Abstract
Objective
To compare switching and discontinuation patterns of patients stable on originator infliximab (IFX) who switched to an IFX biosimilar (switchers) or remained on originator IFX (continuers) in the United States.
Methods
Symphony Health Solutions’ Patient Transactional Datasets (10/2012–03/2019) were used to identify adults with ≥2 claims for either rheumatoid arthritis (RA), psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, or inflammatory bowel disease (IBD); and ≥1 claim for originator or biosimilar IFX. The index date was the first IFX biosimilar claim for switchers or a random originator IFX claim for continuers. All patients were required to have ≥5 originator IFX claims during the 12 months pre-index (prevalent population). The subset of patients with ≥12 months of observation prior to the first originator IFX claim was also analyzed (incident population). Switchers were matched 1:3 to continuers. Discontinuation was defined as having ≥120 days between 2 consecutive index treatment claims.
Results
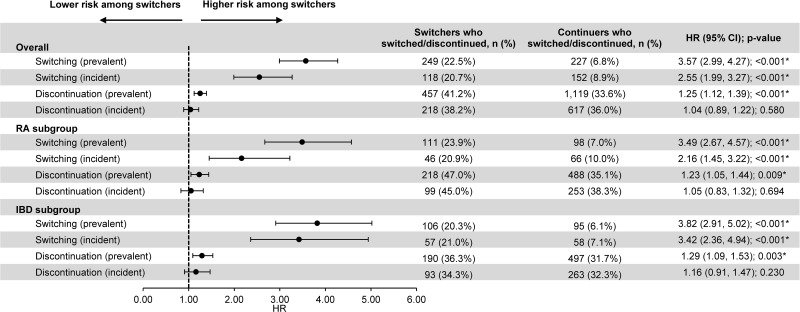
Prevalent switchers (N=1109) were 3.57-times more likely than continuers (N=3327) to switch to another originator biologic (hazard ratio [HR]=3.57, p<0.001). Of 249 prevalent switchers who switched to another originator biologic, 200 (80.3%) switched back to originator IFX. Incident switchers (N=571) were 2.55-times more likely than continuers (N=1713) to switch to another originator biologic (HR=2.55, p<0.001). Of 118 incident switchers who switched to another originator biologic, 90 (76.3%) switched back to originator IFX. Prevalent switchers were 1.25-times more likely than continuers to discontinue index therapy (HR=1.25, p<0.001). Similar results were observed in RA (prevalent population; switching: HR=3.49, p<0.001; discontinuation: HR=1.23, p=0.009) and IBD (prevalent population; switching: HR=3.82, p<0.001; discontinuation: HR=1.29, p=0.003) subgroups.
Conclusion
Patients switching from originator to biosimilar IFX were more likely to switch to another originator biologic (notably back to originator IFX) and discontinue index treatment than those remaining on originator IFX; however, reasons for switching are unknown.
Keywords: biosimilars, chronic inflammatory disease, treatment discontinuation, infliximab, treatment switching
Introduction
Chronic inflammatory diseases (CIDs) that share common inflammatory pathways include conditions such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), plaque psoriasis (PsO), ankylosing spondylitis (AS), Crohn’s disease (CD), and ulcerative colitis (UC).1 The prevalence of CIDs differs according to each condition, with approximately 1.28–1.36 million patients affected with RA, 910,000 affected with UC, and 785,000 affected with CD in the United States (US).2,3 These conditions can have serious detrimental effects on quality of life and health outcomes, in particular, because they are chronic conditions with associated comorbidities.1,4
Despite the significant disease burden, treatment with biologic agents has markedly improved the outcome of the management of CIDs.4,5 Among biologic agents, infliximab (IFX; REMICADE®) was initially approved for the treatment of CD in the US by the Food and Drug Administration (FDA) in 1998 and is currently indicated for a variety of other CIDs, including RA, PsA, PsO, AS, and UC.6,7 Biosimilars of IFX, which have no clinically meaningful differences from the originator biologic,8 are now also available for the treatment of CIDs. The first IFX biosimilar (IFX-dyyb, CT-P13, Inflectra®) was approved on April 5, 2016, and became available for use in clinical practice on November 28, 2016.9 Since then, three more IFX biosimilars have been approved, one of which, IFX-abda (Renflexis®), has been launched.10−12
Switching from originator biologics to biosimilars remains a topic of scientific interest.13 Switching among biologics for reasons unrelated to effectiveness and tolerability, such as cost, is known as non-medical switching (NMS). NMS occurs very rarely among different originator biologics because of poor outcomes.14 In most cases, NMS occurs between two versions of the same biologic (originator and biosimilar).15,16 Characterization of NMS involving an originator and biosimilar presents an opportunity to better understand emerging treatment patterns involving the reference biologic and biosimilar. While randomized controlled trials have shown comparable efficacy, safety, and immunogenicity among patients who switch to IFX biosimilars versus those remaining on originator IFX,17,18 results from real-world studies have suggested that NMS leads to increased rates of treatment discontinuation and switching to yet another therapy, including back to originator IFX.15,16,19–21
The medication utilization patterns of patients stable on originator IFX switching to an IFX biosimilar in the US are not well documented. Specifically, rates of treatment switching and discontinuation after an initial switch to an IFX biosimilar have not been studied in clinical practice in the US. Therefore, the objective of this study was to characterize and compare patterns of switching within a claims database among originator biologics and treatment discontinuation in patients with CIDs stable on originator IFX who switched to an IFX biosimilar versus those remaining on originator IFX. Additionally, subgroups of patients with RA and inflammatory bowel disease (IBD) were evaluated to identify any condition-specific differences in treatment switching and discontinuation patterns.
Patients and Methods
Data Source
Claims data from October 1, 2012 to March 31, 2019 (study period) from the Symphony Health Solutions’ (SHS) Patient Transactional Datasets were used to fulfill the study objectives. The SHS database is a nationally representative, longitudinal, de-identified claims database that captures prescription and medical claims for approximately 280 million patients from across the US. It links healthcare data for the US population from three basic sources: pharmacy point-of-service, switch/network transactions, and additional direct prescription, medical, and hospital claims data. The SHS database covers all payment types, including commercial plans, Medicare Part D, cash, assistance programs, and Medicaid. The SHS database contains only de-identified patient information and is fully compliant with the Health Insurance Portability and Accountability Act (HIPAA); therefore, no ethics review was necessary. Although all personally identifiable information has been removed from the SHS database, the unique identification number assigned to each patient enables assessments to be made over the study timeframe.
Study Design
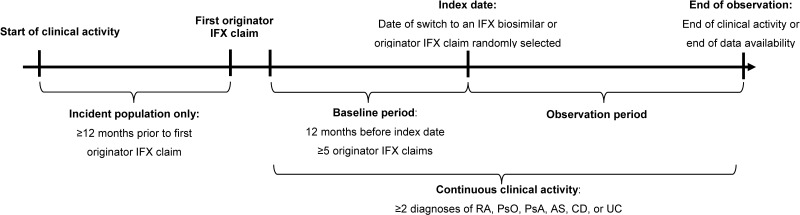
A retrospective cohort study design was used to compare patients stable on originator IFX switching to an IFX biosimilar (ie, switchers) and those remaining on originator IFX (ie, continuers; Figure 1). Stability on originator IFX was defined as having ≥5 claims of originator IFX in the 12 months before the index date, referred to as the baseline period.
