Abstract
Background and aims
Little is known about opioid and gabapentinoid (OPI-GABA) use duration and dose patterns’ associations with adverse outcomes risks. We examined associations between OPI-GABA dose and duration trajectories and subsequent drug overdose.
Design
Retrospective cohort study.
Setting
US Medicare.
Participants
Using a 5% sample (2011–2016), we identified 71,005 fee-for-service Medicare beneficiaries with fibromyalgia, low back pain, neuropathy, and/or osteoarthritis initiating OPIs and/or GABAs (mean age±SD=65.5±14.5 years, female=68.1%, white=76.8%).
Measurements
Group-based multi-trajectory models identified distinct OPI-GABA use patterns during the year of OPI and/or GABA initiation, based on weekly average standardized daily dose (i.e., OPIs=morphine milligram equivalent, GABAs=minimum effective daily dose). We estimated models with three to 12 trajectories and selected the best model based on Bayesian information criterion (BIC) and Nagin’s criteria. We estimated risk of time to first drug overdose diagnosis within 12 months following the index year, adjusting for socio-demographic and health factors using inverse probability of treatment weighted multivariable Cox proportional hazards models.
Findings
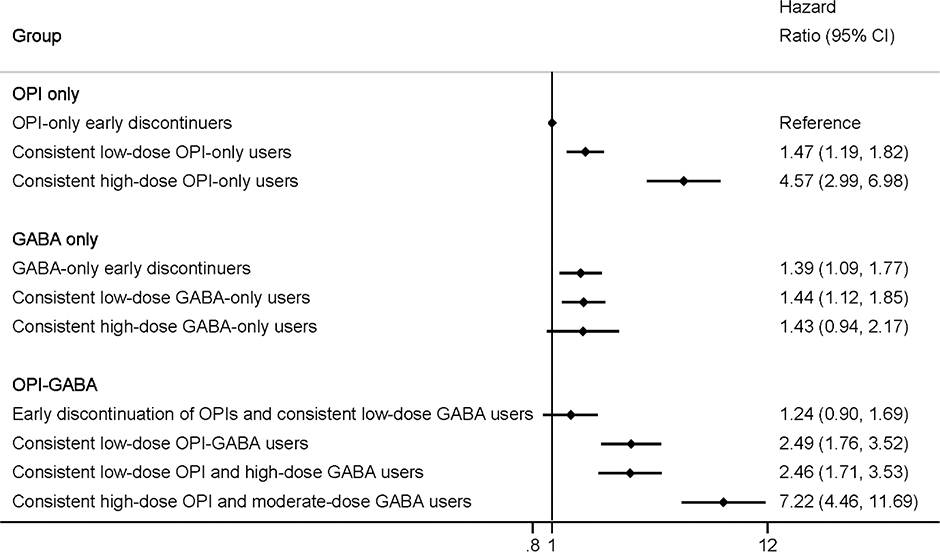
We identified 10 distinct trajectories (BIC=−1,176,954; OPI-only=3, GABA-only=3, OPI-GABA=4). Compared with OPI-only early discontinuers (40.6% of the cohort), 1-year drug overdose risk varied by trajectory group: consistent low-dose OPI-only users (16.6%; HR=1.47, 95%CI=1.19–1.82), consistent high-dose OPI-only users (1.8%; HR=4.57, 95%CI=2.99–6.98), GABA-only early discontinuers (12.5%; HR=1.39, 95%CI=1.09–1.77), consistent low-dose GABA-only users (11.0%; HR=1.44, 95%CI=1.12–1.85), consistent high-dose GABA-only users (3.1%; HR=1.43, 95%CI=0.94–2.17), early discontinuation of OPIs and consistent low-dose GABA users (6.9%; HR=1.24, 95%CI=0.90–1.69), consistent low-dose OPI-GABA users (3.4%; HR=2.49, 95%CI=1.76–3.52), consistent low-dose OPI and high-dose GABA users (3.2%; HR=2.46, 95%CI=1.71–3.53), and consistent high-dose OPI and moderate-dose GABA users (0.9%; HR=7.22, 95%CI=4.46–11.69).
Conclusions
Risk of drug overdose varied substantially among US Medicare beneficiaries on different use trajectories of opioids and gabapentinoids. High-dose opioid-only users and all consistent opioid and gabapentinoid users (regardless of doses) had more than double the risk of subsequent drug overdose compared with opioid-only early discontinuers.
Keywords: gabapentinoids, opioids, trajectories, overdose, Medicare
Introduction
The United States (US) Food and Drug Administration (FDA) has approved gabapentinoids (GABAs), including gabapentin and pregabalin, for the treatment of partial seizures and postherpetic neuralgia.(1, 2) Gabapentin is also approved for restless legs syndrome in adults, while pregabalin has additional indications for diabetic peripheral neuropathy, fibromyalgia, and neuropathic pain associated with spinal cord injury.(1, 2) A study using the Medical Expenditure Panel Survey (MEPS) reported that GABA use, primarily gabapentin, tripled from 1.2% in 2002 to 3.9% in 2015 among US adults.(3) Over 80% of gabapentin prescriptions are for off-label use for a variety of acute and chronic pain conditions (e.g., osteoarthritis, lower back pain) in outpatient care.(4)
Substantial off-label use of GABAs has raised concerns of potential misuse, abuse, dependence, and overdose risk, especially among individuals with concurrent opioid (OPI) use.(5–8) Four observational studies showed that the use of GABAs alone or with OPIs was associated with an increased risk of adverse outcomes (e.g., hospitalizations, OPI-related deaths).(9–12) Possible explanations for the adverse outcomes associated with OPI-GABA use are pharmacokinetic and pharmacodynamic interactions including increases in gabapentin absorption due to co-administration of OPIs,(13) decreased renal function in older adults, comorbid lung disease, and additive central nervous system adverse effects (e.g., dizziness, respiratory depression).(14) Nevertheless, concurrent OPI and GABA (hereafter OPI-GABA) use is common in the US. According to the MEPS data, 52.6% of adults using GABAs reported OPI-GABA use in 2015.(3) In a study of US commercial insurers, a quarter of individuals using GABAs had long-term concurrent OPI use (≥120 days) in a one year period between 2013 and 2015.(15) However, there is a lack of evidence or consensus in clinical practice on the duration and dose patterns of OPI-GABA use that are most associated with an increased risk of adverse health outcomes.
