Abstract
Objective
Systemic chemotherapy has limited efficacy in the treatment of peritoneal metastasis (PM) in gastric cancer (GC). Hyperthermic intraperitoneal chemotherapy (HIPEC) combined with complete cytoreductive surgery (CRS) has shown promising outcomes but remains controversial. The present study aimed to evaluate the safety and efficacy of HIPEC without CRS in GC patients with PM.
Methods
This retrospective propensity score-matched multicenter cohort study included GC patients with PM treated with either chemotherapy alone (Cx group) or with HIPEC combined with chemotherapy (HIPEC-Cx group) in four Chinese high-volume gastric medical centers between 2010 and 2017. The primary outcomes were median survival time (MST) and 3-year overall survival (OS). Propensity score matching was performed to compensate for controlling potential confounding effects and selection bias.
Results
Of 663 eligible patients, 498 were matched. The MST in the Cx and HIPEC-Cx groups was 10.8 and 15.9 months, respectively [hazard ratio (HR)=0.71, 95% confidence interval (95% CI), 0.58−0.88; P=0.002]. The 3-year OS rate was 10.1% (95% CI, 5.4%−14.8%) and 18.4% (95% CI, 12.3%−24.5%) in the Cx and HIPEC-Cx groups, respectively (P=0.017). The complication rates were comparable. The time to first flatus and length of hospital stay for patients undergoing HIPEC combined with chemotherapy was longer than that of chemotherapy alone (4.6±2.4 dvs. 2.7±1.8 d, P<0.001; 14.2±5.8 dvs. 11.4±7.7 d, P<0.001), respectively. The median follow-up period was 33.2 months.
Conclusions
Compared with standard systemic chemotherapy, HIPEC combined with chemotherapy revealed a statistically significant survival benefit for GC patients with PM, without compromising patient safety.
Keywords: Gastric cancer, peritoneal metastasis, hyperthermic intraperitoneal chemotherapy, chemotherapy
Introduction
Peritoneal metastasis (PM) is detected in 10%−30% of patients with gastric cancer (GC) at the time of initial diagnosis, and over 50% of patients with stage II−III tumor develop PM within 5 years after curative surgery (1,2). Also, PM is the most common pattern of disease relapse and is the primary cause of GC-associated mortality (3). The median survival of GC patients with PM has been reported to be 3−9 months, even when treated with standard systemic chemotherapy (4,5). Therefore, PM is often considered incurable in patients with GC, which is attributed to the dismal prognosis of the disease. The current treatment options for these patients are palliative chemotherapy and supportive care; however, they have limited efficacy (6). Intraperitoneal chemotherapy seems to be an intuitive and attractive approach in the treatment of patients with PM.
Hyperthermic intraperitoneal chemotherapy (HIPEC) combined with cytoreductive surgery (CRS) has been proposed over the past 30 years as a treatment modality for PM (7). In some case series studies, it has been suggested that CRS combined with HIPEC could prolong overall survival (OS) of GC patients with PM (8-11). In the recent CYTO-CHIP study, which included 277 GC patients with PM, the propensity score analysis revealed that compared to CRS alone, patients who received HIPEC combined with CRS had significantly better OS (5-year OS, 19.87% vs. 6.43%, respectively) (12). However, CRS-related perioperative morbidity (55.3% vs. 53.7%, for CRS and HIPEC + CRS, respectively) and 90-day mortality (10.1% vs. 7.4%, respectively) seemed to indicate that only highly selected patients with limited PM from GC might benefit from this modality. Standardized CRS is extremely technical demanding and is rarely performed, even by specialized surgeons. In addition, most PM in GC patients is too extensive for CRS to achieve satisfactory cyto-reduction (13,14). Therefore, it is important to evaluate the role of HIPEC alone in improving survival of GC with PM. However, the available evidence has heterogeneous results. Although many studies have suggested the benefits of treatment with HIPEC, others have demonstrated controversial results (8,12,15,16).
To fill this gap, we evaluated the efficacy and safety of HIPEC without CRS combined with systemic chemotherapy in the treatment of 498 GC patients with PM from four members of the Chinese Peritoneal Oncology Study (CPOS) group.
Materials and methods
Definition of P stage
The extent of PM was stratified according to the first English edition of the Japanese Classification of Gastric Carcinoma as follows: P0, no peritoneal seeding; P1, metastasis to the region directly adjacent to the peritoneum of stomach in supracolic department; P2, oligo metastases to distant peritoneum; and P3, numerous metastases throughout the peritoneal cavity (17).
