Graphical abstract
Keywords: Coronavirus, SARS, MERS, SARS-CoV-2, COVID-19, Respiratory infection, Immune response, Male, Female
Abstract
Emerging beta-coronaviruses (β-CoVs), including Severe Acute Respiratory Syndrome CoV-1 (SARS-CoV-1), Middle East Respiratory Syndrome-CoV (MERS-CoV), and Severe Acute Respiratory Syndrome CoV-2 (SARS-CoV-2, the cause of COVID19) are responsible for acute respiratory illnesses in human. The epidemiological features of the SARS, MERS, and new COVID-19 have revealed sex-dependent variations in the infection, frequency, treatment, and fatality rates of these syndromes. Females are likely less susceptible to viral infections, perhaps due to their steroid hormone levels, the impact of X-linked genes, and the sex-based immune responses. Although mostly inactive, the X chromosome makes the female's immune system more robust. The extra immune-regulatory genes of the X chromosome are associated with lower levels of viral load and decreased infection rate. Moreover, a higher titer of the antibodies and their longer blood circulation half-life are involved in a more durable immune protection in females. The activation rate of the immune cells and the production of TLR7 and IFN are more prominent in females. Although the bi-allelic expression of the immune regulatory genes can sometimes lead to autoimmune reactions, the higher titer of TLR7 in females is further associated with a stronger anti-viral immune response. Considering these sex-related differences and the similarities between the SARS, MERS, and COVID-19, we will discuss them in immune responses against the β-CoVs-associated syndromes. We aim to provide information on sex-based disease susceptibility and response. A better understanding of the evasion strategies of pathogens and the host immune responses can provide worthful insights into immunotherapy, and vaccine development approaches.
1. Introduction
Following the 2002 outbreak of SARS and the 2012 outbreak of MERS, COVID-19 is the third 21-century coronavirus-related outbreak that has emerged in the human populations and has made the World Health Organization (WHO) declare the red level alert [1]. Coronaviruses are a group of pathogenic, large (~30 kb), positive- and single- standard RNA viruses with significant effects on animal and human health. Most of them may result in respiratory or enteric infections that can be serious and life-threatening [2]. Many of the well-known pathogenic HCoVs, including SARS-CoV-1, MERS-CoV, and the recently identified SARS-CoV-2, are the species of the Betacoronavirus (Beta-CoV) genus in the coronavirus family. Beta-CoV members are of a zoonotic origin and are associated with a variety of challenging issues such as high human-to-human transmission and low clinical manifestation rates [3], [4], [5]. An epidemiological analysis of SARS, MERS, and COVID-19 diseases indicates that the infection and fatality rates of these three viruses are affected by age and sex distributions in a particular population [6]. According to the previous studies, the fatality rates of all the three mentioned HCoV-related syndromes are higher in males [7], [8], [9]. The underlying mechanisms that mediate these sex-based variations are complicated and may originate from a variety of sex-dependent genetic, epigenetic, and hormonal immunity factors [10].
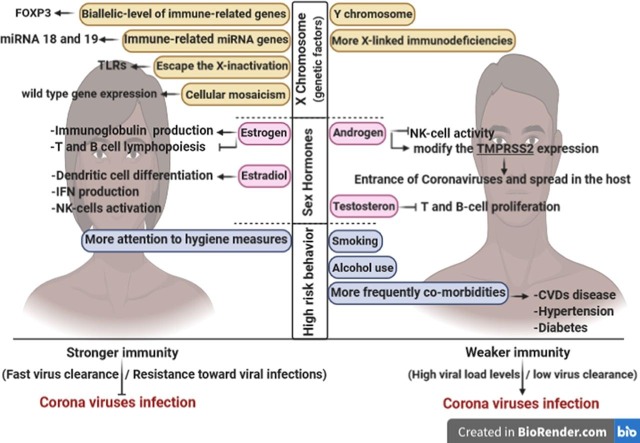
Today, sex is a fundamental biological variable that can affect the intrinsic immune defense against pathogenic agents such as bacteria, fungi, and viruses. We know that both the innate immune system (which has a critical role in the early response against the pathogenic agents) and the adaptive immune system (which is involved in the long-lasting protection and tissue repair) are affected by sex. Many reports have focused on the sex-based differences in immunological pathways activated during the host-pathogen interactions [11], [12]. In this regard, males are more sensitive to several infectious agents, including viruses. This observation can be related to a relatively weaker immune protection in this sex type [13], [14]. It has been proven that females are more protected against viral infections, which can be assigned to both physiological and immunological female-specific responses [14], [15]. Nevertheless, the more robust immune response in females is a double-edged sword, and in some cases, it can contribute to immune deficiencies and autoimmune diseases [10], [16], [17]. Different production rates and metabolisms of the steroid hormones, the effect of the additional X chromosome on the production rate of some essential genes involved in the immune response, and sex-dependent expression of the disease-susceptibility genes are the leading causes which make males more susceptible and vulnerable to viral infections [3], [17].
The X-chromosome is sometimes known as the architect of the immune system. Many immune regulatory genes are located on this chromosome, and it seems that many X-chromosome-linked miRNAs can be substantially involved in the immune system's various functions. However, more shreds of evidence are needed to attribute the sex-specific immune responses to the X-linked miRNAs [12], [18]. Further, physiological differences followed by sex-specific hormone profiles can directly or indirectly affect the immune responses. For example, the male sex hormone (e.g. testosterone) inhibits the innate immune response by decreasing the cytokine, macrophage, and immunoglobulin production rates; in contrast, the female sex hormones (e.g. estrogen) can either suppress the immune system or strengthen its capacity in a dual function at high and low concentrations of the immune modulators, respectively [17], [19], [20]. Until the time of this writing, no effective preventive strategy or treatment approach has been introduced for the SARS-CoV-1 and MERS-CoV infection but recentlysome clinical phase I vaccines have been candidates for the treatment of COVID 19. However, more stringent testing for safety, and evaluation of different immunological responses to these vaccines is required. Hence, having the bulk of previous knowledge on the sex-dependent immune responses, in the present article, after providing a brief introduction to the three rebellious Beta coronaviruse-associated diseases (i.e. SARS, MERS, and COVID-19), we will focus on the sex-dependent immune responses against these HCoVs. We hope that the information provided in this article can help to give an insight into the immunological features of the new and emerging respiratory diseases and offer some clues to alleviate the circulation of these viruses.
2. The Betacoronavirus genus
According to the molecular taxonomic hierarchy, the four genera of the CoVs (i.e. Alpha-CoVs, Beta-CoVs, Gamma-CoVs, and Delta-CoVs) are classified in the subfamily of the coronavirinae and the family of the coronaviridae. Among these four genera, Alpha- and Beta- CoVs mostly infect mammals and humans, while Gamma- and Delta- CoVs generally infect birds and fishes. However, some human infections are also attributed to some members of these species. The genome of the CoVs is a single-stranded positive-sense RNA (+ssRNA) molecule that is about 30 kb in length and contains a 5′‐cap and 3′‐poly-A tail [21]. This genome is wrapped in a phosphorylated nucleocapsid protein, which is further buried in the viral membrane. The CoVs' viral membrane of CoVs is composed of a phospholipid bilayer, specific spike-glycoprotein trimers, glycosylated transmembrane proteins, and envelope proteins [22]. The name corona indicates the shape of this family's particular spike-glycoprotein trimer, which exhibits a crown-like structure on the virus surface under the electron microscope [23].
At least six open reading frames (ORFs) exist in a typical CoV genome [24]. A genome-wide analysis of various species of CoVs reveals a fifty-four percent sequence identity among their genomes, about fifty-eight percent in the nonstructural-protein coding regions, and forty-three percent in the structural protein-coding sequences; this means that nonstructural proteins are more conserved than the structural protein variants among the various species of CoVs. This more considerable variation among the structural viral proteins indicates their determinant role in adapting the virus species to a new host [25].
According to a unique set of ancestry genes, four lineages (A, B, C, and D) are commonly recognized in the Beta-CoV genus. SARS-related CoV belongs to the B lineage, and MERS-CoV belongs to the C lineage [25]. To date, seven HCoV species have been identified, four of which, including NL63, HCoV-229E, OC43, and HKU1, mostly trigger mild respiratory diseases and three of which, including SARS-CoV-1 and MERS-CoV, and the novel SARS-CoV-2, are responsible for the severe respiratory syndromes [26]. SARS-CoV-1, MERS-CoV, and SARS-CoV-2 are known as the Beta-Covs with a zoonotic origin. These three viruses have threatened the world with three severe global outbreaks. The first one (SARS) was reported in November 2002 in Guangdong China, the second (MERS) was first reported in June 2012 in Jeddah, Saudi Arabia, and the third (COVID-19) was first reported in December 2019 in Wuhan, China [22], [27], [28], [29].
The whole-genome comparison of the newly emerged SARS-CoV-2 with its counterparts has shown that the SARS-CoV-2 genome has ~50% similarity with the genome of MERS-CoV and ~70% with SARS-CoV-1. Such a comparison reveals possible similarities and differences among these three pathogens; it can help a better understanding of any possible variations in the relevant responses of their host immune systems, the host-pathogen interactions, and the mechanisms behind any likely anti-immune reactions [29], [30].
