemical classes) via sonication, and finally concentrated under nitrogen gas. Half of each extract’s volume was used for targeted analysis of SVOCs via GC/MS and LC/MS/MS as described previously (Hoffman et al., 2017; Phillips et al., 2018). A portion of the remaining extract’s volume was then utilized for the cell-based assays described herein; these samples were further diluted as necessary to achieve approximately concentrations of 100 μg/mL (20 μg/well in contact with cells). A separate set of isotopically labeled standards were spiked after the extract was split and were not added to the fraction used in cell-based assays. This fraction was evaporated to dryness under nitrogen and reconstituted in 100 μL DMSO for use in bioassays. Laboratory blanks (n=6) were prepared using laboratory solvents and techniques in the absence of dust to ensure that lab procedures did not impart any bioactivities to our assays. None of these samples exhibited significant triglyceride accumulation, pre-adipocyte proliferation, or nuclear receptor bioactivities at any concentration tested (Figure S1). Replicate samples (n=6) were utilized to ensure consistency, and responses were averaged following analysis.
Targeted chemical analysis.
Half of the dust extract volume was used for targeted chemical analysis of 124 organic contaminants. Following concentration using a SpeedVac™ Concentrator, samples were purified as described previously (Van den Eede et al., 2012). Briefly, samples were purified using solid phase extraction with Florisil cartridges (Supel-clean ENVI-Florisil, 6 mL, 500 mg; Supelco) as described previously (Van den Eede et al., 2012). Purified extracts were eluted in three fractions: 6 mL hexane (F1), 10 mL ethyl acetate (F2), and 6 mL methanol (F3). Fractions were concentrated to approximately 1 mL using a SpeedVac™ concentrator and F2 fractions were reconstituted in hexane prior to analysis. Concentrations of 124 chemicals were measured in indoor house dust extracts via LC-MS/MS and GC/MS. Briefly, F1 fractions were analyzed for PAHs, PCBs, PBDEs and other BFRs (Stapleton et al., 2014), and phthalates (Hammel et al., 2019), F2 fractions were analyzed for pesticides (Cooper et al., 2019) and OPFRs (Phillips et al., 2018), and F3 fractions were analyzed for PFAS, phenols, and parabens as described previously (Supplemental Methods, Table S2).
Quality Assurance (QA) and Quality Control (QC) of Dust Measurements.
Standard reference material samples (SRM) 2585 (n=5; National Institute of Standards and Technology [NIST], Gaithersburg, MD) were extracted and analyzed with the dust samples for QA/QC (Table S3). Measured values were generally within 60–140% of the certified SRM 2585 concentrations and were deemed acceptable for QA/QC purposes (Table S3). Detection limits were calculated as a signal/noise ratio of 3.0 if there was no detection in the laboratory blanks. If there was a detection in laboratory blanks, we calculated the average concentration in the laboratory blanks and their standard deviation. All samples were then blank subtracted and the detection limit was set to three times the standard deviation of the laboratory blanks. Method detection limits (MDLs) were calculated on a sample-specific basis using the dust mass extracted for each individual sample. Values below the MDL were replaced with a value representing the MDL divided by two, as described previously (Hammel et al., 2019; Phillips et al., 2018), for statistical analyses.
3T3-L1 Cell Care and Differentiation Assays.
3T3-L1 cells (Zenbio cat# SP-L1-F, lot# 3T3062104; Research Triangle Park, NC) were maintained as described previously in pre-adipocyte media (Dulbecco’s Modified Eagle Medium - High Glucose; DMEM-HG; Gibco cat# 11995, supplemented with 10% bovine calf serum and 1% penicillin and streptomycin; Gibco cat# 15140) (Kassotis et al., 2017b). Cells were utilized between passages 8 (at purchase) and 12 and were maintained in a sub-confluent state until induction of differentiation. Positive and negative controls were included in each assay plate, with no significant changes in control responses observed across passages or the course of the experiment.
3T3-L1 cells were induced to differentiate as described in detail previously (Kassotis et al., 2017a; Kassotis et al., 2017b). Briefly, cells were seeded in pre-adipocyte media into 96-well tissue culture plates (Greiner cat # 655090) at ~30,000 cells per well. Once confluent, cells were allowed 48 hours for growth arrest and to allow initiation of clonal expansion. Media was then replaced with test chemicals, dust extracts, and/or controls using a DMSO vehicle (at 0.1%) in differentiation media (DMEM-HG with 10% fetal bovine serum, 1% penicillin/streptomycin, 1.0 μg/mL human insulin, and 0.5 mM 3-isobutyl-1-methylxanthine, IBMX). After 48 hours of differentiation induction, media was replaced with fresh dilutions of test chemicals, dust extracts, and/or control chemicals in adipocyte maintenance media (differentiation media without IBMX), and this media was refreshed every 2–3 days until assay, ten days after induction.
3T3-L1 Triglyceride Accumulation, Cell Proliferation, and Cell Viability Measurements.
Plates were assayed for triglyceride accumulation, DNA content, and cell viability as described previously (Kassotis et al., 2017a; Kassotis et al., 2017b). Briefly, media was removed and cells rinsed with Dulbecco’s phosphate-buffered saline (DPBS; Gibco cat # 14040) before replacing with 200 μL of a dye mixture (19 mL DPBS, 1 drop/mL NucBlue® Live ReadyProbes® Reagent (cell proliferation/cytotoxicity measure of DNA content; Thermo cat # R37605) and 500 μL AdipoRed™ (intracellular lipid measure of triglyceride accumulation; Lonza cat # PT-7009) per plate). Plates were protected from light and incubated at room temperature for approximately forty minutes; fluorescence was then measured with excitation 485 nm/emission 572 nm for AdipoRed™ and 360/460 for NucBlue®. Following the lipid and DNA readings, cell viability was assessed using the CellTiter-Glo™ (cell viability via ATP content; Promega cat# G7572) assay as described previously (Kassotis et al., 2017a). Briefly, 170 μL of DPBS mixture was removed from all wells, and the remaining 30 μL was mixed with 30 μL of CellTiter-Glo™ reagent. Plates were incubated for ten minutes prior to reading luminescence, and viability was assessed as a percent change from differentiated DMSO controls. Inhibited cell health was assessed via deviations of ≥15% in either cell viability (ATP) and/or cytotoxicity (DNA content) assays. Four technical (replicates within each assay plate) and three biological replicates (separate cell passages/assays) were utilized for every test chemical and concentration herein.
For adipogenic activity, percent activities (efficacies) were calculated relative to the maximal rosiglitazone-induced fold induction over intra-assay differentiated vehicle controls (0.1% DMSO), after correcting for background fluorescence. Rosiglitazone was utilized as the positive control herein due to selective, robust, and potent binding to PPARγ (Lehmann et al., 1995; Seimandi et al., 2005; Spiegelman, 1998) and ease of comparisons across laboratories and studies. DNA content was calculated as percent change from vehicle controls for each chemical at each concentration and was then used to normalize total triglyceride values to obtain triglyceride content per unit DNA (proxy for triglyceride accumulation per cell). As we determined previously that extracted dust mass could be a potential confounder in maximal bioactivity, extracts were normalized to approximately 100 μg/mL prior to testing in the bioassay and were tested at this single concentration only in all bioassays.
Reporter Gene Activity Bioassays.
HEK293/17 cells were obtained through the Duke Cell Culture Facility (ATCC cat# CRL-11268, lot# 3579061) and were maintained in growth media (DMEM-HG with 10% fetal bovine serum and 1% penicillin and streptomycin), ensuring cells did not reach confluency. For transfection, flasks with near confluent cells were switched to white media two days prior (DMEM-HG without phenol red, Gibco cat# 31053; 10% charcoal-stripped fetal bovine serum, Gibco #A33821; and 1% penicillin and streptomycin). Cells were transfected in flask as described in detail previously REFS; briefly, cells were transfected using Lipofectamine LTX & Plus (Invitrogen cat# 15338–100) and receptor (hTRβ1-pSG5 and pcDNA-PPARγ1) and reporter gene plasmids (pGL4-TK-2X-TADR4 and DR1-luc, respectively; receptor response elements linked to the firefly luciferase gene), along with a constitutively-active CMV-β-Gal (all plasmids were generous gifts of the Donald McDonnell Lab). Transfected cells were seeded at approximately 60,000 cells per well into 96-well tissue culture plates (Midsci cat # TP92696) and allowed to settle for four hours. Cells were then induced with dose responses of positive/negative controls, test chemicals, and/or dust extracts at the ~100 μg/mL single concentration using a 0.1% DMSO vehicle.
After approximately 18 hours, cells were lysed and lysate was used for luciferase and beta-galactosidase assays. Receptor bioactivities were calculated as a fold induction relative to the solvent control response (0.1% DMSO) and then were used to assess relative responses to positive control agonists and/or antagonists. For PPARγ agonist bioactivity, vehicle baseline-corrected test chemical values were compared to the maximal positive control response of 1 μM rosiglitazone. For TRβ antagonist bioactivity, corrected test chemical responses were determined as a percent enhancement or suppression of triiodothyronine (T3) at its EC50 concentration. Significant bioactivities were only determined in the absence of significant toxicity (≥15% change in response of constitutively active beta-galactosidase promoter relative to solvent control). Positive and negative controls were utilized to assess efficacy, potency, and sensitivity of assays and responses were compared to historical and literature values to ensure consistency (Figure S2).
Statistical Analysis.
Data for adipogenic and nuclear receptor bioactivities are presented as means ± standard error of the mean (SEM) from four technical replicates of two or three independent biological replicates. Replicate dust extract responses were averaged prior to statistical analyses. Relationships between measures of adipogenic activity and activation or inhibition of specific nuclear receptors were evaluated using Spearman’s correlations when bioactivity was observed in at least 50% of samples. Relationships between measures of bioactivity activity and the concentrations of each of the individual chemicals in dust were assessed using Spearman’s correlations. Kendall’s correlation analyses were also performed (to account for potential impacts of zero activity samples) and were very similar to Spearman’s results (results not shown). As such, Spearman’s correlations were reported herein for all analyses. All correlation analyses were performed using SAS 9.4. To ensure that infrequently detected compounds did not inadvertently skew analyses, correlations were only performed for chemicals detected in at least 75% of the dust extracts. In these analyses, concentrations below the limit of detection were imputed as the limit of detection divided by two.