Patterns of OPI-GABA use may change over time and vary across patient subgroups due to multiple reasons (e.g., pain conditions). Previous studies used arbitrary single-value cutoff points for dose and duration (e.g., having any 120 cumulative days of OPI-GABA use within in a year), which limited the ability to identify heterogeneous utilization patterns over time. Identifying distinct refill patterns that incorporate both OPI and GABA dose and duration changes over time may better guide clinical care. Therefore, our a priori study objective was to apply group-based multi-trajectory models to account for the dynamic nature of simultaneous OPI-GABA use and examine their associations with subsequent risk of adverse outcomes (e.g., drug overdose) among Medicare beneficiaries with fibromyalgia, neuropathy, low back pain, or osteoarthritis who filled ≥1 OPIs or GABAs. We chose Medicare beneficiaries because of the high prevalence of pain conditions and OPI and GABA use among them, the availability of national, longitudinal data, and because the Part D plans will soon be required to implement programs specific for high risk for opioid-related behaviors.(3, 16–18)
Methods
Data Source, Design, and Sample
The University of Arizona Institutional Review Board approved this study (protocol no: 1709791099). The reporting of this study complied with the STROBE guidelines.(19) Although the analysis protocol was developed prior to conducting the study, it was not pre-registered on a publicly available platform and therefore the results should be considered exploratory.
This retrospective cohort study included a 5% nationally representative sample of Medicare beneficiaries from 2011 to 2016.(20, 21) Medicare is the US government health insurance program for individuals in the US aged ≥65, or those aged <65 with certain disabilities or end-stage renal disease.(20) Since the 1970s, Medicare beneficiaries have had the option to receive their Medicare benefits either through the federally administered traditional Medicare program that pays providers for each service they perform for or render to a person (i.e., fee-for-service plans) or Medicare-approved private health plans that receive capitated payments to provide all Medicare covered services (i.e., Medicare Advantage plans). The fee-for-service program includes the Part A (hospital) insurance, Part B (medical) insurance, and Part D (prescription drug coverage). Medicare Advantage plans are referred to as Part C insurance. In 2019, two-thirds of the 64 million Medicare beneficiaries were covered by fee-for-service plans.(22) The completeness of submitting medical claims data to the Centers for Medicare and Medicaid (CMS) varies; we thus limited our analysis to the fee-for-service beneficiaries. The datasets used in this study included Medicare master beneficiary summary files, medical claims of inpatient, outpatient, carrier, skilled nursing facility, home health, hospice, durable medical equipment, and part D drug event files.
From the 5% Medicare sample, we identified fee-for-service beneficiaries who were US residents having ≥1 inpatient or ≥2 other medical claims for fibromyalgia, neuropathy (i.e., diabetic peripheral neuropathy, postherpetic neuralgia, and trigeminal neuralgia), low back pain, or osteoarthritis on different days using the International Classification of Diseases (ICD)-9/10 codes (eTable 1).(23, 24) We focused on these chronic conditions for which OPIs and GABAs are commonly prescribed.(25, 26) We restricted our analytical sample to beneficiaries initiating OPIs or GABAs, who had received no OPI or GABA prescription within six months prior to the index date (i.e., first prescription date of either OPIs or GABAs, whichever occurred first (eFigure 1). As shown in eFigure 2, we excluded beneficiaries who: (1) had end-stage renal disease, seizures or epilepsy, and any type of cancer during the study period (except for non-melanoma skin cancer; eTable 1);(27) (2) were not continuously enrolled in Parts A, B, and D between six months prior to and 12 months post the index date; (3) had a diagnosis of an outcome of interest, including drug overdose, opioid use disorder (OUD), or non-opioid substance use disorders (SUD),(28, 29) six months prior to and 12 months post the index date; and (4) filled opioids likely for acute pain (i.e., with sporadic exposure defined as filling only one OPI prescription, two OPI prescriptions but on the same day, or with <15 days of OPI supply during the index year, based on Pharmacy Quality Alliance’s opioid risk measures that have been used by Part D plan partners for quality improvement).(30)
Exposures: Dual-Trajectories of Concurrent Opioid and Gabapentinoid (OPI-GABA) Use
Our exposure of interest was membership in a distinct dual-trajectory of OPI-GABA use by (1) constructing weekly measures of average standardized daily dose (SDD) for OPIs and GABAs in the 12 months after initiating OPIs or GABAs, respectively, and (2) identifying distinct dose and duration patterns of OPI-GABA use by applying group-based multi-trajectory models with SDD as the outcomes in the model. We chose a 12-month period because (1) it allows us to have sufficient time to identify distinct chronic patterns of OPI-GABA use over time, and (2) prior work has shown that overdose risks generally increase as the duration of concurrent use of OPI and other CNS medications increases but little evidence exists for the clinically meaningful cut points of concurrent use.(31) First, based on dispensing date and days supplied for each OPI and GABA prescription, we calculated SDD for OPIs using morphine milligram equivalent (MME),(32) and for GABAs using 300 mg for gabapentin and 150 mg for pregabalin).(1) Low-, moderate-, and high-dose opioid use was defined as an average daily dosage of <50 MME, 50–90 MME, and >90 MME, respectively.(33) For GABA use, an average daily SDD of <2 (i.e., gabapentin <600 mg or pregabalin <300 mg), 2–3 (i.e., 600≤ gabapentin <900 mg or 300≤ pregabalin <450 mg), and >3 (i.e., gabapentin ≥900 mg or pregabalin ≥450 mg) were considered as low-, moderate-, and high-dose use, respectively. Second, group-based multi-trajectory models were used to identify differential utilization patterns of OPIs, GABAs, or OPI-GABA use based on dose used over time.(34–38) The Appendix Methods explicitly describe the analytical details of identifying dual-trajectories of OPI-GABA use.
Outcome Variables: Drug Overdose, OUD, and Non-Opioid SUD
The primary outcome was defined as the time to the first diagnosis drug overdose (including fatal and non-fatal) that occurred in the 12 months following the first year of OPI or GABA initiation. Similar to prior studies using claims data,(28, 39) we identified any occurrence of fatal or non-fatal drug overdose (e.g., prescription opioids, heroin, and other drugs) from inpatient or ED settings using the ICD-9/10 codes (eTable 1).(28, 29) We chose drug overdose as our primary outcome because misuse or abuse of OPI and/or GABA is likely to involve multiple drugs. We also examined two additional secondary outcomes including time to the first diagnosis of (1) OUD, and (2) non-opioid SUDs from any medical claims in the 12 months following the first year of OPI or GABA initiation.(28, 29)
Covariates
Covariates were measured in the six months prior to the index date, including age, sex, race/ethnicity (White, African American, Hispanic, and others), disability status indicating the original reason for Medicare eligibility, and receipt of low-income subsidy (LIS) and dual Medicaid eligibility (with LIS and dual eligibility, with only LIS or dual eligibility, and no LIS or dual eligibility). Health status factors included the Elixhauser comorbidity index (excluding metastatic cancers and solid tumors with or without metastasis; range 0 to 27), serious mental illness, and anxiety disorders (eTable 1),(40) numbers of outpatient visits, numbers of inpatient or emergency department visits, numbers of prescription fills for benzodiazepines and Z-hypnotics, nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, muscle relaxants, and other prescriptions not mentioned above.
