Abstract
N-methyl-D-aspartate receptors (NMDARs) are glutamate-gated ion channels essential for glutamatergic transmission and plasticity. NMDARs are inhibited by acute ethanol and undergo brain region specific adaptations after chronic alcohol exposure. In previous studies, we reported that knock-in mice expressing ethanol-insensitive GluN1 or GluN2A NMDAR subunits display altered behavioral responses to acute ethanol and genotype-dependent changes in drinking using protocols that do not produce dependence. A key unanswered question is whether the intrinsic ethanol sensitivity of NMDARs also plays a role in determining behavioral adaptations that accompany the development of dependence. To test this, we exposed mice to repeated cycles of chronic intermittent ethanol (CIE) vapor known to produce a robust escalation in ethanol consumption and preference. As expected, wild-type mice showed a significant increase from baseline in ethanol consumption and preference after each of the four weekly CIE cycles. In contrast, ethanol consumption in male GluN2A(A825W) mice was unchanged following cycles 1, 2 and 4 cycles of CIE with a modest increase appearing after cycle 3. Wild-type and GluN2A(A825W) female mice did not show a clear or consistent escalation in ethanol consumption or preference following CIE treatment. In male GluN1(F639A) mice, the increase in ethanol consumption observed with their wild-type littermates was delayed until later cycles of exposure. These results suggest that the acute ethanol sensitivity of NMDARs especially those containing the GluN2A subunit may be a critical factor in the in the escalation of ethanol intake in alcohol dependence.
Keywords: alcohol dependence, glutamate, knock-in mutation, ethanol sensitivity, two-bottle choice
Introduction
As key players in glutamatergic synaptic neuronal transmission and plasticity, ethanol-sensitive NMDARs are hypothesized to play a significant role in the pathophysiology of alcohol use disorders. For example, NMDARs have been shown to mediate the rewarding effects of ethanol and influence the severity of withdrawal symptoms, craving, and relapse in ethanol dependent subjects (Barker et al. 2017; Becker and Lopez 2016; Biala and Kotlinska 1999; Boyce-Rustay and Holmes 2006a; Daut et al. 2015; Jury et al. 2018; Kotlinska et al. 2004; Ron and Wang 2009). NMDAR ion channel activity is suppressed during acute ethanol intoxication and following repeated exposures, neurons show compensatory and subunit-selective upregulation of NMDAR expression and activity. These include changes in synaptic localization, expression and function that are thought to contribute to the escalations in drinking and cognitive deficits seen in alcohol-dependent subjects. (Carpenter-Hyland and Chandler 2007; Carpenter-Hyland et al. 2004). The NMDAR-mediated enhancement in glutamatergic transmission following repeated exposures to alcohol is proposed to contribute to CNS hyperexcitability and excitotoxicity, and it is thought to play an important role in the transition to alcohol dependence (reviewed in (Fritz et al. 2019)). However, it remains unclear whether the intrinsic ethanol sensitivity of NMDARs contributes to the magnitude of such changes or if these adaptations result from alcohol’s actions on other signaling processes that then drive changes in NMDAR expression and/or function.
In humans, enhanced ethanol consumption is a hallmark of the transition from social use of alcohol to that associated with alcohol dependence (American Psychiatric Association. 2013). In animals, this behavior is consistently recapitulated in the chronic intermittent ethanol vapor (CIE) exposure model (Becker and Lopez 2016) in which mice or rats are repeatedly exposed to intoxicating amounts of ethanol vapor for sustained periods of time. Interestingly, previous studies report that mice lacking the GluN2A NMDAR subunit (GluN2A KO) do not show escalations in drinking after CIE exposure and fail to develop tolerance to ethanol-induced ataxia (Daut et al. 2015; Jury et al. 2018). However, these studies are compromised by adaptations in neuronal signaling that follow germ-line deletion of GluN2A subunits such as enhanced dopaminergic and serotonergic tone (Miyamoto et al. 2001) that may influence alcohol-related behaviors.
Previous work from our laboratory and others revealed ethanol-sensitive sites within specific transmembrane domains of GluN1 and GluN2 subunits involved in channel gating and within sub-domains important for channel function. Specific knock-in mutations at these sites produces a significant rightward shift in the ethanol dose-response of these receptors rendering them essentially insensitive to concentrations of alcohol associated with drinking (Ren et al. 2017; Ronald et al. 2001). To minimize complications from compensatory adaptations associated with gene deletions, we developed mice with single amino acid replacements in transmembrane (TM) 1 and 4 domains of the GluN1 (F639A) and GluN2A (A825W) subunits, respectively, that have been shown to reduce the ethanol inhibition of NMDARs (Honse et al. 2004; Smothers and Woodward 2016; Xu et al. 2015). We have recently reported that these mice express ethanol-resistant NMDAR-mediated currents with otherwise normal functional properties (e.g. amplitude, rise/decay time) and show selective alterations in ethanol-induced behaviors (den Hartog et al. 2013; den Hartog et al. 2017; Zamudio et al. 2020; Zamudio-Bulcock et al. 2018).
In the present study we used these mice to test whether the escalation in drinking that develops with alcohol dependence is mediated by the intrinsic ethanol sensitivity of NMDARs.