Patient population
The present retrospective multicenter study was conducted by members of the Chinese Peritoneal Oncology Study group, who analyzed the records of 1,103 patients with stage IV GC treated between January 2010 and May 2017 at four centers (Figure 1 ). Five patients also had other concurrent malignancies, and 256 patients had metastatic disease beyond the peritoneal cavity (including hepatic, lung, brain, bone, and other distant metastases); none of these patients were included in the study. A total of 179 were excluded from the study because of incomplete data. Finally, 663 (60.1%) GC patients with PM who had complete data were enrolled in the study. Peritoneal involvement of gastric origin was confirmed in all patients through laparoscopy, open exploration, imaging, or histological diagnosis.
1.
Flow chart of selection and grouping of stage IV gastric cancer patients with peritoneal metastasis. HIPEC, hyperthermic intraperitoneal chemotherapy; PSM, propensity score matching.
Of the 663 eligible patients, 405 (61.1%) and 258 (38.9%) patients received HIPEC combined with chemotherapy (HIPEC-Cx) and chemotherapy alone (Cx), respectively. Systemic chemotherapy included a 5-fluorouracil-based (90.2%) or paclitaxel-based (9.8%) regimen. Compared with patients in the Cx group, patients in the HIPEC-Cx group were significant younger (51.7±11.8 years old vs. 56.5±13.4 years old, P<0.001), and had more ascites (little: 22.2%vs. 15.5%; moderate: 10.4% vs. 6.2%; large: 16.5% vs. 7.8%, respectively, P<0.001). Therefore, propensity score matching (PSM) was performed to compensate for controlling potential confounding effects and selection bias based on the two variables: age and ascites status. The study was approved by the Institutional Review Boards and the Ethics Committees of Affiliated Cancer Hospital & Institute of Guangzhou Medical University. Written informed consent was waived because of the retrospective design.
Definition of ascites quantity
Ascites was defined as “little” (fluid found only below the pelvic rim), “moderate” (fluid found above the pelvic rim) or “large” (fluid found throughout the abdominal cavity) by computed tomography or sonography (18).
HIPEC procedures
The closed HIPEC technique was standardized among the four participating centers. Two inflow tubes were placed in the upper abdomen, and two outflow tubes were placed in the lower abdomen. Subsequently, the heated perfusate was circulated at a flow rate of 400−600 mL/min and a perfusion volume of 2 L/m2 for 60 min using the BR-TRG-I hyperthermic perfusion intraperitoneal treatment system (Bright Medical Tech, Guangzhou, China). HIPEC was carried out at 43±0.1 °C with paclitaxel (75−100 mg/m2) or platinum (oxaliplatin: 100−130 mg/m2 or cisplatin: 50−75 mg/m2) as chemotherapeutic agents. HIPEC was recommended to be conducted on d 1, 3, and 5, respectively, with a mean frequency of 2.1±1.0 times.
Follow-up
The primary outcomes were median survival time (MST) and 3-year OS. The last patient was seen in June 2019. Data were collected from the outpatients’ visit or via telephone calls and letters for patients who could not attend regular hospital visits. Data of patients who were alive at the cutoff date or who were lost to follow-up was defined as censored data. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Version 4.0).
Statistical analysis
To balance the baseline characteristics and treatment-related factors between treatment groups, we performed a PSM analysis to minimize the potential selection bias. We calculated the propensity score for each patient as the predicted probability of having HIPEC from a multivariable logistic regression that included two confounding factors: age and ascites. One-to-one matching between the two groups was generated using nearest-neighbor matching without replacement and with the use of a caliper width equal to 0.04.
Quantitative variables were described as mean with standard deviation (SD) or median with interquartile range (IQR), and were compared using t-test or Mann-Whitney U test, as appropriate. Categorical variables were described as frequency and percentages, and were compared using Pearson test or Fisher’s exact test. Survival curves were estimated using the Kaplan-Meier method, and differences between curves were compared by using the log-rank test. Hazard ratio (HR) and corresponding 95% confidence interval (95% CI) were estimated with the Cox proportional-hazards model, and the multivariate backward likelihood ratio steps Cox model was chosen to assess the adjusted effect of HIPEC. Subgroup Cox regression analysis of OS was performed to assess the consistency of effect across subgroups, and interactions were evaluated.
Analyses were performed using SPSS statistics software (Version 24.0; IBM Crop., New York, USA). Statistical significance was defined as P<0.05 (two-sided).
