Abstract
Converging evidence suggests that parental exposure to drugs of abuse can affect offspring phenotypes. The impacts of drug abuse on germ cell quality may mediate multigenerational and transgenerational inheritance, although biological pathways underlying this mode of inheritance are not yet characterized. Germline epigenetic marks are modified by drug exposure and have emerged as promising mechanistic candidates in recent work. Drug exposure also impacts overall germline integrity and reproductive functioning, although the role of these consequences in multi/transgenerational inheritance is unclear. This review synthesizes literature on effects of exposure to alcohol, cocaine, and nicotine on the germline with a focus on epigenetic modifications following drug exposure and broader impacts on germline integrity and reproductive functioning. We discuss potential interactions between reproductive functioning, germline integrity, and germline epigenome/transcriptome in pathways underlying multi/transgenerational inheritance. We find that existing data may support independent or interactive contributions of these germline impacts on offspring phenotypes in a manner that may mediate multi/transgenerational inheritance.
1. Introduction
Accumulating evidence pointing to germline-mediated multi- and transgenerational inheritance has renewed interest in the impact of exposure to addictive drugs on the germline. Converging findings show that pre-conception exposure to alcohol, cocaine, and nicotine can result in altered offspring phenotypes. It has long been known that drug abuse can impact fertility through direct and indirect effects on germline quality. It is believed that a subset of these germline modifications may mediate multigenerational and transgenerational inheritance, although the biological pathway underlying this mode of inheritance has yet to be definitively characterized. Germline epigenetic marks, such as DNA cytosine methylation, histone modifications, and small RNAs are modified by drug exposure and have emerged as promising mechanistic candidates in recent work. Exposure to drugs of abuse also impacts overall reproductive functioning and germline integrity, although these consequences have not been discussed extensively in terms of their potential role in multi/transgenerational inheritance. These biological changes may act independently, additively, or in sequence to influence multi/transgenerational inheritance. This review aims to synthesize existing literature on the effects of exposure to alcohol, cocaine, and nicotine on male and female germline with a focus on epigenetic modifications following drug exposure as well as broader impacts on germline integrity. In the context of accompanying evidence of multi/transgenerational phenotypes, we discuss potential interactions between germline integrity and germline epigenome/transcriptome in pathways underlying multi/transgenerational inheritance. We conclude that existing literature may support independent or interactive contributions of these germline impacts on offspring phenotypes in a manner that may mediate multi/transgenerational inheritance.
1.1. Epigenetic features of the male and female germline
Multigenerational and transgenerational inheritance are distinctly defined but related phenomena, both referring to the alteration of organismal phenotype by environmental exposures in parents in a manner unrelated to indirect mechanisms like parental care (Skinner & Guerrero-Bosagna, 2009). Multigenerational inheritance occurs in the first generation conceived from exposed founders. Transgenerational inheritance is more stringently defined as a continuation of multigenerational inheritance beyond the most recently exposed generation. Thus, for exposed male founders (F0), multigenerational inheritance can occur beginning in the F1 generation, which is technically also exposed via the F0 germline, and transgenerational inheritance refers to the F2 generation and beyond. For female founders, multigenerational inheritance begins in the F2 generation, as both the F1 generation and its germline are exposed to the F0 uterine environment during gestation (see Figure 1 for a schematic depiction of maternal vs. paternal multi/transgenerational inheritance).
FIGURE 1.
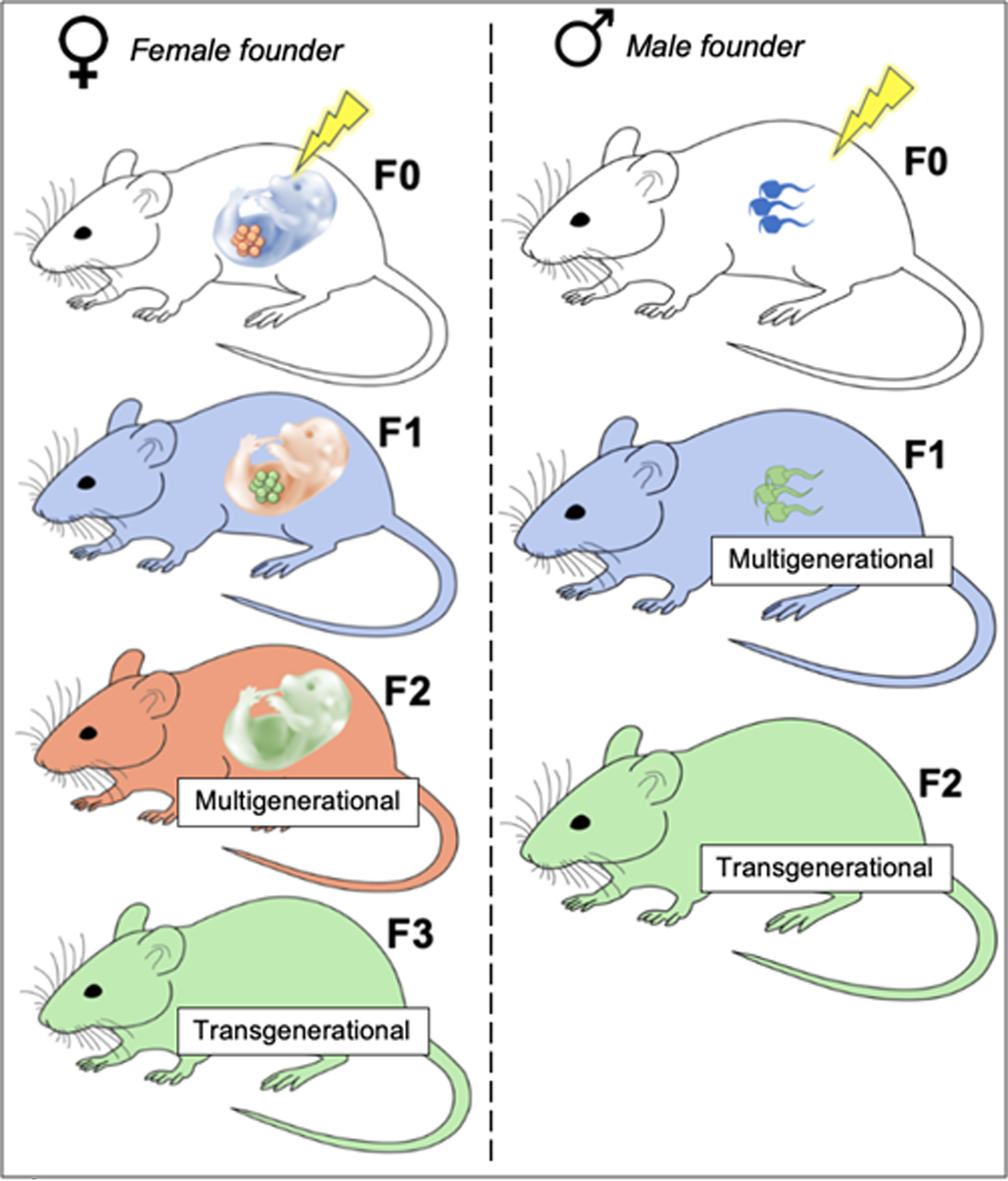
Left: Maternal founder multi/transgenerational inheritance in the female line
Exposure occurs during the lifetime of the F0 generation (white mouse). The F1 generation (blue fetus) and its developing germline (orange germline), which produces the F2 generation, are also exposed. The F1 generation gestates the F2 generation (orange fetus), whose germline (green germline) produces the F3 generation. Multigenerational phenotyping begins in the F2 generation (orange mouse), which is derived from the originally exposed F1 germline (blue fetus, orange germline). Transgenerational phenotyping begins in the F3 generation (green mouse), which is the first that had no direct contact (via gestation or germline) with the original exposure.
Note that the original exposure need not necessarily occur during F0 gestation (F0 pregnancy with F1), as any exposure prior to pregnancy may chronically alter uterine environment or other pregnancy parameters consequential to F1 development.
Right: Paternal founder multi/transgenerational inheritance in the male line
Exposure occurs during the lifetime of the F0 generation (white mouse), also directly exposing the F0 germline (blue germline), which produces the F1 generation. Multigenerational inheritance begins in the F1 generation (blue mouse), which is derived from the originally exposed F0 germline. The F1 germline (green germline), which produces the F2 generation, never contacts the original exposure. Thus, transgenerational phenotyping begins in the F2 generation.
Non-color accessibility image description: Left side, top = white mouse, blue fetus, orange germline; Left side, second to top = Blue mouse, orange fetus, green germline; Left side, third to top = orange mouse, green fetus; Left side, bottom = green mouse; Right side, top = white mouse, blue germline; Right side, middle = blue mouse, green germline; Right side, bottom = green mouse.
Multi/transgenerational inheritance stand in contrast to the classic Darwinian/Mendelian model, whereby organism phenotype is determined by inherited DNA sequence – that is, parental exposures prior to conception are inconsequential to organismal fate in the absence of contact with parents. The classic model thus implies a theoretical barrier between parental somatic cells and germline, such that the germline is protected from environmental insult by one or both of two mechanisms: 1) a physical barrier limiting contact with external agents, and 2) restoration of germline integrity following damage or modification. Both these mechanisms are in fact observed in both the male and female germline. The “blood-testis barrier” and “blood-follicle barrier” represent physical barricades to the male and female reproductive systems, respectively. The blood-testis barrier is formed by Sertoli cells of the testicular seminiferous tubules. Sertoli cells form tight junctions, creating a barrier between the external basal compartment, which contacts blood, and the internal abluminal compartment (Dym & Fawcett, 1970; Russell, 1977). Active barriers, such as drug transporters, are also expressed by sperm and surrounding testicular cells (Bart et al., 2004; Melaine et al., 2002). The blood-follicle barrier is not as well-characterized as the blood-testis barrier, but is known to include cellular tight junctions in vessels surrounding developing follicles in addition to a selective follicular basement membrane (Hess, Chen, & Larsen, 1998; Siu & Cheng, 2013).” As with the blood-brain barrier, the blood-testis and blood-follicle barriers are not absolutely impermeable, making the germline vulnerable under certain conditions. For example, cocaine exposure compromises the blood-testis barrier (Barroso‐Moguel, Méndez‐Armenta, Villeda‐Hernández, 1994), as do other factors like obesity (Fan et al., 2015). The permeability of the blood-follicle barrier to external agents has also been demonstrated using anti-cancer drugs (Bar-Joseph et al., 2010).
Epigenetic marks like DNA and histone modifications can be conceptualized as annotations to the otherwise static DNA sequence. In the germline, flexibility of cell fate (i.e., pluripotency) is of critical importance for fetal development. Accordingly, multiple mechanisms act reset the epigenome so that a “clean slate” is passed on to offspring. A genome-wide near complete erasure of DNA methylation and histone modifications occurs during the formation of primordial germ cells in developing male and female embryos. In males, most histones are replaced with protamines (nuclear basic proteins unique to sperm) during haploid stages of spermatogenesis, resulting in a uniquely ultracompact chromatin structure thought to provide an additional level of protection for sperm DNA. This also serves to eliminate any epigenetic marks associated with histones and renders the cell transcriptionally inert (Kierszenbaum & Tres, 1975; Lescoat, Blanchard, Lavault, Quernee, & Lannou, 1993; Steger, Pauls, Klonisch, Franke, & Bergmann, 2000). In females, active deacetylation of histones has been observed in human and murine secondary oocytes (Kim, Liu, Tazaki, Nagata, & Aoki, 2003; Van den Berg et al., 2011). This global deacetylation occurring prior to ovulation is theorized to facilitate chromosome segregation necessary for the final meiotic division, while also serving to eliminate oocyte histone acetylation that may interfere with embryonic pluripotency (Akiyama, Nagata, & Aoki, 2006).