We also linked the data to the Area Health Resources Files (AHRF) to measure county-level factors, including the standardized numbers of hospitals, non-federally employed physicians, hospitals with pain management programs, and physical medicine/rehabilitation centers per 10,000 population as a proxy for access to health care or certain specialties, population profile (metropolitan or non-metropolitan), annual median household income, and annual unemployment rate.(41)
Statistical Analysis
Characteristics of individuals in each trajectory group were described with means and standard deviations (SD) for continuous variables and frequencies and percentages for categorical variables. Given that the identified trajectory groups were likely to be different by patient characteristic and disease complexity, we used multinomial logistic regression to estimate the inverse probability of treatment weighting (IPTW) for each beneficiary. IPTW was defined as inverse probability of an individual likely to be placed in a specific trajectory group. Weighting subjects with IPTW created a sample in which treatment assignment was independent of measured covariates.(42) We weighted subjects with IPTW in the analyses to minimize confounding by covariates across trajectories. We compared the characteristics across trajectory groups before and after weighting subjects with IPTW, using the standardized mean difference (SMD), wherein SMD>0.1 was considered as non-negligible differences.
The IPTW-weighted multivariable Cox proportional hazards models were used to compare time-to-event (i.e., drug overdose, OUD, or non-opioid SUD) within the 12 months following the first year of OPI or GABA initiation across different OPI-GABA trajectories, adjusting for the covariates with non-negligible differences after IPTW weighting. These models treated beneficiaries switching to Medicare Advantage plans or without any outcomes of interest in the 12 months following the first year of OPI or GABA initiation as censored observations, and treated beneficiaries with deaths in the 12 months following the first year of OPI or GABA initiation as competing events.(43) We assessed the proportional hazard assumption including time dependent covariates.(44) We assessed the validity of the proportional hazards assumption by using Schoenfeld residuals.(45) The cause-specific hazard ratios (HRs) with 95% confidence intervals (CIs) were reported.
We conducted two sensitivity analyses to ensure the robustness of the findings. First, we included beneficiaries having ≥1 inpatient or ≥2 other medical claims with any pain conditions (28) (eTable 2) in addition to fibromyalgia, neuropathy, low back pain, and osteoarthritis. Second, in order to assess potential influences of unmeasured confounders, we calculated the “E-value”, which is defined as the minimum strength of association that an unmeasured confounder would need to have for both the treatment and the outcome to reconcile a specific treatment-outcome association, conditional on the measured covariates.(46) A large E-value implies that considerable unmeasured confounding would be needed to account for an effect estimate; whereas, a small E-value implies little unmeasured confounding would be needed to do so.(46)
The group-based multi-trajectory models were estimated using STATA 15.0 (Stata-Corp LP, College Station, TX) and the TRAJ macro (free download at http://www.andrew.cmu.edu/user/bjones). SMDs were calculated using the R packages tableone and survey and all other analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC, USA).
Results
Dual-Trajectories of Concurrent OPI-GABA Use
Among 71,005 eligible beneficiaries initiating OPI or GABA prescriptions, the overall mean MME and SDD in the 12 months following initiation of OPIs or GABAs were 16.8 (SD=33.9) for OPIs and 1.3 (SD=2.0) for GABAs, respectively (eFigure 3). According to a combination of BIC value (largest BIC=−1,176,954) and Nagin’s criteria, a model with ten distinct dual-trajectories for OPI-GABA use was selected as the final model (eTable 3).
Trajectory Groups Based on Weekly Average Dose Use and Duration Patterns
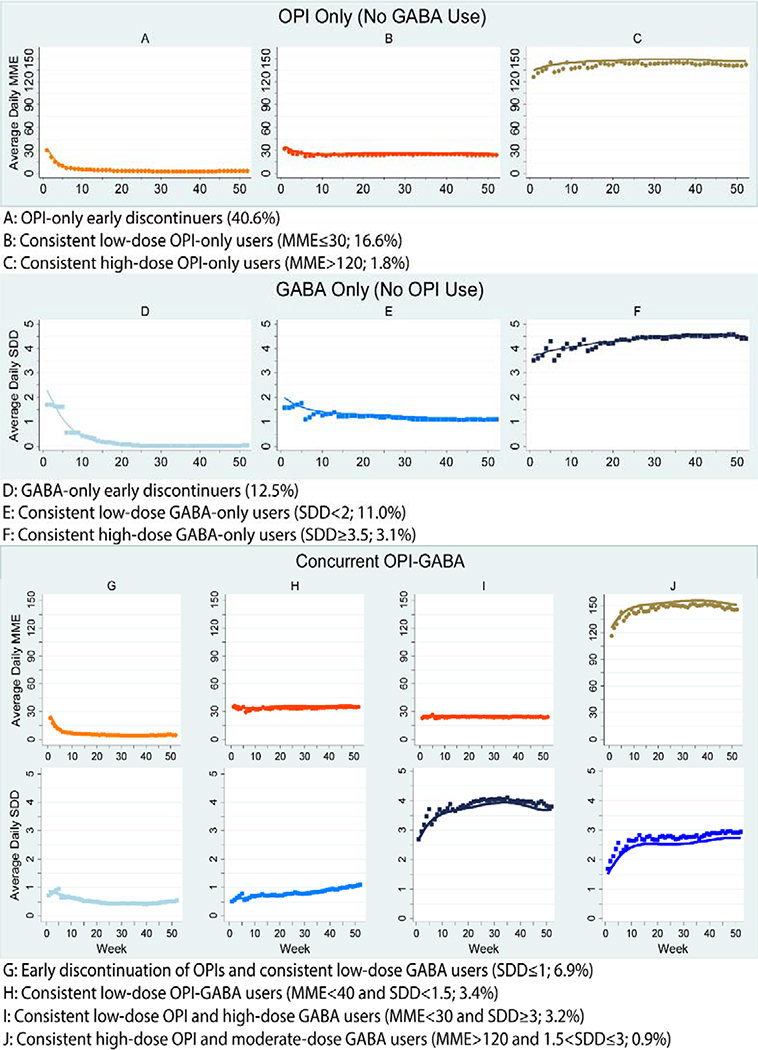
Figure 1 illustrates the predicted weekly dose utilization patterns for OPI and GABA use in the 12 months following initiation of OPIs or GABAs. Three of the ten trajectories comprised OPIs only (59.0% of the cohort); however, there were distinct groups with respect to weekly average dose use and duration. Specifically, 40.6% of the cohort (n=28,842) were OPI-only early discontinuers (Group A); 16.6% were consistent low-dose OPI-only users (Group B; MME ≤30); and 1.8% were consistent high-dose OPI-only users (Group C; MME >120). Similarly, three of the ten trajectories comprised GABAs only (26.6% of the cohort): 12.5% were GABA-only early discontinuers (Group D); 11.0% were consistent low-dose GABA-only users (Group E; SDD <2 [i.e., gabapentin <600 mg or pregabalin <300 mg]);); and 3.1% were consistent high-dose GABA-only users (Group F; SDD ≥3.5 [i.e., gabapentin ≥ 1,050 mg or pregabalin ≥525 mg]). The remaining four trajectories comprised OPI-GABA users, but with distinct dose and duration profiles: 6.9% had early discontinuation of OPIs and consistent low-dose GABA use (Group G; GABA SDD ≤1 [i.e., gabapentin ≤300 mg or pregabalin ≤ 150 mg]); 3.4% were consistent low-dose OPI-GABA users (Group H; MME <40 and SDD <1.5 [i.e., gabapentin ≤450 mg or pregabalin ≤ 225 mg]); 3.2% were consistent low-dose OPI and high-dose GABA users (Group I; MME <30 and SDD ≥3 [i.e., gabapentin ≥900 mg or pregabalin ≤ 450 mg]); and 0.9% were consistent high-dose OPI and moderate-dose GABA users (Group J; MME >120 and 1.5< SDD ≤3 [i.e., 450≤ gabapentin ≤ 900 mg or 225 ≤ pregabalin ≤ 450 mg]).