3. The most severe Betacoronavirus-linked syndromes (SARS, MERS, and COVID-19)
SARS, MERS, and COVID-19 are the well-known Beta-CoV-linked syndromes, causing severe respiratory deficiencies among human populations. Apart from the respiratory system, other tissues, and organs (e.g. tonsils and CNS) can be affected during the course of the Beta-CoV-linked infections [31]. In the following, some of the essential features of these three syndromes will be discussed. Especial attention has been given to providing some information about the immunological functions of these three diseases.
3.1. SARS-CoV-1 infection
The first epidemy of infectious disease (i.e. SARS) was initially reported from Guangdong province, China, at the end of 2002. So far, SARS has affected more than 8000 people worldwide [32]. Fever, nonproductive cough, myalgia, and dyspnea are mainly mentioned as the clinical manifestations of the SARS-CoV-1 infection, which can be approved by two primary laboratory diagnostic approaches, i.e. chest radiography (to detect air-space opacification in the patients' lung) and molecular tests. A variety of molecular diagnostic tests can be used for this purpose, including PCR for detecting the viral genome and enzyme-linked immunosorbent assay (ELISA), and immunofluorescence assay (IFA) for detecting and tracking of the SARS-CoV-1 specific antibodies [33]. Various types of medications can be used to treat SARS-CoV-1 infection. For example, we can mention Ribavirin (a representer of the nucleoside drugs), Lopinavir and Ritonavir (representers of the therapeutic protease inhibitors), several interferon therapies, 80R immunoglobulin G1 (a representer of the therapeutic monoclonal antibodies), convalescent plasma, and systemic corticosteroids [34].
SARS-CoV-1 (the cause of SARS) is among the coronaviruses that are mostly found in bats and transmitted to humans. The ability to choose a different host directly results from the CoVs' high potential for change. The large RNA molecule, the high frequency of the genomic recombination, and the error-prone function of the RNA-dependent RNA polymerase (RNA replicas) are mentioned as the primary sources of the CoVs' potential for change [35].
Although both SARS-CoV-1 and SARS-CoV-2 belong to the B lineage of the Beta-CoV genus, they have different features. For example, the nucleocapsid protein of SARS-CoV-1 mostly acts as the antagonist of the interferon-gamma (IFN-γ). IFN-γ is a critical cytokine that can directly inhibit the viral replication, so its inhibition may result in higher infection and hence increased fatality rates in the populations infected with SARS-CoV-1. The other differing feature is the different lengths of the spike-glycoprotein trimers between these two species. The trimers are larger in the SARS-CoV-2 structure; this leads to different receptor-binding regions of these two viruses [36], [37]. Both SARS-CoVs use their spike-glycoprotein trimers to bind to the receptors of their host cells. This binding facilitates the entrance of the viral genome into the host cell, followed by both the lytic explosion of the cell and stimulation of the immune system to fight against the viral infection. The other feature of these two SARS-CoVs is their contribution to the elevated levels of both IL-6 and IL-8 during the infection cycle. IL-6 is secreted by macrophages and T-lymphocytes and IL-8 is secreted by monocytes, macrophages, fibroblasts, and keratinocytes during the SARS-CoV infections [34].
Numerous studies have reported some sex-specific features of SARS. For example, Karlberg et al. investigated any probable relationship between the case fatality rate and the chronic disease history, age, and sex among the Hong Kong population in 2004. According to this research, the case fatality rate and hence the disease severity were significantly higher among males. A positive correlation was also reported between the severity of SARS and chronic disease history and the infection period [8]. In another study, Channappanavar et al. examined the sex-based susceptibility of mice to the SARS-CoV-1 infection. They discussed the three main factors that seemed to be involved in the more robust immunity observed in the female mice. They discussed i: the sex-dependent level of steroid hormones (unlike estrogen, higher levels of testosterone in males can suppress the immune response), ii: the doubled copy number of the X-linked immune response genes such as TLR7, which plays an essential role in the pathogen recognition and activation of innate immunity, and iii: the different conditions of the disease susceptibility genes in males and females [3].
3.2. MERS-CoV infection
MERS-CoV was formerly known as the human coronavirus EMC/2012 or HCoV-EMC. This virus is the causative agent of MERS. Genomic studies have revealed a close phylogenetic relationship between this virus and the two bat-specific coronaviruses. This relationship indicates the zoonotic origin of this HCoV. The first bat-specific species of these coronaviruses was first discovered in Hong Kong in 2007; hence, even their non-human variants are relatively new for the researchers. The first confirmed case of MERS-CoV in a human was reported in Saudi Arabia in 2012. During that time, a rapidly progressive contagious pneumonia, with a wide range of associated symptoms, was overspreading in this country, and the first confirmed case, who died from the pneumonia-associated acute renal failure, was a 60-year-old man [38].
MERS-CoV causes severe respiratory problems both in the upper and lower parts of the respiratory tract. Severe MERS is more prominent in people with underlying diseases such as diabetes, hypertension, renal diseases, chronic lung diseases, and immunodeficiencies [39]. The typical symptoms of MERS-CoV infection include high-grade fever, pneumonia, coughing, severe shortness of breath, chest pain, non-respiratory symptoms (such as headache, myalgia, and gastrointestinal symptoms of nausea, diarrhea, or vomiting), and death. Most of these symptoms occur only in severe cases and the older people infected with this virus. The most definitive tests for confirming the MERS-CoV infection are laboratory cultivation of the virus (sampled from respiratory, urine, fecal, or other tissue specimens), serum anti-MERS-CoV antibody titers, and chest radiography [38], [40].
Some studies have shown that the combination of ribavirin (a guanosine analog to hamper RNA synthesis) and IFN-α2b can inhibit MERS-CoV infection in vitro [41]. There are also some other reports indicating that ribavirin can enhance the anti-viral effects of alisporivir (the inhibitor of an immunosuppressant protein, cyclophilin) in laboratory experiments [42]. Until now, none of these potential therapeutics (based on the conventional and currently-used drugs) have received final approval; hence, many researchers have focused on developing new therapeutics to fight MERS. An example is the study of Anand et al., in which they worked on the structure of the 3C-like protease or 3CLpro (an essential protease in the HCoVs replication cycle) to facilitate the introduction of the new therapeutic agents [43]. Moreover, when necessary, IFNs, a variety of anti-virals, antimicrobials, and antifungal agents, can reduce the risk of co-infection during the MERS disease in real clinical experiments [44]. Moreover, some latest updates have reported the potential therapeutic effects of the remdesivir and chloroquine against SARS, MERS, and COVID-19 [45].
Remdesivir (GS-5734) is a nucleotide-based prodrug that is converted to its active form (a ribonucleotide analog) during the metabolism; hence, it is known as an anti-viral agent for a broad spectrum of the RNA viruses, including all the Beta-CoV species. This compound was first developed to treat hepatitis C, while its beneficial effects were further approved for the Ebola virus disease [46], [47]. Chloroquine is known for its robust antimalarial effects. The potency of chloroquine and its derivatives (including phosphate and sulfate salts of chloroquine and hydroxychloroquine), as the immune boosters and anti-inflammatory agents, is also well established [48], [49].
In parallel with the efforts to develop new therapeutic products, there are also many types of research focusing on developing preventive and therapeutic immunization programs. An example is a research by Beigel et al., in which a nanostructure MERS-CoV related spike-protein was used as a vaccine to immunize transchromosomic cattle and produce a fully-human polyclonal IgG immunoglobulin (SAB-301) for therapeutic purposes [50].
MERS-CoV is a large, single-stranded, positive-sense RNA virus that consists of over 30,000 nucleotides. The genome of MERS-CoV comprises 10 genes and four codes for structural proteins, including the specific spike-glycoprotein, the nucleocapsid protein, the virus membrane protein, and the viral envelope. One of the main differences between the SARS-CoV and the MERS-CoV can be seen in their specific spike-glycoproteins. The different receptor-binding domains of these viral spike-glycoproteins differentiate the host cells of these viruses. Hence, the dipeptidyl peptidase-4 (DPP4) receptor-positive cells are the preferred targets of the MERS-CoV. In contrast, the angiotensin-converting enzyme 2 (ACE-2) receptor-positive cells are the preferred targets of the SARS-CoV. Because DPP4 (also known as CD26) shows a more diverse expression pattern on the surface of different cells, MERS-CoV has a broader cellular tropism than SARS-CoV [51], [52].
Several studies have revealed that, in many cases, the risk perception of a particular disease is connected with the sex type of infected people. Likewise, similar to the other Beta-CoV-associated diseases, MERS-CoV infection seems to be more common among males. Moreover, disease severity has been proved to be more prominent in patients who are suffering from other underlying diseases. An example of these relevant studies is published by Sherbini et al. in 2017. In this work, Sherbini et al. referred to a report by the WHO and mentioned that 65% of the MERS-CoV confirmed cases during 2012 and July 2015 were males with an average age of 50 years [53]. Overall, we can say that males and females differ in the severity, prevalence, and pathogenesis of the Beta-CoV-related infections, and it is evident that males are more susceptible and more vulnerable to these syndromes than females.
Many researchers worldwide have tried to explain these sex-specific variations in both disease susceptibility and vulnerability. For example, Klein and Flanagan tried to discuss sex as a determinant biological variable that can affect the immune system and hence disease-related features and traits. An explanation provided by these authors was the higher titer of the activated T helper cells (also known as CD4+ T-cells) and higher CD4/CD8 ratios in the females than the males of the same age. T helper cells play an essential role in mediating immune responses by secretion of specific cytokines, affecting antiviral susceptibility and treatment [11].