Figure 1.
Study design.
Abbreviations: AS, ankylosing spondylitis; CD, Crohn’s disease; IFX, infliximab; PsA, psoriatic arthritis; PsO, plaque psoriasis; RA, rheumatoid arthritis; UC, ulcerative colitis.
For switchers, the index date was defined as the date of the first IFX biosimilar claim on or after April 5, 2016 (date of FDA approval of the first IFX biosimilar). For continuers, a random index date was selected among all originator IFX claims starting from the 6th claim to the last one (ie, following stabilization). A patient qualifying for the continuer arm could, if switched to a biosimilar after the index date, also qualify for the switch arm. Based on this design, a single patient could contribute to both cohorts; ie, once as a continuer (using the time before the switch to an IFX biosimilar) and once as a switcher (starting from the moment the patient switched to an IFX biosimilar), following the methodology described by Lund et al.22 By allowing patients who eventually switched to an IFX biosimilar to contribute to the continuer cohort, an unbiased cohort of continuers that is representative of the real-world use of originator IFX was obtained.
The observation period spanned from the index date up to the earliest of the end of clinical activity (defined as having ≥1 claim in the database for a given patient and calendar quarter) or lack of available follow-up data. For patients included in both cohorts, the observation period for time spent in the continuers cohort started at the randomized index date and was censored at the day before the switch to an IFX biosimilar; the observation period for time spent in the switchers cohort then started from the day of the switch to an IFX biosimilar (index date for switchers).
Patient Selection
To be included in the study, patients were required to have ≥2 claims with a diagnosis for either RA (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9 CM] code 714.0; International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10 CM] codes M05.1- M05.9, or M06), PsA (ICD-9 CM code 696.0; ICD-10 CM code L40.5x), PsO (ICD-9 CM code 696.1; ICD-10 CM codes L40.0-L40.4, L40.8, or L40.9), AS (ICD-9 CM code 720.0; ICD-10 CM code M45.x), CD (ICD-9 CM code 555.x; ICD-10 CM code K50.x), or UC (ICD-9 CM code 556.x; ICD-10 CM code K51.x), including ≥1 claim in the 12-month baseline period, and ≥1 claim for originator IFX or an IFX biosimilar on or after April 5, 2016 (Figure 2). Patients were also required to have ≥5 originator IFX claims during the 12-month baseline period, have ≥12 months of continuous clinical activity before the index date, and be ≥18 years of age at the index date.
Figure 2.
Patient selection.
Notes: aDiagnosis was identified using the following ICD-9 CM and ICD-10 CM codes: RA (ICD-9 CM code 714.0; ICD-10 CM code: M05.1-M05.9, M06), PsA (ICD-9 CM code 696.0; ICD-10 CM code: L40.5x), PsO (ICD-9 CM code 696.1; ICD-10 CM code: L40.0-L40.4, L40.8, L40.9), or AS (ICD-9 CM code 720.0; ICD-10 CM code: M45.x), CD (ICD-9 CM code 555.x; IDC-10 CM code K50.x), UC (ICD-9 CM code 556.x; IDC-10 CM code K51.x). bOriginator IFX was identified using the GPI code 5250504000 and HCPCS code J1745. IFX biosimilars were identified using the following codes: infliximab-abda (GPI code 5250504010; HCPCS code Q5102, Q5103), infliximab-dyyb (GPI code 5250504020; HCPCS code Q5102, Q5104), and infliximab-qbtx (GPI code 5250504060). cPregnancy was identified using the ICD-9 CM code V22.x and ICD-10 CM codes Z33.x and Z34.xx. dDiagnosis of heart failure was identified using the ICD-9-CM code 428.xx and ICD-10-CM code I50.x.
Abbreviations: AS, ankylosing spondylitis; CD, Crohn’s disease; GPI, Generic Product Identifier; HCPCS, Healthcare Common Procedure Coding System; ICD-9 CM/ICD-10 CM, international classification of disease, ninth/tenth revision, clinical modification; IFX, infliximab; PsA, psoriatic arthritis; PsO, plaque psoriasis; RA, rheumatoid arthritis; UC, ulcerative colitis.
Patients with a previous transplant procedure at any time, diagnosis of pregnancy (ICD-9 CM code V22.x; ICD-10 CM codes Z33.x Z34.xx) at any time, diagnosis of heart failure (ICD-9-CM code 428.xx; ICD-10-CM code I50.x) at any time, ≥1 claim for originator IFX and an IFX biosimilar on the index date (for switchers only), or ≥1 claim for a biologic agent other than originator IFX between the first claim for originator IFX during the baseline period and the index date were excluded.
Patients selected based on these criteria were termed the “prevalent” population. This population maximized the sample size and all available data at the time by including both long-term users and shorter-term users who initiated originator IFX during the study period. However, since long-term users entered the database already on treatment with originator IFX, the date of initiation and exact duration of treatment could not be determined and appropriately adjusted for. With maturation of the database and accumulation of additional patient data, an “incident” population could also be identified by selecting the subset of prevalent patients with ≥12 months of continuous clinical activity without any originator IFX claims prior to the first claim for originator IFX (ie, patients newly stabilized on originator IFX, with originator IFX initiation on or after October 1, 2013). While the known date of initiation of these patients allowed to appropriately adjust for the duration of treatment on originator IFX prior to the index date, this additional criterion resulted in a smaller sample size and the inclusion of recent initiators of originator IFX only.
Study Outcomes
Switching patterns were measured during the observation period and included the proportion of patients switching to another originator biologic agent (including originator IFX), along with the time to switch. Of note, switches from one IFX biosimilar to another could not be detected, so originator biologics were the only alternative for switching. In addition, since patients who switched to an IFX biosimilar in the continuers cohort were censored the day before they switched (they started contributing to the switcher cohort on that day), switches to an IFX biosimilar were not counted. This only affected a small number of patients, since only 5% of continuers eventually switched to an IFX biosimilar in both the prevalent and incident populations. Time to switch was defined as the time in days between the index date and the date of the first claim for another originator biologic agent (including originator IFX).
Discontinuation patterns were measured during the observation period and included the proportion of patients discontinuing the index therapy and time to discontinuation. Discontinuation was defined as switching to another originator biologic agent (including originator IFX) or having a gap of ≥120 days between 2 consecutive claims of the index therapy. Time to discontinuation was defined as the time in days between the index date and the first between the date of switch or the end of continuous treatment, which was 120 days following the date of the last infusion before a gap ≥120 days in treatment.
Statistical Analysis
Due to the non-experimental nature of this study, patients in the switchers and continuers cohorts may have had different observable baseline characteristics. As such, 1:3 propensity score matching (PSM) and exact matching based on having an RA or IBD (ie, UC or CD) diagnosis during the baseline period was used to minimize confounding. The presence of PsA, PsO, and AS were not used as exact matching covariates given that the sample size for each disease was too small. Propensity scores were estimated using a multivariable logistic regression based on the following baseline covariates: age; gender; year and quarter of index date; US region; type of insurance plan; presence of PsA, PsO, AS, CD, or UC; Quan-Charlson Comorbidity Index (Quan-CCI) score (a measure of comorbidity burden used to predict a patient’s ten-year mortality risk based on a range of conditions);23 pharmacy and medical costs per month; number of outpatient visits per month; place of service for index IFX claim; duration of prior treatment with originator IFX; and specialty of physician prescribing IFX at the index date.