Figure 1. Dual-Trajectories of Opioid and Gabapentinoid Utilization Patterns among Medicare Beneficiaries.
Abbreviations: GABA, gabapentinoid; MME, morphine milligram equivalent; OPI, opioid; SDD, standardized daily dose
We calculated SDDs for OPIs using morphine milligram equivalent (MME) and for GABAs using 300 mg for gabapentin and 150 mg for pregabalin). Low-, moderate-, and high-dose opioid use was defined as an average daily dosage of <50 MME, 50–90 MME, and >90 MME, respectively. For GABA use, an average daily SDD <2 (i.e., gabapentin <600 mg or pregabalin <300 mg), 2–3 (i.e., 600≤ gabapentin <900 mg or 300≤ pregabalin <450 mg), and >3 (i.e., gabapentin ≥900 mg or pregabalin ≥450 mg) were considered as low-, moderate-, and high-dose use, respectively.
Characteristics Overall and by Trajectory Group
Table 1 shows the characteristics by OPI-GABA trajectory group. Among all eligible beneficiaries, the majority had low back pain (78.5%) or osteoarthritis (70.9%), and 20% had fibromyalgia or neuropathy. The mean age was 65.5 (SD=14.5) years, 68.1% were female, and 76.8% were white. The average Elixhauser comorbidity index was 2.7 (SD=2.3) and 72.4% resided in metropolitan counties.
Table 1.
Characteristics of Medicare Beneficiaries Initiating Opioids or Gabapentinoids and by Trajectory Group
| Characteristics | Overall | OPI Only | GABA only | OPI-GABA | SMDb before IPTW | SMDb after IPTW | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa | Ba | Ca | Da | Ea | Fa | Ga | Ha | Ia | Ja | ||||
| No. beneficiaries | 71,005 | 28,842 | 11,793 | 1,284 | 8,854 | 7,802 | 2,179 | 4,786 | 2,408 | 2,303 | 664 | ||
| % of the overall cohort | 100 | 40.6 | 16.6 | 1.8 | 12.5 | 11.0 | 3.1 | 6.9 | 3.4 | 3.2 | 0.9 | ||
| Disease status, % | |||||||||||||
| Any low back pain | 78.5 | 73.6 | 79.5 | 91.7 | 81.2 | 79.5 | 82.2 | 82.0 | 90.2 | 85.8 | 94.3 | 0.22 | 0.08 |
| Any osteoarthritis | 70.9 | 73.4 | 73.0 | 55.3 | 68.7 | 70.4 | 62.1 | 69.8 | 67.8 | 65.5 | 61.0 | 0.15 | 0.07 |
| Any fibromyalgia | 20.2 | 14.4 | 18.7 | 31.1 | 24.5 | 25.1 | 28.7 | 22.7 | 32.1 | 31.0 | 38.0 | 0.20 | 0.04 |
| Any neuropathy | 19.7 | 13.9 | 13.1 | 10.3 | 24.2 | 34.5 | 32.6 | 27.3 | 22.8 | 31.9 | 21.2 | 0.26 | 0.06 |
| Socio-demographics | |||||||||||||
| Age, mean (SD) | 65.5 (14.5) | 68.3 (13.8) | 64.0 (14.6) | 54.1 (3.2) | 65.6 (14.8) | 65.6 (13.9) | 59.8 (13.7) | 66.0 (13.8) | 59.1 (14.5) | 58.9 (13.4) | 52.3 (12.3) | 0.48 | 0.19 |
| Female, % | 68.1 | 69.9 | 64.7 | 52.0 | 69.9 | 70.3 | 61.6 | 71.0 | 64.8 | 65.4 | 54.1 | 0.16 | 0.07 |
| Race/ethnicity, % | 0.20 | 0.09 | |||||||||||
| White | 76.8 | 76.4 | 80.2 | 87.7 | 72.4 | 73.8 | 82.9 | 73.1 | 78.5 | 82.5 | 85.8 | ||
| African American | 13.5 | 13.5 | 13.5 | 8.6 | 14.4 | 14.1 | 10.1 | 14.4 | 15.4 | 10.6 | 8.3 | ||
| Hispanic | 3.8 | 4.0 | 2.5 | 1.4 | 5.3 | 5.1 | 2.4 | 5.0 | 2.2 | 2.1 | 2.3 | ||
| Others | 5.9 | 6.1 | 3.8 | 2.3 | 8.0 | 6.9 | 4.5 | 7.6 | 4.0 | 4.9 | 3.6 | ||
| Disability status, % | 39.3 | 28.9 | 43.8 | 75.3 | 42.5 | 44.3 | 58.0 | 34.0 | 57.6 | 56.9 | 81.9 | 0.44 | 0.08 |
| LIS/dual eligibility, % | 0.14 | 0.06 | |||||||||||
| LIS and dual eligibility | 44.1 | 38.9 | 44.4 | 41.8 | 50.0 | 53.9 | 50.3 | 41.9 | 47.9 | 45.8 | 45.0 | ||
| LIS or dual eligibility | 6.6 | 5.9 | 6.7 | 8.1 | 7.5 | 7.4 | 8.2 | 7.5 | 7.1 | 7.1 | 8.1 | ||
| No LIS/dual eligibility | 49.3 | 55.1 | 49.0 | 50.1 | 42.4 | 38.7 | 41.5 | 44.7 | 44.7 | 47.1 | 46.8 | ||