In another relevant study, Alghamdi et al. assessed the epidemiological features of 425 MERS-approved cases in Saudi Arabia in 2014. This study also showed more vulnerability of males to MERS-CoV infection. Both incidence and death rates were higher in males. They proposed that higher testosterone levels and hence elevated testosterone-mediated immunosuppression could be involved in their observations. They also believed that men spent more time outdoors and thus faced a higher risk of exposure to viral agents. Another feature of this study was the elucidation of a positive correlation between age and the disease rate as well as the impact of the background diseases on the MERS-associated death rate. These researchers showed that the disease and fatality rates were higher for those aged >45 years. Moreover, they showed that young but unhealthy people were also more susceptible and vulnerable to MERS-CoV infection. They believed that the weaker immune system in these two groups could explain some features of their observations [7].
IFN system is a potent weapon of innate immunity which helps fight against viral infections. A variety of mechanisms are involved in this kind of protection. In one scenario, IFNs are secreted by the infected cells and help to inhibit the binding of the viral particles on the neighboring cell surface. Such an IFN immune response is also considered the primary response of mammalian cells to viral infections [54], [55]. This kind of immunity is weaker in MERS-CoV since this virus produces three types of IFN antagonist proteins (i.e. ORF4a, ORF4b, and ORF5). These three proteins are also involved in the inhibition of the NF-kappaB signaling pathways; these pathways are actively involved in the production of the cytokines and cell survival requirements [44].
3.3. SARS-CoV-2 infection
On December 31, 2019, an outbreak of a new pneumonia-like syndrome in Wuhan was reported to the WHO office in China. Further, this new disease (COVID-19) was attributed to a member of a Beta-CoV species nominated as SARS-CoV-2. This new disease's rapid spread made the WHO declare a pandemic situation on March 11, 2020 [56], [57]. Some researchers believe that SARS-CoV-2 may have originated from bats and has been transmitted to humans via an intermediate host (e.g. Pangolin) [58].
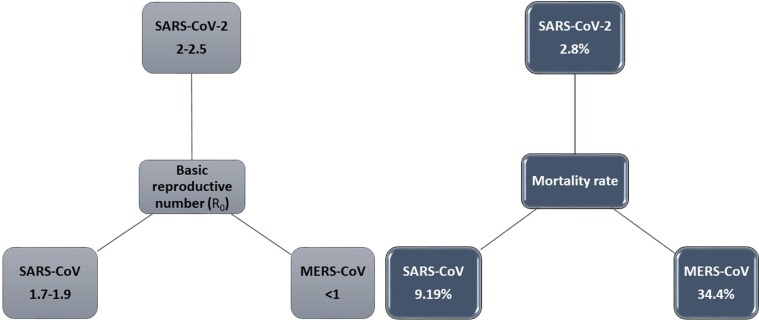
Overall, COVID-19, with approximately 80% asymptomatic or mild infection, is not as severe as its two counterparts (MERS and SARS). A comparison of the mortality rates of these three acute syndromes (Fig. 1 a) provides proof for this claim. However, the extremely contagious nature of COVID-19 (Fig. 1 b) makes this disease more hazardous [5]. As mentioned, an exciting feature of the SARS-CoV-2 is that this viral agent can induce extremely variable responses in the exposed individuals. Not all the individuals exposed to SARS-CoV-2 will be infected; hence, there are a considerable number of COVID-19 asymptomatic carriers, which makes many difficulties in the controlling programs. Moreover, not all the remaining infected patients will experience severe respiratory symptoms, although about 2–6% of the infected people will die from the disease's complications [59]. While more information is still needed to describe these observations, there are some explanations for this variable susceptibility and vulnerability in a number of viral infections. An example is a study by Dutta et al., in which they showed that different major histocompatibility complex (MHC) loci, also known as human leukocyte antigen (HLA) haplotypes, might be involved in such variable disease manifestations [60].
Fig. 1.
Basic reproductive number (R0) (a) and Mortality rate (b) of SARS-CoV-2, SARS-CoV, and MERS-CoV. The basic reproductive number of SARS-CoV-2 is still contentious. It shows that R0 of COVID-19 is a little higher than SARS and MERS (a), but the COVID-19 mortality rate is lower than SARS and much lower than MERS (b) [136].
Both the prevalence and severity of COVID-19 seem to be associated with the sex of the infected people. For example, the study of Yang et al. on 710 patients with the SARS-CoV-2 infection revealed that 67% of their sample size were male, and 33% were female [61]. In another study by Guan et al., on a population of 1099 COVID-19 patients in China, demographic features of the study showed that more share of the patients (about 58%) was males as well [62]. One explanation for such an observation may be the sex-specific expression of the ACE-2. The receptors of ACE-2 are also expressed differently in the sex-specific reproductive organs. It has been shown that ACE-2 titer is more prominent in the testis than in ovaries [63]. Zhao et al. applied the single-cell sequencing technology to assess the ACE-2 expression levels in the healthy human lung tissues. They showed that the ACE-2 expression was higher in Asian males than the females of the same ethnicity. As mentioned earlier, the ACE-2 receptors are the preferred targets of the SARS-CoVs; hence, their higher expression level can be linked with a higher prevalence of the disease [64]. Another important discovery for understanding the mechanism of Infection with SARS-CoV-2 involves the function of serine protease 2 transmembrane (TMPRSS2), a cell surface protein expressed by epithelial cells in different tissues. Coronavirus entry and spread in the host is dependent on the TMPRSS2. In fact, at first, the viral spike protein is attached to the ACE2, and then, viral S protein is trimed by the TMPRSS2 of the host cell to let the internalization of viral particles. Understanding the different expression levels of the TMPRSS2 in the lungs of males and females can give valuable insights into variable infections of coronavirus. Since androgens play a critical role in controlling TMPRSS2, the higher prevalence of coronavirus in males can be described by TMPRSS2 [65].
Some sex-specific habits may also exacerbate these sex-specific observations. For example, smoking is more prevalent in men, and it has been shown that smoking can be involved in the overexpression of the ACE-2 receptors in the lung cells [66].
Interestingly, based on its site of expression, ACE-2 can have two different functions. This protein can be a transmembrane protein located in the specific cell membranes or can be found in plasma. As a transmembrane protein, ACE-2 acts as a gateway for SARS-CoV-2 entry, and its soluble form acts as an anti-inflammatory agent [67].
In general, after exposure to a pathogenic dose of the viral particles, SARS-CoV-2 infection occurs in three main steps in patients with severe symptoms. In the first step, which is also known as the asymptomatic phase of incubation, the virus titer is very low or sometimes unmeasurable. In the second step, the virus titer is increased and can be detected more easily—this higher titer of the virus load is accompanied by non-severe symptoms. In the final step, the titer of the virus load is exceptionally high, and the patient suffers from severe respiratory conditions. During the first two steps of the infection, a specific adaptive immune response is needed to prevent the progression of the disease to its final malignant stage. In these two steps boosting the immune responses can be beneficial for the prevention of the disease progression [68].
The immunology of COVID-19 and hence boosting the immune responses in this disease in some cases are somehow different from other similar respiratory syndromes. For example, a T-cells' critical function is mediating the cytokine release syndrome (CRS), which is often seen in severe respiratory malignancies. There are some pieces of evidence indicating that T-cells are not sufficiently activated during the SARS-CoV-2 infection; alternatively, the unusually high levels of the lymphocytes (known as lymphocytosis) during the severe course of the COVID-19 indicate that CRS may be mediated via activation of leukocytes in the COVID-19 patients. This insufficient activation of T-cells during the SARS-CoV-2 infection is associated with the lower levels of the IFN-γ than the other similar situations. Accordingly, when using the MSC therapy as a boosting strategy to improve the immune responses, it would be necessary to pretreat them with IFN-γ; this makes them more effective in the promotion of tissue repair or suppression of hyperactive immune responses [68], [69], [70].
4. Pharmacotherapy of the CoV-linked syndromes
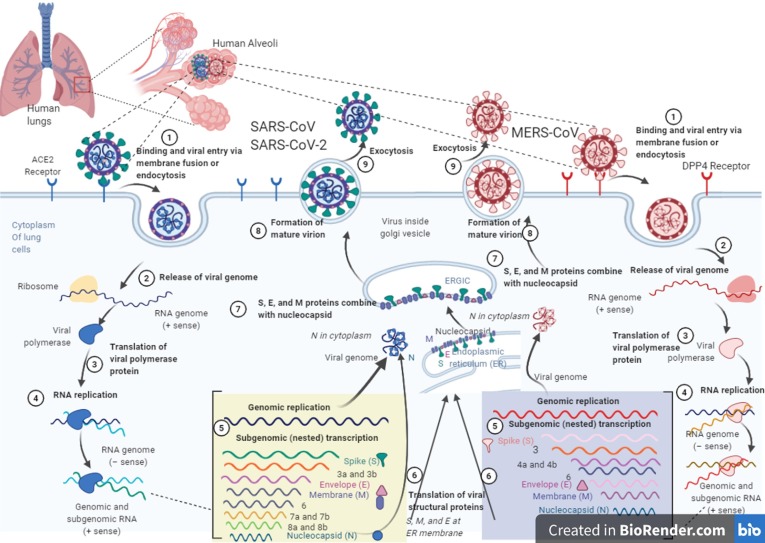
The specific spike-glycoproteins of CoVs, which are essential for their entrance into the host cells, are the critical drug targets of any kind (e.g. antibodies, small chemicals, or any other inhibitory molecules). We know that SARS-CoV infections happen via the attachment of the receptor-binding domain of the viral spike-proteins and a specified cellular receptor on their host cells (i.e. ACE-2) [23]. In the case of MERS, the viral spike-proteins are attached to the dipeptidyl-peptidase 4 (DPP4), which is also known as CD26. DPP4 is the MERS specific host cell receptor, and surprisingly, it does not have any structural similarities with ACE-2 [71], [72]. Such a host cell receptor-viral spike-protein attachment, followed by the viral particles' fusion into the host cells, is the frontline of the viral infection cycle (Fig. 2 ) [73], [74].