Exceptions to this epigenetic reprogramming are observed in both the male and female germline. For example, an additional active genome-wide demethylation of the paternal genome occurs in the zygote immediately following fertilization, but paternally imprinted genes and select regulatory elements are spared (Hackett et al., 2013; Oswald et al., 2000). In contrast, the maternal genome is demethylated passively (through lack of maintenance at replication), resulting in longer retention of methylated regions (Seisenberger et al., 2013). Although previously incorporated sperm protamines are replaced with maternal histones in the developing zygote, a small portion of epigenetically modified sperm histones are never replaced with protamines during spermatogenesis and are subsequently incorporated into zygote chromatin (Brykczynska et al., 2010; van der Heijden et al., 2008; Yamaguchi et al., 2018). Thus, DNA methylation and modified histones from the paternal and maternal genome are both positioned to affect offspring development. The extent to which the contributions of epigenetic factors bypassing germline reprogramming are appreciable within the developmental framework is unknown, and may vary based on both the qualitative (i.e., type) and quantitative (i.e., “dosage”) nature of these modifications. It has been argued that DNA methylation is an unlikely mediator of multi/transgenerational inheritance in paternal exposure models, as variation in sperm DNA methylation due to environmental exposures is minimal relative to stochastic individual differences (Carone et al., 2010; Shea et al., 2015). It has been argued that DNA methylation is an unlikely potential mediator of multi/transgenerational inheritance in paternal exposure models, as variation in sperm DNA methylation due to environmental exposures is minimal relative to stochastic individual differences (Carone et al., 2010; Shea et al., 2015). As these conclusions were based on rodent diet manipulation models, it is unclear whether other exposures, such as addictive drug use, have greater potency to produce penetrant changes in sperm DNA methylation.
Germline RNAs can act as extragenomic regulators with the potential to impact gene transcription during embryonic development. The oocyte is transcriptionally active until the halt of meiosis at metaphase II (Schultz, 1993). At this time, a slow degradation of maternal mRNAs (messenger RNAs) takes place, with those that remain playing a critical role in early embryonic development (Alizadeh, Kageyama, & Aoki, 2005; Bachvarova, De Leon, Johnson, Kaplan, & Paynton, 1985; Yang et al., 2016). Early spermatogenesis requires protein synthesis, but maturing spermatids and mature spermatozoa are transcriptionally inert (Cooper & Yeung, 2003; Kierszenbaum & Tres, 1975). However, recent evidence suggests mature sperm cells harbor RNA that is delivered to the fertilized oocyte and is functional during embryonic development (Guo et al., 2017; Ostermeier, Goodrich, Moldenhauer, Diamond, & Krawetz, 2005; Sendler et al., 2013). The mature sperm RNA population largely consists of small RNAs and transfer RNA fragments, thought to be acquired during epididymal maturation via fusion with “epididymosomes” (i.e., epididymal exosomes) (Conine, Sun, Song, Rivera-Pérez, & Rando, 2018; Krawetz et al., 2011; Sharma et al., 2016; Sharma et al., 2018). Small non-coding RNAs and tRNA fragments primarily act to modulate gene expression through mRNA silencing (RNA interference) (Ghildiyal & Zamore, 2009; Sharma et al., 2016; Yamasaki, Ivanov, Hu, & Anderson, 2009) and may also modulate DNA methylation in mammals (Aravin, Sachidanandam, Girard, Fejes-Toth, & Hannon, 2007). The quantitative contribution of sperm RNA to the zygote is minimal relative to that of the oocyte, although there is evidence of sperm small RNA interference that is critical to early zygote development (Liu et al., 2012; Yang et al., 2016). Thus, environmental exposures altering sperm RNA content can impact offspring development, a mechanism potentially mediating multi/transgenerational phenotypes.
Epigenetic and transcriptional factors have emerged as promising candidates for germline mediators of multi/transgenerational inheritance in recent work. As we discuss in the present review, exposure to drugs of abuse produces changes in germline epigenome and RNA content. Importantly, these modifications occur in the context of broader impacts on reproductive functioning and germline integrity following drug exposure. In characterizing pathways underlying multi/transgenerational inheritance, it is imperative to consider the potential independent or interactive role of germline integrity alongside epigenetic impacts. Given that considerable evidence suggests reproductive functioning, germline integrity, and germline epigenome can interact, discussion of these germline factors together may guide characterization of biological networks underlying multi/transgenerational inheritance. Within this framework, we shall discuss known effects of drug exposure on reproductive functioning/germline integrity and germline epigenome/transcriptome, as well as their potential independent contributions to multi/transgenerational inheritance. Where data are available, we also discuss possible interactions between these factors in a molecular pathway underlying multi/transgenerational inheritance.
1.2. Overview
The present review synthesizes data on the effects of alcohol, nicotine, and cocaine on the germline in rodent and human models. We focus on the following broad discussion points: 1) reproductive proxies (i.e., biological functioning relevant to reproduction), 2) germline integrity, and 3) germline epigenome.” These discussion points are separated into titled sections where reviewed data are sufficient and are otherwise compiled as appropriate. We include discussion of reproductive proxies and germline integrity for the purpose of identifying factors that may act in parallel with or upstream of germline epigenetic and transcriptional changes induced by drug exposure. Reproductive proxies include sex hormones and organs relevant to reproduction. Sex hormones and their effector organs can affect mating behavior and germline development, potentially acting as mediators of germline integrity and epigenetic modifications. We define “epigenome” as inclusive of DNA methylation, histone modifications, and small non-coding RNA content. Where relevant, we also include discussion of related mRNA expression and protein synthesis.
Germline integrity refers to measures of germ cell count and morphology, including motility (in the case of sperm), DNA damage, and general structural degradation. Motility, count, and structural integrity may affect the number and quality of germ cells available for fertilization and subsequent gestation. Germ cell count may be especially consequential to offspring phenotypes in rodent models, as differences in sibling number may affect litter development. At levels below the threshold for inviability, structural damage to the germline can also impact development of offspring derived from damaged germ cells. Abnormal germline morphology also predicts altered germ cell epigenetic profiles (Cassuto et al., 2016; Nanassy & Carrell, 2011; Ibala-Romdhane et al., 2011; Liang, Fu, Li, Yuan, & Zhu, 2014; Malki, van der Heijden, O’Donnell, Martin, & Bortvin, 2014), pointing to the possibility that these germline impacts may interact in a biological pathway underlying multi/transgenerational inheritance.
Although both the mature oocyte and the ovum (i.e., fertilized oocyte) have remarkable capacity for repair of sperm- and oocyte-derived DNA (Brazill, & Masui, 1978; Masui, & Pedersen, 1975; Generoso, Cain, Krishna, & Huff, 1979), sperm cells and oocytes are still viable for fertilization below a certain DNA fragmentation threshold (Ahmadi & Ng, 1999; Sergerie, Laforest, Bujan, Bissonnette, & Bleau, 2005). This raises the possibility that some purported multi/transgenerational phenotypes are in part mediated by heritable modifications to the DNA sequence. Offspring derived from DNA-damaged sperm indeed show altered behavior and physiology, highlighting the importance of co-quantification of DNA integrity in multi/transgenerational models (Fernández-Gonzalez et al., 2008; Kumar et al., 2013; Ramos-Ibeas et al., 2014). Any phenotypes explained by heritable changes to DNA sequence do not represent multi/transgenerational inheritance, but in fact are consistent with traditional Mendelian inheritance. Genome-wide DNA sequencing can further be used to determine whether DNA damage-related changes in sequence are present in both the germline of exposed subjects and in progeny exhibiting multi/transgenerational phenotypes; however, no studies to our knowledge have linked multi/transgenerational phenotypes to changes in DNA sequence. Importantly, it is unclear whether germline damage induced by drug exposure produces random or systematic modifications to DNA sequence, the latter case which may be a greater concern in the context of validating multi/transgenerational models. Relevant to either case, DNA fragmentation may also predispose the germline genome to epigenetic modification and altered non-coding RNA expression (Bahreinian et al., 2015; Montjean et al., 2015; Zhao et al., 2016), representing other avenues by which DNA damage may play a role in multi/transgenerational inheritance.
The current review synthesizes existing data on the effects of exposure to alcohol, cocaine, and nicotine on male and female germline, focusing on epigenetic modifications following drug exposure, broader impacts on reproductive function and germline integrity, and potential interactions between them. Some limitations to these discussions should be noted: Most importantly, although we discuss biological factors that may theoretically interact in a pathway underlying multi/transgenerational inheritance, we stress that the existence and nature of these links will require validation in experimental models of non-Mendelian inheritance. Additionally, data between the discussed studies may not be directly comparable due to their methodological differences. For example, while we generally compile literature across a certain exposure regardless of length of exposure or age at exposure, F0 exposure length and timing may modulate impacts on germline. As this field inevitably continues to expand, future reviews will be better powered to separate data by exposure conditions. We choose to include human studies, from which self-report data necessarily defines exposure conditions; however, self-report data collected from human drug users is subject to omissions and inaccuracies. Finally, because limited data exist on effects of drug exposure on female germline, these sections are generally briefer and consolidated. Nonetheless, we note several converging findings pointing to consistent trends in effects of drug exposure on germline in terms of reproductive proxies, germline integrity, and germline epigenome.
2. Effects of alcohol exposure on male and female germline
2.1. Male germline
2.1.1. Effects of alcohol exposure on male reproductive proxies
Changes in offspring phenotype as a result of paternal alcohol exposure may be explained in part by shifts in reproductive function upstream of germline development and maintenance. For instance, alcohol exposure in rodent models has been associated with reductions in testicle weight, which may precede reduced testosterone levels and declines in sperm production (Bujan et al., 1989; Takihara, Cosentino, Sakatoku, & Cockett, 1987). Indeed, alcohol exposure predicts lowered testosterone in both rodent (Cicero et al., 1990; Willis, Anderson, Oswald, & Zaneveld, 1983) and human (Muthusami, & Chinnaswamy, 2005) studies. Modest alterations to luteinizing (LH) and follicle stimulating (FSH) hormones have also been reported, although the direction of change is inconsistent between published rodent (Cicero et al., 1990) and human (Muthusami, & Chinnaswamy, 2005) studies. In males, FSH and LH act to regulate testosterone levels and testicular function, and thus can impact fertility. Both increases and decreases in male FSH and LH levels are associated with alterations to sperm count, motility, and DNA fragmentation (Arai, Kitahara, Horiuchi, Sumi, & Yoshida, 1998; Biswas et al., 1978; Pasqualotto, Sobreiro, Hallak, Pasqualotto, & Lucon, 2005; Wdowiak, Raczkiewicz, Stasiak, & Bojar, 2014) -- all germline parameters that are impacted by alcohol exposure (discussed below). Thus, despite our designation of these hormones as reproductive “proxies,” it is important to regard them as potential upstream mediators in the biological pathway underlying alcohol-related multi/transgenerational inheritance.
