This editorial refers to ‘MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling’, by B.Y.H. Chan et al., pp. 188–200.
Doxorubicin (DOX) and related anthracyclines are highly effective chemotherapeutic agents used for treating a variety of childhood and adult malignancies. A well-established but poorly understood side effect of DOX is cardiotoxic effects that include arrhythmias, ventricular dysfunction, and heart failure, which translate into poor prognosis and increased mortality.1 At present, there are no effective therapies that mitigate the cardiotoxic effects of DOX without interfering with its chemotherapeutic properties.
At the cellular level, DOX has been shown to disrupt mitochondrial metabolism, signal transduction, and contractile function. Furthermore, many studies support the notion that increased production of reactive oxygen species in response to DOX treatment is the primary mechanism for the underlying cardiotoxicity. Alternative explanations point to the involvement of the BH3-domain like protein Bcl-2-like 19-kDa-interacting protein 3 (Bnip3) which localizes to mitochondria and provokes perturbations to mitochondria that include impaired respiration, loss of membrane potential ΔΨm, and permeability transition pore opening that leads to necrotic cell death.2 Additional plausible mechanisms hold that DOX cardiotoxicity may be attributed to dysregulation of calcium homeostasis, DNA damage, and impaired iron metabolism.1
Notably, it has recently been proposed that matrix metalloproteinases (MMPs) could be a contributing underlying cause of DOX cardiotoxicity. MMPs are responsible for the degradation and remodelling of the extracellular matrix proteins (ECMs), however, they can also target other non-ECM substrates.3 In particular, MMP-2 is abundantly rich in cardiomyocytes and increased circulating levels of MMP-2 have been found in plasma of patients with heart failure and acute ischaemia and reperfusion injury.3,4
A recent study by Chan et al.5 provided evidence that MMP-2, as well as N-terminal truncated (NTT)-MMP-2 were increased in cardiac myocytes treated with DOX. Furthermore, when activated by DOX, MMP-2 was shown to disrupt cardiac contractile function by proteolytic digestion of sarcomeric and myofilament proteins, such as titin, which is critical for maintaining systolic and diastolic cardiac function.6 Despite these observations, there remains a paucity of information regarding how MMP-2 activation in cardiac myocytes treated with DOX impacts sarcomeric titin and cardiac contractile function. Chan et al.7 investigate the possibility as to whether pharmacological inhibition of MMP-2 would suppress the cardiotoxic effects of DOX on cardiac performance. Herein, the authors show that inhibition of MMP-2 attenuated DOX-induced matrix remodelling, titin proteolysis, and interstitial fibrosis. In particular, the authors tested the effects of two orally available MMP inhibitors; doxycycline and ONO-4817, on cardiac endpoints in an acute mouse model of DOX cardiotoxicity. Echocardiography showed that MMP inhibition ameliorated cardiac dysfunction and left ventricular remodelling, induced by DOX, suggesting that the related cardiomyopathy and ensuing heart failure may be attributed in part, to MMP activity. Interestingly, oxidative stress induced by DOX was accompanied by the increased MMP-2, NTT-MMP-2, phenotypic conversion of myofibroblasts, and collagen deposition. Notably, doxycycline and ONO-4817 reduced MMP-2 and NTT-MMP-2 expression, as well as attenuated oxidative stress and myocardial fibrosis. Moreover, the increased MMP-2 activity in cardiac myocytes treated with DOX was accompanied by proteolysis of sarcomeric titin.6 Inhibition of MMP-2 inhibited DOX-induced proteolysis of titin, suggesting that the contractile dysfunction associated with DOX cardiomyopathy is related to MMP-2 activation and proteolysis of sarcomeric titin (Figure 1).
Figure 1.
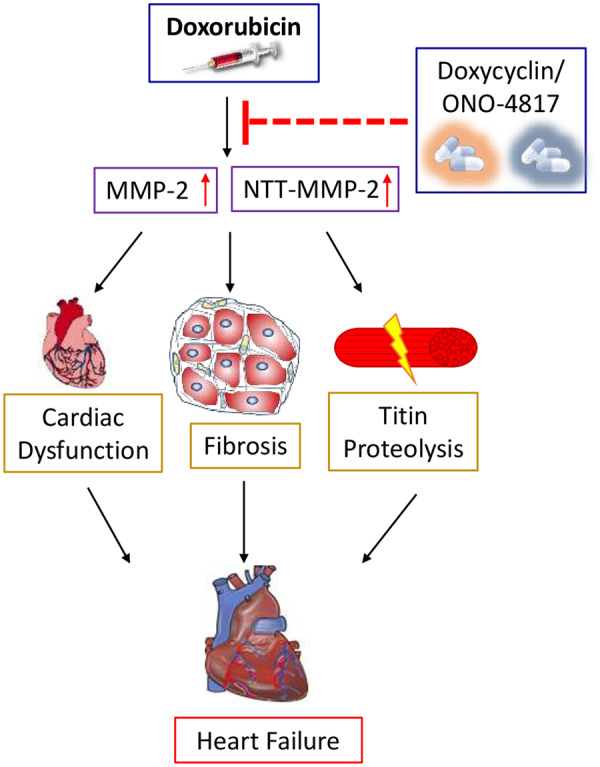
Doxorubicin-induced cardiotoxicity can be attenuated by MMP inhibitors. Doxorubicin (DOX) increases in MMP-2 and NTT-MMP-2 activity, which leads to cardiac dysfunction, fibrosis, titin proteolysis, and eventually heart failure. Inhibition of MMP-2 and NTT-MMP-2 expression with doxycycline or ONO-4817, prevents DOX induced cardiac toxicity and heart failure.