Fig. 2.
The life cycles of MERS-CoV, SARS-CoV, and SARS-CoV-2 in host lung cell. The entrance of SARS-CoV and MERS-CoV viruses into cells are happened by an endosomal pathway through special receptors on the host cells. The receptor of SARS-CoV is ACE2, which binds through S glycoprotein on the surface of viruses. MERS-CoV bind to the DPP4 receptor through S glycoproteins. The viral RNA is released into the cytoplasm after the virus has entered the host cell. Replication of genome leads to the production of full-length (−) RNA copies of the genome as templates for full-length (+) RNA genomes. Also, transcription of the genome leads to the production of 7–9 RNAs, which produces some structural proteins. S, M, and E proteins are produced at the endoplasmic reticulum (ER) membrane. Nucleocapsids are manufactured in the cytoplasm from genomic RNA and N proteins, followed by budding into the lumen of the intermediate ERGIC (endoplasmic reticulum (ER)–Golgi compartment). The virions are then released by exocytosis from the infected host cell. (life cycle of SARS-CoV-2 is similar to SARS-CoV). The artwork is provided by BioRender (https://biorender.com/).
Efficacy of a variety repositioned drugs for the CoV-linked syndromes is under investigation. There are several classification for COVID-19 drugs, include: nucleotide analogs (remdesivir), nucleoside analogs (favipiravir), protease inhibitors (lopinavir/ritonavir.combination therapy), biologics (tocilizumab and sarilumab), and various types of RNA synthesis inhibitors [75], [76]. Biomedical therapies including varity of immunotherapy approaches are being examined by many researchers worldwide. Some of these efforts consist of inhibiting the attachment of the viral particles to their host cells using anti-viral antibodies (either IgM or IgG or both of them) and developing potential vaccine products. Data from animal model and humane were assessed by vaccine developers and manufactures to develop vaccines against COVID-19. There are currently more than 200 developing COVID-19 vaccines candidates and some of them have developed into clinical stages and a few of them have recently moved into final stages of testing [77]. Up to Dec. 11, 2020, according to Coronavirus Vaccine Tracker (By Carl Zimmer, Jonathan Corum and Sui-Lee Wee), five vaccines have introduced for limited use, two vaccines have approved for full use and one vaccine (a vaccine from Australia’s University of Queensland) has abandoned after trials. These vaccines have been produced base on variable types including Inactivated, Non-Replicating Viral Vector, Protein Subunit, mRNA and DNA [78]. According to the U.S. Food and Drug Administration (FDA), Pfizer-BioNTech COVID-19 Vaccine was the first vaccine which has met the statutory criteria for issuance of an EUA. this vaccine contains a small piece of the SARS-CoV-2 virus’s mRNA that instructs cells in the body to make the virus’s distinctive “spike” protein. By receiving this vaccine, body produce copies of the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2 [79]. Despite the diversity of medical interventions, the fact that incidence of COVID-19, its mortality rate, and the effects of medical interventions vary between males and females, has not substantially affected the scientific investigations and very few studies consider various therapeutic approaches for the two sexes [80]. Variations in weight, body mass index, and hormonal changes (during puberty, menstrual cycles, and menopause) can be factors that lead to sexual differences in adverse reactions to antiviral drugs [81] Hence, an appropriate treatment for patients is the use of hormone therapy. Sex hormones such as testosterone, progesterone, and estradiol can be considered in these therapeutic processes (Table 1 ) [80], [82], [83].
Table 1.
Summary of clinical studies related to the sex hormone that may be involved in the different response to SARS‐CoV‐2 between sexes.
| Clinical Trial Study | Intervention/Treatment | Population | Phase | Estimated Enrollment | Ages Eligible for Study | Estimated Study Completion Date | Clinical trial ID |
|---|---|---|---|---|---|---|---|
| Estrogen Therapy in Non-severe COVID-19 Patients | Estrogen Therapy | Male ≥ 18 years of age and female ≥ 55 years of age | Not Applicable | 60 participants | 18 Years to 70 Years (Adult, Older Adult) | March 30, 2021 | NCT04539626 |
| Selective Estrogen Modulation and Melatonin in Early COVID-19 | Toremifene + Melatonin | Clinical testing positive for SARS-Cov-2 by standard RT-PCR or equivalent test | Phase2 | 390 participants | 18 Years and older (Adult, Older Adult) | September 2021 | NCT04531748 |
| Estrogen Patch for COVID-19 Symptoms | Estradiol patch | Male ≥ 18 years of age or female ≥ 55 years of age | Phase2 | 110 participants | 18 Years and older (Adult, Older Adult) | November 15, 2020 | NCT04359329 |
| Phase II RCT to Assess Efficacy of Intravenous Administration of Oxytocin in Patients Affected by COVID-19 (OsCOVID19) | Oxytocin (OT) | Patients Affected by COVID-19 Age | Phase2 | 145 participants | 18 Years and older (Adult, Older Adult) | December 31, 2020 | NCT04386447 |
| COVID-19 In-vitro Diagnostic Test and Androgen Receptor Gene Expression | COVID-19 Androgen Sensitivity Test (CoVAST) | Males over age 18 who have tested positive for SARS-CoV-2 infection within the University of California | – | 200 participants | 18 Years to 90 Years (Adult, Older Adult) | August 31, 2021 | NCT04530500 |
| Anti-Androgen Treatment for COVID-19 | Ivermectin + Azythromycin + Dutasteride | Males Positive SARS-CoV-2 rtPCR test in the past 7 days | Not Applicable | 381 participants | 50 Years and older (Adult, Older Adult) | January 31, 2021 | NCT04446429 |
| Progesterone for the Treatment of COVID-19 in Hospitalized Men | Progesterone 100 MG | Male patients with documented pneumonia | Phase1 | 40 participants | 18 Years and older (Adult, Older Adult) | April 17, 2021 | NCT04365127 |
5. Sex-dependent immunological responses to viruses
Sex is among the main determinants that affects both the innate and adaptive immune responses. Hence, in many cases, immunological responses to viruses differ significantly between men and women. These sex-based differences are evident in a variety of living organisms. It is surprising that, in general, males have a weaker immune system than famels. In this regard, many findings have indicated that fatality rate is higher in males than age-matched females in all 3 viral infections of SARS, MERS, and COVID-19. It should be noted that this higher susceptibility of males to coronavirus infections increase with advancing rise in age [3]. In their study, Ding et al. showed that menstrual status and female hormones, including anti-Müllerian hormone (AMH) and Estradiol (E2), were associated with the severity of COVID-19 infection. Actuallly, nonmenopausal females were milder in the severity and had better results than males who were age-matched. This difference disappeared when postmenopausal females were compared with age-matched males [84].
As the first line of the immune defense, the innate immunity plays a critical role in fighting against CoV-related infections. The viruses are first sensed through an evolutionarily conserved pathogenic structure/sequence known as the pathogen‐associated molecular patterns (PAMPs). In the host organism, each specific PAMP of a group of viruses is recognized by various germline-encoded pattern recognition receptors (PRRs), which induces some relevant immune responses. For example, RLRs (the retinoic acid inducible gene 1 (RIG1) like receptors) can realize the particular RNA sequence of many RNA viruses, including MERS-CoV, SARS-CoVs [85], and Influenza A virus [86]. Upon recognition, the PAMP-TRL (toll like receptors) binding will result in the TRL activation and the recruitment of the adapter proteins within the immune cells. The adapter proteins (such as MyD88 and TRIF) can mediate a series of protein–protein interactions and signaling pathways within the immune cells; hence, they are very important for the immediate action of these cells during the viral infections (e.g. the production of the interferons, inflammatory factors, cytokines, and chemokines) [87], [88]. However, SARS-CoV and MERS-CoV were known to escape the host recogonition of their dsRNA by replicating in the absent vesicles of double membrane PRRs [89].
Type-1 IFN secretion is mediated as the result of the PAMP-PRR interactions and is among the most critical anti-viral responses. Interferons can stimulate the NK cells and macrophages to take part in the clearance of the virus particles, on the one hand, or directly involve in the IRF anti-viral pathways, on the other hand [90], [91], [92]. High expression of IRF-7 in females induces the production of IFN-b by dendritic cells and can decrease the risk for viral infection adverse outcomes in females compared with males [3]. IFN and inflammatory cytokines are higher in females than males, which can contribute to more circulation of T cells in females [11]. Evidence from studies has revealed that SARS-CoV pathogenesis can be controlled by IFN treatment [93]. Like any other kind of pathogens, evasion from the innate immune responses can also be traced among the CoVs. It has been shown that the Beta-CoVs can either control or modify the innate immune responses via several known and unknown mechanisms. Some reports have indicated that the SARS- and MERS- CoVs are able to inhibit the production of the IFNs. This inhibition can lead to limited viral clearance, followed by more severe symptoms. Delayed IFN signaling has also been mentioned as a causative factor for the high replication number, which occurs during the SARS-CoV-2 infection [5], [85], [94]. In the case of the innate immune system, it has been proven that both the titer and activity of the innate immune cells (including dendritic cells, monocytes, and macrophages) are higher in females (Fig. 3 ). Moreover, females show more robust inflammatory immune responses against pathogenic infections [95]. Observations have indicated that lung intense inflammatory response after SARS infection remains even higher in males than in females. In particular, males with SARS-CoV infection show high numbers of IMMs, which are known as a significant origin of proinflammatory cytokines and chemokines in SARS infection [3].