Results
PSM analysis
Of the 663 GC patients with PM who had complete data, those in the HIPEC-Cx group were statistically significantly younger and had more ascites, compared with those in the Cx group. Finally, of the 663 patients with complete data, 498 patients were included in the final analysis after matching (n=249 patients/group; Figure 1 ).
The male-to-female ratio was 1.48 (297/201) and the mean age was 55.3 years old. The Eastern Cooperative Oncology Group performance status (ECOG PS) was 0 or 1 for 450 of 498 patients (90.4%), and 48 of 498 (9.6%) scored 2 and 3. Cancer was undifferentiated in 423 of 498 (84.9%) patients. There were 335 patients (67.3%) with no ascites, 83 patients (16.7%) with little ascites and 80 patients (16.0%) with moderate (36, 7.2%) and large (44, 8.8%) ascites. PM was classified as P1 in 156 patients (31.3%), P2 in 139 patients (27.9%), P3 in 197 patients (39.6%), and was unknown for 6 patients (1.2%). After PSM, there were no statistically significant differences in the baseline or clinical parameters, including sex, age, body mass index, ECOG PS, histology, ascites, or P stage between the Cx and HIPEC-Cx groups, except for the length of hospital stay (P<0.001) and time to first flatus (P<0.001;Table 1 ).
1. Clinical and clinicopathological parameters of gastric cancer with peritoneal metastasis.
| Characteristics | Total patients
[n (%)] |
HIPEC-chemotherapy
(N=249) [n (%)] |
Chemotherapy alone
(N=249) [n (%)] |
P |
| BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; HIPEC, hyperthermic intraperitoneal chemotherapy. | ||||
| Sex | ||||
| Female | 201 (40.4) | 105 (42.2) | 96 (38.6) | |
| Male | 297 (59.6) | 144 (57.8) | 153 (61.4) | 0.411 |
Age (
 ) (year)
) (year)
|
55.3±12.5 | 54.6±11.8 | 56.0±13.1 | 0.230 |
BMI (
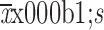 ) (kg/m2)
) (kg/m2)
|
21.4±3.6 | 21.5±3.4 | 21.4±3.9 | 0.742 |
| ECOG PS | ||||
| 0 or 1 | 450 (90.4) | 224 (90.0) | 226 (90.8) | |
| 2 or 3 | 48 (9.6) | 25 (10.0) | 23 (9.2) | 0.761 |
| Histology | ||||
| Differentiated | 75 (15.1) | 41 (16.5) | 34 (13.7) | |
| Undifferentiated | 423 (84.9) | 208 (83.5) | 215 (86.3) | 0.380 |
| Ascites fluid amount | ||||
| None | 335 (67.3) | 161 (64.7) | 174 (69.9) | |
| Little | 83 (16.7) | 43 (17.3) | 40 (16.1) | |
| Moderate/Large | 80 (16.0) | 45 (18.0) | 35 (14.0) | 0.394 |
| P stage | ||||
| P1 | 156 (31.3) | 81 (32.5) | 75 (30.1) | |
| P2 | 139 (27.9) | 70 (28.1) | 69 (27.7) | |
| P3 | 197 (39.6) | 96 (38.6) | 101 (40.6) | |
| Unknown | 6 (1.2) | 2 (0.8) | 4 (1.6) | 0.794 |
Time to first flatus (
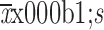 ) (d)
) (d)
|
3.6±2.3 | 4.6±2.4 | 2.7±1.8 | <0.001 |
Hospital stay (
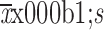 ) (d)
) (d)
|
12.8±7.0 | 14.2±5.8 | 11.4±7.7 | <0.001 |
Survival
The median follow-up period was 33.2 (IQR, 14.2−54.4) months. Survival analysis revealed that patients receiving HIPEC combined chemotherapy had a significantly higher median OS than those receiving chemotherapy alone, with 15.9 (95% CI, 13.2−18.5) months and 10.8 (95% CI, 9.5−12.1) months in the HIPEC-Cx and Cx groups, respectively (HR=0.71; 95% CI, 0.58−0.88; P=0.002;Figure 2 ). The corresponding 3-year OS rate was 18.4% (95% CI, 12.3%−24.5%) and 10.1% (95% CI, 5.4%−14.8%) in the HIPEC-Cx and Cx groups, respectively (P=0.017). Additionally, we found that performing palliative gastrectomy plus HIPEC plus chemotherapy was associated with the highest survival [median OS, 20.8 (95% CI, 15.7−25.8) months, 3-year OS rate, 27.0% (95% CI, 17.6%−36.4%)](Supplementary Figure S1 ).