Baseline characteristics and outcomes were described using means, standard deviations (SDs), and medians for continuous variables and frequencies and proportions for categorical variables. Differences in baseline characteristics were assessed using standardized differences, where a standardized difference <10% indicated balance between cohorts. Kaplan–Meier (KM) survival curves were used to compare time to switch to another originator biologic agent (including originator IFX) and time to discontinuation of index therapy between switchers and continuers. Log-rank tests and 95% confidence intervals (CIs) were used to compare KM curves at 3-month intervals, up to 18 months. Hazard ratios (HRs), along with 95% CIs and p-values, were generated using a Cox proportional hazards model. All analyses were performed with SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC).
Subgroup Analyses
Within the switchers and continuers cohorts, two subgroups were identified based on having ≥1 claim with a diagnosis for RA (RA subgroup) or ≥1 claim with a diagnosis for IBD (ie, UC and CD; IBD subgroup) during the baseline period. All analyses were replicated for the RA and IBD subgroups as described above.
Results
Baseline Demographic and Clinical Characteristics
Prevalent Population
After matching, 1109 switchers and 3327 continuers from the prevalent population were included in the study (Table 1). Among both switchers and continuers, 41.8% had RA and 47.2% had IBD. Mean age was 57.3 (SD=16.3) years for switchers and 56.9 (SD=16.3) years for continuers (standardized difference=2.1%). Among switchers and continuers, 65.3% and 66.1% were female, respectively (standardized difference=1.6%). Mean Quan-CCI score was 1.09 (SD=1.30) among switchers and 1.09 (SD=1.29) among continuers (standardized difference=0.2%). Prior to the index date, patients were treated with originator IFX for an average of ≥1073 (SD=619) days for switchers and ≥1035 (SD=671) days for continuers (standardized difference=6.0%); however, the exact duration of treatment could have been longer, but could not be determined for the prevalent population given that some patients entered the data already on originator IFX treatment. Other baseline characteristics were also well balanced between cohorts (standardized differences <10%).
Table 1.
Baseline Demographic and Clinical Characteristics
| Matched Sample | ||||||
|---|---|---|---|---|---|---|
| Prevalent Population | Incident Population | |||||
| Switchers | Continuers | Standardized Difference | Switchers | Continuers | Standardized Difference | |
| N=1109 | N=3327 | N=571 | N=1713 | |||
| Age in years, mean ± SD [median] | 57.3 ± 16.3 [60.0] | 56.9 ± 16.3 [60.0] | 2.1% | 56.5 ± 15.7 [57.0] | 55.9 ± 15.4 [57.0] | 4.2% |
| Female, n (%) | 724 (65.3%) | 2198 (66.1%) | 1.6% | 380 (66.5%) | 1162 (67.8%) | 2.7% |
| Year of index date, n (%) | ||||||
| 2016 | 8 (0.7%) | 24 (0.7%) | 0.0% | 4 (0.7%) | 12 (0.7%) | 0.0% |
| 2017 | 408 (36.8%) | 1249 (37.5%) | 1.6% | 169 (29.6%) | 563 (32.9%) | 7.1% |
| 2018 | 456 (41.1%) | 1410 (42.4%) | 2.6% | 274 (48.0%) | 810 (47.3%) | 1.4% |
| 2019 | 237 (21.4%) | 644 (19.4%) | 5.0% | 124 (21.7%) | 328 (19.1%) | 6.4% |
| US region, n (%) | ||||||
| South | 309 (27.9%) | 979 (29.4%) | 3.5% | 142 (24.9%) | 428 (25.0%) | 0.3% |
| Midwest | 446 (40.2%) | 1269 (38.1%) | 4.3% | 245 (42.9%) | 713 (41.6%) | 2.6% |
| Northeast | 101 (9.1%) | 324 (9.7%) | 2.2% | 51 (8.9%) | 157 (9.2%) | 0.8% |
| West | 244 (22.0%) | 743 (22.3%) | 0.8% | 132 (23.1%) | 414 (24.2%) | 2.5% |
| Unknown | 9 (0.8%) | 12 (0.4%) | 5.9% | 1 (0.2%) | 1 (0.1%) | 3.4% |
| Type of insurance plan, n (%) | ||||||
| Commercial | 474 (42.7%) | 1422 (42.7%) | 0.0% | 246 (43.1%) | 722 (42.1%) | 1.9% |
| Medicare | 453 (40.8%) | 1385 (41.6%) | 1.6% | 220 (38.5%) | 673 (39.3%) | 1.6% |
| Medicaid | 149 (13.4%) | 424 (12.7%) | 2.1% | 92 (16.1%) | 272 (15.9%) | 0.6% |
| Other | 33 (3.0%) | 96 (2.9%) | 0.5% | 13 (2.3%) | 46 (2.7%) | 2.6% |
| Specialty of physician prescribing IFX at index date, n (%) | ||||||
| Rheumatology | 488 (44.0%) | 1494 (44.9%) | 1.8% | 243 (42.6%) | 750 (43.8%) | 2.5% |
| Gastroenterology | 301 (27.1%) | 906 (27.2%) | 0.2% | 158 (27.7%) | 474 (27.7%) | 0.0% |
| Infectious diseases | 12 (1.1%) | 55 (1.7%) | 4.9% | 10 (1.8%) | 38 (2.2%) | 3.3% |
| Hematology/oncology | 55 (5.0%) | 122 (3.7%) | 6.4% | 32 (5.6%) | 67 (3.9%) | 8.0% |