Recognizing that household dust is a complex mixture of multiple contaminants, additional exploratory analyses were also conducted to evaluate the cumulative effect of multiple dust contaminants on bioactivities for adipogenic and nuclear receptor endpoints. The potential joint effects of multiple compounds were assessed using quantile-based g-computation. Unlike traditional inferential approaches that examine the effects of individual contaminants while holding the values of mixture components constant, quantile g-computation can estimate the effect of an increase in all components of a mixture simultaneously which more closely reflects real-world environmental exposure (Keil et al., 2020). Quantile g-computation can be used for studies with small to moderate sample sizes and highly correlated exposures and has a number of additional advantages over other commonly applied mixtures approaches (e.g. weighted quantile sum regression) in that it allows for both positive and negative relationships between individual exposures and outcomes (Keil et al., 2020). All analyses were conducted in R using the qgcomp package (R 3.6.2,). To address over dispersion in bioactivity measures, a zero-inflated model was specified, and bioactivity outcome measures were treated as counts. As a result, estimated rate ratios represent the percent increase in the expected activity (measured as a percentage) per quantile increase in all exposures. As a comparison with the mixture analysis, analyses were also performed with individual chemicals. Each compound was included in a separate model; thus, individual chemical analyses do not account for patterns of co-exposure to other contaminants in dust.
Results
One hundred eighty-eight unique house dust extracts at concentrations of approximately 100 μg/mL (i.e. 100 ug of dust extracted per mL of media; Table 1) were assessed using 3T3-L1 preadipocytes for pre-adipocyte proliferation (DNA content, relative to vehicle control), triglyceride accumulation (total triglycerides per well and per cell (normalized to DNA content), and cell viability (ATP production) at this single concentration. A randomly selected subset of extracts (n=118) were also assessed for hormone receptor bioactivities using two reporter gene assays (TRβ antagonism and PPARγ agonism). All 188 dust samples were analyzed for organic contaminant concentrations using a combination of LC/MS-MS and GC/MS.
Table 1.
Descriptive Results of Adipogenic Activity Across Dust Extracts
| Dust Sample Metrics | N | Range | Median | Mean |
|---|---|---|---|---|
| Dust extract concentrations (μg/mL) | ||||
| Overall | 188 | 8.5–196.7 | 88.5 | 91.9 |
| Lower tertile | 62 | 8.5–74.9 | 53.1 | 52.3 |
| Middle tertile | 63 | 75.5–100.9 | 88.4 | 88.6 |
| Upper tertile | 63 | 101.0–196.7 | 125.3 | 134.3 |
| Dust-induced triglyceride accumulation (%) | ||||
| Overall | 188 | 0.0–85.29 | 6.6% | 19.6% |
| Inactive samples | 80 | 0.0–4.86 | 0.8% | 1.4% |
| Lower tertile | 36 | 5.0–11.0 | 6.8% | 7.3% |
| Middle tertile | 36 | 11.0–29.3 | 18.0% | 19.1% |
| Upper tertile | 36 | 30.0–152.8 | 69.3% | 73.1% |
| Dust-induced cell proliferation (%) | ||||
| Overall | 188 | 0.0–85.3 | 7.2% | 9.4% |
| Inactive samples | 95 | 0.0–7.0 | 2.2% | 2.3% |
| Lower tertile | 31 | 7.3–10.0 | 9.0% | 8.9% |
| Middle tertile | 31 | 10.2–15.9 | 12.9% | 12.9% |
| Upper tertile | 31 | 16.1–85.3 | 19.6% | 27.7% |
Descriptive statistics for dust extract concentrations and adipogenic activity metrics (both triglyceride accumulation and pre-adipocyte proliferation) for n=188 samples. Tertiles defined based on dust extract concentrations across groups. Dust extract concentrations provided as test concentration, and many samples were diluted to achieve approximate concentrations of 100 μg/mL.
Adipogenic and Nuclear Receptor Activities of Indoor House Dust Extracts.
The majority of dust extracts exhibited adipogenic activity in our assay system, with 65% promoting significant triglyceride accumulation of up to 153% relative to the rosiglitazone-induced maximum, 50% of the samples promoting significant pre-adipocyte proliferation up to 85% relative to the differentiated vehicle control, and ~80% exhibiting one or both activities (Figure 1A, Table 1). Adipogenic metrics exhibited some overlap, with 29% of extracts exhibiting significant triglyceride accumulation only, 14% exhibiting significant pre-adipocyte proliferation only, and 36% exhibiting both adipogenic activities (Figure 1A). Approximately 4% of samples exhibited significant cytotoxicity (significant inhibition of DNA content relative to differentiated vehicle control). Within the random subset of dust extracts, activation of PPARγ ranged from 0–48% relative to the maximal rosiglitazone-induced response, with 21% exhibiting significant activity (Figure 1B). Antagonism of TRβ ranged from 0–74% inhibition of the half maximal triiodothyronine (T3)-induced response, with 76% of the extracts exhibiting significant activity (Figure 1B). Approximately sixteen (9%) of the extracts exhibited both activities and 18% of extracts exhibited neither. No samples exhibited significant inhibition of cell viability in the nuclear receptor bioassays.
Figure 1: Adipogenic and Nuclear Receptor Bioactivities of Indoor House Dust Extracts.
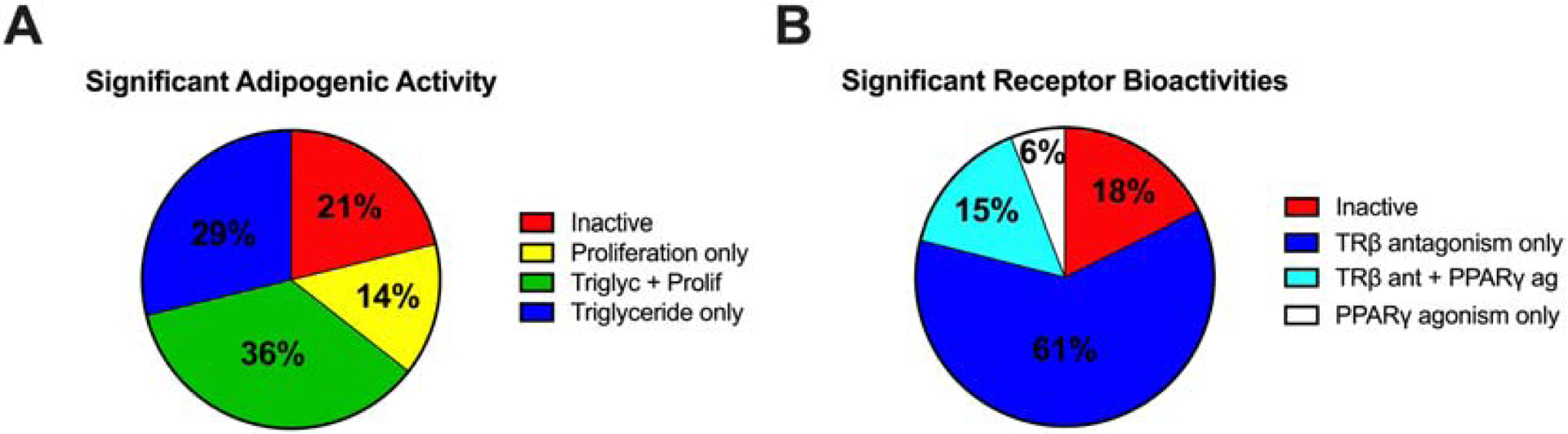
3T3-L1 cells were induced to differentiate as described in Methods. Cells were assessed for degree of adipocyte differentiation after ten days of differentiation while exposed to indoor house dust extracts diluted to approximately 100 μg/mL concentration. (A) The proportion of samples exhibiting significant adipogenic activity relative to the differentiated vehicle control for triglyceride accumulation only, pre-adipocyte proliferation only, or a combination of both triglyceride accumulation and pre-adipocyte proliferation. Reporter gene assays were used to measure activation of the peroxisome proliferator activated receptor gamma (PPARγ) and inhibition of the thyroid receptor beta (TRβ) as described in Methods. A) The proportion of dust extracts exhibiting significant activation of PPARγ, inhibition of TRβ, both, or neither PPARγ agonism nor TRβ antagonism.
Associations of Nuclear Receptor Activities with Adipogenic Activities.
To assess relationships between nuclear receptor bioactivities and adipogenic activities, general descriptive assessments were performed. For extracts exhibiting bioactivity (PPARγ agonism and/or TRβ antagonism), the vast majority (~90%) also exhibited adipogenic activity via triglyceride accumulation and/or pre-adipocyte proliferation (Figure S3A–D). In contrast, for a smaller number of extracts exhibiting neither bioactivity (18% of samples), approximately 80% exhibited adipogenic activity (Figure S3B), suggesting other mechanisms.
Spearman’s correlations were calculated to assess relationships between nuclear receptor bioactivities and adipogenic activities among dust samples, given that they were all tested at the same dust extract concentration. The extent of TRβ antagonism in the reporter gene assay was positively correlated with the extent of triglyceride accumulation in matched extracts in the 3T3-L1 bioassay (rs = 0.57, p<0.001; Figure 2A). TRβ antagonism was not correlated with pre-adipocyte proliferation (Figure 2B). Correlation analyses were not performed for PPARγ agonism due to the high proportion of samples with no activity.
Figure 2: Dust Extract Adipogenic Activity is Correlated with Thyroid Receptor Beta Antagonism.