2.
Kaplan-Meier curves of OS. HIPEC combined with chemotherapy versus chemotherapy for gastric cancer patients with peritoneal metastasis (HR=0.71; 95% CI, 0.58−0.88; P=0.002). OS, overall survival; HIPEC, hyperthermic intraperitoneal chemotherapy; HR, hazard ratio; 95% CI, 95% confidence interval.
S1.
OS of palliative gastrectomy + HIPEC + chemotherapy group for gastric cancer patients with peritoneal metastasis. OS, overall survival; HIPEC, hyperthermic intraperitoneal chemotherapy.
In the multivariable Cox proportional hazard model, patients who received HIPEC combined with chemotherapy were at lower risk of dying from GC than those who received chemotherapy alone (adjusted HR=0.69; 95% CI, 0.56−0.86; P=0.001;Figure 3 ). Subgroup analyses showed that the effect of HIPEC was consistent across the levels of prespecified stratification factors. Patients in the HIPEC-Cx group had statistically significant better survival rate than those in the Cx group for P1/P2 stage (HR=0.71; 95% CI, 0.54−0.93; P=0.015), and those with no or little ascites (HR=0.69, 95% CI, 0.53−0.90, P=0.006; HR=0.58, 95% CI, 0.33−1.00, P=0.050). There was no significant survival difference between the two groups for patients with P3 stage or moderate/large ascites. Notably, HIPEC had a significant impact on the remission of ascites (85.7%) in the moderate/large ascites subgroup (data not shown).
3.
Subgroup analyses of OS. Forest plot showing the impact of HIPEC on OS in patient subgroups. †, Adjusted by ECOG PS, ascites and P stage in multivariate Cox proportional-hazard model. OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; HIPEC, hyperthermic intraperitoneal chemotherapy; HR, hazard ratio; 95% CI, 95% confidence interval.
Safety
Morbidity rates for both groups are shown in Table 2 . The rates of hypoalbuminemia, anemia, lymphopenia, electrolyte disturbance, fever, and abdominal pain were statistically significantly higher. In contrast, the rates of nausea, vomiting, leukopenia, and neutropenia were statistically significantly lower in the HIPEC-Cx group, compared with the Cx group, respectively (P<0.05). The most frequent major complications (grade 3−4) were lymphopenia (21.0%vs. 9.4%, P<0.001), neutropenia (1.6%vs. 8.2%, P=0.001), leukopenia (1.2% vs. 7.8%, P<0.001), and abdominal pain (5.6%vs. 1.2%, P=0.007) in the HIPEC-Cx and Cx groups, respectively. The time to first flatus and length of hospital stay in patients treated with Cx was shorter than that for patients treated with HIPEC-Cx (2.7±1.8 d vs. 4.6±2.4 d, P<0.001; 11.4±7.7 dvs. 14.2±5.8 d, respectively, P<0.001;Table 1 ). No other severe treatment-related complications were recorded.
2. Major toxicities and complications in gastric cancer patients with peritoneal metastasis.
| Adverse event | HIPEC-chemotherapy (n=249) [n (%)] | Chemotherapy alone (n=249) [n (%)] | P | |||||||
| Untested | Grade 1−2 | Grade 3 | Grade 4 | Untested | Grade 1−2 | Grade 3 | Grade 4 | |||
| *, Electrolyte disturbances include hypokalemia, hyperkalemia, hyponatremia, hypernatremia, hypomagnesemia, hypophosphatemia and hypercalcemia; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HIPEC, hyperthermic intraperitoneal chemotherapy. | ||||||||||
| Hypoalbuminemia | 2 (0.8) | 208 (84.2) | 7 (2.8) | 0 (0) | 25 (10.0) | 119 (53.1) | 1 (0.4) | 0 (0) | <0.001 | |