Materials and Methods
Subjects
Adult male and female wild-type and homozygous GluN2A(A825W) mice on a C57BL/6J background (9 to 42 weeks old, average age 20.5 ± 8.2 weeks), and male wild-type and heterozygous GluN1(F639A) mice (17 to 38 week old, average age 22.8 ± 1 weeks) on a mixed C57BL/6J x 129Sv/SvJ background were used in these studies. Mice were generated by Het × Het breeding as described previously (den Hartog et al. 2013; Zamudio et al. 2020). The GluN2A(A825W) mice had an alanine to tryptophan replacement (A to W) at position 825 in the TM4 domain of the GluN2A subunit (Figure 1A). The GluN1(F639A) mice had a phenylalanine to alanine replacement (F to A) at position 639 in the TM3 domain of the GluN1 subunit (Figure 1A). As previously described (den Hartog et al. 2013), mice homozygous for the GluN1(F639A) mutation were not viable post-natally and all experiments with GluN1(F639A) mice used heterozygotes. After weaning, mice were group housed until adulthood with ad libitum access to rodent chow and water with 12-h light/dark cycles (lights off at 9:00 AM). Mice were genotyped by polymerase chain reaction from tail derived DNA. For GluN2A(A825W) genotyping, polymerase chain reactions spanning exon 11 included forward (GCCAAAGGCCAGCAAAGCTCAAGA) and reverse (AACTGCCCTGTGTTGTTCTGCACCT) primers with subsequent Pst1 digestion. For GluN1(F639A) genotyping, primers 5′-TTC ACA GAA GTG CGA TCT GG-3′ and 5′-AGG GGA GGC AAC ACT GTG GAC-3′ amplified a 466-base pair fragment from the wild-type allele; and primers 5′-CTT GGG TGG AGA GGC TAT TC-3′ and 5′-AGG TGA GAT GAC AGG AGA TC-3′ amplified a 280-base pair fragment from the knock-in allele. All experiments were approved by the MUSC Institutional Animal Care and Use Committees and conformed to NIH guidelines for the use of animals in biomedical research.
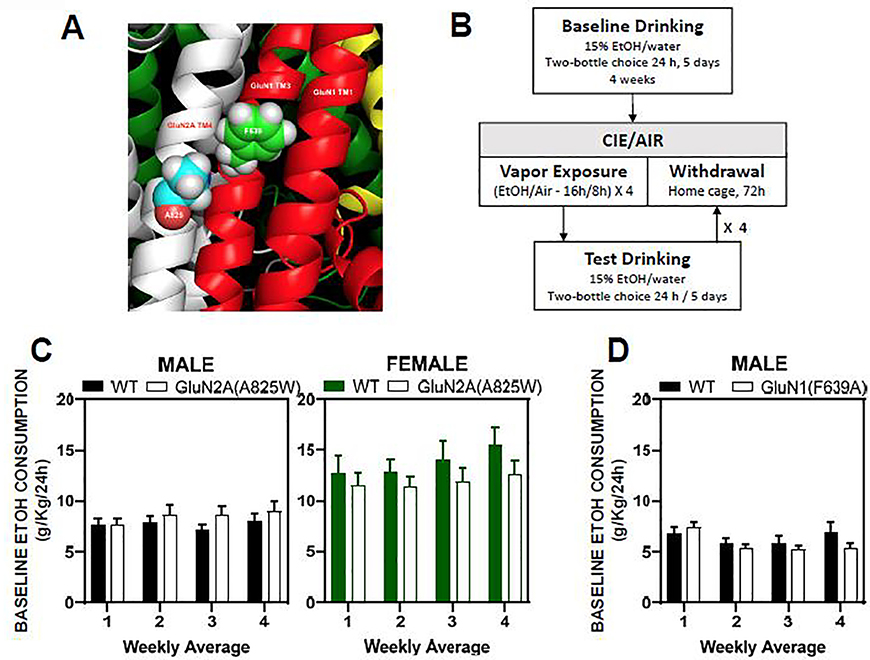
Figure 1. NMDAR mutant sites and protocol for chronic alcohol exposure and drinking.
(A) Locations of single amino acid replacement sites (A, alanine; F, phenylalanine) on transmembrane domains 3 and 4 of GluN1 (red) and GluN2A (white) subunits, respectively. (B) Schematic diagram of ethanol drinking and CIE exposure protocol. Following four weeks of baseline drinking, mice underwent repeated CIE exposures interleaved with weekly drinking tests. (C, D) Summary plots show weekly averages of 24 hr ethanol consumption (mean ± SEM) over the four week baseline drinking period for male WT (N=15) and GluN2A(A825W) (N=16); female WT (N=10) and GluN2A(A825W) (N=12) mice (C) and male WT (N=10) and GluN1(F639A) (N=10) mice (D).
Two-Bottle Choice continuous access drinking paradigm
Prior to beginning the drinking studies, mice were separated by genotype and singly housed and given ad libitum access to food and water and maintained on a reverse light-dark cycle (lights off at 09:00 AM). Two hours after the start of the dark cycle, home cage water bottles were replaced with two drinking bottles containing either ethanol (15% v/v with water) or water and their locations were alternated every 24h to control for individual side preference. Mice were weighed once a week prior to each drinking session and drinking bottles were weighed immediately before the drinking sessions, and every 24 h thereafter for 5 consecutive days (Mon-Fri). The difference in weight was converted to g/kg consumed. At all other times, mice had free access to home cage water bottles. This drinking paradigm was used during baseline drinking (4 weeks) and test drinking sessions interleaved with CIE exposures.