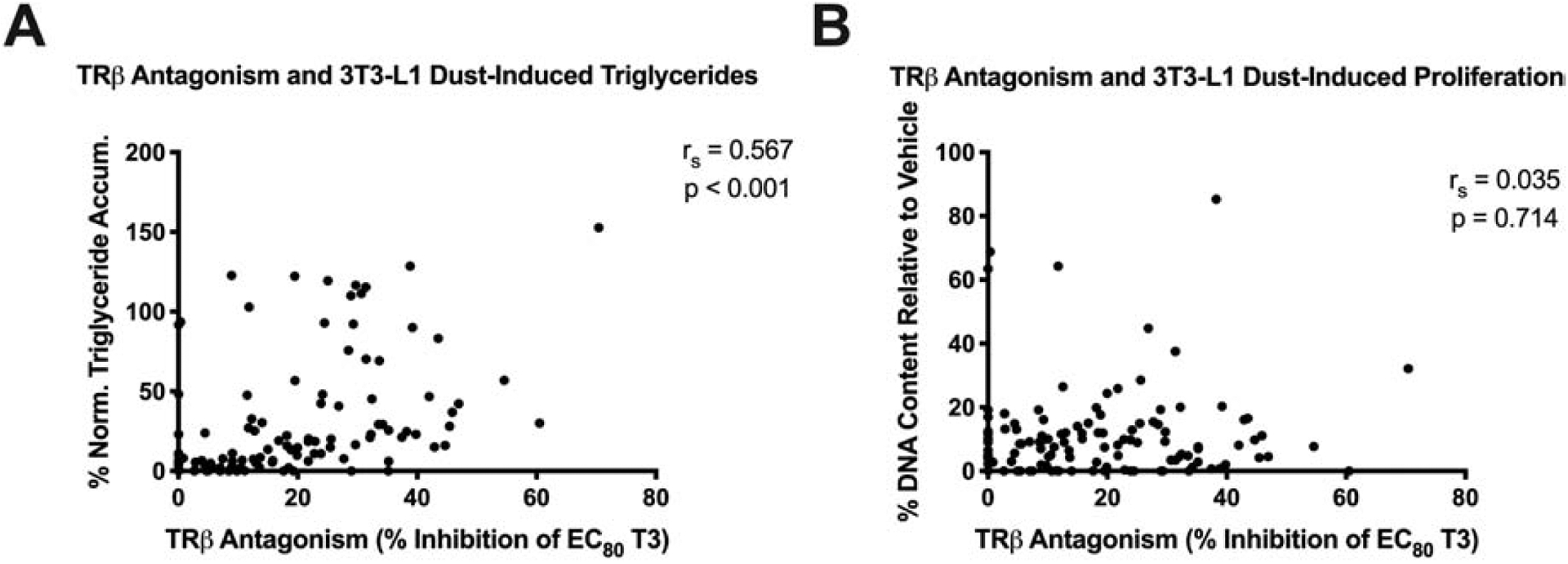
Interrogation of thyroid receptor beta (TRβ) antagonism as putative molecular mechanism that may contribute to normalized triglyceride per cell. (A) Spearman’s correlation comparing normalized triglyceride accumulation per cell relative to maximal intra-assay response for rosiglitazone (normalized to DNA content) versus TRβ antagonism as measured via reporter gene assay in human embryonic kidney cells. (B) Spearman’s correlation comparing pre-adipocyte proliferation relative to a differentiated vehicle control versus TRβ antagonism. Significant correlations were determined using p < 0.05 using GraphPad Prism 8.0.
Associations of Chemical Concentrations in House Dust Extracts.
Concentrations of 124 chemicals were measured in indoor house dust extracts (Table 2), including a range of pesticides, polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), brominated and organophosphate flame retardants (BFRs and OPFRs, respectively), polybrominated diphenyl ethers (PBDEs), phthalates, and perfluoroalkyl substances (PFAS). Sixty-five of these chemicals were detected in ≥75% of dust extracts (for statistical rigor, we focused on these 65 chemicals for all analyses), and concentrations spanned several orders of magnitude: PAHs were reported at median concentrations of between 20–250 ng/g house dust, most at a high frequency of detection; PCBs were all reported at <20% detection frequency; PBDEs and other BFRs were detected frequently and were found at median concentrations ranging from 5–600 ng/g; OPFRs were generally detected frequently at median concentrations of 25–6,000 ng/g; phthalates were detected frequently at median concentrations from 2000–135,000 ng/g; phenolics and parabens were detected frequently at median concentrations from 20–4,000 ng/g; pesticides were generally frequently-detected at median concentrations from 2.5–3,200 ng/g; and most PFAS were detected with high frequency at median concentrations from 1–1,400 ng/g.
Table 2.
Targeted Analysis of Indoor Contaminants in Residential House Dust Samples
| Chemical | % Detect | Concentration Range (ng/g dust) | Median (ng/g) |
|---|---|---|---|
| Pesticides | |||
| Chlorpyrifos | 98.4 | <MDL - 87,960 | 3,182 |
| cis-Chlordane | 80.2 | <MDL - 16,060 | 74.7 |
| trans-Chlordane | 84.0 | <MDL - 22,430 | 23.5 |
| cis-Permethrin | 98.9 | <MDL - 203,600 | 585.2 |
| trans-Permethrin | 100.0 | 171.2 – 176,900 | 1,788 |
| Cypermethrin | 95.7 | <MDL - 106,000 | 1,706 |
| Thiabendazole | 35.3 | <MDL - 211.5 | NR |
| Azoxystrobin | 93.0 | <MDL - 9,635 | 2.5 |
| Fluoxastrobin | 74.9 | <MDL - 37.0 | 0.2 |
| Pyraclostrobin | 34.0 | <MDL - 48.6 | NR |
| Trifloxystrobin | 37.4 | <MDL - 2.5 | NR |
| Polycyclic aromatic hydrocarbons (PAHs) | |||
| Naphthalene | 72.9 | <MDL - 1,865 | 19.2 |
| 1-Methylnaphthalene | 16.0 | <MDL - 587.6 | NR |
| 2,6-Dimethylnaphthalene | 41.5 | <MDL - 1,120 | NR |
| Acenaphthylene | 70.2 | <MDL - 1,017 | 10.0 |
| Acenaphthene | 1.1 | <MDL - 6,763 | NR |
| Dibenzofuran | 20.7 | <MDL - 1,044 | NR |
| Fluorene | 64.9 | <MDL - 2,510 | 36.5 |
| Dibenzothiophene | 96.3 | <MDL - 3,713 | 41.1 |
| Phenanthrene | 98.4 | <MDL - 30,400 | 267.3 |
| Anthracene | 100.0 | 0.2 – 8,005 | 23.5 |
| Carbazole | 69.1 | <MDL - 17,590 | 27.5 |
| 1-Methylphenanthrene | 96.3 | <MDL - 84,060 | 170.7 |
| 2-Methylnaphthalene | 94.7 | <MDL - 36,930 | 138.7 |
| Fluoranthene | 100.0 | 0.7 – 95,150 | 252.7 |
| Pyrene | 100.0 | 0.8 – 70,670 | 251.5 |
| 1,2-Benzofluorene | 97.9 | <MDL - 15,930 | 54.6 |
| Retene | 94.7 | <MDL - 49,420 | 112.5 |
| 3,4-Benzofluorene | 98.9 | <MDL - 9,886 | 44.9 |
| Benzo(c)phenanthrene | 90.4 | <MDL - 9,342 | 19.3 |
| Benzo(b,k)fluoranthene | 92.6 | <MDL - 65,360 | 149.1 |
| Benzo(a)fluoranthene | 66.0 | <MDL - 8,917 | 35.4 |
| Benzo(e)pyrene | 80.9 | <MDL - 40,860 | 55.1 |
| Benzo(a)pyrene | 84.6 | <MDL - 61,730 | 134.3 |
| Perylene | 50.5 | <MDL - 9,669 | 20.6 |
| 3-Methylcholanthrene | 23.9 | <MDL - 18,490 | NR |
| Benzo(g,h,i)perylene | 78.7 | <MDL - 76,460 | 149.8 |
| Phthalates | |||
| DMP | 38.0 | <MDL - 15,160 | NR |
| DEP | 70.6 | <MDL - 64,660 | 1,906 |
| DiBP | 99.5 | <MDL - 149,000 | 4,404 |
| BBP | 99.5 | <MDL - 1,339,000 | 13,630 |
| DBP | 99.5 | <MDL - 213,500 | 9,640 |
| DEHP-adipate | 47.6 | <MDL - 61,580 | NR |
| DEHP | 100.0 | 6,213.3 – 1,812,000 | 118,000 |
| Dioctyl-terephthalate | 100.0 | 4,506.1 – 2,970,000 | 133,100 |
| DINP | 99.5 | <MDL - 1,985,000 | 117,300 |
| TOTM | 81.8 | <MDL - 281,700 | 4,932 |
| Perfluorinated alkyl substances (PFAS) | |||
| 6:2 FTOH | 96.3 | <MDL - 248,900 | 580.1 |
| 8:2 FTOH | 99.5 | <MDL - 44,220 | 1,428 |
| MeFOSE | 19.3 | <MDL - 7,981 | NR |
| EtFOSE | 15.5 | <MDL - 510.9 | NR |
| 6,2-diPAP | 100.0 | 1.1 – 34,360 | 112.5 |
| 8,2-diPAP | 33.3 | <MDL - 6,894 | NR |
| PFBA | 9.3 | <MDL - 545.6 | NR |
| PFBS | 1.1 | <MDL - 577.9 | NR |
| PFDA | 40.4 | <MDL - 4,130 | NR |
| PFHpA | 97.3 | <MDL - 713.4 | 9.1 |
| PFHxA | 97.3 | <MDL - 1,382 | 8.5 |
| PFHxS | 56.3 | <MDL - 694.4 | 0.8 |
| PFNA | 99.5 | <MDL - 207.9 | 3.3 |
| PFOA | 100.0 | <MDL - 2,354 | 8.1 |
| PFOS | 83.6 | <MDL - 2,809 | 4.0 |
| PFPA | 9.8 | <MDL - 134.5 | NR |
| Polybrominated diphenyl ethers (PBDEs) | |||
| BDE-30 | 12.3 | <MDL - 57.2 | NR |
| BDE-17 | 62.6 | <MDL - 6,632 | 2.6 |
| BDE-25 | 58.8 | <MDL - 702.8 | 1.4 |
| BDE-28,33 | 65.8 | <MDL - 659.0 | 8.3 |
| BDE-49 | 97.3 | <MDL - 670.9 | 20.0 |
| BDE-47 | 93.0 | <MDL - 20,200 | 166.4 |
| BDE-66 | 58.3 | <MDL - 311.1 | 4.3 |
| BDE-100 | 95.7 | <MDL - 5,885 | 44.7 |
| BDE-99 | 99.5 | <MDL - 22,730 | 322.8 |
| BDE-119 | 72.7 | <MDL - 850.6 | 13.1 |
| BDE-85,155 | 89.3 | <MDL - 1,443 | 21.1 |
| BDE-154 | 90.9 | <MDL - 1,503 | 24.5 |
| BDE-153 | 96.3 | <MDL - 2,991 | 41.7 |