| Anemia | 0 (0) | 176 (70.7) | 24 (9.6) | 0 (0) | 5 (2.0) | 143 (58.6) | 28 (11.5) | 1 (0.4) | 0.018 | |
| Lymphopenia | 2 (0.8) | 141 (57.1) | 47 (19.0) | 5 (2.0) | 5 (2.0) | 70 (28.7) | 18 (7.4) | 5 (2.0) | <0.001 | |
| Electrolyte disturbances* | 0 (0) | 133 (53.4) | 13 (5.2) | 1 (0.4) | 15 (6.0) | 82 (35.0) | 14 (6.0) | 2 (0.9) | 0.001 | |
| Fever | 0 (0) | 107 (43.0) | 4 (1.6) | 0 (0) | 0 (0) | 41 (16.5) | 1 (0.4) | 0 (0) | <0.001 | |
| Abdominal pain | 0 (0) | 88 (35.3) | 14 (5.6) | 0 (0) | 0 (0) | 39 (15.7) | 3 (1.2) | 0 (0) | <0.001 | |
| Abdominal bloating | 0 (0) | 44 (17.7) | 2 (0.8) | 0 (0) | 0 (0) | 43 (17.3) | 4 (1.6) | 0 (0) | 0.707 | |
| Nausea | 0 (0) | 71 (28.5) | 1 (0.4) | 0 (0) | 0 (0) | 117 (47.0) | 1 (0.4) | 0 (0) | <0.001 | |
| Vomiting | 0 (0) | 64 (25.7) | 2 (0.8) | 0 (0) | 0 (0) | 96 (38.6) | 1 (0.4) | 0 (0) | 0.008 | |
| AST increased | 2 (0.8) | 52 (21.1) | 4 (1.6) | 0 (0) | 15 (6.0) | 46 (19.7) | 1 (0.4) | 0 (0) | 0.370 | |
| ALT increased | 2 (0.8) | 39 (15.8) | 5 (2.0) | 0 (0) | 15 (6.0) | 47 (20.1) | 1 (0.4) | 0 (0) | 0.132 | |
| Pneumonia | 0 (0) | 33 (13.3) | 4 (1.6) | 1 (0.4) | 0 (0) | 20 (8.0) | 2 (0.8) | 0 (0) | 0.098 | |
| Leukopenia | 2 (0.8) | 24 (9.7) | 2 (0.8) | 1 (0.4) | 5 (2.0) | 37 (15.2) | 12 (4.9) | 7 (2.9) | <0.001 | |
| Fatigue | 0 (0) | 24 (9.6) | 0 (0) | 0 (0) | 0 (0) | 38 (15.3) | 1 (0.4) | 0 (0) | 0.058 | |
| Thrombocytopenia | 2 (0.8) | 22 (8.9) | 4 (1.6) | 1 (0.4) | 5 (2.0) | 21 (8.6) | 3 (1.2) | 3 (1.2) | 0.749 | |
| Neutropenia | 2 (0.8) | 19 (7.7) | 3 (1.2) | 1 (0.4) | 5 (2.0) | 41 (16.8) | 7 (2.9) | 13 (5.3) | <0.001 | |
| Creatinine increased | 1 (0.4) | 13 (5.2) | 0 (0) | 0 (0) | 14 (5.6) | 10 (4.3) | 0 (0) | 0 (0) | 0.611 | |
| Ileus | 0 (0) | 12 (4.8) | 2 (0.8) | 0 (0) | 0 (0) | 8 (3.2) | 2 (0.8) | 1 (0.4) | 0.810 | |
| Diarrhea | 0 (0) | 10 (4.0) | 0 (0) | 0 (0) | 0 (0) | 10 (4.0) | 1 (0.4) | 0 (0) | 0.999 | |
| Adhesions | 0 (0) | 2 (0.8) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0.749 | |
| Rash maculopapular | 0 (0) | 2 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0.999 | |
| Hemorrhage | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 3 (1.2) | 0 (0) | 0 (0) | 0.616 | |
| Dyspnea | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 2 (0.8) | 2 (0.8) | 0 (0) | 0.209 | |
| Gastroplegia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0.8) | 0 (0) | 0 (0) | 0.499 | |
| Abdominal infection | 0 (0) | 0 (0) | 7 (2.8) | 1 (0.4) | 0 (0) | 0 (0) | 3 (1.2) | 0 (0) | 0.221 | |
| Thromboembolism | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0.999 | |
| Peripheral neuritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.2) | 0 (0) | 0 (0) | 0.098 | |
Discussion
The National Comprehensive Cancer Network (NCCN) guidelines suggest that systemic chemotherapy is the first-line standard strategy for GC with PM, and chemotherapy combined with trastuzumab for patients with HER-2 positive GC (6,19). While the prognosis of metastatic GC patients has improved in the past decades with the development of new medications (20-24), survival remains poor (median survival: 4.6−8.2 months) (5,25,26) in GC patients with PM compared with other metastatic sites. Poor blood circulation and low drug concentration are purported as the main reasons why tumor cells in the peritoneum have a poorer response to systemic chemotherapy than in other organs (27).