CIE exposure
After 4-weeks of baseline drinking, cohorts of mice, matched for drinking amounts, were assigned to Air or CIE groups. Mice were treated with 4 cycles of CIE vapor exposure or air in their homecage, and each cycle was interleaved with a 5-day test drinking session, as shown in Figure 1B. Each cycle consisted of daily exposure to ethanol vapor or air for 16 hours followed by 8 hours of withdrawal. This was repeated each day for four consecutive days followed by 3 days of abstinence before beginning a test drinking session. For CIE exposures, ethanol (95%) was volatized by passing air through a submerged air stone, and the resulting vapor was mixed with fresh air and delivered to Plexiglas inhalation chambers (5 L/min) to maintain consistent ethanol concentrations between 17 and 21 mg/L of air in the chamber to yield a blood ethanol concentration (BEC) of approximately 200 milligrams of ethanol per deciliter of blood (mg/dL). BEC values were determined with an Analox Instrument analyzer (Lunenburg, MA) from blood samples taken from during each CIE cycle. Prior to entry into vapor chambers, CIE mice were injected intraperitoneally (20 mL/kg of body weight) with ethanol (1.6 g/kg; 8%w/v) and the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) to maintain stable blood ethanol levels. Air control mice were injected with saline and pyrazole and received similar daily handling as CIE exposed mice.
Statistics
Data were analyzed by Prism 8 software (GraphPad, San Diego, CA) using repeated measures ANOVA or Mixed-effects analysis (in cases of missing data points) followed by post-hoc comparisons with the p value corrected for multiple tests where appropriate.
Results
Voluntary ethanol consumption is not altered in GluN2A(A825W) and GluN1(F639A) mice
Male and female GluN2A(A825W) mice and male GluN1(F639A) mice underwent four weeks of voluntary ethanol (15%) drinking using the two-bottle choice continuous access paradigm. During this period there were no significant genotype-dependent differences in the amount of alcohol consumed across groups when compared to their respective WT counterparts (Figure 1C and 1D). Male WT and GluN2A(A825W) mice consumed an average of 8.51 ± 0.77 g/Kg and 7.65 ± 0.61 g/Kg every 24h, respectively, during the 4 weeks of baseline drinking. Female WT and GluN2A(A825W) mice averaged similar amounts of ethanol consumption (WT: 13.74 ± 1.45 g/Kg vs A825W:11.86 ± 1.07 g/Kg) and these were significantly higher than those of males (A825W: female vs male, F(1,26) = 6.84 p<0.05; WT: female vs male, F (1,36) = 61.86, p<0.0001; Mixed-effects analysis). Daily consumption of ethanol by WT and GluN1(F639A) male mice during the baseline period was 6.33 ± 0.59 g/Kg and 5.86 ± 0.87 g/Kg; respectively and these values were not different from one another. After four weeks of baseline voluntary ethanol consumption, mice within each group were separated into Air controls and CIE groups counterbalanced by average ethanol consumption.
BEC values during repeated cycles of CIE
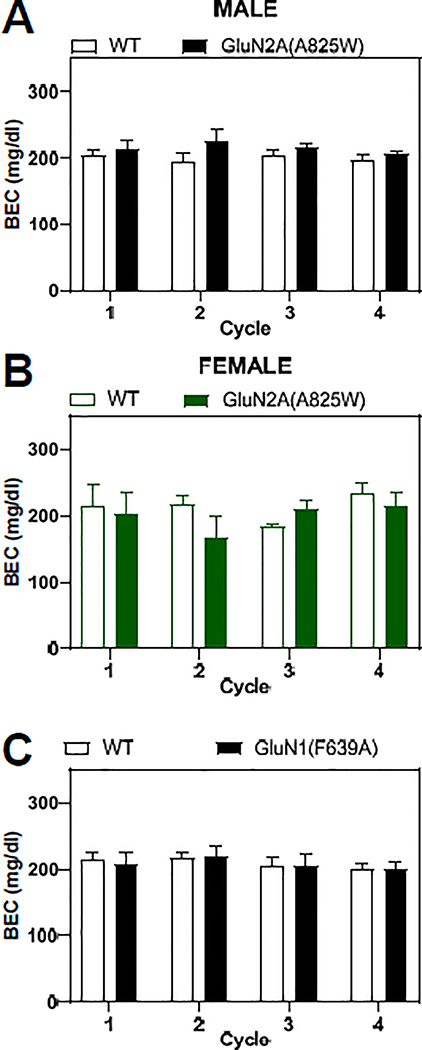
Previous studies have demonstrated that the CIE-induced escalation in ethanol consumption requires a sustained elevation in BEC (>175 mg/dL) during the vapor exposure periods (Griffin et al. 2009). To ensure that these levels were met and did not vary across the genotypes of mice studied, blood samples were taken as mice left the inhalation chambers and BECs were measured. As summarized in Figure 2, BECs averaged approximately 200 mg/dL during each CIE exposure period and were similar between male WT and GluN2A(A825W) mice (F(1,66) = 3.89, p=0.053; Mixed-effects analysis, Fig. 2A) and female WT and GluN2A(A825W) mice (F(1,10) = 0.759, p=0.40; 2-way RM ANOVA, Fig 2B). When compared across male and female GluN2A(A825W) experimental groups, there were no significant differences in BEC values by sex (F(1,102) = 0.005, p=0.94; Three-way Mixed-effects analysis) or genotype (F(1,102) = 0.03, p=0.86; Three-way Mixed-effects analysis). BEC values for male WT and GluN1(F639A) mice were also not different from one another across the CIE treatments (F(1,18) = 0.001, p=0.98; 2-way RM ANOVA, Figure 2C).
Figure 2. Blood ethanol concentrations in CIE treated mice.
Summary plots show weekly blood ethanol concentrations (BEC; mean ± SEM) for wild-type (open columns) and mutant mice (closed columns). (A) Male wild-type (N=10) and GluN2A(A825W) mice (N=10); (B) Female wild-type (N=5) and GluN2A(A825W) mice (N=7); (C) Male wild-type (N=10) and GluN1(F639A) mice (N=10).