| Oncology medical | 20 (1.8%) | 26 (0.8%) | 9.1% | 10 (1.8%) | 13 (0.8%) | 8.9% |
| Other/unknown | 233 (21.0%) | 724 (21.8%) | 1.8% | 118 (20.7%) | 371 (21.7%) | 2.4% |
| Prior history of CID, n (%) | ||||||
| RA | 464 (41.8%) | 1392 (41.8%) | 0.0% | 220 (38.5%) | 660 (38.5%) | 0.0% |
| PsA | 151 (13.6%) | 446 (13.4%) | 0.6% | 86 (15.1%) | 272 (15.9%) | 2.3% |
| PsO | 87 (7.8%) | 262 (7.9%) | 0.1% | 49 (8.6%) | 156 (9.1%) | 1.9% |
| AS | 77 (6.9%) | 226 (6.8%) | 0.6% | 47 (8.2%) | 149 (8.7%) | 1.7% |
| IBD | 523 (47.2%) | 1569 (47.2%) | 0.0% | 271 (47.5%) | 813 (47.5%) | 0.0% |
| CD | 382 (34.4%) | 1141 (34.3%) | 0.3% | 200 (35.0%) | 596 (34.8%) | 0.5% |
| UC | 202 (18.2%) | 587 (17.6%) | 1.5% | 107 (18.7%) | 311 (18.2%) | 1.5% |
| Quan-CCI, mean ± SD [median] | 1.09 ± 1.30 [1.00] | 1.09 ± 1.29 [1.00] | 0.2% | 1.10 ± 1.36 [1.00] | 1.06 ± 1.27 [1.00] | 2.8% |
| Baseline drug use, n (%) | ||||||
| Antidepressants | 429 (38.7%) | 1283 (38.6%) | 0.3% | 242 (42.4%) | 747 (43.6%) | 2.5% |
| NSAIDs | 310 (28.0%) | 947 (28.5%) | 1.1% | 163 (28.5%) | 573 (33.5%) | 10.6% |
| Corticosteroids | 676 (61.0%) | 1998 (60.1%) | 1.8% | 379 (66.4%) | 1108 (64.7%) | 3.6% |
| Biologics | 1109 (100.0%) | 3327 (100.0%) | 0.0% | 571 (100.0%) | 1713 (100.0%) | 0.0% |
| PDE4 inhibitors | 3 (0.3%) | 12 (0.4%) | 1.6% | 2 (0.4%) | 9 (0.5%) | 2.7% |
| Opioids | 573 (51.7%) | 1702 (51.2%) | 1.0% | 311 (54.5%) | 961 (56.1%) | 3.3% |
| Non-narcotic analgesics | 51 (4.6%) | 177 (5.3%) | 3.3% | 28 (4.9%) | 85 (5.0%) | 0.3% |
| DMARDs | 641 (57.8%) | 1962 (59.0%) | 2.4% | 343 (60.1%) | 1082 (63.2%) | 6.4% |
| Duration of treatment with originator IFX prior to index date days mean ± SD [median] | ≥1073 ± 619 [991] | ≥1035 ± 671 [893] | 6.0% | 762 ± 443 [628] | 746 ± 480 [607] | 3.6% |
| Place of service for index claim | ||||||
| Medical | ||||||
| Outpatient | 1006 (90.7%) | 3006 (90.4%) | 1.2% | 516 (90.4%) | 1539 (89.8%) | 1.8% |
| Inpatient | 0 (0.0%) | 0 (0.0%) | - | 0 (0.0%) | 0 (0.0%) | – |
| Emergency room | 2 (0.2%) | 9 (0.3%) | 1.9% | 1 (0.2%) | 4 (0.2%) | 1.3% |
| Other services | 61 (5.5%) | 197 (5.9%) | 1.8% | 37 (6.5%) | 127 (7.4%) | 3.7% |
| Pharmacy | 30 (2.7%) | 84 (2.5%) | 1.1% | 11 (1.9%) | 29 (1.7%) | 1.8% |
| Medical and pharmacy | 10 (0.9%) | 31 (0.9%) | 0.3% | 6 (1.1%) | 14 (0.8%) | 2.4% |
Abbreviations: AS ankylosing spondylitis; CD Crohn’s disease; CID chronic inflammatory disease; DMARD disease modifying anti-rheumatic drug; IBD inflammatory bowel disease; IFX infliximab; NSAID non-steroidal anti-inflammatory drug; PDE4 phosphodiesterase-4; PsA psoriatic arthritis; PsO plaque psoriasis; Quan-CCI Quan-Charlson comorbidity index; RA, rheumatoid arthritis; SD, standard deviation; UC, ulcerative colitis; US, United States.
Incident Population
After matching, 571 switchers and 1713 continuers from the incident population were included in the study (Table 1). Among both switchers and continuers, 38.5% had RA and 47.5% had IBD. Mean age was 56.5 (SD=15.7) years for switchers and 55.9 (SD=15.4) years for continuers (standardized difference=4.2%). Among switchers and continuers, 66.5% and 67.8% were female, respectively (standardized difference=2.7%). Mean Quan-CCI score was 1.10 (SD=1.36) among switchers and 1.06 (SD=1.27) among continuers (standardized difference=2.8%). Prior to the index date, patients were treated with originator IFX for an average of 762 (SD=443) days for switchers and 746 (SD=480) days for continuers (standardized difference=3.6%). Other baseline characteristics were also well balanced between cohorts (standardized differences <10%), except for use of non-steroidal anti-inflammatory drugs (NSAIDs), which was slightly more frequent among continuers (33.5%) than switchers (28.5%; standardized difference=10.6%).
Switching Patterns
Prevalent Population
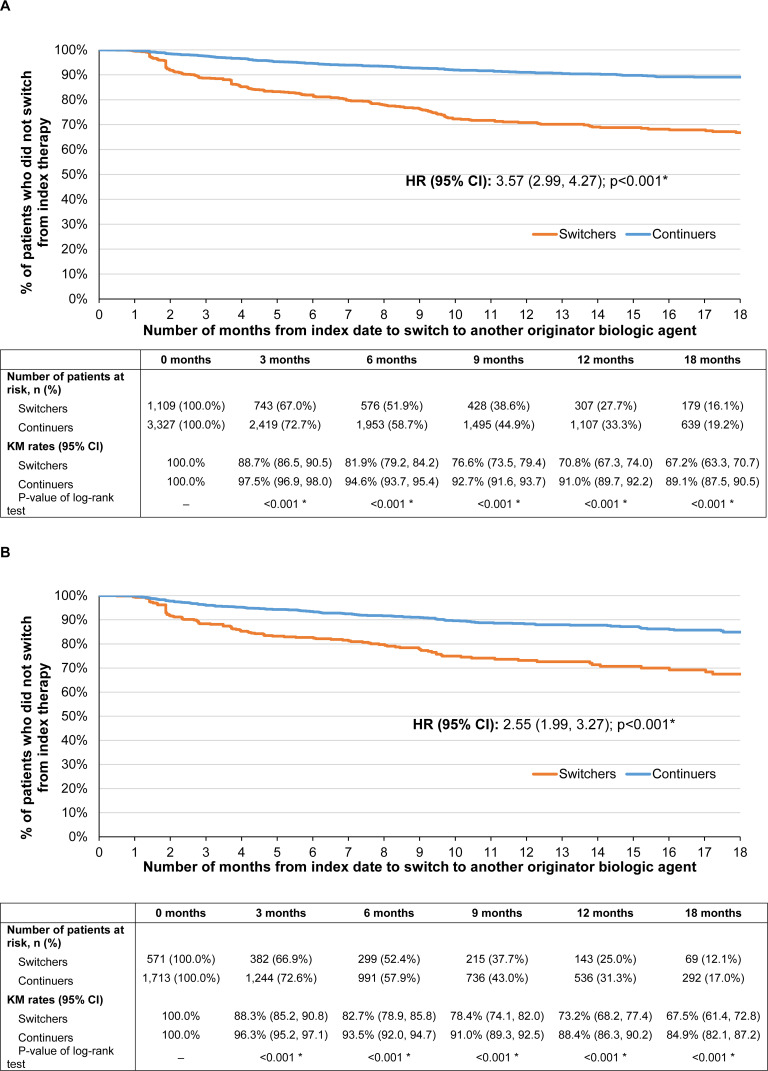
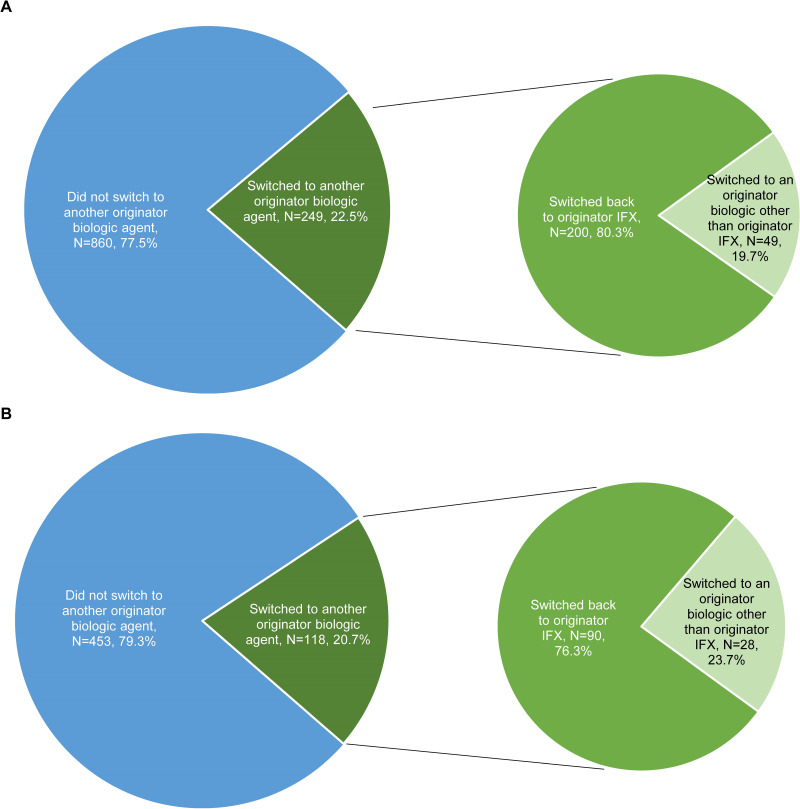