Several recent studies have shown that the multimodality treatment of CRS combined with HIPEC in treating GC with PM has promising results (8,11,12,28). The recent CYTO-CHIP study found that CRS-HIPEC resulted in longer OS than CRS alone (median OS: 18.8 vs. 12.1 months, respectively) (12). The GYMSSA trial found that CRS combined with HIPEC and systemic chemotherapy could prolong survival in selected GC patients with PM (28). In their randomized phase III study, Yang et al. (11) found that the median survival of GC with PM was 11.0 and 6.5 months in patients with CRS-HIPEC and with CRS alone, respectively. This treatment modality is built on the synergistic effect of complete macroscopic removal of all visible tumors within the abdominal cavity, along with the elimination of residual microscopic tumor because of HIPEC. Undeniably, the completeness of CRS was strongly correlated with survival for GC with PM (8). Moreover, HIPEC is more effective when there is limited peritoneal spread or after radical CRS (CC index 0 or 1) (8,12,29).
Unfortunately, as reported in the CYTO-CHIP study, several studies have shown that satisfactory CRS is a highly challenging surgical endeavor with controversial perioperative mortality and morbidity, which limits its use in the treatment of GC with PM (12,30-33). In our study, most of the patients (336/498, 67.5%) had a P2/P3 stage, and therefore might not be suitable for CRS. HIPEC without PM resection was performed as early as 2008, to reduce ascites associated with PM (ascites remission: 100%) (34). Badgwell et al. (35) recently reported a phase II study of 19 patients (6 with positive peritoneal cytology and 13 with PM), who received laparoscopic HIPEC. Median OS was found to be 20.3 months, supporting the use of HIPEC for eliminating peritoneal diseases. In a retrospective study of 71 laparoscopic HIPEC GC patients with PM, the authors concluded that HIPEC was well tolerated, therefore gastrectomy could be performed for long-term survival benefits (36).
The results of our four-center study showed that GC patients with PM could benefit from HIPEC, even if CRS is not performed, with a statistically significantly improved mean survival time 15.9 (95% CI, 13.2−18.5) months in the HIPEC-Cx groupvs. 10.8 (95% CI, 9.5−12.1) months in the Cx group, higher 3-year OS rate 18.4% (95% CI, 12.3%−24.5%)vs. 10.1% (95% CI, 5.4%−14.8%) (P=0.017). As expected, the benefit of HIPEC focused on patients with P1 or P2 disease. This large-scale study, therefore, represents a promising modality for the treatment of GC with PM, with acceptable adverse events when CRS cannot be performed. To the best of our knowledge, HIPEC without CRS for GC with PM has not been previously evaluated in such a large study and may represent an effective and tolerated therapy.
Also, there is currently no standardized modality for HIPEC worldwide, which may explain the heterogeneity of results for GC patients with PM. These modalities involve, among others, the method of HIPEC (closed or open), the ideal temperature, the choice of chemotherapeutic agents, as well as the duration and frequency of treatment. Indeed, with regard to the latter, several studies included only one cycle of HIPEC after CRS, which might mitigate the efficacy of HIPEC (12,37,38). In our study, HIPEC was performed with pre-determined drugs and dosages at a constant temperature (43±0.1 °C) and duration (60 min), and last, repeatedly (mean number of times: 2.1±1.0). Additionally, our study showed that HIPEC was effective in controlling ascites, therefore improving the quality of life of GC patients with PM.
The role of additional palliative gastrectomy in prolonging survival for incurable GC patients with PM remains controversial. Although several previous reports have found that palliative gastrectomy could prolong survival in patients with stage IV GC (39-41), the multicenter REGATTA randomized controlled trial (also in an Asian patient population) failed to find any survival benefit of gastrectomy plus chemotherapy compared with chemotherapy alone in the management of stage IV patients with GC (42). In addition, several small Asian randomized trials have demonstrated a survival benefit for adjuvant HIPEC in high-risk patients undergoing resection with curative intent; however, these results have not been validated in non-Asian patients (43,44). Theoretically, palliative gastrectomy might reduce local symptoms and sensitize HIPEC and systemic chemotherapy by reducing the tumor burden. Interestingly, in the present study, we found that the palliative gastrectomy + HIPEC + Cx had a statistically significant survival benefit (MST: 20.8 months). To the best of our knowledge, this result is better than previous reports of patients managed either with CRS + HIPEC or standard systemic chemotherapy (8,18,42). This finding should be verified in further randomized controlled trials.
The incidence and severity of hematologic and non-hematologic toxicities were well within the range of those of other common chemotherapy and surgery regimens (18,42). Of note, postoperative complications occurred less frequently than in the CYTO-CHIP study (12), possibly because all of the patients in our study did not receive complete CRS. HIPEC did not increase the risk of serious complications in the present study.