| BDE-138 | 96.8 | <MDL - 570.2 | 18.8 |
| BDE-156 | 59.9 | <MDL - 192.0 | 4.6 |
| BDE-183 | 74.3 | <MDL - 2,750 | 8.0 |
| BDE-191 | 66.3 | <MDL - 2,043 | 6.7 |
| BDE-181 | 20.3 | <MDL - 185.6 | NR |
| BDE-190 | 11.2 | <MDL - 181.9 | NR |
| BDE-203,200 | 79.1 | <MDL - 3,762 | 7.7 |
| BDE-205 | 85.0 | <MDL - 576.0 | 25.2 |
| BDE-206 | 96.3 | <MDL - 9,831 | 144.9 |
| BDE-209 | 90.4 | <MDL - 117,700 | 579.6 |
| Novel brominated flame retardants (BFRs) | |||
| TBB | 99.5 | <MDL - 26,520 | 248.8 |
| TBPH | 99.5 | <MDL - 17,720 | 408.1 |
| OBIND | 11.2 | <MDL - 1,121 | NR |
| DBDPE | 67.4 | <MDL - 11,790 | 113.4 |
| TTBP-TAZ | 26.2 | <MDL - 11,900 | NR |
| Organophosphate flame retardants (OPFRs) | |||
| EHDP | 98.4 | <MDL - 3,342 | 215.4 |
| TnBP | 0.0 | <MDL | NR |
| TBOEP | 97.9 | <MDL - 2,209,000 | 6,233 |
| TCEP | 98.4 | <MDL - 167,500 | 793.0 |
| TCPP | 100.0 | 241.2 – 468,800 | 3,696 |
| TDCPP | 100.0 | 98.9 – 257,900 | 4,902 |
| Tpp | 100.0 | 74.8 – 291,000 | 2,132 |
| 2-IPPDPP | 81.3 | <MDL - 2,646 | 93.5 |
| 4-IPPDPP | 54.0 | <MDL - 1,267 | 24.6 |
| 2,4-DIPPDPP | 69.0 | <MDL - 6,775 | 128.4 |
| B2-IPPPP | 21.4 | <MDL - 2,143 | NR |
| B4-IPPPP | 48.1 | <MDL - 1,183 | NR |
| B2,4-DIPPPP | 8.0 | <MDL - 29,640 | NR |
| 4tBPDPP | 95.2 | <MDL - 85,990 | 515.6 |
| 2tBPDPP | 0.0 | <MDL | NR |
| B2tDPP | 0.0 | <MDL | NR |
| B4TBPP | 84.0 | <MDL - 12,780 | 74.9 |
| T4tBPP | 29.9 | <MDL - 822.8 | NR |
| Polychlorinated biphenyls (PCBs) | |||
| PCB-28 | 1.6 | <MDL - 505.3 | NR |
| PCB-52 | 6.4 | <MDL - 476.3 | NR |
| PCB-101 | 6.4 | <MDL - 464.5 | NR |
| PCB-118 | 5.9 | <MDL - 503.8 | NR |
| PCB-153 | 10.2 | <MDL - 807.3 | NR |
| PCB-138 | 15.5 | <MDL - 1,797 | NR |
| PCB-180 | 4.3 | <MDL - 459.4 | NR |
| Phenolics | |||
| 2,4,6-TBP | 77.5 | <MDL - 46,210 | 46.1 |
| BPA | 84.1 | <MDL - 168,000 | 3,810 |
| Triclocarban | 46.7 | <MDL - 6,236 | NR |
| Triclosan | 100.0 | <MDL - 23,620 | 787.1 |
| Parabens | |||
| Butyl paraben | 72.0 | <MDL - 2,728 | 18.4 |
| Ethyl paraben | 72.5 | <MDL - 15,280 | 106.6 |
| Methyl paraben | 92.3 | <MDL - 65,360 | 1,802 |
| Propyl paraben | 99.5 | <MDL - 41,760 | 989.6 |
Descriptive statistics for targeted organic contaminant concentrations by chemical class for n=188 samples. Detection frequency across these dust extracts is provided for all chemicals, the range of concentrations (MDL = method detection limit; this varies based on the concentration of dust able to be collected in each home; as such, the MDL for every chemical is unique for each individual dust sample), and the median concentrations. NR = no reported; for chemicals with <50% detection frequencies.
Spearman’s correlations across chemical classes were performed (Figure 3). PAHs exhibited a high degree of concordance across the class, with strong positive correlations between all PAHs. PAHs were positively correlated with certain BFRs, including PBDEs, several OPFRs, some phthalates, and 6,2-diPAP; PAHs also demonstrated negative correlations with several pesticides, several PBDEs, several PFAS, and a number of phenolics and parabens. PBDEs demonstrated high concordance across the chemical class, though the highly brominated PBDEs (e.g. containing >7 Br atoms) demonstrated less fidelity to the lower brominated PBDEs. PBDEs also had strong positive correlations with some alternate BFRs, several pesticides, and several phthalates. OPFRs demonstrated a lower degree of concordance across the class. Phenolics and parabens were highly correlated with some pesticides, OPFRs, and phthalates, demonstrating a high degree of association, with generally strong negative correlations with PFAS chemicals.
Figure 3: Correlations Among Targeted Chemical Concentrations in Household Dust.
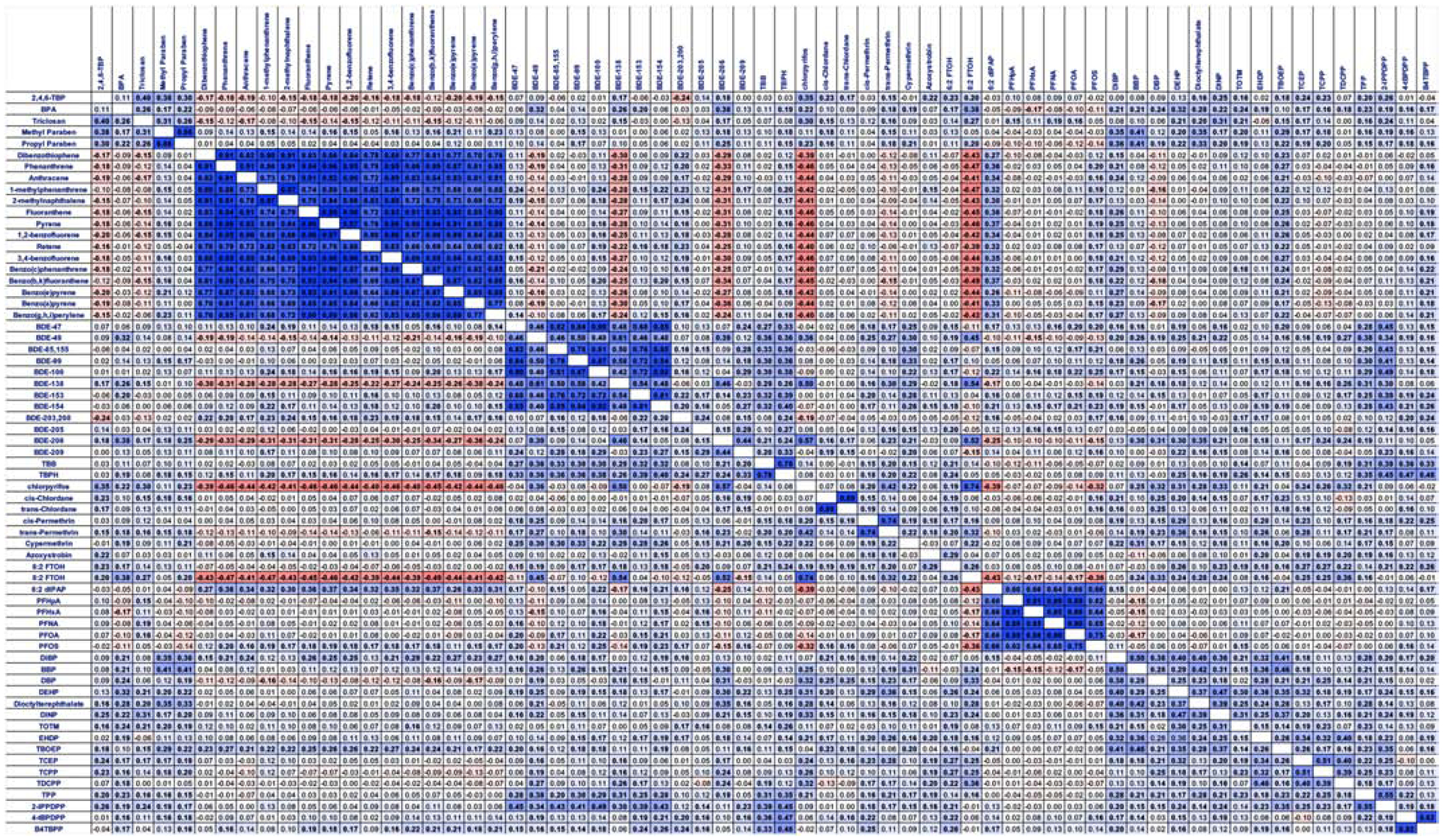
Spearman’s correlations between concentrations of targeted semi-volatile contaminant concentrations (only performed for chemicals with ≥ 75% detection rate across extracts) in house dust extracts. Correlations performed using SAS 9.4; bolded samples represent significant (p<0.05) correlations, and the color depicts the strength and direction of the correlation. Darker colors represent a stronger correlation, blue coloration depicts positive correlations, and red depicts negative correlations.
PFAS chemicals exhibited extremely high concordance for all but the fluorotelomer alcohols (6:2 and 8:2 FTOH), which generally had either no associations (6:2) or negative associations (8:2) with the other PFAS. Notably, PFAS were not appreciably correlated with other chemical classes, suggesting a very different use profile relative to the other chemical classes measured herein (Figure 3). The fluorotelomer alcohols had positive correlations with some pesticides, and several PBDEs, OPFRs, and phthalates.
Associations of Adipogenic and Nuclear Receptor Activities with Chemical Concentrations in House Dust Extracts.
Concentrations of the 65 chemicals with ≥75% detection frequency were further examined for their correlations with bioactivities (PPARγ agonism was not evaluated due to the low percentage of active samples) via Spearman’s correlations. Concentrations of 20 chemicals, across diverse chemical classes, were significantly and positively correlated with triglyceride accumulation (rs: 0.15 – 0.34; Figure 4). Six chemicals were significantly and negatively correlated with pre-adipocyte proliferation (retene, 3,4-benzofluorene, BDE-100, BDE-154, azoxystrobin, and 6,2-diPAP; rs: −0.15 – −0.23). Concentrations of ten chemicals were significantly positively correlated with TRβ antagonism (pesticides, phthalates, phenols, OPEs, PBDEs, and parabens; rs: 0.19 – 0.33).
Figure 4: Correlations of Bioactivities with Targeted Chemical Concentrations in Household Dust.