2.1.2. Effects of alcohol exposure on sperm number and integrity
In rodent and human males, alcohol exposure is associated with mature sperm cell abnormalities. These include sperm malformations, impairment of motility, and DNA damage. Reductions in sperm motility following alcohol exposure have been reported in rodent models (Akang et al., 2017; Anderson, Willis, Oswald, & Zaneveld, 1983) and in humans self-reporting as heavy drinkers (Muthusami & Chinnaswamy, 2005), although conflicting findings and dose response studies suggest this effect may be dose or condition dependent (Anderson et al., 1983; Sánchez et al., 2018). Abnormalities in sperm morphology associated with alcohol exposure are consistently found in rodent (Abel & Moore, 1987; Anderson et al., 1983) and human studies. In rodent models, sperm count and motility can impact the number of sperm available for oocyte fertilization, which may in turn impact litter size. Behavioral phenotypes, including those related to phenotypes altered in alcohol exposure multi/transgenerational inheritance models (e.g. motor and emotional behaviors), are affected by litter size (LaBarba, White, Stewart, & Buckley, 1973; Tanaka, 1998). Paternal alcohol exposure has in fact been associated with decreased litter size (Cicero et al., 1990; Liang et al., 2016; Meek, Myren, Sturm, & Burau, 2007) and weight (Abel & Tan, 1988; Knezovich, & Ramsay, 2012) in rodent studies, although other analyses found no impact or an opposite effect on these parameters (Finegersh & Homanics, 2014; Kim et al., 2014; Willis et al., 1983).
A recent analysis noted DNA fragmentation in sperm of alcohol-exposed rats, which was hypothesized by authors to contribute to infertility in human drinkers (Akang et al., 2017). DNA fragmented sperm may produce altered learning and anxiety-like behaviors in offspring (Fernández-Gonzalez et al., 2008) – offspring phenotypes that are also affected in paternal alcohol multi/transgenerational models (Abel, & Moore, 1987; Wozniak, Cicero, Kettinger, & Meyer, 1991). Thus, DNA fragmentation in response to drug exposure has the potential to directly impact offspring phenotypes in a manner that may confound phenotypes otherwise attributed to multi/transgenerational inheritance. Crucially, however, it is unknown whether alcohol exposure produces sperm DNA damage that is systematic and/or severe enough to impact offspring development. Interestingly, both sperm DNA damage and abnormal sperm morphology/count are associated with changes in sperm epigenetic profile (Bahreinian et al., 2015; Cassuto et al., 2016; Houshdaran et al., 2007) – and, as is discussed below, alcohol exposure produces well-characterized modulation of sperm epigenome. These data in sum suggest that alcohol compromises sperm cell number, motility, and integrity. Further, it is possible that the discussed impacts on male germline integrity may interact with observed shifts in sperm epigenetic profile also induced by alcohol exposure.
2.1.3. Effects of alcohol exposure on sperm RNA and epigenome
Despite the “protected” status of the germline genome, environmental exposures have the potential to impact sperm cell RNA content and epigenome in a manner that may be consequential to offspring (Finegersh & Homanics, 2014; Guo et al., 2017; Sendler et al., 2013; Sharma et al., 2016). In rodent models, alcohol exposure impacted both mRNA and small non-coding RNA populations. Sperm content of three major classes of small non-coding RNAs – mitochondrial small RNAs, microRNAs, and transfer RNA-derived small RNA (tDRs) – was altered following chronic alcohol exposure in mice (Rompala et al., 2018). Enrichment analysis in differentially expressed transcripts suggested changes in pathways involved in transcription, modulation of small RNAs, and post-translational protein modifications. tDRs were found to be similarly affected in the epididymis, suggesting that the sperm tDRs were acquired during epidydimal transit. tDRs are one of the most abundant sperm RNA populations and act to silence endogenous retroelements (self-amplifying genomic regions) involved in germline fate plasticity (Peng et al., 2012; Schoorlemmer, Pérez-Palacios, Climent, Guallar, & Muniesa, 2014; Sharma et al., 2016). One diet manipulation model found that injection of tDRs into a fertilized oocyte was sufficient to partially recapitulate multigenerational phenotypes associated with the altered diet (Chen et al., 2016). Thus, shifts in RNA populations as a result of alcohol exposure may have the capacity to impact development in a manner that mediates multigenerational phenotypes via modulation of early developmental programs. However, it is unclear whether upstream networks impacting reproductive functioning and/or germline integrity must first occur to produce altered sperm RNA content.
Alcohol exposure was also found to impact levels of sperm cytosine methyltransferase mRNA (Bielawski, Zaher, Svinarich, & Abel, 2002), which may point to downstream disturbances of DNA methylation. Multiple human (Ouko et al., 2009) and rodent (Finegersh & Homanics, 2014; Kim et al., 2014; Liang et al., 2014) studies have found changes in sperm DNA methylation associated with alcohol exposure. Notably, these all utilized a targeted approach to assess DNA methylation in sperm samples, meaning that additional changes in genomic methylation status may have gone undetected. In a sample of self-reported heavy drinkers, Ouko and colleagues (2009) found reduced sperm cell DNA methylation at a regulatory region of gene H19 and within differentially methylated regions of genes DLK1 and GTL2, growth factors which are typically hypermethylated in the male germline. Hypomethylation of CpG islands in these genes may affect fetal growth and cell differentiation (Cleaton et al., 2016; St-Pierre at al., 2012). Liang and colleagues (2019) replicated sperm H19 hypomethylation in a rodent model of paternal alcohol exposure. Alcohol exposure additionally predicted increased and decreased sperm Peg3 and Bdnf methylation, respectively, both of which are involved in fetal brain development and whose methylation patterns were inherited to the F1 brain (Finegersh & Homanics, 2014; Liang et al., 2014). Heritable germline epigenetic modifications to genes involved in neural development may in part explain offspring multi/transgenerational phenotypes related to addiction (Abel, 1993; Finegersh & Homanics, 2014; Kim et al., 2014; Rompala, Finegersh, Slater, & Homanics, 2017). In support, Kim and colleagues (2014) found increased methylation of the dopamine transporter (Dat) gene in sperm of male mice exposed to alcohol, as well as decreased alcohol consumption in their offspring.
Collectively, these data suggest a trend of modified male reproductive functioning, sperm integrity, and sperm epigenome following alcohol exposure in a manner that affects developmental pathways in offspring. These germline changes may act independently, additively, or in sequence to influence multi/transgenerational inheritance. Epigenomic and transcriptomic modifications produced by alcohol exposure may point to developmental pathways potentially underlying expression of multi/transgenerational phenotypes; however, because genes involved in development are often pleiotropic and remain active throughout the lifetime, it is difficult to predict their downstream effects on offspring phenotype. Considering the reviewed data and assuming that the affected genomic regions are in fact consequential to offspring development, one would predict a range of potential multi/transgenerational phenotypes, perhaps with a bias to those involved in neural functioning and growth. Although the body of literature on alcohol’s multi/transgenerational effects grows rapidly, additional comprehensive phenotypic batteries are warranted. One early investigation of F1 generation alcohol-sired mice showed no changes in body weight, litter size, or organ weights aside from thymus, but did find a distinct difference in performance on cognitive behavioral paradigms (Abel & Lee, 1988). Available data do appear to confirm a somewhat selective effect on behavior and neural functioning in alcohol-sired animals, although there is clearly a need for additional, comprehensive tests of offspring physiology.
2.2. Female germline
Declines in female fertility associated with heavy alcohol use are explained in part by alcohol’s deleterious effects on oocyte integrity. For example, alcohol exposure produces oocyte degeneration (Cebral, Lasserre, Rettori, & de Gimeno, 1999; Gulyas & Yuan, 1985). Oocyte vitrification, which also produces degeneration (Stachowiak et al., 2009), dysregulates the oocyte epigenome and transcriptome (Liang, Fu, Li, Yuan, & Zhu, 2014; Monzo et al., 2012; Yan, Yan, Qiao, Zhao, & Liu, 2010). Oocyte damage may then precede epigenetic encoding of heritable messages allowing for initiation of multi/transgenerational inheritance in alcohol exposure models, although this possibility can only be explored through direct tests of oocyte integrity and epigenome within multi/transgenerational models.
Alcohol exposure has also been found to increase rates of parthenogenetic activation (abnormal activation in absence of fertilization) of rodent oocytes both after in vivo alcohol ingestion (Cebral et al., 1998) and in vitro application to oocytes (Cuthbertson, 1983). Mammalian parthenogenetic oocytes are not viable for fertilization (McGrath & Solter, 1984), so it is unclear whether the mechanisms mediating this process could contribute to offspring multi/transgenerational phenotypes at a threshold below inviability. For example, inviable oocytes exhibit certain abnormal epigenetic and transcriptional signatures (Ibala-Romdhane et al., 2011; Malki et al., 2014), but it is possible these abnormalities must accumulate to induce inviability – and, thus, that they may be present at below threshold levels in the multigenerational female germline.
Alcohol exposure is known to produce disruptions in menstrual and estrous cycling, likely via modulation of estradiol, LH, and other cycle-related hormones (Eskay, Ryback, Goldman, & Majchrowicz, 1981; Ginsburg et al., 1995; Ryback, 1977; Sanchis, Esquifino, & Guerri, 1985; Sarkola, Mäkisalo, Fukunaga, & Eriksson, 1999). As with the male germline, these hormones modulate development and maintenance of the female germline, so it is possible that their upstream disruption mediates alcohol’s impact on oocyte integrity. The alternative to hormonal mediation of alcohol’s degeneration of oocytes is thus that direct interactions between alcohol and oocytes produce these effects, as is supported by in vitro data. An additional possibility is that these two processes interact to produce oocyte modifications that mediate multi/transgenerational inheritance. For example, culturing oocytes with LH favors oocyte repair (Rossi et al., 2017), but alcohol exposure is associated with reductions in LH (Sanchis et al., 1985). Thus, oocytes already damaged by alcohol may also be less likely to undergo repair.
In summary, alcohol exposure broadly affects reproductive functioning and germline integrity in both males and females. As shifts in reproductive hormones can influence germ cell integrity in the male and female germline, reproductive disturbances produced by alcohol exposure may occur upstream of germline degradation found in alcohol exposure models. We previously noted the possibility that compromised germ cell integrity may further associate with epigenome modifications: In male exposure models, sperm epigenome and transcriptome modifications have been observed following alcohol exposure, while female exposure models have found alcohol-induced oocyte damage, which may itself predict altered extragenomic factors. A tentative model of germline impacts following alcohol exposure may predict compromised germline integrity following changes in upstream reproductive functioning, which triggers modifications to germline epigenome. Future inquiries should aim to clarify potential links between alcohol-induced changes in reproductive function/germline integrity and germline epigenome.
3. Effects of nicotine exposure on male and female germline
3.1. Male germline
Smoking impacts fertility, sperm integrity, and sperm epigenome, although whether this is the result of nicotine exposure or other chemical constituents of tobacco is difficult to deduce from studies sampling human tobacco users. Such research has noted abnormal sperm morphology and DNA fragmentation as well as reductions in sperm motility/count and fertilizing capability in association with smoking (Belcheva, Ivanova‐Kicheva, Tzvetkova, & Marinov, 2004; Fraga, Motchnik, Wyrobek, Rempel, & Ames, 1996; Mulla, Köhn, Beheiry, & Schill, 1995; Nie et al., 2016; Shaarawy & Mahmoud, 1982; Sofikitis et al., 1995; Vine, Chiu-Kit, Hu, & Truong, 1996). Recent analyses additionally found changes in smoker sperm miRNA content (Marczylo, Amoako, Konje, Gant, & Marczylo, 2012) and histone to protamine ratio (Hamad, Shelko, Kartarius, Montenarh, & Hammadeh, 2014). As sperm histones can harbor modifications that are transmitted to the developing zygote, a shift in their abundance relative to protamines increases or decreases the number available for encoding heritable histone modifications (Brykczynska et al., 2010; González et al., 2018; van der Heijden et al., 2008; Yamaguchi et al., 2018). Decreased protamine 2 levels have also been associated with sperm DNA damage (Cho et al., 2003), raising the possibility that changes in histone:protamine ratio may precede DNA damage associated with nicotine exposure.