The present study has several limitations. First, although larger than previous studies on GC with PM, our study is retrospective in nature. Second, the peritoneal cancer index, a major prognostic factor for GC patients with PM, was not used in all the centers participating in our study. Therefore, we adopted the Japanese guidelines, which are not universally used. Finally, of the 663 eligible patients, 498 were matched. We did not analyze the 165 patients that were not included in the PSM. The exclusion of large number of patients is known to reduce the statistical power and may lead to a type II error if the number of unmatched cases or controls is large or the treatment effects are small (45). Moreover, current treatment regimens for HIPEC are not uniform concerning the choice of intraperitoneal chemotherapeutic agents, temperature, and duration and frequency of treatment, so our modality may not be ideal for all patients. Therefore, it is critical to launch well-designed, prospective, multi-center, large-scale randomized controlled clinical trials to resolve the above-mentioned problems.
Conclusions
Compared with standard systemic chemotherapy, HIPEC combined with chemotherapy revealed a statistically significant survival benefit for GC patients with PM, without additional serious morbidity or mortality. This treatment modality may represent an effective and more acceptable therapy option for GC patients with PM.
Acknowledgements
This study was supported by the Guangzhou Key Medical Discipline Construction Project Fund, the Guangzhou High-Level Clinical Key Specialty Construction, the Clinical Research Promotion Project of Guangzhou Medical University for Building High Level University, the National Natural Science Foundation of China (No. 81972918), and the Guangzhou Major Clinical Technology Program (No. 2019ZD16).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Han Liang, Email: tjlianghan@126.com.
Shuzhong Cui, Email: cuishuzhong@gzhmu.edu.cn.
References
- 1.Yonemura Y, Endou Y, Shinbo M, et al Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009;100:311–6. doi: 10.1002/jso.21324. [DOI] [PubMed] [Google Scholar]
- 2.Yonemura Y, Bandou E, Kawamura T, et al Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol. 2006;32:602–6. doi: 10.1016/j.ejso.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Isobe Y, Nashimoto A, Akazawa K, et al Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–16. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner AD, Syn NL, Moehler M, et al Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koizumi W, Narahara H, Hara T, et al S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (spirits trial): A phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, D’Amico TA, Almhanna K, et al Gastric cancer, Version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH, Jablonski KA Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–32. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glehen O, Gilly FN, Arvieux C, et al Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370–7. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 9.Scaringi S, Kianmanesh R, Sabate JM, et al Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: A single western center experience. Eur J Surg Oncol. 2008;34:1246–52. doi: 10.1016/j.ejso.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Glehen O, Schreiber V, Cotte E, et al Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139:20–6. doi: 10.1001/archsurg.139.1.20. [DOI] [PubMed] [Google Scholar]
- 11.Yang XJ, Huang CQ, Suo T, et al Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–81. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnot PE, Piessen G, Kepenekian V, et al Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis. J Clin Oncol. 2019;37:2028–40. doi: 10.1200/JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Yan M, Zhu ZG Nonpalliative surgical resection for gastric cancer patients with distant metastasis. J Invest Surg. 2012;25:100–6. doi: 10.3109/08941939.2011.607225. [DOI] [PubMed] [Google Scholar]
- 14.Xia X, Li C, Yan M, et al Who will benefit from noncurative resection in patients with gastric cancer with single peritoneal metastasis? Am Surg. 2014;80:124–30. doi: 10.1177/000313481408000219. [DOI] [PubMed] [Google Scholar]
- 15.Paredes AZ, Guzman-Pruneda FA, Abdel-Misih S, et al Perioperative morbidity of gastrectomy during CRS-HIPEC: An ACS-NSQIP analysis. J Surg Res. 2019;241:31–9. doi: 10.1016/j.jss.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Chua TC, Saxena A, Schellekens JF, et al Morbidity and mortality outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy at a single tertiary institution: Towards a new perspective of this treatment. Ann Surg. 2010;251:101–6. doi: 10.1097/SLA.0b013e3181b5ae43. [DOI] [PubMed] [Google Scholar]
- 17.Japanese research society for gastric cancer: Japanese classification of gastric carcinoma (1st English ed). Tokyo: Kanehara & Co. Ltd, 1995.