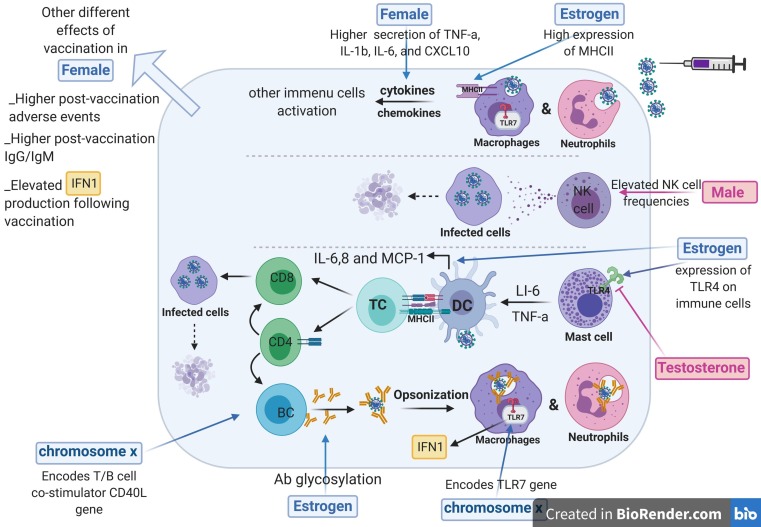
Fig. 3.
Sex-specific immune responses to vaccination. Innate and adaptive immune responses to vaccination are overall different in males and females, including sex chromosomal and hormonal differences. Sex-based effects of vaccination are more pronounced in females than males. At the first step of immune responses, neutrophils and macrophages release more inflammatory cytokines and chemokines in females. Actually, females have more levels of neutrophils and macrophages, but in contrast NK cell frequencies are more in males than females. Sex hormones of estrogen mostly influence responses to vaccination. Major histocompatibility complex (MHCII) that exists on antigen-presenting cells of dendritic cells, macrophages and B cells, is upregulated by estrogen. Also, while testosterone has an inhibitory effect on the expression of TLR7, estrogen exerts a stimulatory impact on them. Also, estrogen leads to more expression of IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) in the dendritic cells. In adaptive immunity, estrogen result in antibody glycosylation levels. X-chromosome encodes the genes involved in immune responses to vaccination. TLR7 and CD40L locate on the X-chromosome and more expressed in females than males.
Adaptive immune responses are the second line of defense against viruses. It has been proven that in the case of viral infections (including Beta-CoVs), the function of the Th1 (T helper 1) immune cells is the leading adaptive immune response against the viral pathogens [5]. During the infection cycles, the cytotoxic T-cells are involved in the production of the anti-viral cytokines. They can also kill the infected cells [96]. The high rate of genetic variability in the Beta-CoVs and inhibition of antigen presentation pathways (which are mediated by MHC class 1 and 2), besides many other factors, are responsible for their escape from the pre-existing adaptive immune responses [97]. The adaptive immune responses are stronger in famales, as well; they elicit more robust humoral immune responses and have a higher titer of the Th1 cells [95], [98]. However, this strength of the immune system makes females more susceptible to autoimmune diseases and increased adverse biological reactions in vaccination [10], [99].
Notably, Sex-based differences in both innate and adaptive immune responses are among the most determinant factors affecting prophylaxis, exposure, pathogenesis, intension, and clearance of the CoVs. Overall, famales are less susceptible and less vulnerable to viral infections, although, as a result, protective responses upon vaccination programs are more severe in females [3], [98], [100]. There are many biological mechanisms underlying all of these different sex-based functions against corona viruses. In general, it should be said that sex-based immune responses can be affected by the individuals' genetic profile, sex hormone expression levels, age, and many environmental variables [11]. In the following, we will discuss the main mechanisms involved in all these sex-based immunological variations. These pieces of information may be beneficial for a better understanding of the success of the therapeutic approaches as well as mitigation policies against Beta-Covs.
5.1. Effects of the sex chromosomes on the host immune responses
The X chromosome encodes a variety of immune system-related genes. The biallelic expression of the X-linked genes dramatically contributes to many immune-based diseases; most of which are the autoimmune abnormalities. The biallelic expression involves in both susceptibility and vulnerability against viral infections, as well [18]. Apart from the biallelic expression, we know that the X-linked immune-related genes are more active in females than in males [101].
Recent studies on the sex-based variability of the innate immune system have revealed a different activation pattern for the genes involved in the innate immunity between males and females. TLR7, TLR8, FOXP3, CD40L (CD154), and the interleukin-1 receptor-associated kinase 1 (IRAK1) are some examples of these differently activated genes. Among these genes, TLR7 and IRAK1 are the key players of the pro-inflammatory immune responses [11], [12]. TLR7 is among the genes which can escape the X-inactivation and hence is overexpressed in females. The sex-based overexpression of the TLR7 gene is associated with a higher expression of the IFNs and the IFN-stimulated genes (ISGs) and will ultimately result in higher levels of activation of CD8+ T-cells in females [102].
It has been proven that females have a diverse cell population with preferential expression of either maternal or paternal X-linked genes. This phenomenon, which occurs as a result of uneven X-inactivation, is known as cellular mosaicism. Cellular mosaicism can lead to reduced immune dysregulation (which is associated with a stronger immunity) and an increase the number of the wild type gene expression (which is followed by increased diversity in the immune responses) in females [103], [104], [105].
In contrast to the Y chromosome, the X chromosome is among the most miRNA gene containing chromosomes [11], [106]. In addition to the immune-related genes, the X chromosome encodes several critical microRNAs that can be involved in the regulation of many immune responses, as well [14], [107]. miRNA-18 and miRNA-19, which are encoded by X chromosome, have been reported to regulate some immunological reactions. Moreover, miR-233 is considered a regulator of neutrophil differentiation, and miR-424, miR-503, and miR-542 have been reported to downregulate the differentiation of monocytes [11], [14]. More concentration of miRNAs on the X chromosome can probably result in the importance of miRNAs in the sex-based differences in the immune system.
Moreover, the immune system is influenced by age-dependent alterations in hormone levels. Studies on SARS-CoV-1, MERS-CoV, and SARS-CoV-2 epidemics have revealed that viral infection-linked adverse outcomes are lower in younger males and females. There were also more infection susceptibility and case fatality rates (CFRs) in older patients than in younger patients [108]. This could not be associated with mere genetics or chromosomal components and is thought to be related to the hormonal effects. The level of sex hormones varies over the life course of individuals. Generally, sex steroid levels decrease with a rise in age in males and females, which leads to the attenuation of immunity function. Estrogen levels in females are low after menopause, which reflects weaker immune responses and result in increased susceptibility to viral infection in old age [11]. The protective effect of estrogen, which causees stronger clearance of viruses in females than males, is lost with a rise in age, and the immunological advantage dwindles in the aged females [109]. Considering those findings, administration of hormone replacement therapy may assist for treating older cases of SARS-CoVs.
5.2. Impacts of the sex hormones on the host immunity
Sex hormones, also known as sex steroids, consist of three main classes of hormones, including estrogens, progestogens, and androgens. They are responsible for a substantial part of the sex-specific differences. All of them can be found in both sex types. The expression levels of the androgens are higher in males, while estrogens are more expressed in females. Hence, androgens and estrogens are known as the male and female sex hormones, respectively. Progesterones are involved in a variety of sex-specific functions in both males and females. The sex-specific hormones as well as their sex-specific functions can also affect the immune system of these two sex types and participate in the sex-specific immune responses [63], [110], [111]. Expression of a variety of sex hormone receptors (of any kind) on the surface of the immune cells is a proof for this process [14].
Estrogens with a dual role in the immune system result in immune suppression at a high dose and lead to immune induction at a low dose [3], [112]. More precisely, in addition to estrogen concentration, there are many other factors affecting estrogen activity. Therefore, the effects of estrogen can be verified in different cell types and organs and also variable conditions in the immune system. Because of the large quantity of data about the dual role of estrogen in the immune system, and according to the purpose of our study, we focus more on its tendency to immunoenhancing. The estrogen-related downstream signaling in females leads to neutrophil accumulation and also stimulation of adaptive immune responses in T-cells [113]. Several studies have revealed that estrogens and their receptors (ERα and ERβ) contribute to the regulation of immune responses. During IAV (influenza A virus) infection, ERα decreases the inflammatory cytokine responses and mortality rate in the female mice [113], [114]. Likewise, the activation of endothelial estrogen receptors has been proven to inhibit the angiotensin II-mediated inflammation and ROS generation [115]. ERs were also detected in PBMCs and T cell lymphocytes and estrogen could modulate the lymphocytes by binding to these specific receptors. It was demonstrated that estrogen reduce TNF-α-induced toxicity by inhibition of TNF-α-induced signaling in T cells and increase cell survival by apoptosis prevention [116]. Generally, via ERS-mediated mechanisms, estrogen modulates the Immune activities like inhibition of T suppressor, helping the T helper maturation, induction of CD5 + B cells, production of autoantibodies, and regulation of macrophage activity [112], [117]. Recent studies have shown that mice treated with estrogen receptor antagonists are more susceptible to SARS-COVs. Thus, it can be inferred that estrogen may have a protective role against coronaviruse infections [3]. Estrogen has also been revealed to alleviate lung myeloperoxidase expression, which may happen during extensive inflammation in the coronavirus patients [112].