| Health status factors | |||||||||||||
| Elixhauser index, mean (SD) | 2.7 (2.3) | 2.7 (2.2) | 2.5 (2.4) | 1.4 (19) | 2.9 (2.3) | 2.9 (2.5) | 2.9 (2.5) | 2.7 (2.3) | 2.3 (2.3) | 2.4 (2.5) | 1.7 (2.2) | 0.31 | 0.16 |
| Mental disorders, % | 4.5 | 3.9 | 4.5 | 2.8 | 5.3 | 5.6 | 8.1 | 3.4 | 3.2 | 3.3 | 4.2 | 0.08 | 0.03 |
| Anxiety, % | 12.6 | 10.4 | 12.5 | 13.5 | 15.7 | 16.3 | 18.8 | 10.9 | 15.0 | 11.9 | 14.6 | 0.09 | 0.03 |
| No. outpatient visits, mean (SD) | 2.5 (3.5) | 2.5 (3.3) | 2.1 (3.2) | 1.4 (3.0) | 2.9 (3.9) | 3.1 (4.0) | 3.0 (4.0) | 2.3 (3.0) | 2.1 (3.3) | 2.0 (3.3) | 1.5 (2.9) | 0.22 | 0.10 |
| No. inpatient/ED visits, mean (SD) | 0.6 (1.1) | 0.6 (1.0) | 0.5 (1.0) | 0.3 (1.0) | 0.6 (1.4) | 0.6 (1.2) | 0.6 (1.2) | 0.5 (1.0) | 0.5 (1.0) | 0.5 (1.1) | 0.4 (0.9) | 0.14 | 0.06 |
| No. benzodiazepines or Z-hypnotics, mean (SD) | 0.5 (3.7) | 0.4 (3.4) | 0.4 (3.4) | 0.1 (1.2) | 0.7 (1.3) | 0.8 (4.9) | 0.8 (4.9) | 0.5 (3.4) | 0.1 (2.4) | 0.2 (1.8) | 0.1 (0.6) | 0.11 | 0.07 |
| No. NSAIDs, mean (SD) | 0.6 (1.4) | 0.6 (1.4) | 0.5 (1.3) | 0.1 (0.5) | 0.7 (1.5) | 0.9 (1.7) | 0.8 (1.7) | 0.6 (1.3) | 0.3 (0.9) | 0.3 (0.9) | 0.1 (0.4) | 0.31 | 0.18 |
| No. of antidepressants, mean (SD) | 1.1 (2.5) | 1.1 (3.4) | 0.9 (2.3) | 0.3 (0.9) | 1.4 (2.7) | 1.9 (3.3) | 2.0 (3.3) | 1.0 (2.2) | 0.7 (1.9) | 0.6 (1.6) | 0.4 (0.8) | 0.32 | 0.13 |
| No. muscle relaxants, mean (SD) | 0.3 (1.0) | 0.2 (0.7) | 0.2 (0.7) | 0.2 (0.5) | 0.5 (1.4) | 0.8 (1.9) | 0.8 (1.9) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.4) | 0.19 | 0.06 |
| No. other prescription fills, mean (SD) | 16.0 (16.7) | 16.0 (15.1) | 11.4 (15.4) | 3.9 (6.5) | 21.1 (17.2) | 24.4 (20.5) | 21.6 (21.2) | 14.5 (15.4) | 7.6 (12.2) | 6.7 (9.3) | 3.7 (5.9) | 0.72 | 0.40 |
| County-level factors | |||||||||||||
| No. hospitalsc, mean (SD) | 2.6 (3.4) | 2.6 (3.4) | 2.7 (3.3) | 2.5 (3.3) | 2.6 (3.2) | 2.6 (3.7) | 2.7 (3.2) | 2.5 (3.3) | 2.6 (3.0) | 2.7 (3.0) | 2.6 (3.4) | 0.03 | 0.03 |
| No. physiciansc, mean (SD) | 70.3 (30.9) | 70.8 (31.0) | 69.1 (30.2) | 72.4 (28.9) | 70.8 (31.5) | 70.2 (31.5) | 69.8 (30.6) | 71.0 (30.9) | 69.3 (30.2) | 69.1 (29.8) | 70.9 (30.6) | 0.04 | 0.01 |
| No. hospitals with pain management programc, mean (SD) | 0.8 (1.4) | 0.8 (1.5) | 0.9 (1.4) | 0.8 (1.4) | 0.8 (1.4) | 0.8 (1.4) | 0.9 (1.4) | 0.8 (1.3) | 0.9 (1.5) | 0.9 (1.3) | 0.8 (1.1) | 0.04 | 0.04 |
| No. physical medicine/rehabilitation centersc, mean (SD) | 0.4 (0.6) | 0.4 (0.6) | 0.4 (0.6) | 0.4 (0.7) | 0.4 (0.6) | 0.4 (0.6) | 0.4 (0.7) | 0.4 (0.6) | 0.4 (0.7) | 0.4 (0.6) | 0.4 (0.6) | 0.03 | 0.03 |
| Resided in metropolitan counties, % | 72.4 | 72.8 | 71.4 | 75.9 | 73.2 | 72.0 | 67.0 | 74.6 | 72.2 | 70.0 | 78.0 | 0.08 | 0.01 |
| Median household incomed, mean (SD) | 51 (14) | 51 (14) | 50 (13) | 53 (14) | 52 (14) | 51 (14) | 50 (14) | 52 (14) | 50 (13) | 50 (13) | 52 (13) | 0.10 | 0.01 |
| % unemployment, mean (SD) | 5.7 (1.7) | 5.7 (1.7) | 5.7 (1.7) | 5.6 (1.6) | 5.7 (1.8) | 5.7 (1.8) | 5.7 (1.7) | 5.7 (1.9) | 5.7 (1.7) | 5.7 (1.6) | 5.6 (1.5) | 0.04 | 0.03 |
Abbreviations: ED, emergency department; GABA, gabapentinoid; IPTW, inverse probability of treatment weighting; LIS, low-income subsidy; MME, morphine milligram equivalent; No., number of; NSAID, nonsteroidal anti-inflammatory drug; OPI, opioid; SD, standard deviation; SDD, standardized daily dose; SMD: standardized mean difference.