Human in vitro models and rodent studies can be used to isolate the effect of nicotine (vs. other tobacco constituents) on sperm integrity. Studies for which nicotine was applied to human sperm cells in vitro have confirmed nicotine’s independent reduction of sperm motility (Arabi, 2004; Condorelli et al., 2013), although others found this effect was only detectable at concentrations of nicotine well above those expected with in vivo recreational use (Gandini et al., 1997). Sperm DNA damage associated with nicotine application has also been noted using this model (Arabi, 2004). Rodent in vivo models resulting in blood nicotine levels comparable to human smokers additionally replicated reductions in sperm count, motility, and integrity (Asiyah, Syazana, Hashida, Sharifah, & Kamaruddin, 2011; Oyeyemi, Shittu, Kolawole, Ubanecheand, & Akinola, 2015; Oyeyipo, Raji, Emikpe, & Bolarinwa, 2010). One study contradicted these findings, however, noting an increase in sperm motility following nicotine, along with promoter hypomethylation and increased protein expression of sperm motility factor PFN1 (Dai et al., 2015). A lower nicotine dose and shorter treatment period in the aforementioned study may in part explain this discrepancy, but additional data are required to investigate the possibility of a dose-response effect of nicotine on sperm integrity. In rodent models, litter size may be impacted by sperm count and motility, and behavioral phenotypes are affected by litter size (LaBarba et al., 1973; Tanaka, 1998). Data on paternal nicotine’s effect on litter number would clarify whether there is a need to consider the potential impact of litter size on nicotine-related multi/transgenerational phenotypes; however, these are limited: One rodent study noted no impact of paternal nicotine exposure on litter size, while another found decreased F0 sperm motility in conjunction with smaller F1 litters (Oyeyemi et al., 2015). As we discuss in relation to alcohol exposure, changes in testosterone, LH, and FSH may feasibly mediate the effects of nicotine on sperm integrity and motility, as these hormones modulate sperm development and are lowered by nicotine administration (Oyeyemi et al., 2015; Oyeyipo et al., 2010).
Rodent in vivo models further allow for testing of nicotine’s independent effects on the male germline following systematic administration. Studies utilizing this design have corroborated nicotine-induced changes in F0 sperm epigenome in genes associated with nervous system functioning, in conjunction with altered behavior in F1 offspring. In sperm of nicotine-exposed mice, targeted methylation approaches found hypermethylation in CGI regions of the gene encoding mmu-miR-15b, a microRNA known to target genes involved nervous system functioning (Dai et al., 2017). mRNA transcripts of a gene targeted by mmu-miR-15b were upregulated in F0 sperm as well as in F1 brains, and F1 offspring exhibited altered behavior related to these biological pathways. Non-targeted methylation quantification noted increased global methylation in the sperm of mice exposed to nicotine (McCarthy et al., 2018). An additional targeted analysis in the same study found decreased methylation in F0 sperm at the Drd2 (dopamine receptor 2) promoter, which was accompanied by altered monoamine signaling and related behavior in F1 offspring. The discrepancy between the direction of change in methylation between the global assay and the targeted approach further highlights the need to supplement targeted assays with genome-wide assessments when feasible. That is, global assays may reveal meaningful general trends in methylation patterns, while targeted approaches are important for testing phenotype-related hypotheses. As with alcohol exposure models, neuro-behavioral associated pathways appear to be enriched among sperm epigenetic alterations following nicotine exposure.
The reviewed data suggest that nicotine exposure impacts reproductive hormones, sperm integrity/motility, and sperm epigenome. Shifts in reproductive hormones can affect germline integrity (Arai et al., 1998; Biswas et al., 1978; Pasqualotto et al., 2005; Wdowiak et al., 2014), and epigenomic factors are reactive to sperm integrity (Cho et al., 2003). Thus, future analyses may consider prioritizing co-assessment of these variables, which may act within the same biological network to produce multi/transgenerational inheritance in paternal nicotine exposure models.
3.2. Female germline
The impact of nicotine on the female germline is reflected at the population level by the common occurrence of fertility issues in female smokers, who are reported to produce fewer viable oocytes and have lower successful pregnancy rates in comparisons of smoking and non-smoking in vitro fertilization patients (Harrison, Breen, & Hennessey, 1990; Rosevear et al., 1992; Oyesanya, Zenzes, Wang, & Casper, 1995; Van Voorhis, Dawson, Stovall, Sparks, & Syrop, 1996). Increased rates of abnormal oocytes derived from female smokers, including cases of aberrant ploidy, have also been reported (Oyesanya et al., 1995). In vitro application of nicotine to human ovaries has confirmed a reduction in oocyte number in addition to upregulated ovarian nAChR subunit gene expression (Cheng et al., 2018). Whether oocyte nAChR expression is also affected is unknown, although detectable expression of nAChR subunit mRNA has been found in human oocytes (Grøndahl et al., 2010). It has been suggested that select nAChRs may play a role in cell development and death in early developing oocytes (Grøndahl et al., 2013), pointing to a potential mediator of nicotine’s effects on oocyte number/integrity.
In vivo rodent nicotine exposure models suggest changes in female sex hormones estradiol and progesterone (Halder, Trauth, & Pearce, 2015; Holloway, Kellenberger, & Petrik, 2006), disruptions that may persist into the F1 generation (Holloway et al., 2006). They also replicate oocyte degradation and reductions in oocyte number (Gorkem et al., 2016; Mohammadghasemi, Jahromi, Hajizadeh, Homafar, & Saadat, 2012; Noor, Bahkriansyah, Widjiati, & Santoso, 2015). Recent rodent models have found no effect of preconceptional nicotine exposure on F1 litter size, however, suggesting that the number of oocytes available for fertilization is not significantly reduced by nicotine (Holloway, Cuu, Morrison, Gerstein, & Tarnopolsky, 2007; Zhang, Spencer, Biederman, & Bhide, 2018).
No work to our knowledge has characterized the oocyte epigenome following nicotine exposure; however, the above findings point to oocyte degradation in response to nicotine, and oocyte degradation is itself associated with altered oocyte epigenome and transcriptome (Liang et al., 2014; Monzo et al., 2012; Yan et al., 2010; Ibala-Romdhane et al., 2011; Malki et al., 2014). Future research may thus aim to identify oocyte epigenetic modifications alongside oocyte integrity following nicotine exposure.
In males and females, nicotine’s effects on fertility are demonstrative of its broad impacts on reproductive hormones, germline integrity, and germline epigenome. Through their combined regulation of offspring development, these factors may contribute to nicotine’s ability to initiate multi/transgenerational inheritance. Nicotine impacts reproductive hormones in males and females, and these shifts in reproductive functioning may associate with degradation of male and female germline following nicotine exposure. In the male germline, nicotine affects the epigenome and transcriptome. In females, nicotine-induced oocyte degradation may itself predict oocyte epigenome modifications, although this will require direct testing in models of female nicotine exposure.
4. Effects of cocaine exposure on male and female germline
4.1. Effects of cocaine exposure on reproductive proxies and germline integrity
Literature on cocaine’s effects on both the male and female germline is limited, with the bulk of existing data derived from rodent models. Early investigations of cocaine exposure in rodent models found abnormal sperm morphology but no impact on DNA fragmentation or male sex organs (Abel, Moore, Waselewsky, Zajac, & Russell, 1989; He, Lidow, & Lidow, 2006). Data on cocaine’s in vivo effects on testosterone are inconsistent, and may be dose- and time-dependent (Abel et al., 1989; Gordon, Mostofsky, & Gordon, 1980; Heesch et al., 1996). Cocaine administration is known to stimulate FSH and LH release in both females and males (Heesch et al., 1996; Mello, Mendelson, Drieze, & Kelly, 1990; Mendelson, Sholar, Siegel, & Mello, 2001), as well as estradiol release in females (Kaufmann, Savoy-Moore, Sacco, & Subramanian, 1990; Mello et al., 2004). Dysregulation of these reproductive hormones can impact germline integrity (Arai et al., 1998; Beker-van Woudenberg, van Tol, Roelen, Colenbrander, & Bevers, 2004; Biswas et al., 1978; Kreiner, Liu, Itskovitz, Veeck, & Rosenwaks, 1987; Pasqualotto et al., 2005; Wdowiak et al., 2014). Analyses of human self-reported users point to reduced sperm count and motility as well as general infertility in cocaine users (Bracken et al., 1990), whereas in vivo application of cocaine to sperm has indicated a potential reduction in oocyte penetration capability but minimal or no effect on sperm motility (Yazigi, Odem, & Polakoski, 1991; Yelian, Sacco, Ginsburg, Doerr, & Armant, 1994). Culturing of developing murine oocytes in cocaine produced moderate shifts in meiotic dynamics, although this was only seen at concentrations well above those observed in recreational users (Combelles, Carabatsos, London, Mailhes, & Albertini, 2000; Sobel, Goldstein, & Dannenhoffer, 1996). A rabbit cocaine exposure model found no change in oocyte number or cell division after in vitro fertilization (Kaufmann et al., 1990). Thus, it is unclear whether cocaine’s impacts on upstream reproductive functioning have the potential to modulate the male or female germline.
While these findings point to clear dysregulation of reproductive hormones following cocaine exposure, they also suggest a less prominent effect of cocaine exposure on germline integrity (as compared to alcohol and nicotine exposure); however, this may only reflect limited data in this developing field of study. Additional assessment of cocaine’s effects on germline integrity will be necessary to determine whether epigenomic modifications associated with cocaine exposure can in fact be decoupled from its impact on germline integrity – and whether shifts in reproductive hormones necessarily precede declines in germline integrity.
4.2. Effects of cocaine exposure on germline epigenome
Rodent exposure models have shown cocaine alters sperm cell epigenome, with many of these modifications showing heritability to the F1 generation. In rats, increased association of acetylated histone h3 with the Bdnf promoter was observed in sperm of cocaine-exposed males, a pattern that was inherited to the F1 generation brain. This was observed in conjunction with upregulation of brain-derived neurotrophic factor (BDNF) protein and decreased cocaine self-administration (Vassoler, White, Schmidt, Sadri-Vakili, & Pierce, 2013). These multigenerational changes in BDNF, a neurotrophin involved in cognition and plasticity, were hypothesized to mediate decreased cocaine self-administration in cocaine-sired animals. In support, administration of a BDNF receptor antagonist reversed the multigenerational cocaine self-administration phenotype.
González and colleagues (2018) noted increases in acetylated histone h4 and decreases in histone deacetylase mRNA, along with increased global methylation and dysregulation of DNA methyltransferase mRNA in sperm of cocaine-exposed males. In support, a genome-wide DNA methylation analysis confirmed broadly altered DNA methylation in cocaine-exposed male sperm in a rodent self-administration paradigm (Le et al., 2017). Interestingly, Le and colleagues (2017) noted that sperm methylation patterns segregated by F0 addiction phenotype – that is, exposed males showed overall sperm DNA hypomethylation vs. non-exposed controls, and males with higher cocaine self-administration were further distinguishable in terms of methylation patterns from males with lower administration. Altered methylation was heritable to F1 offspring sperm cells and was also dependent upon F0 phenotype and corresponding sperm methylation – that is, F1 offspring derived from high administering fathers had a methylome that matched sperm of high-administering males, while F1 offspring from low-administering fathers had a sperm methylome matching their low-administering fathers. Finally, F1 offspring of high-administering F0 males showed increased cocaine self-administration relative to offspring of low-administering F0 males. Phenotype segregation of F1 animals in this study was only impacted by sire phenotype (i.e., high vs. low self-administration) -- not by the total quantity of cocaine administered in sires. In line with previously discussed research suggesting that multigenerational phenotypes may relate to the parental exposure, Le and colleagues’ (2017) found altered incentive to administer cocaine in offspring of cocaine-exposed rats. However, this effect was mediated by the parental addictive phenotype. Multi/transgenerational inheritance may thus be dynamic between, and even within, populations depending on unknown individual characteristics. An interesting future area of inquiry may involve tests of this phenomenon in the context of links between reproductive functioning, germline integrity, and germline epigenome (i.e., do reproductive functioning and germline integrity additionally segregate by addictive phenotype in the exposed generation?)