Male GluN2A(A825W) mice show blunted escalation in ethanol drinking after CIE treatment
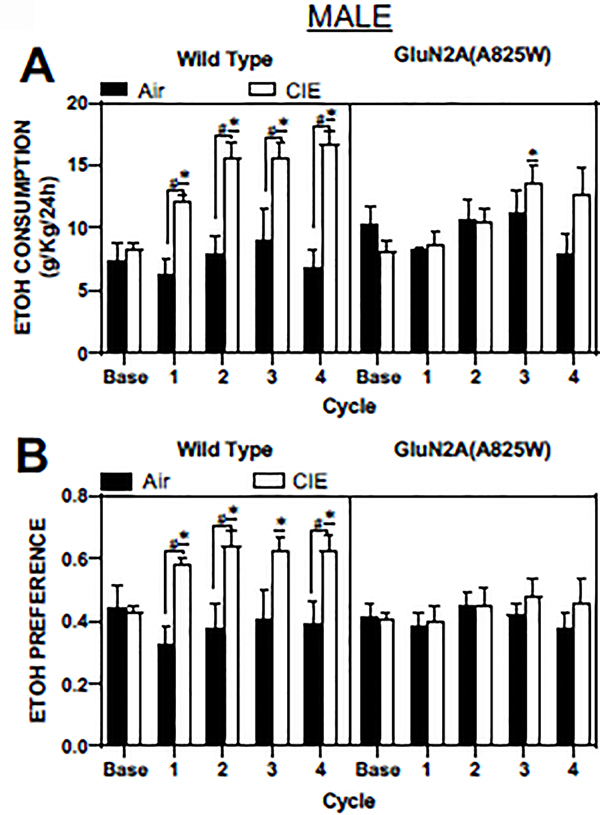
Each of the four CIE cycles was followed by a 5-day drinking session that typically reveals a significant increase in ethanol intake (Becker and Lopez 2016). As shown in Figure 3A (left panel), CIE treated WT male GluN2A(A825) mice showed a significant increase in ethanol consumption (g/Kg) after CIE treatment cycles one through four when compared to WT air controls (CIE vs Air; F(1,13) = 19.73, p=0.0007; Mixed-effects analysis). Moreover, in the CIE treated WT mice, post-hoc comparisons showed that ethanol consumption was significantly increased compared to baseline drinking (p<0.05; Dunnett’s post-hoc) and higher than that of Air controls (p<0.01; Sidak’s post-hoc) during each of the drinking sessions. In contrast to WT male mice, there was no effect of CIE on ethanol consumption in male GluN2A(A825W) mice (F(1,13) = 0.48, p=0.50, Figure 3A, right panel). Post-hoc comparisons revealed that ethanol consumption in GluN2A(A825W) mice was not significantly different from baseline after CIE treatments one, two and four while there was a significant increase in ethanol intake from baseline drinking after cycle 3 (p<0.05; Dunnett’s post-hoc).
Figure 3. Ethanol consumption and preference after repeated cycles of CIE exposure in WT and GluN2A(A825W) male mice.
Summary plots show weekly averages (mean ± SEM) of 24 hr ethanol consumption (A) and preference (B) in male WT (air: N=5, CIE: N=10) and GluN2A(A825W) mice (air: N=5, CIE: N=10). Base is average 24 hr ethanol consumption during the pre-CIE exposure drinking tests. Symbols: (*) value statistically different (p<0.05) from baseline; (#) value significantly different (p<0.05) from Air controls.
CIE exposed WT mice also showed significantly enhanced preference for ethanol, with respect to water, when compared to WT Air controls (main effect of treatment: F(1,13) = 10.08, p=0.007; Mixed-effects analysis). In addition, ethanol preference during each of the 4 drinking test sessions was significantly higher than baseline ethanol preference (p<0.05; Dunnett’s post-hoc) and that of the Air controls (p<0.05, Sidak’s post-hoc; Figure 3B, left panel). In contrast, GluN2A(A825W) male mice showed no change in ethanol preference following any of the CIE cycles when compared to GluN2A(A825W) Air controls (main effect of treatment: F(1,14) = 0.30, p=0.59; Mixed-effects analysis; Figure 3B, right panel). Noteworthy, increases in ethanol consumption were mirrored by decreases in water consumption (data not shown).
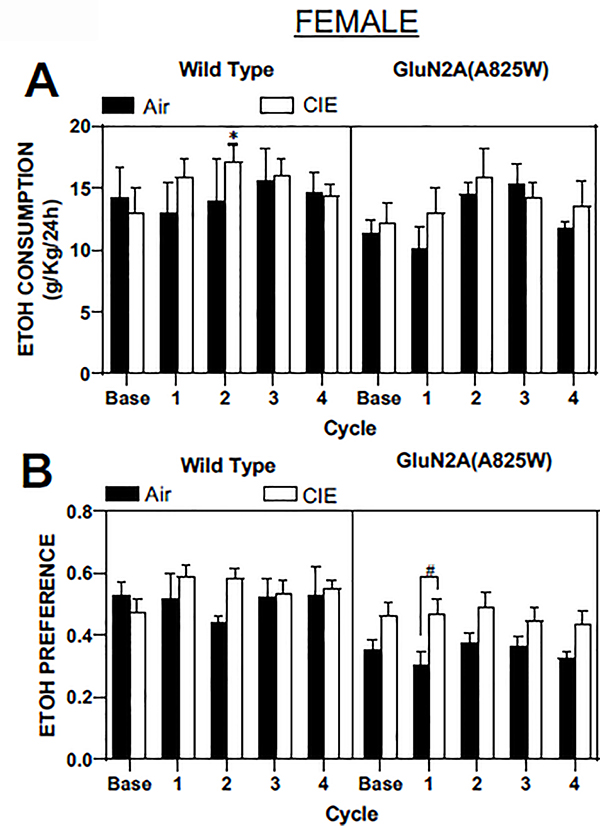
In contrast to male mice, female WT mice did not show a consistent increase in ethanol drinking following CIE treatment (main effect of treatment: F(1,8) = 0.14, p=0.71; Mixed-effects analysis), although there was a significant increase in drinking after CIE cycle two as compared to baseline drinking (p<0.05; Dunnett’s post-hoc; Figure 4A, left panel). Additionally, there were no significant changes in ethanol preference by WT female mice after any of the four CIE cycles (Figure 4B, left panel). GluN2A(A825W) female mice also showed no changes in ethanol consumption after CIE exposures (main effect of treatment: F(1,10) = 0.28, p=0.61; 2-way RM ANOVA, Figure 4A, right panel). There was a main effect of CIE on ethanol preference in female GluN2A(825W) mice (F(1,10) = 5.97, p=0.035) and post-hoc comparisons indicated a significant difference during the first test drinking session (p<0.05; Sidak’s post-hoc; Figure 4B, right panel). However, this effect was likely due to an apparent difference in preference between the Air and CIE groups rather than a CIE-induced increase as there were no significant differences in preference across the CIE test cycles when values were compared to baseline (p>0.05; Dunnett’s post-hoc).