Collectively, this novel work demonstrates that MMP-2 contributes to DOX cardiotoxicity by promoting interstitial fibrosis, sarcomeric disruption, and pathological cardiac remodelling. Inhibition of MMP activity by doxycycline or ONO-4817 reversed the cardiotoxic effects of DOX, suggesting that MMP inhibitors may be of potential therapeutic benefit in cancer patients undergoing DOX treatment.
While the data presented by Chan et al. provides intriguing evidence that MMP inhibition may attenuate DOX-induced cardiotoxicity, it also raises several important questions for consideration. For example, one of the most intriguing questions arising from this study is that over the years, many of the MMP inhibitors tested failed to reduce tumour burden or enhance overall survival rates. This may be explained by the fact that most drugs used to inhibit MMP activity are broad-spectrum with limited specificity of action. Thus, global inhibition of MMP activity, may inadvertently effect other physiological properties of MMPs including tumour inhibition.8 Additionally, MMPs contribute to early stage tumour development, therefore inhibiting MMPs in late stages of disease may be of limited clinical value8 in mitigating the cardiotoxic effects of DOX.
Another point of consideration is whether the actions of DOX with MMP inhibitors would interfere with the individual anti-tumour properties of each drug when used in combination. According to previous studies, doxycycline treatment can reduce oxidative stress and apoptosis, suggesting it can potentially counteract the cardiotoxic effects of DOX. However, emerging evidence indicated that when doxycycline was administered along with DOX, the G2/M cell cycle arrest, which is a key feature of DOX ‘only’ treatment, was abrogated,9 causing antagonistic effect on tumour growth. Although the counteracting effects of DOX and ONO-4817 have not been reported, further investigation for potential interaction of drugs used in combination for cancer therapy is warranted.
Furthermore, another question that should be considered is the model used for the present study which assessed the effects of cumulative dose of 24 mg/kg in relatively young 8 weeks old male mice. Observations from other rodent models and human clinical studies have shown that unlike malignancies observed in adults, younger aged females are more at risk for DOX-induced cardiotoxicity.10 Hence the effects of MMP inhibitors while very intriguing need to be addressed in a more chronic and clinically relevant model to better assess the translational potential of the present finding.
Nevertheless, the study by Chan et al. takes a fresh look and provides novel insight into DOX-cardiotoxicity by exploring the extracellular matrix and MMP-2 as a contributing underlying cause of cardiac dysfunction in DOX cardiotoxicity. The work herein provides an excellent opportunity to address the potential cardioprotecive effects of MMP inhibitors in DOX treatment, as well as to unravel the possible mechanisms by which MMP inhibitors attenuate interstitial fibrosis, and disruption of sarcomeric proteins such as titin proteolysis that contribute to ventricular remodelling and heart failure. Addressing these questions in future studies may prove beneficial in establishing a role for MMP inhibitors as an adjuvant therapy for attenuating the cardiac dysfunction in cancer patients undergoing DOX treatment.
Conflict of interest: none declared.
Funding
This work was supported by a Foundation grant to L.A.K from the Canadian Institute for Health Research (CIHR), grant number FRN148480; I.R.N holds a post-doctoral fellowship from the CIHR; L.A.K. holds a Canada Research Chair in Molecular Cardiology.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Chatterjee K, Zhang J, Honbo N, Karliner JS.. Doxorubicin cardiomyopathy. Cardiology 2010;115:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Dorn GW, Kirshenbaum LA.. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci USA 2014;111:E5537–E5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Altieri P, Brunelli C, Garibaldi S, Nicolino A, Ubaldi S, Spallarossa P, Olivotti L, Rossettin P, Barsotti A, Ghigliotti G.. Metalloproteinases 2 and 9 are increased in plasma of patients with heart failure. Eur J Clin Invest 2003;33:648–656. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R.. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 2002;106:1543–1549. [DOI] [PubMed] [Google Scholar]
- 5. Chan BYH, Roczkowsky A, Moser N, Poirier M, Hughes BG, Ilarraza R, Schulz R.. Doxorubicin induces de novo expression of n-terminal-truncated matrix metalloproteinase-2 in cardiac myocytes. Can J Physiol Pharmacol 2018;96:1238–1245. [DOI] [PubMed] [Google Scholar]
- 6. Ali MAM, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R.. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 2010;122:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan BYH, Chan A, Roczkowsky WJ, Cho M, Poirier C, Sergi V, Keschrumrus JM, Churko H, Granzier R, Schulz. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling. Cardiovasc Res 2021;117:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winer A, Adams S, Mignatti P.. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther 2018;17:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foroodi F, Wilhelmina CD, Singh G.. Interactions of doxycycline with chemotherapeutic agents in human breast adenocarcinoma MDA-MB-231 cells. Anticancer Drugs 2009;20:115–122. [DOI] [PubMed] [Google Scholar]
- 10. Meiners B, Shenoy C, Zordoky BN.. Clinical and preclinical evidence of sex-related differences in anthracycline-induced cardiotoxicity. Biol Sex Differ 2018;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]