As the most potent estrogenic derivative in human, estradiol [118] has been reported to elevate innate and adaptive immune responses. Estradiol leads to more activation of NK cells and upregulation pro-inflammatory cytokines [119]. In a study, estradiol was reported as a mitigation agent of coronavirus. The results revealed that estradiol might be a candidate as a potetnt drug for controlling coronavirus infection by downregulating the ACE-2, a gene that is needed for coronavirus cell entry [120]. Moreover, 17β-estradiol was suggested as a stimulator of B cell differentiation and antibody secretion by inhibiting the activity of suppressor T cells, which suppress the B cells. Estradiol can also cause the accumulation of immuneglobin cells (IgM-containing and IgM-secreting). However, the high dose of this hormone has been shown to be toxic with immunosuppressive effects [112], [121].
Based on the results of a recent study, the menopausal females had more severe COVID-19 infection with increased mortality than the nonmenopausal ones. In the above-mentioned experiment, the menopausal patients displayed worse outcomes and higher hospitalization proportion than the nonmenopausal patients. These results might be associated to estradiol and its related inflammation and immunity modulation roles. During a viral infection, estradiol contributes to immunopathology by regulating the expression of cytokines and chemokines and can help treat the individuals diagnosed with COVID-19 [122].Testosterone as a male hormone acts differently and leads to the inhibition of innate immune responses. Testosterone exerts its immunosuppressive effects by limiting the T- and B-cell proliferation and TLR expression [105]. It has also been reported as a potential agent involved in the promotion of coronavirus infection [120]. Although testosterone generally results in immune suppression and may lead to high susceptibility to virus infection in males, it can exert a protective impact on respiratory muscles by its anti-catabolic properties. In light of that, the coronavirus patients are substantially damaged in lung and reveal acute hypoxemic respiratory failure, testosterone may lead to less assisted ventilation in patients [123].
Gaskins et al., showed that CRP concentrations is fluctuating during menstrual period with a peak on menses. CRP is inversely related with levels of endogenous estradiol but positively related with levels of luteal progesterone. They provided a comprehensive assay of interplay between CRP and endogenous hormones in reproductive age woman [124].
Both estrogen and testosterone have been reported to be associated with inflammatory signaling pathways, which are the most theoretical accepted pathways involved in corona virus-related damage to the respiratory tract. The anti-inflammatory effects of estrogen and testosterone have been proved to be useful for therapeutic purposes. Estrogen inhibits the pro-inflammatory cytokines, including IL-6, IL-8, and TNFa, and causes inflammation suppression in a dose-dependent manner [112], [124]. Additionally, estrogen restricts the activity of free radicals and consequent inflammation because of generation of esterogen-induced nitric oxide and regulation of leukocyte recruitment. Noticeably, many researches have reported a negative relationship between endogenous estrogen and inflammatory hallmarks by investigating the anti-inflammatory activities of estrogen, including antioxidant activity, antiapoptotic effects on various cell types, and suppression of cytokines and the renin‐angiotensin system [124], [125]. It has also been indicated that testosterone has anti-inflammatory effects and can decrees the IL-6 and TNF-a inflammatory markers [126].
The SARS-CoV-2 entry to the cells mediates through ACE2 receptor abundantly present on pneumocytes and leads to negative regulation of ACE2 which converts Angiotensin (Ang) II to less immune stimulating variants of Ang. Ang2 could bind to type 1 Ang receptors that causes activation of NF-KB pathway. NF-KB signaling increases cytokine production, contraction of the vessels (vasoconstriction), and immune reactions. The activated estrogen alpha receptor has been shown to prevent the starting of immune responses due to suppression of NF-KB signaling [127], [128], [129]. Besides as mentioned above, testosterone (Androgen) also like estrogen could slap down humoral and cellular immune responses where reduces IL-6 and TNF-α by restricting NF-KB signaling, similar to estrogen [126].Therefore, both estrogen and testosterone as sex hormones could shut down NF-KB signaling as master active signaling in COVID-19 disease, can be very promising strategy for hormone replacement therapy [123].
6. Sex differences in vaccine-induced immunity
Vaccination is a public health intervention with the greatest impact on reducing different diseases and mortality rate and is recognized as a cost-benefit public health investment. The first vaccine was used in 1980 to eradicate smallpox and then poliovirus, Haemophilus influenza type B (Hib), etc. [130]. Sexual differences in immune reactions affect both innate and acquired immune responses, thereby contributing to variations in susceptibility to infectious diseases and vaccine responses (Fig. 3) [131]. Biological differences related to a person's sex are the principal source of change in the immune response to vaccination. Clinical results show that males and females have different reactions to viral vaccines in their intrinsic, humoral, and cellular aspects. Sex affects the frequency and severity of the harmful side effects of vaccination, such as fever, pain, and inflammation [98]. In other words, these adverse reactions are more frequent in females. In addition, females have higher antibody responses to vaccines than males in equal doses, resulting in more inflammatory responses and increased adverse biological reactions [99]. Different studies have shown a sex disparity in vaccination against different viruses such as seasonal influenza virus [132], HBV, Hepatitis A, and Herpes simplex-2. All of these studies have indicated that both adverse reactions to viruses and post-vaccination antibody titers are higher in females than males [98], [133], [134]. In a recent study by Fink et al., males and females were immunized with an inactivated H1N1 (a type of influenza) vaccine that primarily caused antibody-mediated immunity. After vaccination, females had superior antibody responses and demanding defense with an H1N1 drift variant than males [135]. In the case of CoVs related disease, vaccines study is constantly evolving, but more studies and experiments will be needed to exact determination of approval vaccines effects in different sexes. According to sex related variation impact in response to vaccination, considering sex based immune studies in vaccine design and production will be very helpful and we think that addressing sex-immune profile differences can result in more efficient vaccines development.
7. Conclusion
With emergence and pandemic spread of new human coronaviruses (HCoVs), SARS-CoV-2 (the cause of the COVID-19 disease), which is the origin of the serious and occasionally fatal respiratory complaints in human, scientists are trying to focus more on different aspects of COVID-19 disease to find some strategies to improve the management and design specific therapeutic programs. In this regard, the body's antiviral immune fight has great importance. As mentioned previously, the antiviral immune responses ear dissimilar between females and males. This concept, as the focus of this review, can affect the immunotherapy programs and effective vaccine-development against COVID-19. The prevalence of Beta-CoV-related infections is usually higher in males. Females exhibit more robust innate and adaptive immune responses against pathogens, which is associated with lower susceptibility, less vulnerability, and faster clearance of the viral particles. However, such a strength of the immune system also enhances the immunopathology development. In reaction to viral vaccines, females produce more antibody and severe responses than males. The efficiency of anti-viral drugs and vaccines in decreasing the viral content and suppressing their effects differ between the sexes, and the side effects of these anti-viral drugs and vaccines are usually higher in females than males. These different immunological responses observed between males and females have been attributed to the X chromosome. The human X chromosome encodes multiple key genes involved in immune response regulation. Further, different activation patterns of some genes like TLR7 in males and females lead to the sexual variation of pro-inflammatory responses against different pathogens related to the stimulation of cytokins like IFNs. Therefore, understanding the sex-related differences in COVID-19 outbreaks will be a critical tool to recognize the impact of a health emergency on individuals and populations and to implement efficient strategies, public health interventions, and proper therapies.
Author contributions
Golbarg Rahimi and Bahareh Rahimi contributed equally and shared the first co-authorship. They wrote all sections of the manuscript. Mohammad Panahi wrote some sections of the original draft. Shadi Abkhiz worked on the literature research and original draft preparation.