Figure 4. Ethanol consumption and preference after repeated cycles of CIE exposure in WT and GluN2A(A825W) female mice.
Summary plots show weekly averages (mean ± SEM) of 24 hr ethanol consumption (A) and preference (B) in female WT (air: N=5, CIE: N=5) and GluN2A(A825W) (air: N=5, CIE: N=7) mice. Base is average 24 hr ethanol consumption during the pre-CIE exposure drinking tests. Symbols: (*) value statistically different (p<0.05) from baseline; (#) value significantly different (p<0.05) from Air controls.
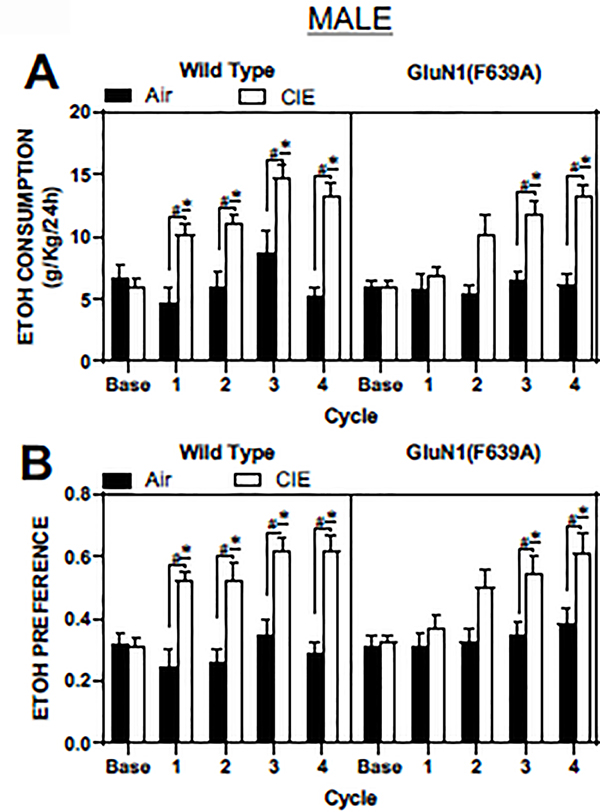
Male GluN1(F639A) mice show a delayed increase in ethanol drinking after CIE vapor treatments
Because only male WT mice showed a clear and consistent CIE-induced increase in drinking (Figure 3A), we examined the effect of CIE on drinking in males using a second line of knock-in mice that express an ethanol-resistant GluN1 subunit. After four weeks of baseline drinking, male GluN1(F639A) and their WT littermates were subjected to four cycles of CIE treatments, interleaved with test drinking sessions. As expected, male WT mice from the GluN1(F639A) line displayed a robust escalation in ethanol drinking after each CIE vapor treatment (main effect of treatment; F(1,18) = 12.57, p=0.002; 2-way RM ANOVA; Figure 5A, left panel). Ethanol consumption was significantly higher in CIE exposed male WT mice when compared to their baseline drinking (p<0.05; Dunnett’s post-hoc) and was higher when compared to drinking in WT Air controls (p<0.05; Sidak’s post-hoc). Male GluN1(F639A) mice also showed a main effect of CIE on ethanol drinking (F(1,18) = 11.67, p=0.003; 2-way RM ANOVA), but significant increases in ethanol drinking over baseline (p<0.05; Dunnett’s post-hoc) and Air controls (p<0.05; Sidak’s post-hoc) occurred only after the third and fourth CIE exposures (Figure 5A, right panel).
Figure 5. Figure 4. Ethanol consumption and preference after repeated cycles of CIE exposure in WT and GluN1(F639A) mice.
Summary plots show weekly averages (mean ± SEM) of 24 hr ethanol consumption (A) and preference (B) in male WT (air: N=10, CIE: N=10) and GluN1(F639A) (air: N=10, CIE: N=10) mice. Base is average 24 hr ethanol consumption during the pre-CIE exposure drinking tests. Symbols: (*) value statistically different (p<0.05) from baseline; (#) value significantly different (p<0.05) from Air controls.
In wild-type male mice, ethanol preference was also increased by CIE exposure (main effect of treatment; F(1,18) = 18, p=0.0005; 2-way RM ANOVA) and was significantly different from baseline values (p<0.05; Dunnett’s post-hoc) and Air controls (p<0.05; Sidak’s post-hoc; Figure 5B, left panel). Similarly, while there was a main effect of CIE treatment on ethanol preference in male GluN1(F639A) animals (F(1,18) = 6.85, p=0.017; 2-way RM ANOVA), differences between baseline (p<0.05; Dunnett’s post-hoc) and Air controls (p<0.05; Sidak’s post-hoc) appeared only after the third and fourth CIE exposure cycles (Figure 5B, right panel).