Over a mean post-index observation period length of 337 (SD=235) and 314 (SD=230) days for switchers and continuers in the prevalent population, respectively, 249 (22.5%) switchers and 227 (6.8%) continuers switched to another originator biologic (including originator IFX) (HR [95% CI]=3.57 [2.99, 4.27]; p<0.001). Of note, since switches from one IFX biosimilar to another could not be detected, all other alternative treatments for switching were originator biologics. Among patients who switched to another originator biologic, the mean time to switch was 151 (SD=126) days for switchers and 183 (SD=145) days for continuers. The KM rates representing the proportion of switchers and continuers who did not switch to another originator biologic were 88.7% and 97.5% at 3 months post-index, 81.9% and 94.6% at 6 months, 70.8% and 91.0% at 12 months, and 67.2% and 89.1% at 18 months, respectively (all log-rank p<0.001; Figure 3A). As of the last follow-up available in the data, of the 249 switchers who switched to another originator biologic over the entire duration of the observation period (mean duration=337 days), 200 (80.3%) switched back to originator IFX, over a mean time to switch of 140 days (SD=124), and 49 (19.7%) switched to another originator biologic (Figure 4A). Among the 200 patients who switched back to originator IFX, 89 (44.5%) eventually discontinued originator IFX within a mean of 173 days (SD=135).
Figure 3.
KM curves of (A) time to switch to another originator biologic among the prevalent population and (B) time to switch to another originator biologic among the incident population.
Note: *p<0.05. Abbreviations: CI, confidence interval; HR, hazard ratio; KM, Kaplan–Meier.
Figure 4.
Proportion of patients switching to another originator biologic agent among switchers in the (A) prevalent population (N=1109; mean observation period of 337 days) and (B) incident population (N=571; mean observation period of 314 days).
Abbreviations: IFX, infliximab.
Incident Population
Similar results were found in the incident population. Over a mean post-index observation period length of 314 (SD=222) and 309 (SD=227) days for switchers and continuers, respectively, 118 (20.7%) switchers and 152 (8.9%) continuers switched to another originator biologic (including originator IFX) (HR [95% CI]=2.55 [1.99, 3.27]; p<0.001). Among patients who switched to another originator biologic, the mean time to switch was 142 (SD=120) days for switchers and 182 (SD=147) days for continuers. The KM rates representing the proportion of switchers and continuers who did not switch to another originator biologic were 88.3% and 96.3% at 3 months post-index, 82.7% and 93.5% at 6 months, 73.2% and 88.4% at 12 months, and 67.5% and 84.9% at 18 months, respectively (all log-rank p<0.001; Figure 3B). As of the last follow-up available in the data, of the 118 switchers who switched to another originator biologic over the entire duration of the observation period (mean duration=314 days), 90 (76.3%) switched back to originator IFX, over a mean time to switch of 130 days (SD=113), and 28 (23.7%) switched to another originator biologic (Figure 4B). Among the 90 patients who switched back to originator IFX, 34 (37.8%) eventually discontinued originator IFX within a mean of 170 days (SD=129).
Discontinuation Patterns
Prevalent Population
As of the last follow-up available in the data, 457 (41.2%) switchers and 1119 (33.6%) continuers in the prevalent population discontinued their index therapy (HR [95% CI]=1.25 [1.12, 1.39]; p<0.001). Among patients who discontinued their index therapy, the mean time to discontinuation was 211 (SD=162) days for switchers and 220 (SD=134) days for continuers.
Incident Population
In the incident population, discontinuation rates were more similar, with 218 (38.2%) switchers and 617 (36.0%) continuers discontinuing their index therapy (HR [95% CI]=1.04 [0.89, 1.22]; p=0.580) as of the last follow-up available in the data. Among patients who discontinued their index therapy, the mean time to discontinuation was 199 (SD=154) days for switchers and 207 (SD=130) days for continuers.
Subgroup Analyses
Identical analyses within subgroups of patients with RA and IBD yielded similar results as in the main analysis (Figure 5 and Table 2).
Figure 5.
Switching and discontinuation rates for prevalent (switchers, N=1109; continuers, N=3327) and incident (switchers, N=571; continuers, N=1713) patients in the overall population, prevalent (switchers, N=464; continuers, N=1392) and incident (switchers, N=220; continuers, N=660) patients in the RA subgroup, as well as prevalent (switchers, N=523; continuers, N=1569) and incident (switchers, N=271; continuers, N=813) patients in the IBD subgroup.
Note: *p<0.05. Abbreviations: CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; RA, rheumatoid arthritis.
Table 2.