Trajectory groups: A:OPI-only early discontinuers (40.6% of the cohort); B: Consistent low-dose OPI-only users (MME ≤30; 16.6%); C: Consistent high-dose OPI-only users (MME>120; 1.8%); D: GABA-only early discontinuers (12.5%); E: Consistent low-dose GABA-only users (SDD <2; 11.0%); F: Consistent high-dose GABA-only users (SDD ≥3.5; 3.1%); G: Early discontinuation of OPIs and consistent low-dose GABA users (SDD ≤1; 6.9%); H: Consistent low-dose OPI-GABA users (MME <40 and SDD <1.5; 3.4%); I: Consistent low-dose OPI and high-dose GABA users (MME <30 and SDD ≥3; 3.2%); J: Consistent high-dose OPI and moderate-dose GABA users (MME >120 and 1.5< SDD ≤3; 0.9%). Low-, moderate-, and high-dose opioid use was defined as an average daily dosage of <50 MME, 50–90 MME, and >90 MME, respectively. For GABA use, an average daily SDD of <2 (i.e., gabapentin <600 mg or pregabalin <300 mg), 2–3 (i.e., 600≤ gabapentin <900 mg or 300≤ pregabalin <450 mg), and >3 (i.e., gabapentin ≥900 mg or pregabalin ≥450 mg) were considered as low-, moderate-, and high-dose use, respectively.
Average SMD of 45 SMDs from group comparisons (the number of 2-combinations from given 10 elements: ; e.g., group A vs B, group A vs C, group A vs D). The maximum and minimum SMD were presented in eTable 3.
Per 10,000 population
Annual median household income was represented in units of thousands ($)
The identified trajectory groups had significantly different characteristics before including the IPTW. For example, compared to the overall study cohort, individuals in the consistent high-dose OPI and moderate-dose GABA group were more likely to have low back pain (94.3% vs 78.5%) and fibromyalgia (38.0% vs 20.2%), to be younger (52.3±12.3 years vs 65.5±14.5 years), male (45.9% vs 31.9%), white (85.8% vs 76.8%), and to have a disability (81.9% vs 39.3%). After accounting for the IPTW for each beneficiary, most characteristics were comparable across trajectories, except for age, Elixhauser comorbidity index, and number of prescriptions for NSAIDs, antidepressants, and all other prescriptions (SMD >0.1). The minimum and maximum SMD across the 45 group comparisons (; e.g., A vs B, A vs C, B vs C) are presented in eTable 4.
Inverse Probability Treatment Weighted Multivariable Cox Proportional Hazards Model for Drug Overdose, OUD, Non-Opioid SUD
As shown in Figure 2 (also eTable 5), compared with OPI-only early discontinuers (crude rate: 0.8 per 100 person-years), greater than double the risk of drug overdose was observed among individuals in the following trajectories: consistent high-dose OPI-only users (adjusted HR [aHR]=4.57, 95% CI=2.99–6.98), consistent low-dose OPI-GABA users (aHR=2.49, 95% CI=1.76–3.52), consistent low-dose OPI and high-dose GABA users (aHR=2.46, 95% CI=1.71–3.53), and consistent high-dose OPI and moderate-dose GABA users (aHR=7.22, 95% CI=4.46–11.69). Similar findings were observed for the risk of OUD and non-opioid SUDs (eFigures 4–5, eTable 6). Compared with OPI-only early discontinuers, consistent high-dose OPI-only users (OUD: aHR=8.11, 95% CI=5.85–11.24; non-opioid SUD: aHR=2.14, 95% CI=1.61–2.86) and consistent high-dose OPI and moderate-dose GABA users (OUD: aHR=10.89, 95% CI=7.43–15.96; non-opioid SUD: aHR=2.42, 95% CI=1.66–3.53) were associated with the highest risk of OUD and non-opioid SUDs.
Figure 2. Dual-Trajectories of Opioid and Gabapentinoid Utilization Patterns and Risk of Drug Overdose among Medicare Beneficiaries.
Abbreviations: CI, confidence interval; GABA, gabapentinoid; OPI, opioid
Sensitivity analysis
The sensitivity analysis including beneficiaries with any pain conditions yielded similar findings (eFigure 6).(28) eTable 7 shows the robustness of our findings to unmeasured confounders. E-values indicated that the estimated HRs for consistent high-dose OPI-only users and all consistent OPI-GABA users were more robust to the unmeasured confounding. For example, the observed drug overdose risk (aHR=7.22) for high-dose OPI and moderate-dose GABA users could only be explained by an unmeasured confounder that was associated with both this trajectory group and drug overdose by a HR of 13.92-fold each, beyond the current measured confounders, but could not be explained by a weaker confounder.
Discussion
Our study yielded three important findings regarding OPI-GABA use among fee-for-service Medicare beneficiaries with fibromyalgia, neuropathy, low back pain, or osteoarthritis. First, we identified ten distinct dual-trajectories of OPI-GABA use in the 12 months following initiation of OPIs and GABAs. This high variability likely arises from a combination of patient factors (e.g., pain diagnosis, chronicity and severity; medication preferences), prescriber factors (e.g., prescribing preferences), and payer factors (e.g., formulary tiers). Second, the vast majority of beneficiaries received monotherapy, with 59.0% of the beneficiaries using OPIs only, 26.6% using GABAs only, and only 14.4% using OPIs and GABAs, with distinct dose and duration patterns. Third, trajectories characterized by consistent high-dose OPI-only use (MME >120) and consistent OPI-GABA use (regardless of doses) were associated with more than double the risk of drug overdose as compared to OPI-only early discontinuers (i.e., discontinued within a month of initiation).
Our findings are generally consistent with the four previous studies that suggested an increased risk of adverse health outcomes associated with concurrent OPI-GABA use.(9–12) However, the definitions of concurrent OPI-GABA use varied substantially in previous studies, including (1) any overlapping OPI-GABA use in the 120 days preceding the outcome of opioid-related death,(10, 11) (2) any pregabalin use during opioid use,(9) and 3) concurrent OPI-GABA use ≥120 days in 12 months.(12) Using single values (e.g., only focusing on GABA doses exceeding the FDA’s maximum recommendation) over a fixed time period to define medication use only provides a gross measure that may mask heterogeneity in concurrent use and corresponding risk. Alternatively, data-driven group-based multi-trajectory models have advantages and may be valuable to better characterize dynamic changes in concurrent OPI-GABA use over time.(47, 48) Our findings that risk magnitudes vary by OPI-GABA dose over time may help to more effectively identify individuals at highest risk for further management (e.g., case manager follow- up).