Discussion
While it is well established that homeostatic changes in NMDAR function and expression caused by chronic alcohol exposure play an important role in the pathophysiology of alcohol dependence (Ron and Wang 2009; Szumlinski and Woodward 2014); it has been unclear whether these changes are driven by inhibition of ethanol-sensitive NMDARs. Here, using a well-established preclinical model of alcohol dependence, we report that mice expressing genetically engineered GluN2A(A825W) or GluN1(F639A) NMDAR subunits with reduced ethanol sensitivity (den Hartog et al. 2013; Zamudio et al. 2020) display a blunted or delayed escalation in ethanol consumption following CIE exposure as compared to their WT littermates. These results are important for understanding the underlying mechanisms that drive the development of alcohol dependence and suggest that factors that influence the intrinsic alcohol sensitivity of NMDARs may contribute to individual differences in the severity of symptoms in individuals with alcohol use disorder.
We have previously characterized alterations in alcohol-induced behaviors in GluN2A(A825W) and GluN1(F639A) mutant mice. For GluN2A(A825W) mice, we showed that male, but not female, mice are less sensitive to the sedative and motor-incoordinating effects of ethanol and show a rightward shift in ethanol’s locomotor stimulating effect (Zamudio et al. 2020). In contrast, there was no effect of the A825W mutation on acute ethanol-induced anxiolysis or voluntary drinking in either male or female mice. Baseline drinking in the current study was also not different between wild-type and mutant mice although as others have shown, female mice consumed significantly more ethanol than males. Our previous studies also showed that, like male GluN2A(A825W) mice, male GluN1(F639A) mice also showed faster recovery of ethanol-induced motor impairment (den Hartog et al. 2013) and these effects were mirrored by a similar rightward shift in the locomotor enhancing effects of ethanol (den Hartog et al. 2013). Interestingly, voluntary ethanol consumption was not altered in male and female GluN2A(A825W) mice using an intermittent access drinking protocol that, while not considered a dependence model, generates relatively high levels of consumption (Zamudio et al. 2020). On the other hand, GluN1(F639A) mice showed altered patterns of ethanol consumption that varied depending on which drinking model was used. Compared to their wild-type littermates, drinking in the GluN1(F639A) mutant mice was reduced in the 2-hr limited access, 2-bottle choice assay but was slightly increased when ethanol was available every other day (den Hartog et al. 2013). In the present study, GluN2A(A825W) and GluN1(F639A) mice ingested comparable amounts of ethanol as compared to their WT littermates, during the 4 weeks of baseline consumption that used the 24-hr access, 2-bottle choice drinking paradigm. Although longer periods of access may reveal an effect of genotype on drinking, these findings suggest that ethanol-sensitive NMDARs do not appear to be critical for regulating voluntary ethanol consumption in non-dependent animals.
Interestingly, while in the present study the CIE-induced escalation in alcohol consumption was blunted in both homozygous GluN2A(A825W) and heterozygous GluN1(F639A) male mice, the timing and magnitude of this change was different between the two genotypes. GluN1(F639A) mice showed a delay in escalation with significant increases in drinking only observed following the third and fourth CIE cycles. In contrast, GluN2A(A825W) mice showed no consistent changes in drinking over baseline with only a modest increase in drinking appearing after the third CIE cycle. This difference could reflect the presence of the wild-type GluN1 subunit in the heterozygous GluN1(F639A) mice that may allow for CIE-induced compensations in NMDAR function to occur upon repeated CIE treatments. Moreover, given that in GluN1(F639A) mice, the mutation is present in all functional NMDARs, it is possible that the difference in drinking escalation seen between GluN2A(A825W) and GluN1(F639A) mice is mediated by opposing behavioral roles of NMDARs containing the GluN2A subunit and those expressing other GluN2 subunits. Ongoing studies in this laboratory are evaluating this idea using additional lines of NMDAR knock-in mice.
The GluN2A subunit has been proposed to play an influential role on alcohol consumption and dependence. High resolution genome screening studies identified quantitative trait loci linked to alcohol preference in alcohol preferring rodents within chromosome 16, where the Grin2A gene resides (Carr et al. 2003; Colville et al. 2017; Lo et al. 2016). Intriguingly, a polymorphism in the promoter region of the GluN2A gene suggested a decrease in GluN2A-containing NMDAR function in human alcoholics (Domart et al. 2012). In a human study examining the association between single nucleotide polymorphisms and alcohol dependence, the GluN2A subunit was found to have the greatest relevance among a group of 10 different glutamatergic genes (Schumann et al. 2008). Concomitantly, GluN2A knockout mice show impaired ability to form or express learned reward-related responses to ethanol (Boyce-Rustay and Holmes 2006a), impaired tolerance to ethanol intoxication after CIE exposure (Daut et al. 2015), unaltered alcohol consumption in non-dependent animals and a lack of CIE-induced escalation in ethanol drinking (Boyce-Rustay and Holmes 2006a; Jury et al. 2018). Nonetheless, it is possible that GluN2A knockout mice may undergo developmental compensations that could either mask effects produced by the lack of the GluN2A subunit or unveil effects driven by compensatory mechanisms. For example, GluN2A knockout mice display elevated levels of monoaminergic tone that in turn mediates a resistance to the hypnotic effect of nitrous oxide that is independent of NMDAR dysfunction (Miyamoto et al. 2001; Petrenko et al. 2013).