KM Analysis of Time to Switch to Another Originator Biologic (Including Originator IFX) for the RA and IBD Subgroups
| 0 Months | 3 Months | 6 Months | 9 Months | 12 Months | 18 Months | |
|---|---|---|---|---|---|---|
| RA subgroup | ||||||
| Prevalent population | ||||||
| KM rates (95% CI) | ||||||
| Switchers | 100.00% | 91.1% (88.0, 93.4) | 83.4% (79.4, 86.8) | 76.3% (71.5, 80.4) | 70.3% (65.0, 75.0) | 67.2% (61.5, 72.3) |
| Continuers | 100.00% | 97.3% (96.3, 98.1) | 94.3% (92.7, 95.5) | 92.2% (90.3, 93.7) | 90.8% (88.7, 92.5) | 89.2% (86.8, 91.2) |
| P-value of Log rank test | – | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
| Incident population | ||||||
| KM rates (95% CI) | ||||||
| Switchers | 100.00% | 91.7% (86.9, 94.7) | 83.4% (77.1, 88.1) | 75.7% (68.2, 81.7) | 73.8% (66.0, 80.1) | 69.5% (60.5, 76.9) |
| Continuers | 100.00% | 96.2% (94.3, 97.4) | 93.0% (90.5, 94.9) | 90.0% (86.9, 92.4) | 88.6% (85.2, 91.3) | 82.4% (77.3, 86.5) |
| P-value of Log rank test | – | 0.008* | <0.001* | <0.001* | <0.001 * | <0.001* |
| IBD subgroup | ||||||
| Prevalent population | ||||||
| KM rates (95% CI) | ||||||
| Switchers | 100.00% | 86.1% (82.6, 89.0) | 80.9% (76.8, 84.4) | 78.0% (73.6, 81.9) | 73.3% (68.1, 77.8) | 70.1% (64.2, 75.3) |
| Continuers | 100.00% | 97.9% (97.0, 98.5) | 95.3% (93.9, 96.4) | 93.7% (92.1, 95.0) | 92.1% (90.2, 93.7) | 90.3% (87.9, 92.2) |
| P-value of Log rank test | – | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
| Incident population | ||||||
| KM rates (95% CI) | ||||||
| Switchers | 100.00% | 84.7% (79.4, 88.7) | 80.4% (74.4, 85.0) | 78.2% (71.9, 83.3) | 72.3% (64.7, 78.6) | 65.0% (54.8, 73.4) |
| Continuers | 100.00% | 96.6% (95.0, 97.7) | 94.7% (92.6, 96.2) | 92.9% (90.4, 94.7) | 89.2% (86.0, 91.8) | 87.6% (83.8, 90.6) |
| P-value of Log rank test | – | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
Note: *p<0.05.
Abbreviations: CI, confidence interval; IBD, inflammatory bowel disease; KM, Kaplan–Meier; RA, rheumatoid arthritis.
Switching Patterns
Among patients with RA in the prevalent population, 464 switchers were matched to 1392 continuers. Over a mean post-index observation period length of 374 (SD=237) and 325 (SD=227) days for switchers and continuers, respectively, 111 (23.9%) switchers and 98 (7.0%) continuers switched to another originator biologic (including originator IFX) (HR [95% CI]=3.49 [2.67, 4.57]; p<0.001) as of the last follow-up available in the data. Of the 111 switchers who switched to another originator biologic, 82 (73.9%) switched back to originator IFX. Similarly, in the incident population, 220 switchers were matched to 660 continuers. Over a mean post-index observation period length of 338 (SD=219) and 314 (SD=225) days for switchers and continuers, respectively, 46 (20.9%) switchers and 66 (10.0%) continuers switched to another originator biologic (including originator IFX) (HR [95% CI]=2.16 [1.45, 3.22]; p<0.001). Of the 46 switchers who switched to another originator biologic, 30 (65.2%) switched back to originator IFX.
Among patients with IBD in the prevalent population, 523 switchers were matched to 1569 continuers. Over a mean post-index observation period length of 306 (SD=233) and 303 (SD=233) days for switchers and continuers, respectively, 106 (20.3%) switchers and 95 (6.1%) continuers switched to another originator biologic (including originator IFX) (HR [95% CI]=3.82 [2.91, 5.02]; p<0.001) as of the last follow-up available in the data. Of the 106 switchers who switched to another originator biologic, 93 (87.7%) switched back to originator IFX. Similarly in the incident population, 271 switchers were matched to 813 continuers. Over a mean post-index observation period length of 296 (SD=227) and 304 (SD=231) days for switchers and continuers, respectively, 57 (21.0%) switchers and 58 (7.1%) continuers switched to another originator biologic (including originator IFX) (HR [95% CI]=3.42 [2.36, 4.94]; p<0.001). Of the 57 switchers who switched to another originator biologic, 48 (84.2%) switched back to originator IFX.
Discontinuation Patterns
Among patients with RA in the prevalent population, 218 (47.0%) switchers and 488 (35.1%) continuers discontinued their index therapy during the observation period (HR [95% CI]=1.23 [1.05, 1.44]; p=0.009). In the incident population, 99 (45.0%) switchers and 253 (38.3%) continuers discontinued their index therapy during the observation period (HR [95% CI]=1.05 [0.83, 1.32]; p=0.694).
Among patients with IBD in the prevalent population, 190 (36.3%) switchers and 497 (31.7%) continuers discontinued their index therapy during the observation period (HR [95% CI]=1.29 [1.09, 1.53]; p=0.003). In the incident population, 93 (34.3%) switchers and 263 (32.3%) continuers discontinued their index therapy during the observation period (HR [95% CI]=1.16 [0.91, 1.47]; p=0.230).
Discussion
In these US retrospective cohort studies with a mean observation period of almost one year, both prevalent and incident patients with CIDs switching from originator to biosimilar IFX were 2-to-3-times more likely to switch to another originator biologic (including originator IFX) compared to those remaining on originator IFX. Among those who switched to an IFX biosimilar and then to another originator biologic, more than 75% returned to originator IFX, on average within 5 months. This is noteworthy because it has been reported in the literature that patients may request a return to originator IFX, suggesting that this may not be a non-medical decision.16,20,21,24,25 Additionally, prevalent patients switching from originator to biosimilar IFX were 1.25-times more likely to discontinue their index therapy compared to those remaining on originator IFX. Similar switching and discontinuation patterns were observed among the subgroups of patients with RA and IBD.
There is limited evidence in the literature regarding real-world switching and discontinuation patterns following a switch from originator to biosimilar IFX in the US. One recent matched-cohort study of a US integrated healthcare system found that a higher proportion of patients with IBD who switched from originator IFX to IFX biosimilar switched therapy again to another biologic compared to patients who remained on originator IFX (15.7% versus 11.6%, p<0.01),26 corroborating the current findings. A number of non-US real-world studies have also evaluated switching patterns with IFX and other originator biologics like etanercept and adalimumab,15,16,19–21,27–31 with results that are consistent with the current analyses. Of note, in the few studies that compared patients switching from originator IFX to IFX biosimilar and patients remaining on originator IFX, the latter patients were more likely to continue with their respective treatment.15,16,27,29 For instance, in a French study of patients with RA, AS, or PsA conducted by Scherlinger et al, the drug retention rate was significantly lower in patients who switched to IFX biosimilar (72%) compared to a historic cohort of patients who were treated with originator IFX (88%; p<0.001).16 In addition, numerous studies have also shown that among patients who discontinue an IFX biosimilar, 79% to 92% return to originator IFX,16,19,21,29 which is in line with the findings of the current study, where 76.3% to 80.3% switch back to originator IFX after initially switching to an IFX biosimilar. Interestingly, in three studies that evaluated outcomes after the switch back to originator IFX, 71% to 100% of patients who reinitiated originator IFX after discontinuing IFX biosimilar due to disease relapse, lack of treatment efficacy, or adverse events subsequently achieved partial or full clinical improvement after the switch back.16,20,32 The small proportion of patients not regaining a clinical response suggests that switching itself may also be an issue. Furthermore, the lower drug costs of IFX biosimilars may be offset by additional costs related to implementation of new treatments and dose adjustments when switching.33 Indeed, higher healthcare resource utilization and costs have been reported in studies of real-world switches from originator biologics to biosimilars.27,34
In contrast to the previously mentioned studies showing negative effects associated with switching, other, non-US studies have demonstrated well-tolerated switching to IFX biosimilars and limited effects on long-term clinical outcomes.35–37 Given the diverse evidence regarding biologic treatment switching in the literature, further research is warranted to examine the reasons for treatment switching and discontinuations using real-world data.