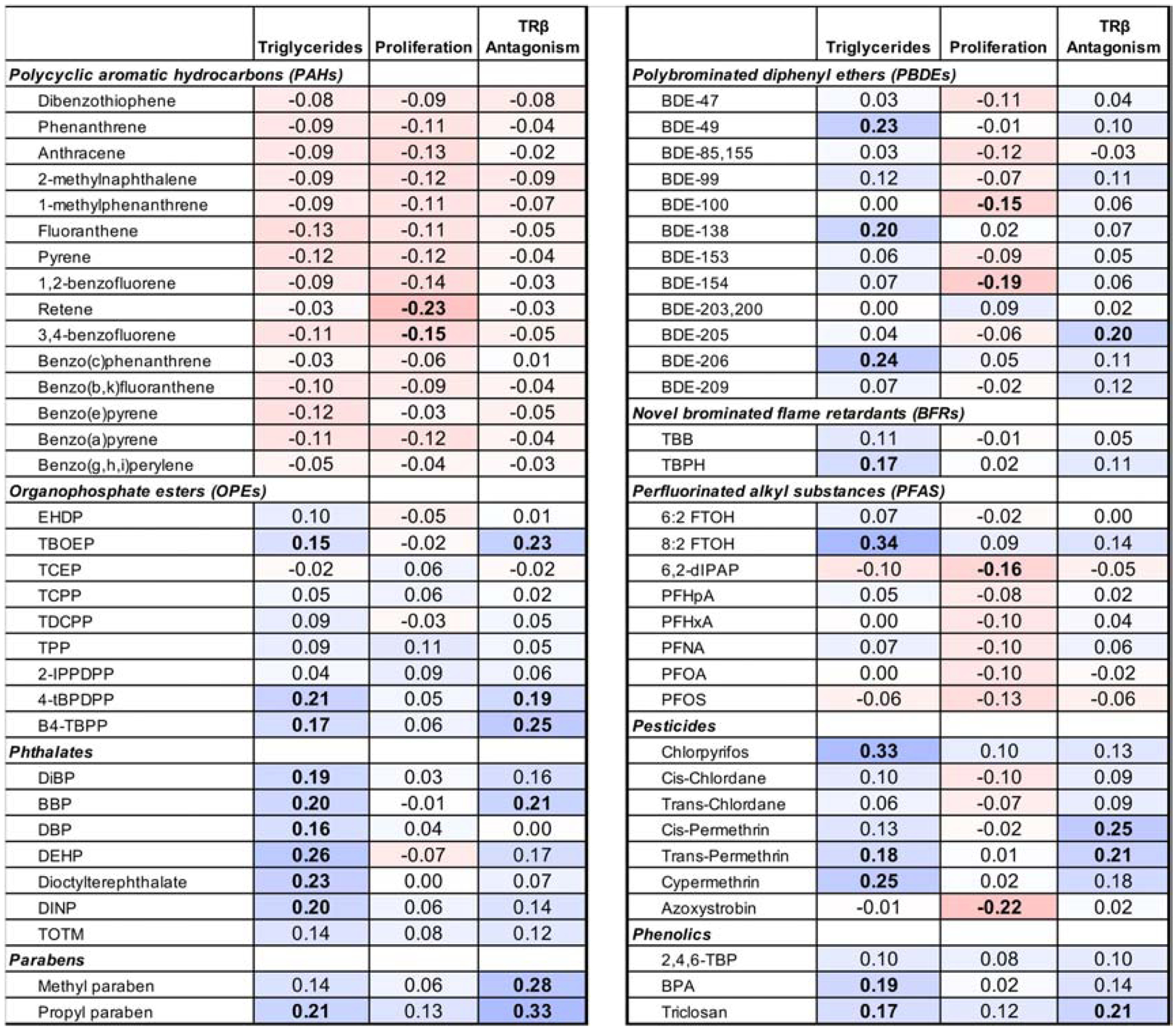
Spearman’s correlations between concentrations of targeted semi-volatile contaminant concentrations (only performed for chemicals with ≥ 75% detection rate across extracts) in house dust extracts and the bioactivities exhibited by these paired dust extracts. Bioactivities examined include adipogenic activity (normalized triglyceride accumulation per cell and pre-adipocyte proliferation) and antagonism of thyroid receptor beta. Correlations performed using SAS 9.4; bolded samples represent significant (p<0.05) correlations, and the color depicts the strength and direction of the correlation. Darker colors represent a stronger correlation, blue coloration depicts positive correlations, and red depicts negative correlations.
Concentrations of each contaminant measured in dust were also assessed for their relationship with adipogenic and receptor bioactivities via individual regression models (rate ratio estimates used to determine the percent increase in bioactivities with each quartile increase in each individual contaminant concentration). Because dust samples included a mixture of contaminants, individual compound analyses are likely confounded by other exposures.
Nonetheless, these results are presented for comparison with mixtures analyses and previous literature. Concentrations of 20 chemicals were positively associated with triglyceride accumulation (Figure 5), with 8:2 FTOH exhibiting the greatest promoting effect. Percent triglyceride accumulation was predicted to increase by 46% for a one quartile increase in 8:2 FTOH (RR=1.46 and 95% CI: 1.21, 1.78; p<0.001) (Figure 5). In individual analyses, six compounds were significantly and negatively associated with pre-adipocyte proliferation, with retene exhibiting the greatest inhibitory effect. Ten compounds were positively associated with TRβ antagonism, with propyl paraben exhibiting the greatest effect.
Figure 5: House dust mixtures and triglyceride accumulation.
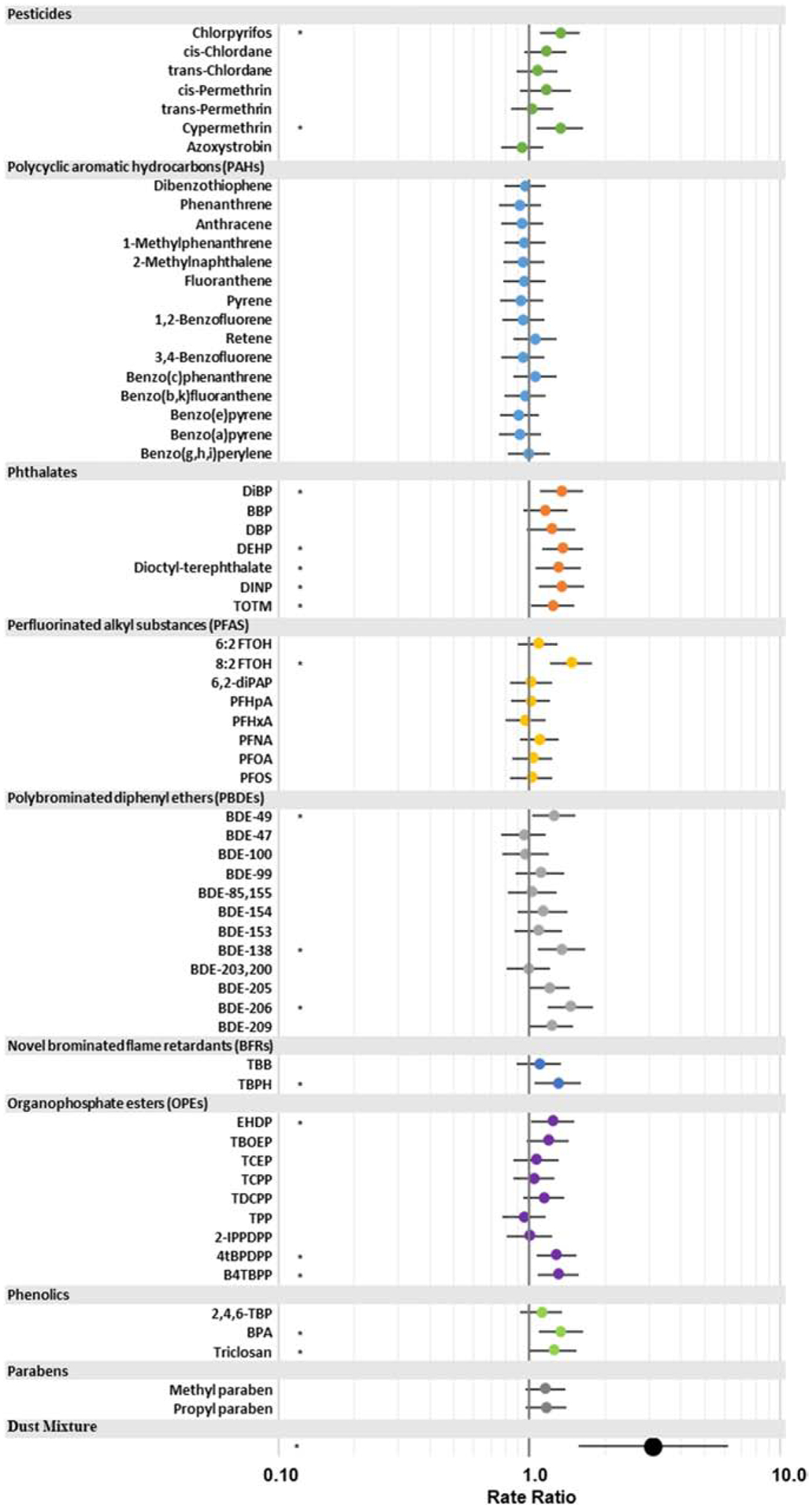
Quantile g-computation was performed with all frequently detected contaminants in dust and triglyceride accumulation. Analyses were first performed for each contaminant individually and then for all contaminants in mixture. Rate ratios estimates (and 95% confidence intervals) represent the expected change in percent triglyceride accumulation for a one quartile increase in all exposures. For example, one quartile in the contaminant mixtures corresponds to a 212 percent increase in the percent triglyceride accumulation of dust samples (RR=3.12; 95% CI: 1.58, 6.17; p<0.001). Different colors represent classes of contaminants and * denotes statistical significance at p<0.05.
Exploratory Mixtures Assessment.
Quantile based g-computation approach was used to evaluate associations between contaminants in dust and bioactivities. Total contaminants measured in dust were significantly associated with normalized triglyceride accumulation. Percent triglyceride accumulation was predicted to increase by 212% for a one quartile increase in all contaminants simultaneously (RR=3.12 and 95% confidence interval: 1.58, 6.17; p<0.001) (Figure 5). The gqcomp package provides a set of weights that describe the contribution of each contaminant to the overall effect estimate; 8:2 FTOH, PFNA, and 2-methylnaphthalene were the three highest weighting compounds in the positive direction, suggesting they may be important drivers of adipogenic activity. For proliferation and TRβ antagonism, we did not observe statistically significant associations with mixtures of contaminants in house dust (RR=0.96 and 1.12 and 95% CI: 0.67, 1.38 and 0.73, 1.69, respectively).