The GluN2A(A825W) mutation in the knock-in mice used in this study results in GluN2A-containing NMDARs with reduced sensitivity to ethanol but otherwise normal function (Honse et al. 2004; Smothers and Woodward 2006; Zamudio et al. 2020). Importantly, the reduction in ethanol sensitivity generated by this mutation is exclusive to ethanol, as GluN2A(A825W) NMDARs retain normal sensitivity to other central nervous system depressants such as benzene and the abused inhalant toluene (Ogata et al. 2006; Smothers and Woodward 2016). Noteworthy, in GluN2A knockout mice, the aforementioned elevation in monoaminergic tone was associated with hyperlocomotion, and reductions in anxiety-like and depressant-related behaviors (Boyce-Rustay and Holmes 2006a; b; Miyamoto et al. 2001). Although monoamine levels were not measured in the present study, we previously reported no changes in locomotion or anxiety in GluN2A(A825W) mice (Zamudio et al. 2020) suggesting that altered levels of catecholamines are unlikely to explain the observed changes in drinking by these mice after CIE.
In the present study, the blunted CIE-induced increase in drinking in GluN2A(A825W) mice was limited to male mice. This is perhaps not surprising as although female rodents typically consume more ethanol than males in either operant or home cage drinking paradigms (Anderson and Spear 2011; Bertholomey et al. 2016; Cailhol and Mormede 2001; Piano et al. 2005; Priddy et al. 2017), they do not show a reliable increase in ethanol consumption after CIE exposure (Jury et al. 2017; Lopez et al. 2017; Morales et al. 2015; Schweitzer et al. 2016). The lack of a consistent elevation in ethanol drinking by females following CIE exposure may be due to a ceiling effect as they already consume higher levels of ethanol during baseline periods. Other important aspects of dependence such as reward seeking, habit development and behavioral inflexibility have also been shown to differ between female and male rodents (Barker et al. 2017; Xie et al. 2019). Interestingly, our previous study on GluN2A(A825W) mice reported that alterations in ethanol-induced behaviors were also only observed in male mice despite a similar loss of ethanol sensitivity of synaptic NMDARs in prefrontal cortex and cerebellum in male and female GluN2A(A825W) mice (Zamudio et al. 2020). Importantly, studies proposing a prominent role for GluN2A containing NMDARs in substance abuse and alcohol use disorders included women and men (Domart et al. 2012; Levran et al. 2009; Zhao et al. 2013) and the relevance of GluN2A genetic variation for human alcohol dependence was similar among men and women (Schumann et al. 2008). Thus, future studies with male and female NMDA mutant mice should investigate additional hallmarks of dependence, such as shifts in the rewarding effects of ethanol, withdrawal symptoms, craving, tolerance and relapse in order to better elucidate the role of GluN2A-containing NMDA receptor in alcohol dependence. In addition, these studies could examine whether dependence-induced changes in glutamatergic signaling in brain areas such as prefrontal cortex and extended amygdala that may drive escalated drinking (reviewed in Hwa et al. 2017) are blunted in mice expressing ethanol-resistant NMDARs.
In closing, the results of the present study suggest that the ethanol sensitivity of GluN2A containing NMDARs may be important in mediating the escalation in drinking observed in alcohol dependent individuals. Ongoing studies are focused on identifying the brain regions and neural circuits that mediate this effect.
Acknowledgments
Funding and Disclosure
This work was supported by NIH grants K01AA028059 (PAZ), F32AA026774 (DAG), R37AA009986 (JJW), R37AA10422 (GEH), AA020889 (GEH) and P50AA010761 (JJW, ML).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- American Psychiatric Association. (2013) Desk reference to the diagnostic criteria from DSM-5. American Psychiatric Publishing, Washington, DC [Google Scholar]
- Anderson RI, Spear LP (2011) Autoshaping in adolescence enhances sign-tracking behavior in adulthood: impact on ethanol consumption. Pharmacol Biochem Behav 98: 250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Bryant KG, Osborne JI, Chandler LJ (2017) Age and Sex Interact to Mediate the Effects of Intermittent, High-Dose Ethanol Exposure on Behavioral Flexibility. Front Pharmacol 8: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2016) An Animal Model of Alcohol Dependence to Screen Medications for Treating Alcoholism. Int Rev Neurobiol 126: 157–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Nagarajan V, Torregrossa MM (2016) Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 233: 2277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Kotlinska J (1999) Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol 34: 175–82. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A (2006a) Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 187: 455–66. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A (2006b) Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology 31: 2405–14. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P (2001) Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res 25: 594–9. [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ (2007) Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav 86: 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ (2004) Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci 24: 7859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LG, Habegger K, Spence J, Ritchotte A, Liu L, Lumeng L, Li TK, Foroud T (2003) Analyses of quantitative trait loci contributing to alcohol preference in HAD1/LAD1 and HAD2/LAD2 rats. Alcohol Clin Exp Res 27: 1710–7. [DOI] [PubMed] [Google Scholar]