In sum, cocaine exposure disrupts reproductive hormones in males and females, although its effect on germline integrity may be limited. Rodent models of cocaine exposure find changes in sperm histone modifications and DNA methylation, suggesting that detectable degradation of germline integrity need not necessarily precede modifications to germ cell epigenome.
5. Conclusions
Alcohol, nicotine, and cocaine produce measurable alterations to the male and female germline. Thus, we broadly conclude that the germline’s protected status is not immutable in the face of exposure to drugs of abuse. Further, the consequences of such exposure can extend to offspring in a manner that may subvert traditional assumptions of Mendelian inheritance. Multi/transgenerational inheritance by definition implies a germline mediator, although the precise biological pathways allowing for this astonishing phenomenon have yet to be delineated.
Across the reviewed drug exposures, both males and females show dysregulated reproductive hormones, declines in germline integrity, and modified germline epigenome. Although they have not been studied in the context of multi/transgenerational drug exposure models, known links between these germline impacts allow for the construction of a tentative theoretical pathway underlying germline modifications contributing to multi/transgenerational inheritance. Theoretical variants of this pathway are depicted in Figure 1. Pathway #1 depicts an additive model of germline impacts downstream of drug exposure, wherein changes in reproductive functioning, germline integrity, and/or germline epigenome additively mediate multi/transgenerational inheritance. In this model, at least two separate impacts are necessary to produce multi/transgenerational inheritance, but they need not occur in a specific sequence (i.e., a “double-hit” model). Pathway #2 depicts a sequential model of germline impacts downstream of drug exposure, wherein changes in reproductive functioning cause altered germline integrity, which then triggers germline epigenomic modifications that mediate multi/transgenerational inheritance. Data potentially supporting both of these models include the presently discussed literature finding altered germline integrity in association with disturbances in reproductive functioning (Arai et al., 1998; Biswas et al., 1978; Pasqualotto et al., 2005; Wdowiak et al., 2014; Rossi et al., 2017) and modified germ cell epigenome/transcriptome in association with declines in germline integrity (Bahreinian et al., 2015; Liang at el., 2014; Montjean et al., 2015; Monzo et al., 2012; Yan et al., 2010; Ibala-Romdhane et al., 2011; Malki et al., 2014; Pacheco et al., 2011). Importantly, these findings may support both the additive and sequential pathways, as the depicted sequences are based upon existing literature only associatively linking pathway components.
Pathways #3 and 4 show alternative sequential models, wherein impacts of drug exposure on one or more, but not all, of the discussed factors in sequence are sufficient to produce multi/transgenerational phenotypes. Factors omitted from pathways #3 and 4 would then be inconsequential to multi/transgenerational inheritance in drug exposure models, only occurring in parallel with the true mediating factor. Finally, pathways #5 and 6 depict a single independent mediator of multi/transgenerational inheritance in drug exposure models (altered germline integrity in pathway #5 and modifications to germline epigenome/transcriptome in pathway #6). Pathway #5 may be supported by evidence of direct impacts of germline degradation on offspring phenotypes (cell count impacting litter size and/or DNA damage producing heritable sequence changes) (Fernández-Gonzalez et al., 2008; LaBarba et al.,1973; Kumar et al., 2013; Ramos-Ibeas et al., 2014; Tanaka, 1998). Pathway #6 is supported by ample evidence of germline epigenome modifications in association with drug-related multi/transgenerational inheritance (Bielawski et al., 2002; Dai et al., 2017; Finegersh, & Homanics, 2014; Govorko et al., 2012; Kim et al., 2014; Le et al., 2017; Liang et al., 2014; McCarthy et al., 2018; Vassoler et al., 2013). However, in absence of testing for other pathway components, these findings cannot be said to definitively point to an independent contribution of germline integrity or epigenome to multi/transgenerational inheritance.
In addition to simultaneously examining reproductive functioning, germline integrity, and germline epigenome, future experiments testing these theoretical models within multi/transgenerational designs may consider systematic manipulation of these factors in order to assess their impact on the pathway as a whole. We emphasize, however, that mechanisms underlying multi/transgenerational inheritance may differ between different drug exposures and perhaps even between individuals. For example, certain drugs of abuse may be able to directly impact germline integrity, bypassing upstream reproductive functioning. The reviewed in vitro drug application models (Arabi, 2004; Cheng et al., 2018; Condorelli et al., 2013; Cuthbertson, 1983) support this possibility – although in the context of the additive pathway model (Figure 1, pathway #1), germline integrity impacts in isolation may be insufficient to initiate multi/transgenerational inheritance. Individual variability in hormonal responses to drug (perhaps mediated by addictive phenotype; see Le et al., 2017 discussed above) may also predispose only a subset of a heterogenous population to multi/transgenerational inheritance by a pathway involving altered reproductive function. Indeed, certain drug exposures may have the ability to initiate multi/transgenerational inheritance through multiple pathways with different resultant impacts on offspring phenotype. Future work may thus necessitate testing in models of genetic and phenotypic variability (e.g. inbred rodent strain panels) to characterize the full repertoire of multi/transgenerational effects initiated by specific exposures. We do not intend to suggest that this work has not been performed previously due to negligence of these important concepts. Rather, multi/transgenerational inheritance is a burgeoning field that required initial proof-of-concept research. What now follows should be development and testing of falsifiable theory. We believe first priority should be given to co-assessment of reproductive functioning, germline integrity, and germline epigenome in models known to produce multi/transgenerational inheritance. Given that the number of biological factors within these categories are near unlimited, selection of variables should be guided by prior knowledge of the exposure. For example, the known interaction of alcohol with LH and female germline integrity (Rossi et al., 2017; Sanchis et al., 1985) makes LH a promising candidate for a reproductive proxy possibly acting within the biological pathway leading to alcohol-induced multi/transgenerational inheritance. “Reverse-engineering” of multi/transgenerational inheritance models in which offspring effects segregate by parental phenotype (as in Le et al., 2017) may also aid in identification of candidate variables that covary with offspring phenotype. Subsequent work may aim to determine if each of the identified factors are able to independently reproduce multi/transgenerational inheritance – and whether induction of one is followed by impacts on the other.
We conclude by noting that the reviewed literature can only directly support that alcohol, nicotine, and cocaine exposure all produce distinct impacts on reproductive functioning, germline integrity, and germline epigenome. Additional evidence suggests that these factors may exist in an interacting biological network that mediates multi/transgenerational inheritance. Characterization of germline impacts following drug exposure in the full context of affected biological pathways may serve to facilitate identification of true causal factors in germline-mediated multigenerational and transgenerational inheritance.
FIGURE 2:
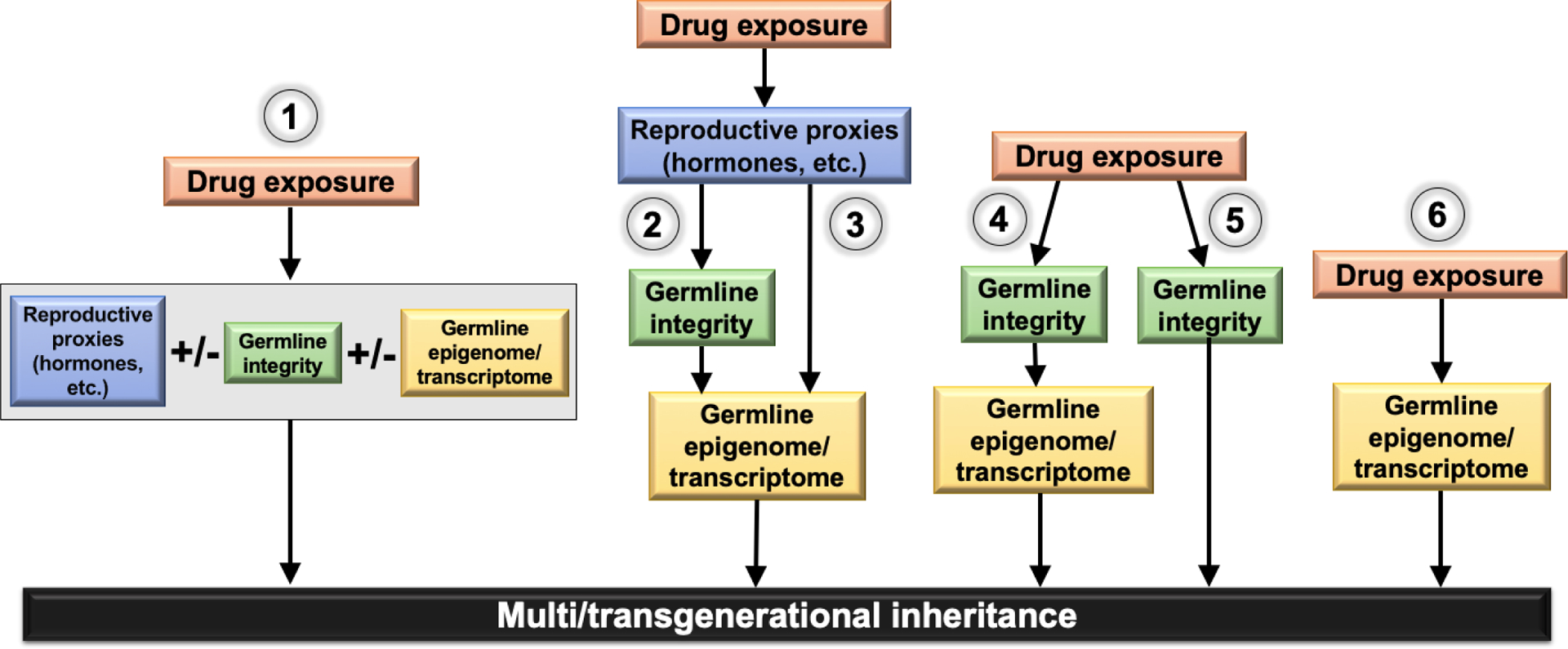
Theoretical biological pathways underlying multi/transgenerational inheritance: 1) Additive model of germline impacts downstream of drug exposure, wherein changes in reproductive functioning, germline integrity, and/or germline epigenome additively mediate multi/transgenerational inheritance; 2) Sequential model of germline impacts downstream of drug exposure, wherein changes in reproductive functioning cause altered germline integrity, which then triggers germline epigenomic modifications that mediate multi/transgenerational inheritance; 3) & 4) Alternative sequential models, wherein impacts of drug exposure on one or more, but not all, of the discussed factors in sequence are sufficient to produce multi/transgenerational phenotypes; 5) and 6) Pathways depicting a single independent mediator of multi/transgenerational inheritance in drug exposure models.
Highlights.
Parental exposure to nicotine, alcohol, and cocaine can affect offspring phenotypes.
Drug abuse impacts male and female germline epigenome and general integrity.
Germline integrity & epigenome may interact to produce multi/transgenerational inheritance.