A notable strength of this study is the evaluation of both prevalent (ie, patients stable on originator IFX for an extended period of time, with mean duration of pre-index originator IFX treatment of at least 1035–1073 days) and incident patients (ie, patients newly stabilized on originator IFX, with mean duration of pre-index originator IFX treatment of 746–762 days). While results were similar between the two patient populations, the differences in switching and discontinuation patterns were less pronounced among incident patients. This may be due to the smaller sample size of the incident population (N=571 switchers, N=1713 continuers) compared to the prevalent population (N=1109 switchers N=3327 continuers), thereby limiting the statistical power of the comparison. However, additional research is needed to further evaluate the differences between patients newly stabilized on originator IFX and those who have maintained long-term stability.
While the present study found that patients switching from originator to biosimilar IFX are more likely to switch again to another originator biologic, the reasons why patients switch treatments after initiating IFX biosimilars or switch back to originator IFX remain unclear. One potential reason for switching that has been the focus of several studies is the “nocebo” effect.21,28,38 The nocebo effect, which is the negative equivalent of the better-known placebo effect, occurs when a patient has a negative subjective feeling towards a treatment despite acceptable pharmacological outcomes. The source of the nocebo effect in the context of biosimilars may be related to a lack of awareness or understanding of the product, prompting patients to react to perceived minor adverse events or to discontinue treatment.28,38 To this effect, in the survey-based study of US patients with CIDs by Teeple et al, 85% of patients were concerned that biosimilars would not treat their disease as well as the originator biologic.39 Additionally, several observational studies have reported patients discontinuing biosimilars despite no change in disease activity and/or a lack of objective adverse events, suggesting subjective reasons for treatment discontinuation.16,19,21 Alternatively, it is possible that switches away from biosimilars may just be a reflection of originator and biosimilar product availability.
In addition to evaluating switching patterns among patients with CIDs, the present study also assessed subgroups of patients with RA or IBD. The subgroup analyses yielded similar findings as the main analysis, suggesting that the switching and discontinuation patterns observed are consistent regardless of CID indication. These RA- and IBD-specific results contribute important insight to the literature regarding two of the most common CIDs,40 which had thus far been mostly evaluated in combination with other IFX indications in studies of switching and discontinuation patterns.15,19–21,27
Limitations
The results of this study should be interpreted in light of some limitations. As with all claims-based studies, there is the possibility of data omissions or coding errors within the database. In addition, prescriptions or healthcare services that were processed through different claims transactions than those captured by the SHS database may not be reflected in the data; therefore, a treatment interruption observed in the data may in fact be a patient receiving healthcare services outside of the networks that were not captured in the database. While formal assessments have not been conducted, in the event patients change providers to obtain different medications, they tend to obtain the same medication through the same provider, making the analysis of switching patterns for a specific medication possible. Treatment patterns were analyzed based on claims for a filled prescription, which does not guarantee the actual consumption of the medication by patients. The SHS database also has no information on disease severity or the reason for medication switching or discontinuation. Because of this lack of clinical context, switching to another originator biologic and switching back to originator IFX were considered together; despite possible differences between these two outcomes, insights on the clinical decision-making that may differentiate between the two types of switches were not available. Moreover, since the same procedure code is used to identify all claims for IFX biosimilars, switches from one IFX biosimilar to another could not be detected and, as such, patients moving from one IFX biosimilar to the other were considered as not switching to another treatment. Since medical claims in the SHS database may be less well-populated than in a traditional claims database, patients who used pharmacy benefits to acquire IFX may not have had medical claims. However, most patients received their IFX through medical benefits, thus mitigating this limitation. Additionally, stability on originator IFX was defined as having ≥5 claims of originator IFX in the 12 months prior to the index date; however, patients’ clinical stability could not be determined in this database. While differences in observable characteristics between the two cohorts were accounted for using PSM based on selected covariates measured during the baseline period, residual confounding may have remained due to unobserved factors. Lastly, for patients already being treated with originator IFX when entering the database (ie, >40% of patients in the prevalent switchers and continuers cohorts), the full duration of treatment with originator IFX prior to the index date could not be determined, since data prior to October 1, 2012, were not available. For these patients, the start date of treatment with originator IFX was assumed to be October 1, 2012. Additionally, the prevalent population was subject to immortal time bias, whereby the population may have been biased towards patients less likely to switch or discontinue treatment due to duration of stability on originator IFX that was longer than the duration of treatment observed in the data. As more data became available, an incident population was analyzed, whereby the full duration of treatment with originator IFX could be assessed. Since this analysis included patients who were recently initiated on originator IFX on or after October 1, 2013, it resulted in a smaller sample size.
Conclusion
This retrospective study found that patients switching from originator to biosimilar IFX were 2-to-3-times more likely to switch to another originator biologic, notably back to originator IFX, than those remaining on originator IFX. Additionally, prevalent patients switching from originator to biosimilar IFX were more likely to discontinue their index therapy than those remaining on originator IFX. Because the reasons for switching and discontinuation are unknown, additional research is needed to understand the higher rates of switching and discontinuation among patients who switched to an IFX biosimilar and its potential impact on clinical outcomes. Reasons for switching back to originator IFX versus to another originator biologic also need to be investigated.
Acknowledgments
Medical writing assistance was provided by Isabelle Ghelerter and Christine Tam, employees of Analysis Group, Inc.
Funding Statement
This research was funded by Janssen Scientific Affairs, LLC. The sponsor was involved in the study design, interpretation of results, manuscript preparation, and publication decisions.
Abbreviations
AS, ankylosing spondylitis; CD, Crohn’s disease; CI, confidence interval; CID, chronic inflammatory disease; DMARD, disease modifying anti-rheumatic drug; FDA, Food and Drug Administration; GPI, Generic Product Identifier; HCPCS, Healthcare Common Procedure Coding System; HIPAA, Health Insurance Portability and Accountability Act; HR, hazard ratio; ICD-9/10-CM, International Classification of Diseases, 9th/10th Revision, Clinical Modification; IBD, inflammatory bowel disease; IFX, infliximab; KM, Kaplan–Meier; NMS, non-medical switching; NSAID, non-steroidal anti-inflammatory drug; PDE4, phosphodiesterase-4; PsA, psoriatic arthritis; PSM, propensity score matching; PsO, plaque psoriasis; Quan-CCI, Quan-Charlson Comorbidity Index; RA, rheumatoid arthritis; SD, standard deviation; SHS, Symphony Health Solutions; UC, ulcerative colitis; US: United States.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
LM, MHL, BE, and PL are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. IL, RM, KW, and TF are employees of Janssen Scientific Affairs, LLC and stockholders of Johnson & Johnson, the parent company of Janssen Pharmaceuticals, which manufactures and sells Remicade (infliximab). The authors report no other conflicts of interest for this work.