Several other findings in our study are also noteworthy for discussion. Individuals in the GABA only trajectory groups were associated with ~1.5 times increased risk of drug overdose compared to opioid-only early discontinuers, even for those having GABAs below the maximum dose approved by the FDA. Although gabapentinoids may be good non-NSAID and opioid substitutes for some patients, clinicians should not assume that gabapentinoids are safer and effective for all pain conditions.(49) Furthermore, we observed consistent weekly exposures to OPIs and/or GABAs among beneficiaries with fibromyalgia, neuropathy, low back pain, or osteoarthritis after initiating OPIs or GABAs. There is a lack of evidence supporting long-term effectiveness for OPI and GABA use (especially for off-label conditions). OPIs and GABAs are often prescribed with medications (e.g., benzodiazepines) that have central nervous system side effects among older adults, and it may not be possible to totally avoid co-prescribing for certain patients. Regularly evaluating clinically relevant polypharmacy with appropriate de-prescribing and dose adjustment plans, along with integrating non-pharmacological pain management approaches should be considered.
Recently, several clinical trials have suggested the efficacy of GABA use for off-label pain conditions (e.g., chronic sciatica; irritable bowel syndrome) and for reducing acute and chronic postoperative pain and OPI use.(50–55) However, given the safety concerns about GABAs, there have been calls for placing more stringent regulations on gabapentin at the federal level and to include gabapentin monitoring in state prescription drug monitoring programs (PDMPs) to promote safety. Our study findings support these actions. At the state level, several states, including Kentucky, Minnesota, Ohio, and Virginia, have required mandatory reporting for gabapentin dispensing in PDMPs.(56) Kentucky further classified gabapentin as a Schedule V Controlled Substance and restricts the amounts of gabapentin that can be prescribed.(56) Other target interventions suggested for clinical practice include promoting health provider education and awareness of the potential risk associated with OPI-GABA use, implementing auto-alert electronic health systems, and providing risk stratification and risk-informed monitoring of individuals with OPI-GABA use. Our trajectory subgroups (e.g., consistent high-dose OPI and moderate-dose GABA use) may be valuable to better guide target interventions.
This study has several limitations. First, our claims-based analyses have limited clinical and socio-behavioral information, such as indications for GABAs and pain severity and pain relief with medication use that may influence OPI-GABA use. Second, we could not determine whether individuals used prescription OPIs and GABAs as prescribed or whether they have obtained additional drugs through case payments, illicit purchases, or from other sources. Although unmeasured confounding could not be ruled out, our E-value results showed that the risk estimates were robust to unmeasured confounders. Third, our diagnosis-based SUD-related outcomes are likely to be underestimated due to under-coding issues because of reasons such as stigma in clinical practice. Prior studies have shown high specificity but low sensitivity in SUD-related outcomes.(57, 58) Fourth, we were not able to link to death certificate data and thus could not distinguish fatal from non-fatal overdoses. Finally, the study results have limited generalizability to other populations (e.g., Medicaid) and Medicare beneficiaries using OPIs or GABAs for diagnoses other than the four included in the current study.
Conclusions
Risk of drug overdose, OUD, and non-opioid SUDs varied substantially by different OPI-GABA trajectory groups among fee-for-service Medicare beneficiaries. High-dose OPI use and consistent OPI-GABA use, especially high-dose OPI and moderate-dose GABA use, were associated with the highest risk of adverse health outcomes. Clinicians should consider relative risks and benefits before prescribing OPIs and GABAs. When co-administration is medically necessary, clinicians should regularly monitor the patient’s benefit-risk profiles for continuation of OPI-GABA use and adjust OPI and GABA doses carefully.
Supplementary Material
Acknowledgments
Funding: NIH/NIA R21AG060308
Conflict of interest
Dr. Kwoh has received grant funding from Abbvie and EMD Serono. Dr. Kwoh also serves as a consultant for EMD Serono.
References
- 1.Gabapentin, Pregabalin. In: Micromedex®2.0, (electronic version). Greenwood Village (CO): Truven Health Analytics; 2019. [Google Scholar]
- 2.Drugs@FDA: FDA Approved Drug Products.
- 3.Johansen ME Gabapentinoid Use in the United States 2002 Through 2015, JAMA Intern Med 2018: 178: 292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radley DC, Finkelstein SN, Stafford RS Off-label prescribing among office-based physicians, Arch Intern Med 2006: 166: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 5.Smith RV, Lofwall MR, Havens JR Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky, Am J Psychiatry 2015: 172: 487–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RV, Havens JR, Walsh SL Gabapentin misuse, abuse and diversion: a systematic review, Addiction 2016: 111: 1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet U, Scherbaum N How addictive are gabapentin and pregabalin? A systematic review, Eur Neuropsychopharmacol 2017: 27: 1185–1215. [DOI] [PubMed] [Google Scholar]
- 8.Buttram ME, Kurtz SP, Dart RC, Margolin ZR Law enforcement-derived data on gabapentin diversion and misuse, 2002–2015: diversion rates and qualitative research findings, Pharmacoepidemiol Drug Saf 2017: 26: 1083–1086. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsson T, Berge J, Ojehagen A, Hakansson A Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study, Drug Alcohol Depend 2017: 174: 58–64. [DOI] [PubMed] [Google Scholar]
- 10.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study, PLoS Med 2017: 14: e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: A nested case–control study, Ann Intern Med 2018. [DOI] [PubMed] [Google Scholar]
- 12.Peckham AM, Fairman KA, Sclar DA All-Cause and Drug-Related Medical Events Associated with Overuse of Gabapentin and/or Opioid Medications: A Retrospective Cohort Analysis of a Commercially Insured US Population, Drug Saf 2018: 41: 213–228. [DOI] [PubMed] [Google Scholar]
- 13.Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G Gabapentin enhances the analgesic effect of morphine in healthy volunteers, Anesth Analg 2000: 91: 185–191. [DOI] [PubMed] [Google Scholar]
- 14.Vashchinkina E, Piippo O, Vekovischeva O, Krupitsky E, Ilyuk R, Neznanov N et al. Addiction-related interactions of pregabalin with morphine in mice and humans: reinforcing and inhibiting effects, Addict Biol 2017. [DOI] [PubMed] [Google Scholar]
- 15.Peckham AM, Fairman KA, Sclar DA Prevalence of Gabapentin Abuse: Comparison with Agents with Known Abuse Potential in a Commercially Insured US Population, Clin Drug Investig 2017: 37: 763–773. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AW, Gellad WF, Skinner AC Lock-In Programs and the Opioid Epidemic: A Call for Evidence, Am J Public Health 2016: 106: 1918–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The US Congressional Research Services: The SUPPORT for Patients and Communities Act (P.L.115–271): Medicare Provisions.; 2019.