References
- Abel EL (1993). Paternal alcohol exposure and hyperactivity in rat offspring: effects of amphetamine. Neurotoxicology and teratology, 15(6), 445–449. [DOI] [PubMed] [Google Scholar]
- Abel EL, & Lee JA (1988). Paternal alcohol exposure affects offspring behavior but not body or organ weights in mice. Alcoholism: Clinical and Experimental Research, 12(3), 349–355. [DOI] [PubMed] [Google Scholar]
- Abel EL, & Moore C (1987). Effects of paternal alcohol consumption in mice. Alcoholism: Clinical and Experimental Research, 11(6), 533–535. [DOI] [PubMed] [Google Scholar]
- Abel EL, Moore C, Waselewsky D, Zajac C, & Russell LD (1989). Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. Journal of andrology, 10(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Abel EL, & Tan SE (1988). Effects of paternal alcohol consumption on pregnancy outcome in rats. Neurotoxicology and teratology, 10(3), 187–192. [DOI] [PubMed] [Google Scholar]
- Ahmadi A, & Ng SC (1999). Fertilizing ability of DNA‐damaged spermatozoa. Journal of experimental zoology, 284(6), 696–704. [DOI] [PubMed] [Google Scholar]
- Akang EN, Oremosu AA, Osinubi AA, James AB, Biose IJ, Dike SI, & Idoko KM (2017). Alcohol-induced male infertility: Is sperm DNA fragmentation a causative? Journal of Experimental and Clinical Anatomy, 16(1), 53. [Google Scholar]
- Akiyama T, Nagata M, & Aoki F (2006). Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proceedings of the National Academy of Sciences, 103(19), 7339–7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh Z, Kageyama SI, & Aoki F (2005). Degradation of maternal mRNA in mouse embryos: selective degradation of specific mRNAs after fertilization. Molecular Reproduction and Development: Incorporating Gamete Research, 72(3), 281–290. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Furby JE, Oswald C, & Zaneveld LJ (1981). Tetratological evaluation of mouse fetuses after paternal alcohol ingestion. Neurobehavioral toxicology and teratology, 3(2), 117–120 [PubMed] [Google Scholar]
- Anderson RA, Willis BR, Oswald C, & Zaneveld LJ (1983). Ethanol-induced male infertility: impairment of spermatozoa. Journal of Pharmacology and Experimental Therapeutics, 225(2), 479–486. [PubMed] [Google Scholar]
- Aoki VW, Moskovtsev SI, Willis J, Liu L, Mullen JBM, & Carrell DT (2005). DNA integrity is compromised in protamine‐deficient human sperm. Journal of Andrology, 26(6), 741–748. [DOI] [PubMed] [Google Scholar]
- Arabi M (2004). Nicotinic infertility: assessing DNA and plasma membrane integrity of human spermatozoa. Andrologia, 36(5), 305–310. [DOI] [PubMed] [Google Scholar]
- Arai T, Kitahara S, Horiuchi S, Sumi S, & Yoshida KI (1998). Relationship of testicular volume to semen profiles and serum hormone concentrations in infertile Japanese males. International journal of fertility and women’s medicine, 43(1), 40–47. [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, & Hannon GJ (2007). Developmentally regulated piRNA clusters implicate MILI in transposon control. Science, 316(5825), 744–747. [DOI] [PubMed] [Google Scholar]
- Asiyah HA, Syazana NS, Hashida NH, Durriyyah Sharifah HA, & Kamaruddin MY (2011). Effects of nicotine and Gelam honey on testis parameters and sperm qualities of juvenile rats. Sci. Res. Essays, 6(26), 5471–5474. [Google Scholar]
- Bachvarova R, De Leon V, Johnson A, Kaplan G, & Paynton BV (1985). Changes in total RNA, polyadenylated RNA, and actin mRNA during meiotic maturation of mouse oocytes. Developmental biology, 108(2), 325–331. [DOI] [PubMed] [Google Scholar]
- Bahreinian M, Tavalaee M, Abbasi H, Kiani-Esfahani A, Shiravi AH, & Nasr-Esfahani MH (2015). DNA hypomethylation predisposes sperm to DNA damage in individuals with varicocele. Systems biology in reproductive medicine, 61(4), 179–186. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph H, Ben-Aharon I, Rizel S, Stemmer SM, Tzabari M, & Shalgi R (2010). Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reproductive toxicology, 30(4), 566–572. [DOI] [PubMed] [Google Scholar]
- Barroso‐Moguel R, Méndez‐Armenta M, & Villeda‐Hernández J (1994). Testicular lesions by chronic administration of cocaine in rats. Journal of Applied Toxicology, 14(1), 37–41. [DOI] [PubMed] [Google Scholar]
- Beker-van Woudenberg AR, van Tol HT, Roelen BA, Colenbrander B, & Bevers MM (2004). Estradiol and its membrane-impermeable conjugate (estradiol-bovine serum albumin) during in vitro maturation of bovine oocytes: effects on nuclear and cytoplasmic maturation, cytoskeleton, and embryo quality. Biology of reproduction, 70(5), 1465–1474. [DOI] [PubMed] [Google Scholar]
- Belcheva A, Ivanova‐Kicheva M, Tzvetkova P, & Marinov M (2004). Effects of cigarette smoking on sperm plasma membrane integrity and DNA fragmentation. International journal of andrology, 27(5), 296–300. [DOI] [PubMed] [Google Scholar]
- Bart J, Hollema H, Groen HJM, De Vries EGE, Hendrikse NH, Sleijfer DT, … & Van Der Graaf WTA (2004). The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood–testis barrier and in primary testicular tumours. European journal of cancer, 40(14), 2064–2070. [DOI] [PubMed] [Google Scholar]
- Bielawski DM, Zaher FM, Svinarich DM, & Abel EL (2002). Paternal alcohol exposure affects sperm cytosine methyltransferase messenger RNA levels. Alcoholism: Clinical and Experimental Research, 26(3), 347–351. [PubMed] [Google Scholar]
- Biswas S, Ferguson KM, Stedronska J, Baffoe G, Mansfield MD, & Kosbab MH (1978). Fructose and hormone levels in semen: their correlations with sperm counts and motility. Fertility and Sterility, 30(2), 200–204. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Eskenazi B, Sachse K, McSharry JE, Hellenbrand K, & Leo-Summers L (1990). Association of cocaine use with sperm concentration, motility, and morphology. Fertility and Sterility, 53(2), 315–322. [DOI] [PubMed] [Google Scholar]
- Brazill JL, & Masui Y (1978). Changing levels of UV light and carcinogen-induced unscheduled DNA synthesis in mouse oocytes during meiotic maturation. Experimental cell research, 112(1), 121–125. [DOI] [PubMed] [Google Scholar]
- Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, … & Peters AH (2010). Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nature structural & molecular biology, 17(6), 679. [DOI] [PubMed] [Google Scholar]
- Bujan L, Mieusset R, Mansat A, Moatti JP, Mondinat C, & Pontonnier F (1989). Testicular size in infertile men: relationship to semen characteristics and hormonal blood levels. British journal of urology, 64(6), 632–637. [DOI] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, … & Meissner A (2010). Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell, 143(7), 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, … & Yazbeck C (2012). Correlation between DNA defect and sperm-head morphology. Reproductive biomedicine online, 24(2), 211–218. [DOI] [PubMed] [Google Scholar]
- Cassuto NG, Montjean D, Siffroi JP, Bouret D, Marzouk F, Copin H, & Benkhalifa M (2016). Different levels of DNA methylation detected in human sperms after morphological selection using high magnification microscopy. BioMed research international, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, … & Qian J (2016). Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science, 351(6271), 397–400. [DOI] [PubMed] [Google Scholar]
- Cheng SF, Qin XS, Han ZL, Sun XF, Feng YN, Yang F, … & Zou SH (2018). Nicotine exposure impairs germ cell development in human fetal ovaries cultured in vitro. Aging (Albany NY), 10(7), 1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, … & Eddy EM (2003). Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biology of reproduction, 69(1), 211–217. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Adams ML, O’Connor L, Nock B, Meyer ER, & Wozniak D (1990). Influence of chronic alcohol administration on representative indices of puberty and sexual maturation in male rats and the development of their progeny. Journal of pharmacology and experimental therapeutics, 255(2), 707–715. [PubMed] [Google Scholar]
- Cebral E, Lasserre A, Motta A, & De Gimeno MF (1998). Mouse oocyte quality and prostaglandin synthesis by cumulus oocyte complex after moderate chronic ethanol intake. Prostaglandins, leukotrienes and essential fatty acids, 58(5), 381–387. [DOI] [PubMed] [Google Scholar]
- Cebral E, Lasserre A, Rettori V, & de Gimeno MA (1999). Deleterious effects of chronic moderate alcohol intake by female mice on preimplantation embryo growth in vitro. Alcohol and Alcoholism, 34(4), 551–558. [DOI] [PubMed] [Google Scholar]
- Cleaton MA, Dent CL, Howard M, Corish JA, Gutteridge I, Sovio U, … & Powell TL (2016). Fetus-derived DLK1 is required for maternal metabolic adaptations to pregnancy and is associated with fetal growth restriction. Nature genetics, 48(12), 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles CM, Carabatsos MJ, London SN, Mailhes JB, & Albertini DF (2000). Centrosome-specific perturbations during in vitro maturation of mouse oocytes exposed to cocaine. Experimental cell research, 260(1), 116–126. [DOI] [PubMed] [Google Scholar]
- Condorelli RA, La Vignera S, Giacone F, Iacoviello L, Vicari E, Mongioi L, & Calogero AE (2013). In vitro effects of nicotine on sperm motility and bio-functional flow cytometry sperm parameters. International journal of immunopathology and pharmacology, 26(3), 739–746. [DOI] [PubMed] [Google Scholar]
- Conine CC, Sun F, Song L, Rivera-Pérez JA, & Rando OJ (2018). Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Developmental cell, 46(4), 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, & Yeung CH (2003). Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microscopy research and technique, 61(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Cuthbertson KR (1983). Parthenogenetic activation of mouse oocytes in vitro with ethanol and benzyl alcohol. Journal of experimental Zoology, 226(2), 311–314 [DOI] [PubMed] [Google Scholar]
- Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, … & Qiao Z (2017). Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b. Scientific reports, 7(1), 7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Zhan C, Xu W, Wang Z, Nie D, Zhao X, … & Qiao Z (2015). Nicotine elevates sperm motility and induces Pfn1 promoter hypomethylation in mouse testis. Andrology, 3(5), 967–978. [DOI] [PubMed] [Google Scholar]
- Dym M, & Fawcett DW (1970). The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biology of reproduction, 3(3), 308–326. [DOI] [PubMed] [Google Scholar]
- Eskay RL, Ryback RS, Goldman M, & Majchrowicz E (1981). Effect of chronic ethanol administration on plasma levels of LH and the estrous cycle in the female rat. Alcoholism: Clinical and Experimental Research, 5(2), 204–206. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu Y, Xue K, Gu G, Fan W, Xu Y, & Ding Z (2015). Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PloS one, 10(4), e0120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, … & Gutiérrez-Adán A (2008). Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biology of reproduction, 78(4), 761–772. [DOI] [PubMed] [Google Scholar]
- Finegersh A, & Homanics GE (2014). Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PloS one, 9(6), e99078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, & Ames BN (1996). Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 351(2), 199–203. [DOI] [PubMed] [Google Scholar]
- Gandini L, Lombardo F, Lenzi A, Culasso F, Pacifici R, Zuccaro P, & Dondero F (1997). The in-vitro effects of nicotine and cotinine on sperm motility. Human reproduction (Oxford, England), 12(4), 727–733. [DOI] [PubMed] [Google Scholar]