Discussion
These results demonstrate that complex mixtures of chemicals present in house dust induce adipogenic activity in vitro, at environmentally relevant concentrations. The majority of extracts induced significant effects at approximate concentrations of 100 μg/mL, or 20 μg/well. The EPA estimates that children ingest 60–100 mg of dust per day from indoor environments (Agency, 2017), substantiating the relevance of the low doses used in this study. A large proportion (80%) of dust extracts induced significant adipogenic activity via either triglyceride accumulation (65%) and/or pre-adipocyte proliferation (50%). Further, two thirds of extracts antagonized TRβ and one fifth acted as agonists for PPARγ. Notably, adipogenic activity (triglyceride accumulation) was significantly correlated with antagonism of TRβ, suggesting a contributory role for TR (supporting our previous findings (Kassotis et al., 2019)). More than 30 semi-volatile indoor contaminants from various chemical classes were significantly correlated with the extent of adipogenic activity exhibited by the household dust extracts. Several of these were also correlated with nuclear receptor bioactivities, suggesting that these pathways are potential contributory promoters of the metabolic outcomes induced by these environmental mixtures.
Concentrations of 65 contaminants were detected in in ≥75% of dust extracts, from diverse contaminant classes, and were generally similar to previous levels reported by other researchers. As our laboratory has reported previously (Phillips et al., 2018), OPEs were similar to or higher than previous studies, particularly relative to other cohorts in the United States. We have also previously reported concentrations of phthalates and plasticizers (Hammel et al., 2019), reporting similar levels and similar pattern of occurrence relative to other recent studies. Levels of pesticides were similar to or greater than concentrations (often greater detection frequencies) reported in previous studies (Colt et al., 2004; Rudel et al., 2003; Simcox et al., 1995). PFAS were present at similar or lower concentrations relative to those reported previously in diverse countries (Fraser et al., 2013; Karaskova et al., 2016; Winkens et al., 2018; Zhang et al., 2020). BFRs were similar to those reported in some previous studies (Johnson et al., 2013; Karlsson et al., 2007; Stapleton et al., 2008), though were one or two orders of magnitude lower than reported in certain other countries (Ali et al., 2011; Fromme et al., 2014; Kalachova et al., 2012; Stuart et al., 2008). PAHs were detected at concentrations between 2–10-fold higher than reported previously in California (Whitehead et al., 2011; Whitehead et al., 2013) and Nigeria (Sulong et al., 2019), and generally at equivalent or lower levels than concentrations reported in Italy (Yang et al., 2015) and Jordan (Maragkidou et al., 2016). Phenolics and parabens were generally present at equivalent concentrations to those reported previously across diverse countries (Fan et al., 2010; Wan-Li Ma et al., 2014), but greater than those reported in Vietnam (Tran et al., 2016) and China (Wang et al., 2012).
Only one previous study has assessed the adipogenic activity associated with household dust extracts, and though from our lab and the same geographic region, participants had notably different demographics and socioeconomic differences compared to the current study (Kassotis et al., 2019). Bioactivity levels of activity were quite similar across these cohorts, though >20% fewer samples exhibited significant pre-adipocyte proliferation in the current study. Our previous work evaluated dose responses in dust extracts, with five test concentrations and concentrations up to ~4500 μg/mL; in this study we tested a single concentration of each dust extract at concentrations normalized to approximately 100 μg/mL. It is possible that by utilizing a single concentration we have limited identification of samples that would exhibit significant bioactivity at greater concentrations, but the similarity of our metrics between these studies and the previous finding that pre-adipocyte proliferation was a more potent metric than triglyceride accumulation (Kassotis et al., 2019) suggests that this is likely to be minimal contribution (if tested concentration was the cause of lowered proliferation, we would expect lowered triglyceride accumulation as well).
While adipogenic activity induced by environmental samples has not been well-reported previously, several studies have reported nuclear receptor bioactivities in dust. Previous research by our laboratory measured TRβ antagonism in approximately 40% of dust extracts (Kollitz et al., 2018). Herein we reported activity for approximately twice this at the single concentration examined, potentially due to the greater dynamic range of the transient transfection reporter used here. Other researchers have assessed TR bioactivities in dust extracts, reporting antagonism from samples collected in both indoor and outdoor environments (Chou et al., 2015), but at considerably higher concentrations than we examined herein (>5 mg dust equivalence/mL), potentially due to the yeast bioassay utilized and the maximal concentration of T3 used for antagonism assessment (EC50 concentrations allow for much more sensitive detection of inhibition). Importantly, we did not assess receptor-specific TR inhibition herein, so it is possible that the antagonism reported here is (at least in part) non-specific; we did assess this previously (Kassotis et al., 2019), and reported that the TR antagonism from dust from a separate (though geographically similar) cohort was receptor-specific through ligand recovery and siRNA knock-down experiments (Kassotis et al., 2019). We reported low PPARγ agonism across dust extracts, which may be due to the concentrations of dust examined. Previous research from our laboratory reported much greater prevalence for PPARγ activation (Fang et al., 2015a; Fang et al., 2015b; Fang et al., 2015c), but generally at much higher concentrations than we examined herein. Other research evaluating a range of dust extracts from diverse countries reported minimal PPARγ activity and none from US samples (Suzuki et al., 2013), instead reporting more widespread PPARγ antagonist activities. This study utilized a stably transfected human construct in human bone cells, which may have contributed to differing responses. More research is needed to assess the nuclear receptor and adipogenic bioactivities associated with indoor dust samples.
We previously reported that antagonism of TR appeared to be a contributory causative mechanism promoting the adipogenic activities exhibited by dust samples (Kassotis et al., 2019). We also demonstrated a clear significant and positive correlation between the extent of TRβ antagonism and the extent of triglyceride accumulation by matched indoor house dust extracts. Previous research has delineated a clear role for TR antagonism in adipogenesis, purportedly through reciprocal regulation with PPARγ (Lu and Cheng, 2010). We previously reported that antagonism of TR was a particularly efficacious/potent adipogenic pathway (Kassotis et al., 2017b), and TR antagonism of dust may disrupt metabolic health at least in part through dysregulation of thyroid hormones (Kollitz et al., 2018). Thyroid hormones are generally considered anti-obesogenic, and hypothyroid-induced adiposity can be reduced through thyroid hormone supplementation (Bryzgalova et al., 2008). Both TRα/β are expressed in adipocytes, with TRα playing a larger role in regulating thermogenesis and TRβ in cholesterol metabolism, lipogenesis, pre-adipocyte proliferation, and differentiation (Obregon, 2008). We also evaluated a potential contributory role for PPARγ activation and reported no significant association with adipogenic activity, though the fact that only a small number of samples were active for PPARγ agonism may have hindered this assessment. There are hundreds of chemicals reported in house dust that might contribute to numerous receptor bioactivities, many that have been previously reported by other researchers (Chou et al., 2015; Kollitz et al., 2018; Suzuki et al., 2007; Suzuki et al., 2013). Notably, we have not evaluated GR activation or AR inhibition, both of which are adipogenic mechanisms that have been reported previously in house dust extracts (Chou et al., 2015; Suzuki et al., 2013), nor do we know of any research evaluating the retinoid X receptor (RXR), farnesoid X receptor (FXR), liver X receptor (LXR), or other adipogenic pathways in dust. It’s important to note that there are also known adipogenic impacts of heavy metals (Lee et al., 2012; Martini et al., 2014; Martini et al., 2018), and other non-nuclear receptor mediated adipogenic mechanisms that could be involved in these dust-mediated effects (Lee et al., 2009; Lian et al., 2018; Luz et al., 2018; Phillips and Stapleton, 2019; Vankoningsloo et al., 2006; Vankoningsloo et al., 2005).
We also reported that concentrations of 26 chemicals were significantly correlated with triglyceride accumulation and/or pre-adipocyte proliferation. We previously reported that concentrations of 12 BFRs and OPEs in dust extracts were all positively correlated with triglyceride accumulation but none with pre-adipocyte proliferation (Kassotis et al., 2019). Despite these significant correlations, we suspected that it was likely mixture effects or co-occurring causal contaminants promoting these effects, as most of these chemicals were independently inactive in 3T3-L1 cells. In our current study, TBPH was again significantly and positively correlated with triglyceride accumulation, substantiating that association, and we have demonstrated that TBPH is active in promoting adipogenesis (Kassotis et al., 2017a). The other 11 previously examined BFRs and OPEs were not correlated in this cohort, though BDE-49 was correlated; we previously reported a correlation for BDE-47 and adipogenic activity (Kamstra et al., 2014; Tung et al., 2014), though BDE-49 has not been tested to the best of our knowledge.
Given the non-overlapping diversity of chemicals associated herein, this bolsters our previous hypothesis that complex mixtures might be promoting these adipogenic endpoints. Among the chemicals with the strongest correlations in the research presented herein: chlorpyrifos has been previously demonstrated active both in vitro (Kassotis et al., 2017a; Taxvig et al., 2012) and in vivo (Meggs and Brewer, 2007); 8:2 FTOH has been demonstrated inactive in our hands previously (Kassotis et al., 2017a), but can be metabolized to perfluorooctanoic acid (PFOA) (Butt et al., 2014), which subsequently can promote adipogenic activity in vitro (Watkins et al., 2015) and in vivo (Hines et al., 2009); and both DEHP and cypermethrin have been previously demonstrated active in vitro (Kassotis et al., 2017a) and in vivo (Jin et al., 2014; Schmidt et al., 2012). Additionally, chemical metabolism in these cells has not been definitively characterized, though it is possible that some chemicals may be metabolized to varying degrees throughout the differentiation process, and we cannot rule out the possibility that is playing a role in our observations.
Here we also evaluated relationships between chemicals in dust and bioactivities using an exploratory g-computation approach. And as a comparison, logistic regression analyses of single chemical measure in house dust were performed. Seventeen individual contaminants were associated with triglyceride accumulation using single chemical logistic regression modes, eight chemicals were positively and five negatively associated with pre-adipocyte proliferation, and eleven chemicals were positively associated with TRβ antagonism; however, these relationships may be confounded by co-exposures as described above. Contaminant mixtures were strongly and positively associated with triglyceride accumulation, and the magnitude of the effect (RR = 3.12 and 95% confidence interval: 1.58, 6.17) was larger than that for any individual chemical.