- 18.Campbell CI, Weisner C, Leresche L, Ray GT, Saunders K, Sullivan MD et al. Age and gender trends in long-term opioid analgesic use for noncancer pain, Am J Public Health 2010: 100: 2541–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, J Clin Epidemiol 2008: 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 20.Mues KE, Liede A, Liu J, Wetmore JB, Zaha R, Bradbury BD et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US, Clin Epidemiol 2017: 9: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chronic Condition Data Warehouse. CCW Medicare Administrative Data User Guide [Internet]. 2019. [cited June 2019]. Available from: https://www2.ccwdata.org/web/guest/user-documentation
- 22.Kaiser Family Foundation. Medicare Advantage [Internet]. 2019. [cited 4 April 2020]. Available from: https://www.kff.org/medicare/fact-sheet/medicare-advantage/
- 23.Kim SC, Landon JE, Lee YC Patterns of health care utilization related to initiation of amitriptyline, duloxetine, gabapentin, or pregabalin in fibromyalgia, Arthritis Res Ther 2015: 17: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolis JM, Cao Z, Onukwugha E, Sanchez RJ, Alvir J, Joshi AV et al. Healthcare utilization and cost effects of prior authorization for pregabalin in commercial health plans, Am J Manag Care 2010: 16: 447–456. [PubMed] [Google Scholar]
- 25.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain, J Pain 2009: 10: 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pergolizzi J, Boger RH, Budd K, Dahan A, Erdine S, Hans G et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone), Pain practice : the official journal of World Institute of Pain 2008: 8: 287–313. [DOI] [PubMed] [Google Scholar]
- 27.Ip Q, Malone DC, Chong J, Harris RB, Labiner DM An update on the prevalence and incidence of epilepsy among older adults, Epilepsy Res 2018: 139: 107–112. [DOI] [PubMed] [Google Scholar]
- 28.Lo-Ciganic WH, Huang JL, Zhang HH, Weiss JC, Wu Y, Kwoh CK et al. Evaluation of Machine-Learning Algorithms for Predicting Opioid Overdose Risk Among Medicare Beneficiaries With Opioid Prescriptions, JAMA network open 2019: 2: e190968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang Y, Zhao W, Xiang H, Smith GA ED visits for drug-related poisoning in the United States, 2007, The American Journal of Emergency Medicine 2012: 30: 293–301. [DOI] [PubMed] [Google Scholar]
- 30.Pharmacy Quality Alliance: Opioid Core Measure Set-2019.
- 31.Hernandez I, He M, Brooks MM, Zhang Y Exposure-Response Association Between Concurrent Opioid and Benzodiazepine Use and Risk of Opioid-Related Overdose in Medicare Part D Beneficiaries, JAMA network open 2018: 1: e180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Center for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain.
- 33.Centers for Disease Control and Prevention. Calculating Total Daily Dose of Opioids for Safer Dosage..
- 34.Jones BL, Nagin DS A Stata Plugin for Estimating Group-Based Trajectory Models; 2012.
- 35.Twisk J, Hoekstra T Classifying developmental trajectories over time should be done with great caution: a comparison between methods, J Clin Epidemiol 2012: 65: 1078–1087. [DOI] [PubMed] [Google Scholar]
- 36.Daniel SN, Bobby LJ, Valéria Lima P, Richard ET Group-based multi-trajectory modeling, Stat Methods Med Res 2016: 0962280216673085. [Google Scholar]
- 37.Bobby LJ, Daniel SN Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them, Sociol Methods Res 2007: 35: 542–571. [Google Scholar]
- 38.Lo-Ciganic WH, Gellad Walid F, Gordon Adam J, Cochran G, Zemaitis Michael A, Cathers T et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization, Addiction 2016: 111: 892–902. [DOI] [PubMed] [Google Scholar]
- 39.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD et al. Opioid prescriptions for chronic pain and overdose: a cohort study, Ann Intern Med 2010: 152: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley AJ et al. State Legal Restrictions and Prescription-Opioid Use among Disabled Adults, N Engl J Med 2016: 375: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. Area Health Resources Files (AHRF) 2013–2014., Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. [Google Scholar]
- 42.Austin PC An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res 2011: 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dignam JJ, Zhang Q, Kocherginsky M The use and interpretation of competing risks regression models, Clin Cancer Res 2012: 18: 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.UCLA Institute for Digital Research and Education. Testing the Proportional Hazard Assumption in Cox Models. .
- 45.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals., Biometrika 1994: 81: 515–526. [Google Scholar]
- 46.VanderWeele TJ, Ding P Sensitivity Analysis in Observational Research: Introducing the E-Value, Ann Intern Med 2017: 167: 268–274. [DOI] [PubMed] [Google Scholar]
- 47.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence, Med Care 2013: 51: 789–796. [DOI] [PubMed] [Google Scholar]
- 48.Modi AC, Rausch JR, Glauser TA Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy, JAMA 2011: 305: 1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodman CW, Brett AS Gabapentin and Pregabalin for Pain - Is Increased Prescribing a Cause for Concern?, N Engl J Med 2017: 377: 411–414. [DOI] [PubMed] [Google Scholar]
- 50.Saito YA, Almazar AE, Tilkes KE, Choung RS, Van Norstrand MD, Schleck CD et al. Randomised clinical trial: pregabalin vs placebo for irritable bowel syndrome, Aliment Pharmacol Ther 2019: 49: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson K, Marshman LAG, Plummer D, Downs E Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults WIth Chronic Sciatica: A Randomized Clinical Trial, JAMA neurology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Dong Y, Zhang J, Tan H The efficacy of gabapentin in reducing pain intensity and postoperative nausea and vomiting following laparoscopic cholecystectomy: A meta-analysis, Medicine (Baltimore) 2017: 96: e8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han C, Kuang MJ, Ma JX, Ma XL The Efficacy of Preoperative Gabapentin in Spinal Surgery: A Meta-Analysis of Randomized Controlled Trials, Pain physician 2017: 20: 649–661. [PubMed] [Google Scholar]
- 54.Jiang Y, Li J, Lin H, Huang Q, Wang T, Zhang S et al. The efficacy of gabapentin in reducing pain intensity and morphine consumption after breast cancer surgery: A meta-analysis, Medicine (Baltimore) 2018: 97: e11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hesami O, Haghighatzadeh M, Lima BS, Emadi N, Salehi S The effectiveness of gabapentin and exercises in the treatment of carpal tunnel syndrome: a randomized clinical trial, Journal of exercise rehabilitation 2018: 14: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peckham AM, Fairman KA, Sclar DA Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed?, Expert Opin Drug Saf 2018: 17: 519–523. [DOI] [PubMed] [Google Scholar]
- 57.Kim HM, Smith EG, Stano CM, Ganoczy D, Zivin K, Walters H et al. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use, BMC Health Serv Res 2012: 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO Performance Measures of Diagnostic Codes for Detecting Opioid Overdose in the Emergency Department, Acad Emerg Med 2017: 24: 475–483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.