- Generoso WM, Cain KT, Krishna M, & Huff SW (1979). Genetic lesions induced by chemicals in spermatozoa and spermatids of mice are repaired in the egg. Proceedings of the National Academy of Sciences, 76(1), 435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M, & Zamore PD (2009). Small silencing RNAs: an expanding universe. Nature reviews genetics, 10(2), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg ES, Walsh BW, Shea BF, Gao X, Gleason RE, & Barbieri RL (1995). The effects of ethanol on the clearance of estradiol in postmenopausal women. Fertility and sterility, 63(6), 1227–1230. [PubMed] [Google Scholar]
- González B, Pantoja CRG, Sosa MH, Vitullo AD, Bisagno V, & González CR (2018). Cocaine alters the mouse testicular epigenome with direct impact on histone acetylation and DNA methylation marks. Reproductive biomedicine online, 37(3), 269–278. [DOI] [PubMed] [Google Scholar]
- Gordon LA, Mostofsky DI, & Gordon GG (1980). Changes in testosterone levels in the rat following intraperitoneal cocaine HCl. International Journal of Neuroscience, 11(2), 139–141. [DOI] [PubMed] [Google Scholar]
- Gorkem U, Togrul C, Seckin KD, Karsli MF, Deveci E, & Gungor T (2016). Effect of nicotine exposure during gestation on neonatal rat ovaries. Int J Clin Exp Med, 9(2), 4158–4162. [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, & Sarkar DK (2012). Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biological psychiatry, 72(5), 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøndahl ML, Borup R, Vikeså J, Ernst E, Andersen CY, & Lykke-Hartmann K (2013). The dormant and the fully competent oocyte: comparing the transcriptome of human oocytes from primordial follicles and in metaphase II. MHR: Basic science of reproductive medicine, 19(9), 600–617. [DOI] [PubMed] [Google Scholar]
- Grøndahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, & Borup R (2010). Gene expression profiles of single human mature oocytes in relation to age. Human Reproduction, 25(4), 957–968. [DOI] [PubMed] [Google Scholar]
- Guo L, Chao SB, Xiao L, Wang ZB, Meng TG, Li YY, … & Ou XH (2017). Sperm-carried RNAs play critical roles in mouse embryonic development. Oncotarget, 8(40), 67394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas BJ, & Yuan LC (1985). Cortical reaction and zona hardening in mouse oocytes following exposure to ethanol. Journal of Experimental Zoology, 233(2), 269–276. [DOI] [PubMed] [Google Scholar]
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, & Surani MA (2013). Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science, 339(6118), 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S, Trauth S, & Pearce AR (2015). Oral nicotine alters uterine histo-morphology but does not disrupt the estrous cycle in female rats. Nicotine & Tobacco Research, 18(5), 590–595. [DOI] [PubMed] [Google Scholar]
- Hamad MF, Shelko N, Kartarius S, Montenarh M, & Hammadeh ME (2014). Impact of cigarette smoking on histone (H2B) to protamine ratio in human spermatozoa and its relation to sperm parameters. Andrology, 2(5), 666–677. [DOI] [PubMed] [Google Scholar]
- Harrison KL, Breen TM, & Hennessey JF (1990). The effect of patient smoking habit on the outcome of IVF and GIFT treatment. Australian and New Zealand Journal of Obstetrics and Gynaecology, 30(4), 340–342. [DOI] [PubMed] [Google Scholar]
- He F, Lidow IA, & Lidow MS (2006). Consequences of paternal cocaine exposure in mice. Neurotoxicology and teratology, 28(2), 198–209. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Bost JE, Keffer JH, Snyder RW, & Eichhorn EJ (1996). Effects of cocaine on anterior pituitary and gonadal hormones. Journal of Pharmacology and Experimental Therapeutics, 278(3), 1195–1200. [PubMed] [Google Scholar]
- Hess KA, Chen L, & Larsen WJ (1998). The ovarian blood follicle barrier is both charge-and size-selective in mice. Biology of reproduction, 58(3), 705–711. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Cuu DQ, Morrison KM, Gerstein HC, & Tarnopolsky MA (2007). Transgenerational effects of fetal and neonatal exposure to nicotine. Endocrine, 31(3), 254–259. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Kellenberger LD, & Petrik JJ (2006). Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine, 30(2), 213–216. [DOI] [PubMed] [Google Scholar]
- Houshdaran S, Cortessis VK, Siegmund K, Yang A, Laird PW, & Sokol RZ (2007). Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PloS one, 2(12), e1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibala-Romdhane S, Al-Khtib M, Khoueiry R, Blachère T, Guérin JF, & Lefèvre A (2011). Analysis of H19 methylation in control and abnormal human embryos, sperm and oocytes. European Journal of Human Genetics, 19(11), 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann RA, Savoy-Moore RT, Sacco AG, & Subramanian MG (1990). The effect of cocaine on oocyte development and the follicular microenvironment in the rabbit. Fertility and sterility, 54(5), 921–926. [PubMed] [Google Scholar]
- Kierszenbaum A, & Tres LL (1975). Structural and transcriptional features of the mouse spermatid genome. The Journal of cell biology, 65(2), 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Choi CS, Park JH, Joo SH, Kim SY, Ko HM, … & Ryu JH (2014). Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder‐like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. Journal of neuroscience research, 92(5), 658–670. [DOI] [PubMed] [Google Scholar]
- Kim JM, Liu H, Tazaki M, Nagata M, & Aoki F (2003). Changes in histone acetylation during mouse oocyte meiosis. The Journal of cell biology, 162(1), 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezovich JG, & Ramsay M (2012). The effect of preconception paternal alcohol exposure on epigenetic remodeling of the h19 and rasgrf1 imprinting control regions in mouse offspring. Frontiers in genetics, 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, & Diamond MP (2011). A survey of small RNAs in human sperm. Human reproduction, 26(12), 3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner D, Liu HC, Itskovitz J, Veeck L, & Rosenwaks Z (1987). Follicular fluid estradiol and progesterone are markers of preovulatory oocyte quality. Fertility and sterility, 48(6), 991–994. [PubMed] [Google Scholar]
- Kumar D, Upadhya D, Salian SR, Rao SB, Kalthur G, Kumar P, & Adiga SK (2013). The extent of paternal sperm DNA damage influences early post-natal survival of first generation mouse offspring. European Journal of Obstetrics & Gynecology and Reproductive Biology, 166(2), 164–167. [DOI] [PubMed] [Google Scholar]
- LaBarba RC, White JL, Stewart A, & Buckley N (1973). The effects of litter size on emotional reactivity in BALB/c mice. Bulletin of the Psychonomic Society, 1(1), 37–38. [Google Scholar]
- Le Q, Yan B, Yu X, Li Y, Song H, Zhu H, … & Ma L (2017). Drug-seeking motivation level in male rats determines offspring susceptibility or resistance to cocaine-seeking behaviour. Nature communications, 8, 15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescoat D, Blanchard Y, Lavault MT, Quernee D, & Lannou DL (1993). Ultrastructural and immunocytochemical study of P1, protamine localization in human testis. Andrologia, 25(2), 93–99. [DOI] [PubMed] [Google Scholar]
- Liang F, Diao L, Liu J, Jiang N, Zhang J, Wang H, … & Ma D (2014). Paternal ethanol exposure and behavioral abnormities in offspring: associated alterations in imprinted gene methylation. Neuropharmacology, 81, 126–133. [DOI] [PubMed] [Google Scholar]
- Liang Y, Fu XW, Li JJ, Yuan DS, & Zhu SE (2014). DNA methylation pattern in mouse oocytes and their in vitro fertilized early embryos: effect of oocyte vitrification. Zygote, 22(2), 138–145. [DOI] [PubMed] [Google Scholar]
- Liu WM, Pang RT, Chiu PC, Wong BP, Lao K, Lee KF, & Yeung WS (2012). Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proceedings of the National Academy of Sciences, 109(2), 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki S, van der Heijden GW, O’Donnell KA, Martin SL, & Bortvin A (2014). A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Developmental cell, 29(5), 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczylo EL, Amoako AA, Konje JC, Gant TW, & Marczylo TH (2012). Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern?. Epigenetics, 7(5), 432–439. [DOI] [PubMed] [Google Scholar]
- Masui Y, & Pedersen RA (1975). Ultraviolet light-induced unscheduled DNA synthesis in mouse oocytes during meiotic maturation. Nature, 257(5528), 705. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Morgan TJ Jr, Lowe SE, Williamson MJ, Spencer TJ, Biederman J, & Bhide PG (2018). Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS biology, 16(10), e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, & Solter D (1984). Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell, 37(1), 179–183. [DOI] [PubMed] [Google Scholar]
- Meek LR, Myren K, Sturm J, & Burau D (2007). Acute paternal alcohol use affects offspring development and adult behavior. Physiology & behavior, 91(1), 154–160. [DOI] [PubMed] [Google Scholar]
- Melaine N, Liénard MO, Dorval I, Le Goascogne C, Lejeune H, & Jégou B (2002). Multidrug resistance genes and P-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biology of reproduction, 67(6), 1699–1707. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Drieze J, & Kelly M (1990). Cocaine effects on luteinizing hormone-releasing hormone-stimulated anterior pituitary hormones in female rhesus monkey. The Journal of Clinical Endocrinology & Metabolism, 71(6), 1434–1441. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Negus SS, Kelly M, Knudson I, & Roth ME (2004). The effects of cocaine on gonadal steroid hormones and LH in male and female rhesus monkeys. Neuropsychopharmacology, 29(11), 2024. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Siegel AJ, & Mello NK (2001). Effects of cocaine on luteinizing hormone in women during the follicular and luteal phases of the menstrual cycle and in men. Journal of Pharmacology and Experimental Therapeutics, 296(3), 972–979. [PubMed] [Google Scholar]
- Mohammadghasemi F, Jahromi SK, Hajizadeh H, Homafar MA, & Saadat N (2012). The protective effects of exogenous melatonin on nicotine-induced changes in mouse ovarian follicles. Journal of reproduction & infertility, 13(3), 143. [PMC free article] [PubMed] [Google Scholar]
- Montjean D, Zini A, Ravel C, Belloc S, Dalleac A, Copin H, … & Benkhalifa M (2015). Sperm global DNA methylation level: association with semen parameters and genome integrity. Andrology, 3(2), 235–240. [DOI] [PubMed] [Google Scholar]
- Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, & Hamamah S (2012). Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Human Reproduction, 27(7), 2160–2168. [DOI] [PubMed] [Google Scholar]
- Mulla KE, K¨ ohn FM, Beheiry AE, & Schill WB (1995). The effect of smoking and varicocele on human sperm acrosin activity and acrosome reaction. Human Reproduction, 10(12), 3190–3194. [DOI] [PubMed] [Google Scholar]
- Muthusami KR, & Chinnaswamy P (2005). Effect of chronic alcoholism on male fertility hormones and semen quality. Fertility and sterility, 84(4), 919–924. [DOI] [PubMed] [Google Scholar]
- Nanassy L, & Carrell DT (2011). Abnormal methylation of the promoter of CREM is broadly associated with male factor infertility and poor sperm quality but is improved in sperm selected by density gradient centrifugation. Fertility and sterility, 95(7), 2310–2314. [DOI] [PubMed] [Google Scholar]
- Nie D, Zhang D, Dai J, Zhang M, Zhao X, Xu W, … & Qiao Z (2016). Nicotine induced murine spermatozoa apoptosis via up-regulation of deubiquitinated RIP1 by Trim27 promoter hypomethylation. Biology of reproduction, 94(2), 31–1. [DOI] [PubMed] [Google Scholar]
- Noor MS, Bahkriansyah HM, Widjiati W, & Santoso B (2015). Nicotine supplementation blocks oocyte maturation in Rattus norvegicus. Universa Medicina, 32(2), 92–98. [Google Scholar]
- Ostermeier GC, Goodrich RJ, Moldenhauer JS, Diamond MP, & Krawetz SA (2005). A suite of novel human spermatozoal RNAs. Journal of andrology, 26(1), 70–74. [PubMed] [Google Scholar]
- Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, … & Walter J (2000). Active demethylation of the paternal genome in the mouse zygote. Current Biology, 10(8), 475–478. [DOI] [PubMed] [Google Scholar]
- Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, & Ramsay M (2009). Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG‐DMR in male gametes—Implications for fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 33(9), 1615–1627. [DOI] [PubMed] [Google Scholar]
- Oyesanya OA, Zenzes MT, Wang P, & Casper RF (1995). Cigarette smoking may affect meiotic maturation of human oocytes. Human Reproduction, 10(12), 3213–3217. [DOI] [PubMed] [Google Scholar]
- Oyeyemi WA, Shittu ST, Kolawole TA, Ubanecheand P, & Akinola AO (2015). Protective effect of vitamin E on nicotine induced reproductive toxicity in male rats. Nigerian Journal of Basic and Applied Sciences, 23(1), 7–13. [Google Scholar]
- Oyeyipo IP, Raji Y, Emikpe BO, & Bolarinwa AF (2010). Effects of oral administration of nicotine on organ weight, serum testosterone level and testicular histology in adult male rats. Nigerian Journal of Physiological Sciences, 25(1), 81–86. [PubMed] [Google Scholar]
- Pacheco SE, Houseman EA, Christensen BC, Marsit CJ, Kelsey KT, Sigman M, & Boekelheide K (2011). Integrative DNA methylation and gene expression analyses identify DNA packaging and epigenetic regulatory genes associated with low motility sperm. PloS one, 6(6), e20280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, & Lucon AM (2005). Sperm concentration and normal sperm morphology decrease and follicle‐stimulating hormone level increases with age. BJU international, 96(7), 1087–1091. [DOI] [PubMed] [Google Scholar]
- Peng H, Shi J, Zhang Y, Zhang H, Liao S, Li W, … & Zhou Q (2012). A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell research, 22(11), 1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ibeas P, Calle A, Fernández-González R, Laguna-Barraza R, Pericuesta E, Calero A, … & Gutiérrez-Adán A (2014). Intracytoplasmic sperm injection using DNA-fragmented sperm in mice negatively affects embryo-derived embryonic stem cells, reduces the fertility of male offspring and induces heritable changes in epialleles. PloS one, 9(4), e95625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Slater M, & Homanics GE (2017). Paternal preconception alcohol exposure imparts intergenerational alcohol-related behaviors to male offspring on a pure C57BL/6J background. Alcohol, 60, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, & Homanics GE (2018). Heavy chronic intermittent ethanol exposure alters small noncoding RNAs in mouse sperm and epididymosomes. Frontiers in genetics, 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosevear SK, Ford WCL, Wardle PG, Hull MGR, Holt DW, & Lee TD (1992). Smoking and decreased fertilisation rates in vitro. The Lancet, 340(8829), 1195–1196. [DOI] [PubMed] [Google Scholar]
- Russell L (1977). Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. American Journal of Anatomy, 148(3), 313–328. [DOI] [PubMed] [Google Scholar]
- Rossi V, Lispi M, Longobardi S, Mattei M, Di Rella F, Salustri A, … & Klinger FG (2017). LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell death and differentiation, 24(1), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryback RS (1977). Chronic alcohol consumption and menstruation. JAMA, 238(20), 2143–2143. [PubMed] [Google Scholar]
- Sánchez MC, Fontana VA, Galotto C, Cambiasso MY, Sobarzo CM, Calvo L, … & Cebral E (2018). Murine sperm capacitation, oocyte penetration and decondensation following moderate alcohol intake. Reproduction, 155(6), 529–541. [DOI] [PubMed] [Google Scholar]
- Sanchis R, Esquifino A, & Guerri C (1985). Chronic ethanol intake modifies estrous cyclicity and alters prolactin and LH levels. Pharmacology Biochemistry and Behavior, 23(2), 221–224. [DOI] [PubMed] [Google Scholar]
- Sarkola T, Mäkisalo H, Fukunaga T, & Eriksson CP (1999). Acute effect of alcohol on estradiol, estrone, progesterone, prolactin, cortisol, and luteinizing hormone in premenopausal women. Alcoholism: Clinical and Experimental Research, 23(6), 976–982. [PubMed] [Google Scholar]
- Schoorlemmer J, Pérez-Palacios R, Climent M, Guallar D, & Muniesa P (2014). Regulation of mouse retroelement MuERV-L/MERVL expression by REX1 and epigenetic control of stem cell potency. Frontiers in oncology, 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM (1993). Regulation of zygotic gene activation in the mouse. Bioessays, 15(8), 531–538. [DOI] [PubMed] [Google Scholar]
- Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, & Reik W (2013). Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1609), 20110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, & Krawetz SA (2013). Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic acids research, 41(7), 4104–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie M, Laforest G, Bujan L, Bissonnette F, & Bleau G (2005). Sperm DNA fragmentation: threshold value in male fertility. Human reproduction, 20(12), 3446–3451. [DOI] [PubMed] [Google Scholar]
- Shaarawy M, & Mahmoud KZ (1982). Endocrine profile and semen characteristics in male smokers. Fertility and Sterility, 38(2), 255–7. [DOI] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, … & Song L (2016). Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science, 351(6271), 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, … & Rando OJ (2018). Small RNAs are trafficked from the epididymis to developing mammalian sperm. Developmental cell, 46(4), 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea JM, Serra RW, Carone BR, Shulha HP, Kucukural A, Ziller MJ, … & Meissner A (2015). Genetic and epigenetic variation, but not diet, shape the sperm methylome. Developmental cell, 35(6), 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MK, & Cheng CY (2013). The blood-follicle barrier (BFB) in disease and in ovarian function In Biology and Regulation of Blood-Tissue Barriers (pp. 186–192). Springer, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, & Guerrero-Bosagna C (2009). Environmental signals and transgenerational epigenetics. Epigenomics, 1(1), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J, Goldstein EG, & Dannenhoffer R (1996). Induction by cocaine of defective spindle formation in cultured mouse oocytes. Toxicology in vitro, 10(3), 377–382. [DOI] [PubMed] [Google Scholar]
- Sofikitis N, Miyagawa I, Dimitriadis D, Zavos P, Sikka S, & Hellstrom W (1995). Effects of smoking on testicular function, semen quality and sperm fertilizing capacity. The Journal of urology, 154(3), 1030–1034. [PubMed] [Google Scholar]
- Stachowiak EM, Papis K, Kruszewski M, Iwaneńko T, Bartłomiejczyk T, & Modliński JA (2009). Comparison of the level (s) of DNA damage using Comet assay in bovine oocytes subjected to selected vitrification methods. Reproduction in domestic animals, 44(4), 653–658. [DOI] [PubMed] [Google Scholar]
- Steger K, Pauls K, Klonisch T, Franke FE, & Bergmann M (2000). Expression of protamine-1 and-2 mRNA during human spermiogenesis. Molecular human reproduction, 6(3), 219–225. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Hivert MF, Perron P, Poirier P, Guay SP, Brisson D, & Bouchard L (2012). IGF2 DNA methylation is a modulator of newborn’s fetal growth and development. Epigenetics, 7(10), 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takihara H, Cosentino MJ, Sakatoku J, & Cockett AT (1987). Significance of testicular size measurement in andrology: II. Correlation of testicular size with testicular function. The Journal of urology, 137(3), 416–419. [DOI] [PubMed] [Google Scholar]
- Tanaka T (1998). Effects of litter size on behavioral development in mice. Reproductive toxicology, 12(6), 613–617. [DOI] [PubMed] [Google Scholar]
- Van den Berg IM, Eleveld C, Van Der Hoeven M, Birnie E, Steegers EAP, Galjaard RJ, … & van Doorninck JH (2011). Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Human reproduction, 26(5), 1181–1190. [DOI] [PubMed] [Google Scholar]
- van der Heijden GW, Ramos L, Baart EB, van den Berg IM, Derijck AA, van der Vlag J, … & de Boer P (2008). Sperm-derived histones contribute to zygotic chromatin in humans. BMC developmental biology, 8(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis BJ, Dawson JD, Stovall DW, Sparks AE, & Syrop CH (1996). The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstetrics & Gynecology, 88(5), 785–791. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, & Pierce RC (2013). Epigenetic inheritance of a cocaine-resistance phenotype. Nature neuroscience, 16(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine MF, Chiu-Kit JT, Hu PC, & Truong KY (1996). Cigarette smoking and semen quality. Fertility and sterility, 65(4), 835–842. [DOI] [PubMed] [Google Scholar]
- Willis BR, Anderson RA, Oswald C, & Zaneveld LJ (1983). Ethanol-induced male reproductive tract pathology as a function of ethanol dose and duration of exposure. Journal of Pharmacology and Experimental Therapeutics, 225(2), 470–478. [PubMed] [Google Scholar]
- Wdowiak A, Raczkiewicz D, Stasiak M, & Bojar I (2014). Levels of FSH, LH and testosterone, and sperm DNA fragmentation. Neuroendocrinology Letters, 35(1). [PubMed] [Google Scholar]
- Wozniak DF, Cicero TJ, Kettinger L, & Meyer ER (1991). Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology, 105(2), 289–302. [DOI] [PubMed] [Google Scholar]
- Xie Z, Zhao Z, Yang X, Pei L, Luo H, Ni Q, … & Chen L (2018). Prenatal nicotine exposure intergenerationally programs imperfect articular cartilage via histone deacetylation through maternal lineage. Toxicology and applied pharmacology, 352, 107–118. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Hada M, Fukuda Y, Inoue E, Makino Y, Katou Y, … & Okada Y (2018). Re-evaluating the localization of sperm-retained histones revealed the modification-dependent accumulation in specific genome regions. Cell reports, 23(13), 3920–3932. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Ivanov P, Hu GF, & Anderson P (2009). Angiogenin cleaves tRNA and promotes stress-induced translational repression. The Journal of cell biology, 185(1), 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LY, Yan J, Qiao J, Zhao PL, & Liu P (2010). Effects of oocyte vitrification on histone modifications. Reproduction, Fertility and Development, 22(6), 920–925. [DOI] [PubMed] [Google Scholar]
- Yang Q, Lin J, Liu M, Li R, Tian B, Zhang X, … & Shi H (2016). Highly sensitive sequencing reveals dynamic modifications and activities of small RNAs in mouse oocytes and early embryos. Science advances, 2(6), e1501482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazigi RA, Odem RR, & Polakoski KL (1991). Demonstration of specific binding of cocaine to human spermatozoa. Jama, 266(14), 1956–1959. [PubMed] [Google Scholar]
- Yelian FD, Sacco AG, Ginsburg KA, Doerr PA, & Armant DR (1994). The effects of in vitro cocaine exposure on human sperm motility, intracellular calcium, and oocyte penetration. Fertility and sterility, 61(5), 915–921. [DOI] [PubMed] [Google Scholar]
- Zhao K, Chen Y, Yang R, Bai Y, Li C, Li H, & Xiong C (2016). miR-424/322 is downregulated in the semen of patients with severe DNA damage and may regulate sperm DNA damage. Reproduction, Fertility and Development, 28(10), 1598–1607. [DOI] [PubMed] [Google Scholar]
- Zhang L, Spencer TJ, Biederman J, & Bhide PG (2018). Attention and working memory deficits in a perinatal nicotine exposure mouse model. PloS one, 13(5), e0198064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lee KP, Spencer TJ, Biederman J, & Bhide PG (2014). Transgenerational transmission of hyperactivity in a mouse model of ADHD. Journal of Neuroscience, 34(8), 2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]