We did not observe significant associations with mixtures of contaminants and pre-adipocyte proliferation or TRβ antagonism. G-computation also provides weights describing the contribution of each individual contaminant to the overall mixture effect. For triglyceride accumulation, 8:2 FTOH, PFNA, and 2-methylnaphthalene were the highest weighted compounds for promoting triglyceride accumulation, suggesting they might be important contributors to the adipogenic activity of the total mixtures. Individually, PFNA has been demonstrated to promote triglyceride accumulation (Watkins et al., 2015), though 8:2 FTOH has been found to be inactive (Kassotis et al., 2017a). To the best of our knowledge, 2-methylnaphthalene has not been directly tested, but the sum of naphthalene metabolites was reported to be associated with BMI z-scores and obesity in children in the NHANES cohort (Scinicariello and Buser, 2014). However, chemicals can respond differently in mixtures. Several studies have demonstrated the “something from nothing” and “a lot from a little” effects in vitro (Rajapakse et al., 2002; Silva et al., 2002) and in vivo (Runnalls et al., 2015; Thrupp et al., 2018), which suggests the potential for synergistic effects of complex mixtures. Other work reported TRβ antagonism for a specific PCB congener only when present in a mixture with others (Giera et al., 2011), demonstrating the complexity of even simple mixtures. House dust contains thousands of chemicals (Ferguson et al., 2015; Hilton et al., 2010), so it is likely that there are other active constituents that have yet to be identified and measured, and future research should evaluate other potential contributory contaminants via non-target chemical analyses.
This study is the first to perform a comprehensive targeted analytical assessment of chemicals in indoor house dust extracts that are likely to be contributing to mixture-induced bioactivities (either adipogenic activity or activation/inhibition of specific nuclear receptors). We report that a large proportion of household dust appear to be capable of inducing adipogenic activity at low, environmentally relevant concentrations, and that these adipogenic activities seem to be due, in part at least, by inhibition of the thyroid receptor. Our exploratory g-computation assessment demonstrates that while several individual chemicals measured herein were strongly associated with the adipogenic and nuclear receptor activities, accounting for mixtures of compounds in dust provides a more complete picture of the association with bioactivity, at least for triglyceride accumulation. While this study consisted of a heterogeneous study population representing the central NC area, future research should attempt to reproduce these effects utilizing dust from other regions to assess reproducibility. Future research should also attempt to substantiate these findings using other in vitro models such as human mesenchymal cell models (Foley et al., 2015; Foley et al., 2017; Janderova et al., 2003) (which may provide more relevance to human health following rigorous validation) and to characterize potential metabolic disruption in vivo following exposure to complex mixtures, or even house dust extracts, as has been performed previously for other environmental matrices previously (Biasiotto et al., 2016; Galus et al., 2013).
Supplementary Material
Highlights:
Adipogenic and nuclear receptor in vitro activity were tested in house dust extracts (HDE)
120+ organic contaminants were quantified in HDEs
HDE adipogenic activity was positively correlated with thyroid receptor antagonism
Contaminant mixtures were positively associated with triglyceride accumulation
Contaminant mixtures had stronger associations than individual chemicals
Acknowledgements:
We wish to thank Albert Chen, Amelia Lorenzo, and Stephanie Hammel for their help with home data collection. Dr. Alexander Keil provided significant statistical support for the g computation mixtures analyses used herein. We also thank our participants for opening their homes to our study team and helping us gain a better understanding of exposures to SVOCs.
Funding: Project supported by grants [R01 ES016099 to HMS; K99 ES030405 to CDK; R01ES028800 and R01ES027813 to TFW] and a fellowship [F32 ES027320 to CDK] from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests declaration: The authors have nothing to disclose.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agency UEP. Exposure Factors Handbook Chapter 5 (Update): Soil and Dust Ingestion In: Agency UEP, editor. 2011 Edition (Final), 2017 update, Washington, DC, 2017. [Google Scholar]
- Ali N, Harrad S, Goosey E, Neels H, Covaci A. “Novel” brominated flame retardants in Belgian and UK indoor dust: implications for human exposure. Chemosphere 2011; 83: 1360–5. [DOI] [PubMed] [Google Scholar]
- Biasiotto G, Zanella I, Masserdotti A, Pedrazzani R, Papa M, Caimi L, et al. Municipal wastewater affects adipose deposition in male mice and increases 3T3-L1 cell differentiation. Toxicol Appl Pharmacol 2016; 297: 32–40. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, et al. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem Mol Biol 2008; 111: 262–7. [DOI] [PubMed] [Google Scholar]
- Butt CM, Muir DC, Mabury SA. Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: a review. Environ Toxicol Chem 2014; 33: 243–67. [DOI] [PubMed] [Google Scholar]
- Chou P-H, Lee C-H, Ko F-C, Lin Y-J, Kawanishi M, Yagi T, et al. Detection of Hormone-Like and Genotoxic Activities in Indoor Dust from Taiwan Using a Battery of in Vitro Bioassays. Aerosol and Air Quality Research 2015; 15: 1412–1421. [Google Scholar]
- Colt JS, Lubin J, Camann D, Davis S, Cerhan J, Severson RK, et al. Comparison of pesticide levels in carpet dust and self-reported pest treatment practices in four US sites. J Expo Anal Environ Epidemiol 2004; 14: 74–83. [DOI] [PubMed] [Google Scholar]
- Cooper EM, Rushing R, Hoffman K, Phillips AL, Hammel SC, Zylka MJ, et al. Strobilurin fungicides in house dust: is wallboard a source? Journal of Exposure Science and Environmental Epidemiology 2019; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, Dewailly E, Muckle G, Ayotte P. Time trends of persistent organic pollutants and heavy metals in umbilical cord blood of Inuit infants born in Nunavik (Quebec, Canada) between 1994 and 2001. Environ Health Perspect 2003; 111: 1660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009; 30: 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SL, Gaitens JM, Jacobs DE, Strauss W, Nagaraja J, Pivetz T, et al. Exposure of U.S. children to residential dust lead, 1999–2004: II. The contribution of lead-contaminated dust to children’s blood lead levels. Environ Health Perspect 2009; 117: 468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Kubwabo C, Rasmussen P, Jones-Otazo H. Simultaneous quantitation of parabens, triclosan, and methyl triclosan in indoor house dust using solid phase extraction and gas chromatography-mass spectrometry. J Environ Monit 2010; 12: 1891–7. [DOI] [PubMed] [Google Scholar]
- Fang M, Webster TF, Ferguson PL, Stapleton HM. Characterizing the peroxisome proliferator-activated receptor (PPARgamma) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ Health Perspect 2015a; 123: 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Webster TF, Stapleton HM. Activation of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) by Semi-Volatile Compounds (SVOCs) and Chemical Mixtures in Indoor Dust. Environ Sci Technol 2015b; 49: 10057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Webster TF, Stapleton HM. Effect-Directed Analysis of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) Ligands in Indoor Dust. Environ Sci Technol 2015c; 49: 10065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson PL, Vogler B, Stapleton HM. Non-targeted analysis to assess human exposure to semi-volatile organic contaminants in the indoor environment Proceedings of the 63rd ASMS Conference on Mass Spectrometry and Allied Topics, St. Louis, MO, 2015. [Google Scholar]
- Foley B, Clewell RA, Deisenroth C. Development of a Human Adipose-Derived Stem Cell Model for Characterization of Chemical Modulation of Adipogenesis. Applied in Vitro Toxicology 2015; 1: 66–78. [Google Scholar]
- Foley B, Doheny DL, Black MB, Pendse SN, Wetmore BA, Clewell RA, et al. Screening ToxCast Prioritized Chemicals for PPARG Function in a Human Adipose-Derived Stem Cell Model of Adipogenesis. Toxicol Sci 2017; 155: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, et al. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ Sci Technol 2012; 46: 1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, et al. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environ Int 2013; 60: 128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Hilger B, Kopp E, Miserok M, Volkel W. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int 2014; 64: 61–8. [DOI] [PubMed] [Google Scholar]
- Galus M, Jeyaranjaan J, Smith E, Li H, Metcalfe C, Wilson JY. Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat Toxicol 2013; 132–133: 212–22. [DOI] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology 2011; 152: 2909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine Reviews 2015; 36: E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Levasseur JL, Hoffman K, Phillips AL, Lorenzo AM, Calafat AM, et al. Children’s exposure to phthalates and non-phthalate plasticizers in the home: The TESIE study. Environ Int 2019; 132: 105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DC, Jones RS, Sjodin A. A method for rapid, non-targeted screening for environmental contaminants in household dust. J Chromatogr A 2010; 1217: 6851–6. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 2009; 304: 97–105. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ Health Perspect 2015; 123: 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Hammel SC, Phillips AL, Lorenzo AM, Chen A, Calafat AM, et al. Biomarkers of exposure to SVOCs in children and their demographic associations: The TESIE Study. Environ Int 2018; 119: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, et al. Exposure to flame retardant chemicals and the occurrence and severity of papillary thyroid cancer. Environment International 2017; 107: 235–242. [DOI] [PubMed] [Google Scholar]
- Houlihan J, Kropp T, Wiles R, Gray S, Campbell C. BodyBurden: The Pollution in Newborns. Environmental Working Group, 2005. [Google Scholar]
- Janderova L, McNeil M, Murrell AN, Mynatt RL, Smith SR. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes Res 2003; 11: 65–74. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lin X, Miao W, Wu T, Shen H, Chen S, et al. Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism. Toxicol Lett 2014; 225: 392–400. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Mukherjee B, Hauser R, Meeker JD. Associations between brominated flame retardants in house dust and hormone levels in men. Sci Total Environ 2013; 445–446: 177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kademoglou K, Xu F, Padilla-Sanchez JA, Haug LS, Covaci A, Collins CD. Legacy and alternative flame retardants in Norwegian and UK indoor environment: Implications of human exposure via dust ingestion. Environ Int 2017; 102: 48–56. [DOI] [PubMed] [Google Scholar]
- Kalachova K, Hradkova P, Lankova D, Hajslova J, Pulkrabova J. Occurrence of brominated flame retardants in household and car dust from the Czech Republic. Sci Total Environ 2012; 441: 182–93. [DOI] [PubMed] [Google Scholar]
- Kamstra JH, Hruba E, Blumberg B, Janesick A, Mandrup S, Hamers T, et al. Transcriptional and epigenetic mechanisms underlying enhanced in vitro adipocyte differentiation by the brominated flame retardant BDE-47. Environ Sci Technol 2014; 48: 4110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaskova P, Venier M, Melymuk L, Becanova J, Vojta S, Prokes R, et al. Perfluorinated alkyl substances (PFASs) in household dust in Central Europe and North America. Environ Int 2016; 94: 315–324. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Julander A, van Bavel B, Hardell L. Levels of brominated flame retardants in blood in relation to levels in household air and dust. Environ Int 2007; 33: 62–9. [DOI] [PubMed] [Google Scholar]