Neda Saraygord-Afshari, Morteza Milani, and Effat Alizadeh contributed to the conceptualization, literature research, writing the discussion and conclusion, editing, reviewing, and organization of the final draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.107365.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N. Engl. J. Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 2.A.H. de Wilde, E.J. Snijder, M. Kikkert, M.J. van Hemert, Host factors in coronavirus replication, Roles of Host Gene and Non-coding RNA Expression in Virus Infection, Springer2017, pp. 1–42. [DOI] [PMC free article] [PubMed]
- 3.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsen K.H. Will COVID-19 generate global preparedness? The Lancet. 2020;395(10229):1013–1014. doi: 10.1016/S0140-6736(20)30559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 6.Park M., Thwaites R.S., Openshaw P.J. COVID-19: Lessons from SARS and MERS. Eur. J. Immunol. 2020;50(3):308–311. [Google Scholar]
- 7.Alghamdi I.G., Hussain I.I., Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014;7:417. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlberg J., Chong D., Lai W. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159(3):229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respirat. Med. 2020 doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amur S., Parekh A., Mummaneni P. Sex differences and genomics in autoimmune diseases. J. Autoimmun. 2012;38(2–3):J254–J265. doi: 10.1016/j.jaut.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 12.Markle J., Fish E.N. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Bouman A., Heineman M.J., Faas M.M. Sex hormones and the immune response in humans. Human Reprod. Update. 2005;11(4):411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 14.Fischer J., Jung N., Robinson N., Lehmann C. Sex differences in immune responses to infectious diseases. Infection. 2015;43(4):399–403. doi: 10.1007/s15010-015-0791-9. [DOI] [PubMed] [Google Scholar]
- 15.Robinson D.P., Lorenzo M.E., Jian W., Klein S.L. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7(7) doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karnam G., Rygiel T.P., Raaben M., Grinwis G.C., Coenjaerts F.E., Ressing M.E., Rottier P.J., de Haan C.A., Meyaard L. CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS Pathog. 2012;8(5) doi: 10.1371/journal.ppat.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson D.P., Huber S.A., Moussawi M., Roberts B., Teuscher C., Watkins R., Arnold A.P., Klein S.L. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex Diff. 2011;2(1):8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libert C., Dejager L., Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 2010;10(8):594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 19.Malkin C.J., Pugh P.J., Jones R.D., Kapoor D., Channer K.S., Jones T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metabol. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 20.Rettew J.A., Huet-Hudson Y.M., Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008;78(3):432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020 doi: 10.1002/jmv.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R. Madhugiri, M. Fricke, M. Marz, J. Ziebuhr, Coronavirus Cis-acting RNA Elements, Advances in Virus Research, Elsevier, 2016, pp. 127–163. [DOI] [PMC free article] [PubMed]
- 25.Yang D., Leibowitz J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge X.-Y., Yang W.-H., Zhou J.-H., Li B., Zhang W., Shi Z.-L., Zhang Y.-Z. Detection of alpha-and betacoronaviruses in rodents from Yunnan, China. Virol. J. 2017;14(1):98. doi: 10.1186/s12985-017-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.K. Nakagawa, K. Lokugamage, S. Makino, Viral and cellular mRNA translation in coronavirus-infected cells, Advances in virus research, Elsevier, 2016, pp. 165–192. [DOI] [PMC free article] [PubMed]
- 29.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 30.Finlay B.B., McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Constable P., Hinchcliff K., Done S., Grünberg W. 11th ed. WB Saunders; Philadelphia, Pennsylvania: 2017. Diseases of the Alimentary Tract: Nonruminant, Veterinary Medicine; pp. 175–435. [Google Scholar]
- 32.Leung G.M., Medley A.J., Ho L.M., Chau P., Wong I.O.L., Thach T.Q., Ghani A.C., Donnelly C.A., Fraser C., Riley S., Ferguson N.M., Anderson R.M., Tsang T., Leung P.Y., Wong V., Chan J.C.K., Tsui E., Lo S.V., Lam T.H. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann. Intern. Med. 2004;141(9):662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 33.Nie Q.-H., Luo X.-D., Hui W.-L. Advances in clinical diagnosis and treatment of severe acute respiratory syndrome. World J. Gastroenterol. 2003;9(6):1139–1143. doi: 10.3748/wjg.v9.i6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan Paul K.S., Tang Julian W., Hui David S.C. SARS: clinical presentation, transmission, pathogenesis and treatment options. Clin. Sci. 2006;110(2):193–204. doi: 10.1042/CS20050188. [DOI] [PubMed] [Google Scholar]
- 35.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 36.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.G.S. Randhawa, M.P.M. Soltysiak, H.E. Roz, C.P.E. de Souza, K.A. Hill, L. Kari, Machine learning-based analysis of genomes suggests associations between Wuhan 2019-nCoV and bat Betacoronaviruses, bioRxiv, 2020. 2020.02.03.932350.
- 38.Chan J.F.W., Lau S.K.P., Woo P.C.Y. The emerging novel Middle East respiratory syndrome coronavirus: the “knowns” and “unknowns”. J. Formos. Med. Assoc. 2013;112(7):372–381. doi: 10.1016/j.jfma.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali M.A. Gender dynamics and socio-cultural determinants of middle east respiratory syndrome coronavirus (MERS-Cov) in Saudi Arabia. Univ. Toronto Med. J. 2017;94(1):32–37. [Google Scholar]
- 40.Degnah A.A., Al-amri S.S., Hassan A.M., Almasoud A.S., Mousa M., Almahboub S.A., Alhabbab R.Y., Mirza A.A., Hindawi S.I., Alharbi N.K., Azhar E.I., Hashem A.M. Seroprevalence of MERS-CoV in healthy adults in western Saudi Arabia, 2011–2016. J. Infect. Publ. Health. 2020 doi: 10.1016/j.jiph.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falzarano D., De Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., Brining D., Bushmaker T., Martellaro C., Baseler L. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV–infected rhesus macaques. Nat. Med. 2013;19(10):1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Wilde A.H., Falzarano D., Zevenhoven-Dobbe J.C., Beugeling C., Fett C., Martellaro C., Posthuma C.C., Feldmann H., Perlman S., Snijder E.J. Alisporivir inhibits MERS-and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13. doi: 10.1016/j.virusres.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 44.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2) doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020 doi: 10.1074/jbc.AC120.013056. jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinha N., Balayla G. Hydroxychloroquine and covid-19. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beigel J.H., Voell J., Kumar P., Raviprakash K., Wu H., Jiao J.-A., Sullivan E., Luke T., Davey R.T. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet. Infect. Dis. 2018;18(4):410–418. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabaan A.A., Alahmed S.H., Bazzi A.M., Alhani H.M. A review of candidate therapies for Middle East respiratory syndrome from a molecular perspective. J. Med. Microbiol. 2017;66(9):1261–1274. doi: 10.1099/jmm.0.000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherbini N., Iskandrani A., Kharaba A., Khalid G., Abduljawad M., Al-Jahdali H. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: demographic, clinical and survival data. J. Epidemiol. Global Health. 2017;7(1):29–36. doi: 10.1016/j.jegh.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89(1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 55.García-Sastre A., Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312(5775):879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 56.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.W.H. Organization, WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020, Geneva, Switzerland, 2020.
- 58.Cyranoski D. Mystery deepens over animal source of coronavirus. Nature. 2020;579(7797):18–19. doi: 10.1038/d41586-020-00548-w. [DOI] [PubMed] [Google Scholar]
- 59.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dutta M., Dutta P., Medhi S., Borkakoty B., Biswas D. Polymorphism of HLA class I and class II alleles in influenza A (H1N1) pdm09 virus infected population of Assam, Northeast India. J. Med. Virol. 2018;90(5):854–860. doi: 10.1002/jmv.25018. [DOI] [PubMed] [Google Scholar]
- 61.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma G., Volgman A.S., Michos E.D. Sex differences in mortality from COVID-19 pandemic: are men vulnerable and women protected? JACC: Case Reports. 2020 doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ambrosino I., Barbagelata E., Ortona E., Ruggieri A., Massiah G., Giannico O.V., Politi C., Moretti A.M. Gender differences in patients with COVID-19: a narrative review. Monaldi Arch. Chest Dis. 2020;90(2) doi: 10.4081/monaldi.2020.1389. [DOI] [PubMed] [Google Scholar]
- 66.G. Cai, Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov, medRxiv, 2020.
- 67.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. 2020;134(5):543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 68.Y. Shi, Y. Wang, C. Shao, J. Huang, J. Gan, X. Huang, E. Bucci, M. Piacentini, G. Ippolito, G. Melino, COVID-19 infection: the perspectives on immune responses, Nature Publishing Group, 2020. [DOI] [PMC free article] [PubMed]
- 69.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15(11):1009. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 70.Wang G., Cao K., Liu K., Xue Y., Roberts A.I., Li F., Han Y., Rabson A.B., Wang Y., Shi Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25(7):1209–1223. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., Guo D., Fu L., Cui Y., Liu X., Arledge K.C., Chen Y.-H., Zhang L., Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park Y.-J., Walls A.C., Wang Z., Sauer M.M., Li W., Tortorici M.A., Bosch B.-J., DiMaio F., Veesler D. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat. Struct. Mol. Biol. 2019;26(12):1151–1157. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaimes J.A., Millet J.K., Stout A.E., André N.M., Whittaker G.R. A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses. 2020;12(1):83. doi: 10.3390/v12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehta N., Mazer-Amirshahi M., Alkindi N., Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am. J. Emerg. Med. 2020;38(7):1488–1493. doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haynes B.F., Corey L., Fernandes P., Gilbert P.B., Hotez P.J., Rao S., Santos M.R., Schuitemaker H., Watson M., Arvin A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020;12(568) doi: 10.1126/scitranslmed.abe0948. [DOI] [PubMed] [Google Scholar]
- 78.Corum J., Grady D., Wee S.-L., Zimmer C. Coronavirus vaccine tracker. The New York Times. 2020;5 [Google Scholar]
- 79.U.S.F.a.D. Administration, FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine, 2020.