References
- 1.Vangeli E, Bakhshi S, Baker A, et al. A systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseases. Adv Ther. 2015;32(11):983–1028. doi: 10.1007/s12325-015-0256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551–1557. [DOI] [PubMed] [Google Scholar]
- 3.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and prevalence of Crohn’s Disease and ulcerative colitis in Olmsted county, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15(6):857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83(978):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanauer SB. The expanding role of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol. 2010;7(2):63–64. [DOI] [PubMed] [Google Scholar]
- 6.Janssen Pharmaceutical Companies. REMICADE (Infliximab) Highlights of Prescribing Information; 2018. [Google Scholar]
- 7.Melsheimer R, Geldhof A, Apaolaza I, Schaible T. Remicade® (infliximab): 20 years of contributions to science and medicine. Biologics. 2019;13:139–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Drug Administration (FDA). Biosimilar and interchangeable products. 2017; https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products. Accessed December17, 2019.
- 9.CELLTRION, Inc. INFLECTRA® (Infliximab-Dyyb) Highlights of Prescribing Information; 2019. [Google Scholar]
- 10.Amgen Inc. AVSOLA (Infliximab-Axxq) Highlights of Prescribing Information; 2019. [Google Scholar]
- 11.Samsung Bioepis Co. RENFLEXIS (Infliximab-Abda) Highlights of Prescribing Information; 2019. [Google Scholar]
- 12.Pfizer Ireland Pharmaceuticals. IXIFI (Infliximab-Qbtx) Highlights of Prescribing Information; 2020. [Google Scholar]
- 13.Teeple A, Ellis LA, Huff L, et al. Physician attitudes about non-medical switching to biosimilars: results from an online physician survey in the United States. Curr Med Res Opin. 2019;35(4):611–617. doi: 10.1080/03007995.2019.1571296 [DOI] [PubMed] [Google Scholar]
- 14.Wolf D, Skup M, Yang H, et al. Clinical outcomes associated with switching or discontinuation from Anti-TNF inhibitors for nonmedical reasons. Clin Ther. 2017;39(4):849–862. doi: 10.1016/j.clinthera.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 15.Glintborg B, Sorensen IJ, Loft AG, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76(8):1426–1431. [DOI] [PubMed] [Google Scholar]
- 16.Scherlinger M, Germain V, Labadie C, et al. Switching from originator infliximab to biosimilar CT-P13 in real-life: the weight of patient acceptance. Joint Bone Spine. 2018;85(5):561–567. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet (London, England). 2017;389(10086):2304–2316. [DOI] [PubMed] [Google Scholar]
- 18.Smolen JS, Choe JY, Prodanovic N, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, Phase III transition study. Ann Rheum Dis. 2018;77(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avouac J, Molto A, Abitbol V, et al. Systematic switch from innovator infliximab to biosimilar infliximab in inflammatory chronic diseases in daily clinical practice: the experience of Cochin University Hospital, Paris, France. Semin Arthritis Rheum. 2018;47(5):741–748. [DOI] [PubMed] [Google Scholar]
- 20.Gentileschi S, Barreca C, Bellisai F, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15:1677-1683. Expert Opin Biol Ther. 2016;16(10):1311–1312.27266338 [Google Scholar]
- 21.Tweehuysen L, van den Bemt BJF, van Ingen IL, et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol. 2018;70(1):60–68. [DOI] [PubMed] [Google Scholar]
- 22.Lund JL, Horvath-Puho E, Komjathine Szepligeti S, et al. Conditioning on future exposure to define study cohorts can induce bias: the case of low-dose acetylsalicylic acid and risk of major bleeding. Clin Epidemiol. 2017;9:611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 24.Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7(1):35–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmmod S, Schultheiss J, Mahmmod N, Tan A, Dijkstra G, Fidder H. P1809 - Reverse switching to originator infliximab may be considered in patients with inflammatory bowel diseases experiencing new side effects or loss of response after switching to a CT-P13 biosimilar. Paper presented at: United European Gastroenterology Week 2019.
- 26.Ho SL, Niu F, Pola S, Velayos FS, Ning X, Hui RL. Effectiveness of switching from reference product infliximab to infliximab-Dyyb in patients with inflammatory bowel disease in an integrated healthcare system in the United States: a retrospective, propensity score-matched, non-inferiority cohort study. BioDrugs. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips K, Juday T, Zhang Q, Keshishian A. SAT0172 Economic outcomes, treatment patterns, and adverse events and reactions for patients prescribed infliximab or ct-p13 in the Turkish population. Ann Rheum Dis. 2017;76(Suppl 2):835. [Google Scholar]
- 28.Reuber K, Kostev K. Prevalence of switching from two anti-TNF biosimilars back to biologic reference products in Germany. Int J Clin Pharmacol Ther. 2019;57(6):323–328. [DOI] [PubMed] [Google Scholar]
- 29.Yazici Y, Xie L, Ogbomo A, et al. Analysis of real-world treatment patterns in a matched rheumatology population that continued innovator infliximab therapy or switched to biosimilar infliximab. Biologics. 2018;12:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madenidou A, Jeffries A, Varughese S, et al. Switching patients with inflammatory arthritis from Etanercept (Enbrel®) to the biosimilar drug, SB4 (Benepali®): a single-centre retrospective observational study in the UK and a review of the literature. Mediterranean J Rheumatol. 2019;30(Supp 1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alten R, Neregard P, Jones H, et al. SAT0161 Preliminary real world data on switching patterns between etanercept, its recently marketed biosimilar counterpart and its competitor adalimumab, using swedish prescription registry. Ann Rheum Dis. 2017;76(Suppl):2. [Google Scholar]
- 32.Mahmmod S, Schultheiss J, Tan A, et al. Reasons for and effectiveness of switching back to originator infliximab after a prior switch to CT-P13 biosimilar. J Crohn’s Colitis. 2020;14(Suppl):1.31605526 [Google Scholar]
- 33.Brown CN, McCann E. Cost of switching from an originator biologic (Remicade) to a biosimilar. Value Health. 2016;19(7):A581. [Google Scholar]
- 34.Liu Y, Yang M, Garg V, Wu EQ, Wang J, Skup M. Economic impact of non-medical switching from originator biologics to biosimilars: a systematic literature review. Adv Ther. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra Veloz MF, Belvis Jimenez M, Valdes Delgado T, et al. Long-term follow up after switching from original infliximab to an infliximab biosimilar: real-world data. Therap Adv Gastroenterol. 2019;12:1756284819858052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoivik ML, Buer LCT, Cvancarova M, et al. Switching from originator to biosimilar infliximab - real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol. 2018;53(6):692–699. [DOI] [PubMed] [Google Scholar]
- 37.Kaltsonoudis E, Pelechas E, Voulgari PV, Drosos AA. Maintained clinical remission in ankylosing spondylitis patients switched from reference infliximab to its biosimilar: an 18-month comparative open-label study. J Clin Med. 2019;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther. 2017;4(2):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teeple A, Ginsburg S, Howard L, et al. Patient attitudes about non-medical switching to biosimilars: results from an online patient survey in the United States. Curr Med Res Opin. 2019;35(4):603–609. [DOI] [PubMed] [Google Scholar]
- 40.El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2–10. [DOI] [PubMed] [Google Scholar]