- Colville AM, Iancu OD, Oberbeck DL, Darakjian P, Zheng CL, Walter NA, Harrington CA, Searles RP, McWeeney S, Hitzemann RJ (2017) Effects of selection for ethanol preference on gene expression in the nucleus accumbens of HS-CC mice. Genes Brain Behav 16: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, Camp M, Holmes A (2015) Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addict Biol 20: 259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog CR, Beckley JT, Smothers TC, Lench DH, Holseberg ZL, Fedarovich H, Gilstrap MJ, Homanics GE, Woodward JJ (2013) Alterations in ethanol-induced behaviors and consumption in knock-in mice expressing ethanol-resistant NMDA receptors. PLoS One 8: e80541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog CR, Gilstrap M, Eaton B, Lench DH, Mulholland PJ, Homanics GE, Woodward JJ (2017) Effects of Repeated Ethanol Exposures on NMDA Receptor Expression and Locomotor Sensitization in Mice Expressing Ethanol Resistant NMDA Receptors. Front Neurosci 11: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domart MC, Benyamina A, Lemoine A, Bourgain C, Blecha L, Debuire B, Reynaud M, Saffroy R (2012) Association between a polymorphism in the promoter of a glutamate receptor subunit gene (GRIN2A) and alcoholism. Addict Biol 17: 783–5. [DOI] [PubMed] [Google Scholar]
- Fritz M, Klawonn AM, Zahr NM (2019) Neuroimaging in alcohol use disorder: From mouse to man. J Neurosci Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Becker HC (2009) Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res 33: 1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honse Y, Ren H, Lipsky RH, Peoples RW (2004) Sites in the fourth membrane-associated domain regulate alcohol sensitivity of the NMDA receptor. Neuropharmacology 46: 647–54. [DOI] [PubMed] [Google Scholar]
- Hwa L, Besheer J, Kash T (2017) Glutamate plasticity woven through the progression to alcohol use disorder: a multi-circuit perspective. F1000Res 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A (2017) Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol 58: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, Radke AK, Pati D, Kocharian A, Mishina M, Kash TL, Holmes A (2018) NMDA receptor GluN2A subunit deletion protects against dependence-like ethanol drinking. Behav Brain Res 353: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J, Biala G, Rafalski P, Bochenski M, Danysz W (2004) Effect of neramexane on ethanol dependence and reinforcement. Eur J Pharmacol 503: 95–8. [DOI] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Randesi M, Rotrosen J, Casadonte P, Linzy S, Ott J, Adelson M, Kreek MJ (2009) Heroin addiction in African Americans: a hypothesis-driven association study. Genes Brain Behav 8: 531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CL, Lossie AC, Liang T, Liu Y, Xuei X, Lumeng L, Zhou FC, Muir WM (2016) High Resolution Genomic Scans Reveal Genetic Architecture Controlling Alcohol Preference in Bidirectionally Selected Rat Model. PLoS Genet 12: e1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Miles MF, Williams RW, Becker HC (2017) Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol 58: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T (2001) Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor epsilon1 subunit. J Neurosci 21: 750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, McCool BA (2015) Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol Biochem Behav 139: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA (2006) Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 318: 434–43. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Kohno T, Sakimura K, Baba H (2013) Increased brain monoaminergic tone after the NMDA receptor GluN2A subunit gene knockout is responsible for resistance to the hypnotic effect of nitrous oxide. Eur J Pharmacol 698: 200–5. [DOI] [PubMed] [Google Scholar]
- Piano MR, Carrigan TM, Schwertz DW (2005) Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol 35: 113–8. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Wu M, Dwyer DS, Peoples RW (2017) Two adjacent phenylalanines in the NMDA receptor GluN2A subunit M3 domain interactively regulate alcohol sensitivity and ion channel gating. Neuropharmacology 114: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Wang J (2009) The NMDA Receptor and Alcohol Addiction In: Van Dongen AM (ed) Biology of the NMDA Receptor (Frontiers in Neuroscience), Boca Raton (FL) [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ (2001) Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem 276: 44729–35. [DOI] [PubMed] [Google Scholar]
- Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K (2008) Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry 65: 826–38. [DOI] [PubMed] [Google Scholar]
- Schweitzer P, Cates-Gatto C, Varodayan FP, Nadav T, Roberto M, Lasek AW, Roberts AJ (2016) Dependence-induced ethanol drinking and GABA neurotransmission are altered in Alk deficient mice. Neuropharmacology 107: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ (2006) Effects of amino acid substitutions in transmembrane domains of the NR1 subunit on the ethanol inhibition of recombinant N-methyl-D-aspartate receptors. Alcohol Clin Exp Res 30: 523–30. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ (2016) Differential effects of TM4 tryptophan mutations on inhibition of N-methyl-d-aspartate receptors by ethanol and toluene. Alcohol 56: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski K, Woodward JJ (2014) Glutamate Signaling in Alcohol Abuse and Dependence In: Noronha ABC, Cui C, Harris RA, Crabbe JC (eds) Neurobiology of Alcohol Dependence. Academic Press, London, pp 173–206 [Google Scholar]
- Xie Q, Buck LA, Bryant KG, Barker JM (2019) Sex Differences in Ethanol Reward Seeking Under Conflict in Mice. Alcohol Clin Exp Res 43: 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Smothers CT, Woodward JJ (2015) Cysteine substitution of transmembrane domain amino acids alters the ethanol inhibition of GluN1/GluN2A N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 353: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio PA, Smothers TC, Homanics GE, Woodward JJ (2020) Knock-in Mice Expressing an Ethanol-Resistant GluN2A NMDA Receptor Subunit Show Altered Responses to Ethanol. Alcohol Clin Exp Res 44: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio-Bulcock PA, Homanics GE, Woodward JJ (2018) Loss of Ethanol Inhibition of N-Methyl-D-Aspartate Receptor-Mediated Currents and Plasticity of Cerebellar Synapses in Mice Expressing the GluN1(F639A) Subunit. Alcohol Clin Exp Res 42: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Zhu Y, Wang W, Cui HM, Wang YP, Lai JH (2013) Analysis of variations in the glutamate receptor, N-methyl D-aspartate 2A (GRIN2A) gene reveals their relative importance as genetic susceptibility factors for heroin addiction. PLoS One 8: e70817. [DOI] [PMC free article] [PubMed] [Google Scholar]