- 80.Penna C., Mercurio V., Tocchetti C.G., Pagliaro P. Sex-related differences in COVID-19 lethality. Br. J. Pharmacol. 2020;177(19):4375–4385. doi: 10.1111/bph.15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.M. Ighovwerha Ofotokun, and Claire Pomeroy, MD, Sex Differences in Adverse Reactions to Antiretroviral Drugs, 2003. [PubMed]
- 82.Cattrini C., Bersanelli M., Latocca M.M., Conte B., Vallome G., Boccardo F. Sex hormones and hormone therapy during COVID-19 pandemic: implications for patients with cancer. Cancers (Basel) 2020;12(8) doi: 10.3390/cancers12082325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Lami R.A., Urban R.J., Volpi E., Algburi A.M.A., Baillargeon J. Sex hormones and novel corona virus infectious disease (COVID-19) Mayo Clin. Proc. 2020;95(8):1710–1714. doi: 10.1016/j.mayocp.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding T., Zhang J., Wang T., Cui P., Chen Z., Jiang J., Zhou S., Dai J., Wang B., Yuan S., Ma W., Ma L., Rong Y., Chang J., Miao X., Ma X., Wang S. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome coronavirus 2 infection: a cross-sectional multicenter study in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M., Bortolotti P., Martinez L., Dubucquoi S., Dessein R. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoneyama M., Fujita T. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 2010;20(1):4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- 87.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11(5):373. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 89.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taniguchi T., Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat. Rev. Mol. Cell Biol. 2001;2(5):378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 91.Welsh R.M., Waggoner S.N. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology. 2013;435(1):37–45. doi: 10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okamoto M., Tsukamoto H., Kouwaki T., Seya T., Oshiumi H. Recognition of viral RNA by pattern recognition receptors in the induction of innate immunity and excessive inflammation during respiratory viral infections. Viral Immunol. 2017;30(6):408–420. doi: 10.1089/vim.2016.0178. [DOI] [PubMed] [Google Scholar]
- 93.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.R. Channappanavar, S. Perlman, Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Seminars in immunopathology, Springer, 2017, pp. 529–539. [DOI] [PMC free article] [PubMed]
- 95.Klein S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays. 2012;34(12):1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maloir Q., Ghysen K., Louis R., Guiot J. Acute respiratory distress revealing antisynthetase syndrome. Rev. Med. Liege. 2018;73(7–8):370–375. [PubMed] [Google Scholar]
- 97.Schmolke M., García-Sastre A. Evasion of innate and adaptive immune responses by influenza A virus. Cell. Microbiol. 2010;12(7):873–880. doi: 10.1111/j.1462-5822.2010.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet. Infect. Dis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015;109(1):9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engler R.J., Nelson M.R., Klote M.M., VanRaden M.J., Huang C.-Y., Cox N.J., Klimov A., Keitel W.A., Nichol K.L., Carr W.W. Half-vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008;168(22):2405–2414. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 101.Stamova B., Tian Y., Jickling G., Bushnell C., Zhan X., Liu D., Ander B.P., Verro P., Patel V., Pevec W.C. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43(2):326–334. doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meier A., Chang J.J., Chan E.S., Pollard R.B., Sidhu H.K., Kulkarni S., Wen T.F., Lindsay R.J., Orellana L., Mildvan D. Sex differences in the Toll-like receptor–mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009;15(8):955. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brooks E.G., Schmalstieg F.C., Wirt D.P., Rosenblatt H.M., Adkins L.T., Lookingbill D.P., Rudloff H.E., Rakusan T.A., Goldman A.S. A novel X-linked combined immunodeficiency disease. J. Clin. Investig. 1990;86(5):1623–1631. doi: 10.1172/JCI114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmalstieg F.C., Goldman A.S. Immune consequences of mutations in the human common γ-chain gene. Mol. Genet. Metab. 2002;76(3):163–171. doi: 10.1016/s1096-7192(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 105.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghorai A., Ghosh U. miRNA gene counts in chromosomes vary widely in a species and biogenesis of miRNA largely depends on transcription or post-transcriptional processing of coding genes. Front. Genet. 2014;5:100. doi: 10.3389/fgene.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pinheiro I., Dejager L., Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays. 2011;33(11):791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 108.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 109.Giefing-Kröll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.C.D. Ciccone, Pharmacology in rehabilitation, FA Davis, 2015.
- 111.Copland J.A., Sheffield-Moore M., Koldzic-Zivanovic N., Gentry S., Lamprou G., Tzortzatou-Stathopoulou F., Zoumpourlis V., Urban R.J., Vlahopoulos S.A. Sex steroid receptors in skeletal differentiation and epithelial neoplasia: is tissue-specific intervention possible? BioEssays. 2009;31(6):629–641. doi: 10.1002/bies.200800138. [DOI] [PubMed] [Google Scholar]
- 112.Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 113.Robinson D.P., Hall O.J., Nilles T.L., Bream J.H., Klein S.L. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J. Virol. 2014;88(9):4711–4720. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Castles C., Oesterreich S., Hansen R., Fuqua S. Auto-regulation of the estrogen receptor promoter. J. Steroid Biochem. Mole. Boil. 1997;62(2–3):155–163. doi: 10.1016/s0960-0760(97)00023-x. [DOI] [PubMed] [Google Scholar]
- 115.Froldi G., Dorigo P. Endothelial dysfunction in Coronavirus disease 2019 (COVID-19): gender and age influences. Med. Hypotheses. 2020;144:110015. doi: 10.1016/j.mehy.2020.110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takao T., Kumagai C., Hisakawa N., Matsumoto R., Hashimoto K. Effect of 17β-estradiol on tumor necrosis factor-α-induced cytotoxicity in the human peripheral T lymphocytes. J. Endocrinol. 2005;184(1):191–197. doi: 10.1677/joe.1.05914. [DOI] [PubMed] [Google Scholar]
- 117.Cutolo M., Accardo S., Villaggio B., Clerico P., Bagnasco M., Coviello D.A., Carruba G., Casto M.L., Castagnetta L. Presence of estrogen-binding sites on macrophage-like synoviocytes and CD8+, CD29+, CD45RO+ T lymphocytes in normal and rheumatoid synovium. Arth. Rheum.: Off. J. Am. College Rheumatol. 1993;36(8):1087–1097. doi: 10.1002/art.1780360809. [DOI] [PubMed] [Google Scholar]
- 118.Ruiz-Cortés Z.T. Gonadal sex steroids: production, action and interactions in mammals. Steroids-From Physiol. Clin. Med. 2012:3–44. [Google Scholar]
- 119.Lambert K., Curran E.M., Judy B.M., Milligan G.N., Lubahn D.B., Estes D.M. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J. Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 120.G. Glinsky, Genomics-guided molecular maps of coronavirus targets in human cells: a path toward the repurposing of existing drugs to mitigate the pandemic, arXiv preprint arXiv:2003.13665, 2020.
- 121.Paavonen T., Andersson L., Adlercreutz H. Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen stimulated cultures. J. Exp. Med. 1981;154:1935–1945. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.T. Ding, J. Zhang, T. Wang, P. Cui, Z. Chen, J. Jiang, S. Zhou, J. Dai, B. Wang, S. Yuan, Potential Influence of Menstrual Status and Sex Hormones on Female Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Cross-sectional Multicenter Study in Wuhan, China, Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 2020. [DOI] [PMC free article] [PubMed]
- 123.R.A. Al-Lami, R.J. Urban, E. Volpi, A.M. Algburi, J. Baillargeon, Sex Hormones and Novel Corona Virus Infectious Disease (COVID-19), Mayo Clinic proceedings, Elsevier, 2020. [DOI] [PMC free article] [PubMed]
- 124.Gaskins A.J., Wilchesky M., Mumford S.L., Whitcomb B.W., Browne R.W., Wactawski-Wende J., Perkins N.J., Schisterman E.F. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am. J. Epidemiol. 2012;175(5):423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chakrabarti S., Lekontseva O., Davidge S.T. Estrogen is a modulator of vascular inflammation. IUBMB Life. 2008;60(6):376–382. doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- 126.Traish A., Bolanos J., Nair S., Saad F., Morgentaler A. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. J. Clin. Med. 2018;7(12):549. doi: 10.3390/jcm7120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46(3):239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 128.K. Sriram, E. Insel, R. Loomba, What is the ACE2 receptor, how is it connected to coronavirus and why might it be key to treating COVID-19? The experts explain, The Conversation, 2020.
- 129.Biswas D.K., Singh S., Shi Q., Pardee A.B., Iglehart J.D. Crossroads of estrogen receptor and NF-κB signaling. Science's STKE. 2005;2005(288):pe27-pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 130.Rémy V., Zöllner Y., Heckmann U. Vaccination: the cornerstone of an efficient healthcare system. J. Market Access Health Policy. 2015;3(1):27041. doi: 10.3402/jmahp.v3.27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Živković I., Petrović R., Arsenović-Ranin N., Petrušić V., Minić R., Bufan B., Popović O., Leposavić G. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals. 2018;52:18–24. doi: 10.1016/j.biologicals.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 132.Furman D., Hejblum B.P., Simon N., Jojic V., Dekker C.L., Thiébaut R., Tibshirani R.J., Davis M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vermeiren A.P., Hoebe C.J., Dukers-Muijrers N.H. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J. Clin. Virol. 2013;58(1):262–264. doi: 10.1016/j.jcv.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 134.Cook I.F., Barr I., Hartel G., Pond D., Hampson A.W. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine. 2006;24(13):2395–2402. doi: 10.1016/j.vaccine.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 135.Fink A.L., Engle K., Ursin R.L., Tang W.-Y., Klein S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. 2018;115(49):12477–12482. doi: 10.1073/pnas.1805268115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.