- Kassotis CD, Hoffman K, Stapleton HM. Characterization of Adipogenic Activity of Semi-volatile Indoor Contaminants and House Dust. Environ Sci Technol 2017a; In 51: 8735–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Kollitz EM, Hoffman K, Sosa JA, Stapleton HM. Thyroid Receptor Antagonism as a Contributory Mechanism for Adipogenesis Induced by Environmental Mixtures in 3T3-L1 Cells. Science of the Total Environment 2019; 666: 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Masse L, Kim S, Schlezinger JJ, Webster TF, Stapleton HM. Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling. Scientific Reports 2017b; 7: 42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Tillitt DE, Lin CH, McElroy JA, Nagel SC. Endocrine-Disrupting Chemicals and Oil and Natural Gas Operations: Potential Environmental Contamination and Recommendations to Assess Complex Environmental Mixtures. Environ Health Perspect 2016; 124: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 2020; 128: 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollitz EM, Kassotis CD, Hoffman K, Ferguson PL, Sosa JA, Stapleton HM. Chemical mixtures isolated from house dust disrupt thyroid receptor β (TRβ) signaling. Environ Sci Technol 2018; 52: 11857–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Mattison D, McCally M, Garg A. Chemical contaminants in breast milk and their impacts on children’s health: an overview. Environ Health Perspect 2002; 110: A313–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Moon JY, Yoo BS. Cadmium inhibits the differentiation of 3T3-L1 preadipocyte through the C/EBPalpha and PPARgamma pathways. Drug Chem Toxicol 2012; 35: 225–31. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 2009; 284: 10601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor y (PPARy). The Journal of Biological Chemistry 1995; 270: 12953–12956. [DOI] [PubMed] [Google Scholar]
- Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol 2011; 127: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J, Nelson R, Lehner R. Carboxylesterases in lipid metabolism: from mouse to human. Protein Cell 2018; 9: 178–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Cheng SY. Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signaling with peroxisome proliferator-activated receptors. J Mol Endocrinol 2010; 44: 143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz AL, Kassotis CD, Stapleton HM, Meyer JN. The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARgamma activation, in 3T3-L1 cells. Toxicology 2018; 393: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Nourizadeh-Lillabadi R, Almaas C, Stavik B, Berg V, Skare JU, et al. Natural mixtures of persistent organic pollutants (POP) increase weight gain, advance puberty, and induce changes in gene expression associated with steroid hormones and obesity in female zebrafish. J Toxicol Environ Health A 2010; 73: 1032–57. [DOI] [PubMed] [Google Scholar]
- Maragkidou A, Ma Y, Jaghbeir O, Faouri D, Harrad S, Al-Hunaiti A, et al. PAHs in Household Floor Dust Collected in Amman, Jordan. Journal of Chemical Engineering & Process Technology 2016; 7: e1000292. [Google Scholar]
- Martini CN, Brandani JN, Gabrielli M, Vila Mdel C. Effect of hexavalent chromium on proliferation and differentiation to adipocytes of 3T3-L1 fibroblasts. Toxicol In Vitro 2014; 28: 700–6. [DOI] [PubMed] [Google Scholar]
- Martini CN, Gabrielli M, Bonifacino G, Codesido MM, Vila MDC. Lead enhancement of 3T3-L1 fibroblasts differentiation to adipocytes involves ERK, C/EBPbeta and PPARgamma activation. Mol Cell Biochem 2018; 437: 37–44. [DOI] [PubMed] [Google Scholar]
- Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J Med Toxicol 2007; 3: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, et al. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ Sci Technol 2016; 50: 10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol 2015; 49: 10466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon M-J. Thyroid Hormone and Adipocyte Differentiation. Thyroid 2008; 18: 185–195. [DOI] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, et al. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ Int 2018; 116: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Stapleton HM. Inhibition of Human Liver Carboxylesterase (hCE1) by Organophosphate Ester Flame Retardants and Plasticizers: Implications for Pharmacotherapy. Toxicol Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect 2002; 110: 917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen PE, Levesque C, Chenier M, Gardner HD, Jones-Otazo H, Petrovic S. Canadian House Dust Study: population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. Sci Total Environ 2013; 443: 520–9. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 2003; 37: 4543–53. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Dodson RE, Perovich LJ, Morello-Frosch R, Camann DE, Zuniga MM, et al. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ Sci Technol 2010; 44: 6583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnalls TJ, Beresford N, Kugathas S, Margiotta-Casaluci L, Scholze M, Scott AP, et al. From single chemicals to mixtures - Reproductive effects of levonorgestrel and ethinylestradiol on the fathead minnow. Aquat Toxicol 2015; 169: 152–167. [DOI] [PubMed] [Google Scholar]
- Schilmann A, Lacasana M, Blanco-Munoz J, Aguilar-Garduno C, Salinas-Rodriguez A, Flores-Aldana M, et al. Identifying pesticide use patterns among flower growers to assess occupational exposure to mixtures. Occup Environ Med 2010; 67: 323–9. [DOI] [PubMed] [Google Scholar]
- Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect 2012; 120: 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001–2006). Environ Health Perspect 2014; 122: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimandi M, Lemaire G, Pillon A, Perrin A, Carlavan I, Voegel JJ, et al. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal Biochem 2005; 344: 8–15. [DOI] [PubMed] [Google Scholar]
- Shao X, Wang M, Wei X, Deng S, Fu N, Peng Q, et al. Peroxisome Proliferator-Activated Receptor-gamma: Master Regulator of Adipogenesis and Obesity. Curr Stem Cell Res Ther 2016; 11: 282–9. [DOI] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”--eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol 2002; 36: 1751–6. [DOI] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ Health Perspect 1995; 103: 1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47: 507–14. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, et al. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol 2008; 42: 6910–6. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ Sci Technol 2005; 39: 925–31. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect 2012; 120: 1049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Harner T, Shoeib M, Keller JM, Schantz MM, Leigh SD, et al. Determination of polybrominated diphenyl ethers in indoor dust standard reference materials. Anal Bioanal Chem 2006; 384: 791–800. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol 2009; 43: 7490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014; 116: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart H, Ibarra C, Abdallah MA, Boon R, Neels H, Covaci A. Concentrations of brominated flame retardants in dust from United Kingdom cars, homes, and offices: causes of variability and implications for human exposure. Environ Int 2008; 34: 1170–5. [DOI] [PubMed] [Google Scholar]
- Sulong NA, Latif MT, Sahani M, Khan MF, Fadzil MF, Tahir NM, et al. Distribution, sources and potential health risks of polycyclic aromatic hydrocarbons (PAHs) in PM2.5 collected during different monsoon seasons and haze episode in Kuala Lumpur. Chemosphere 2019; 219: 1–14. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Takigami H, Nose K, Takahashi S, Asari M, Sakai S. Dioxin-like and transthyretin-binding compounds in indoor dusts collected from Japan: average daily dose and possible implications for children. Environ Sci Technol 2007; 41: 1487–93. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Tue NM, Malarvannan G, Sudaryanto A, Takahashi S, Tanabe S, et al. Similarities in the endocrine-disrupting potencies of indoor dust and flame retardants by using human osteosarcoma (U2OS) cell-based reporter gene assays. Environ Sci Technol 2013; 47: 2898–908. [DOI] [PubMed] [Google Scholar]
- Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol 2012; 361: 106–15. [DOI] [PubMed] [Google Scholar]
- Thrupp TJ, Runnalls TJ, Scholze M, Kugathas S, Kortenkamp A, Sumpter JP. The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Science of the Total Environment 2018; 619–620: 1482–1492. [DOI] [PubMed] [Google Scholar]
- Tran TM, Minh TB, Kumosani TA, Kannan K. Occurrence of phthalate diesters (phthalates), p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE) and their derivatives in indoor dust from Vietnam: Implications for exposure. Chemosphere 2016; 144: 1553–9. [DOI] [PubMed] [Google Scholar]
- Tung EW, Boudreau A, Wade MG, Atlas E. Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells. PLoS One 2014; 9: e94583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 2012; 89: 292–300. [DOI] [PubMed] [Google Scholar]
- Vankoningsloo S, De Pauw A, Houbion A, Tejerina S, Demazy C, de Longueville F, et al. CREB activation induced by mitochondrial dysfunction triggers triglyceride accumulation in 3T3-L1 preadipocytes. J Cell Sci 2006; 119: 1266–82. [DOI] [PubMed] [Google Scholar]
- Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P, et al. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res 2005; 46: 1133–49. [DOI] [PubMed] [Google Scholar]
- Wan-Li Ma, Subedi Bikram, Kannan K. The Occurrence of Bisphenol A, Phthalates, Parabens and Other Environmental Phenolic Compounds in House Dust: A Review. Current Organic Chemistry 2014; 18: 2182–2199. [Google Scholar]
- Wang L, Liao C, Liu F, Wu Q, Guo Y, Moon HB, et al. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environ Sci Technol 2012; 46: 11584–93. [DOI] [PubMed] [Google Scholar]
- Watkins AM, Wood CR, Lin MT, Abbott BD. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 2015; 400: 90–101. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, et al. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ Health Perspect 2011; 119: 1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, et al. Impact of dust from multiple microenvironments and diet on PentaBDE body burden. Environ Sci Technol 2012; 46: 1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Webster TF. Associations between PBDEs in office air, dust, and surface wipes. Environ Int 2013; 59: 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead T, Metayer C, Gunier RB, Ward MH, Nishioka MG, Buffler P, et al. Determinants of polycyclic aromatic hydrocarbon levels in house dust. J Expo Sci Environ Epidemiol 2011; 21: 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Metayer C, Petreas M, Does M, Buffler PA, Rappaport SM. Polycyclic aromatic hydrocarbons in residential dust: sources of variability. Environ Health Perspect 2013; 121: 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkens K, Giovanoulis G, Koponen J, Vestergren R, Berger U, Karvonen AM, et al. Perfluoroalkyl acids and their precursors in floor dust of children’s bedrooms - Implications for indoor exposure. Environ Int 2018; 119: 493–502. [DOI] [PubMed] [Google Scholar]
- Yang Q, Chen H, Li B. Polycyclic aromatic hydrocarbons (PAHs) in indoor dusts of Guizhou, southwest of China: status, sources and potential human health risk. PLoS One 2015; 10: e0118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, He Y, Huang Y, Hong D, Yao Y, Wang L, et al. Novel and legacy poly- and perfluoroalkyl substances (PFASs) in indoor dust from urban, industrial, and e-waste dismantling areas: The emergence of PFAS alternatives in China. Environ Pollut 2020; 263: 114461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