- 18.Ishigami H, Fujiwara Y, Fukushima R, et al Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36:1922–9. doi: 10.1200/JCO.2018.77.8613. [DOI] [PubMed] [Google Scholar]
- 19.Ajani JA, Bentrem DJ, Besh S, et al Gastric cancer, version 2.2013: Featured updates to the nccn guidelines. J Natl Compr Canc Netw. 2013;11:531–46. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 20.Thomassen I, van Gestel YR, van Ramshorst B, et al Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–8. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 21.Muro K, Chung HC, Shankaran V, et al Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 22.Janmaat VT, Steyerberg EW, van der Gaast A, et al Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev. 2017;11:CD004063. doi: 10.1002/14651858.CD004063.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen WQ, Zheng RS, Zeng HM, et al Trend analysis and projection of cancer incidence in China between 1989 and 2008. Zhonghua Zhong Liu Za Zhi. 2012;34:517–24. doi: 10.3760/cma.j.issn.0253-3766.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chau I, Norman AR, Cunningham D, et al Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer-pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 26.Kim JG, Ryoo BY, Park YH, et al Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301–7. doi: 10.1007/s00280-007-0476-x. [DOI] [PubMed] [Google Scholar]
- 27.Geng X, Liu H, Lin T, et al Survival benefit of gastrectomy for gastric cancer with peritoneal carcinomatosis: A propensity score-matched analysis. Cancer Med. 2016;5:2781–91. doi: 10.1002/cam4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudloff U, Langan RC, Mullinax JE, et al Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J Surg Oncol. 2014;110:275–84. doi: 10.1002/jso.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desiderio J, Chao J, Melstrom L, et al The 30-year experience — a meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1–14. doi: 10.1016/j.ejca.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias D, Blot F, El Otmany A, et al Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–6. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Sayag-Beaujard AC, Francois Y, Glehen O, et al Intraperitoneal chemo-hyperthermia with mitomycin C for gastric cancer patients with peritoneal carcinomatosis. Anticancer Res. 1999;19:1375–82. [PubMed] [Google Scholar]
- 32.Elias DM, Ouellet JF Intraperitoneal chemohyperthermia: Rationale, technique, indications, and results. Surg Oncol Clin N Am. 2001;10:915–33,xi. doi: 10.1016/S1055-3207(18)30039-5. [DOI] [PubMed] [Google Scholar]
- 33.Sugarbaker PH, Alderman R, Edwards G, et al Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13:635–44. doi: 10.1245/ASO.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 34.Facchiano E, Scaringi S, Kianmanesh R, et al Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol. 2008;34:154–8. doi: 10.1016/j.ejso.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Badgwell B, Blum M, Das P, et al Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24:3338–44. doi: 10.1245/s10434-017-6047-4. [DOI] [PubMed] [Google Scholar]
- 36.Newhook TE, Agnes A, Blum M, et al Laparoscopic hyperthermic intraperitoneal chemotherapy is safe for patients with peritoneal metastases from gastric cancer and may lead to gastrectomy. Ann Surg Oncol. 2019;26:1394–400. doi: 10.1245/s10434-018-07140-7. [DOI] [PubMed] [Google Scholar]
- 37.Rihuete Caro C, Manzanedo I, Pereira F, et al Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44:1805–10. doi: 10.1016/j.ejso.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 38.Yang XJ, Li Y, Yonemura Y Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol. 2010;101:457–64. doi: 10.1002/jso.21519. [DOI] [PubMed] [Google Scholar]
- 39.Lasithiotakis K, Antoniou SA, Antoniou GA, et al Gastrectomy for stage IV gastric cancer. A systematic review and meta-analysis. Anticancer Res. 2014;34:2079–85. [PubMed] [Google Scholar]
- 40.Sun J, Song Y, Wang Z, et al Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: A systematic review and meta-analysis. BMC Cancer. 2013;13:577. doi: 10.1186/1471-2407-13-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang YR, Han DS, Kong SH, et al The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol. 2012;19:1231–9. doi: 10.1245/s10434-011-2056-x. [DOI] [PubMed] [Google Scholar]
- 42.Fujitani K, Yang HK, Mizusawa J, et al Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 43.Fujimoto S, Takahashi M, Mutou T, et al Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–34. doi: 10.1002/(SICI)1097-0142(19990201)85:3<529::AID-CNCR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Fujimura T, Yonemura Y, Muraoka K, et al Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: Randomized controlled study. World J Surg. 1994;18:150–5. doi: 10.1007/BF00348209. [DOI] [PubMed] [Google Scholar]
- 45.Fujita T Propensity score-matched analysis to assess the outcome of surgical procedures. Surgery. 2019;165:1247. doi: 10.1016/j.surg.2018.12.005. [DOI] [PubMed] [Google Scholar]






