Abstract
Introduction
Local anesthetics (LAs) are frequently used during anesthesia; however, they may influence granulocyte function which in turn could modify immune responses in the perioperative period. Therefore, the aim of this study was to investigate the impact of clinically used doses of bupivacaine and lidocaine on granulocyte function with regard to migration, reactive oxygen species (ROS) production, neutrophil extracellular traps (NETosis) formation, and viability.
Methods
A total of 38 granulocyte-enriched samples from healthy subjects were obtained by whole blood lysis. Polymorphonuclear neutrophil (PMN) samples were incubated simultaneously with different concentrations of either bupivacaine (0.03–3.16 mmol/L) or lidocaine (0.007–14.21 mmol/L), or without drug (control). Live cell imaging was conducted in order to observe granulocyte chemotaxis, migration, ROS production, and NETosis. Flow cytometry was used to analyze viability and antigen expression.
Results
The track length (TL) of PMNs exposed to bupivacaine concentrations of 0.16 mmol/L and above significantly decreased compared to the control. Low concentrations of lidocaine were associated with slight but significant increases in TL, whereas this changed with concentrations above 1.4 mmol/L, showing a significant decrease in TL. PMN incubated with bupivacaine concentrations of 1.58 mmol/L and above or lidocaine concentrations of at least 3.6 mmol/L showed no migration or chemotaxis at all. Time to onset of maximal ROS production and time for half-maximal NETosis decreased in a dose-dependent manner for both substances. Equipotency in NETosis induction was reached by bupivacaine (1.1 mmol/L) at significantly lower concentrations than lidocaine (7.96 mmol/L). Cell viability and oxidative burst were unaffected by LAs.
Conclusion
Local anesthetics in clinically used doses ameliorate granulocyte defense mechanisms, thus indicating their potentially decisive effect during the perioperative period.
Keywords: local anesthetics, granulocytes, immune modulation, surgical trauma, inflammation
Introduction
Ever since the discovery of cocaine’s local anesthetic properties in the mid-19th century, local anesthetics (LAs) have been used in surgery and modern anesthesiologic interventions (eg, epidural or spinal anesthesia, local nerve and wound infiltration) would not be possible without them.1–3 In recent years, several studies have shown significant immunomodulation with LAs caused by interactions with polymorphonuclear granulocytes (PMNs). Although PMNs are considered to be electrically non-excitable, the predominant mechanisms4–8 for the modulations are thought to be the inactivation of voltage-gated sodium channel 1.3 (NaV 1.3), non-canonical effects such as the blocking of inflammatory mediators, antiapoptotic effects or reduced vascular adhesion, and trans-endothelial migration capacity. The amide type LAs bupivacaine and lidocaine are on the WHO’s list of essential drugs9,10 and may also have antitumor effects for several tumor entities.11,12 Furthermore, a recent study by Tohme et al observed that neutrophil extracellular traps (NETs), which are chromatin DNA released by PMNs, were implicated in colorectal carcinoma liver metastasis after surgical stress.13 Galos et al could observe reduced NETosis in women treated with intravenous lidocaine during breast cancer surgery which might result in lower recurrence rates.14
The perioperative period is a decisive time in which it turns out if the patient has benefitted from the surgery. Depending on the country, mortality rates due to surgical traumata are up to 4%, severe morbidity rates range between 5% and 15%, and around 15% of patients have to be readmitted to hospital within 30 days. It has been estimated that up to 50% of these problems could be avoided.15,16 After surgical trauma, the anti-inflammatory response is designed to maximize host defense. As a consequence, an anti-inflammatory state with reduced immunocompetence follows the host initially having a highly activated immune system.16,17 Therefore, perioperative immunomodulation could result in deleterious effects when this fragile equilibrium is disturbed.
As PMNs represent the majority of circulating inflammatory cells and are the first line of host defense, they inherently play a pivotal role in this perioperative period. As such, we investigated the impact of clinically used doses of amide type LAs bupivacaine and lidocaine on the function of isolated granulocytes by performing in vitro assays for the comparative and time-resolved analysis of granulocyte chemotactic migration capacity, reactive oxygen species (ROS) production, neutrophil extracellular trap formation (NETosis), time-dependent viability, and expression of adhesion and activation antigens.
Materials and Methods
Granulocyte Preparation
After approval by the local ethics committee of the University of Regensburg (Vote No: 12–101-0192) and informed consent was obtained, 7.5 mL of whole blood was drawn from 17 healthy blood donors (Table 1) and anti-coagulated in test-tubes with lithium heparin. The samples were purified by short-term hypotonic lysis for 30 seconds at ambient temperature (21°C) using a purified aq. dest. and sodium chloride solution for reconstitution (Merck, Darmstadt, Germany). The lysed blood samples were centrifuged for 5 minutes at 425 g, obtaining a leucocyte-enriched cellular sediment. Each sample was split into aliquots for treatment (variables: concentrations of local anesthetics) and detection (methods: live cell imaging and flow cytometry).
Table 1.
Characteristics of the Study Population
| Characteristics | Value |
|---|---|
| Number of subjects | 17 |
| Gender (female/male) | 8/9 |
| Age [years] | 27 [22–63] |
| Height [cm] | 172 [161–191] |
| Weight [kg] | 76 [37–91] |
| BMI [kg/m2] | 26 [21–29] |
Note: Mean values and [range].
Microscopy and Live Cell Imaging
This experimental setup was based on previous experiments.18–20 In brief, the cells were examined in temporal resolution by light and fluorescence microscopy over 8 hours. 3D-µ-slides (ibidi© GmbH, Martinsried, Germany) were used to perform a chemotactic assay. Each µ-slide consisted of three separate channels with each channel bordered by two reservoirs. Following the manufacturer’s instructions, the cells were embedded in collagen gel (1.5 mg/mL PureCol, Advanced BioMatrix, Carlsbad, USA) and followed by fluorescent stains. This cell-enriched gel matrix was filled into the µ-slide channels and incubated under humid conditions for 30 minutes at 37°C and 5% CO2. Immediately before microscopy, a chemoattractant (CA) N-formyl-met-leu-phe (fMLP, Sigma Aldrich, St. Louis, USA) was introduced to the reservoir on the left of each channel, instantly creating a linear gradient provoking chemotactic PMN movement. Along with the CA, medium RPMI 1640 (PAN-Biotech GmbH, Aidenbach, Germany) with 10% fetal calf serum (FCS, Sigma Aldrich, Steinheim, Germany) and the chosen local anesthetic concentration of either bupivacaine hydrochloride monohydrate or lidocaine hydrochloride monohydrate (both Sigma Aldrich, Steinheim, Germany) were applied. The intracellular ROS production was visualized using 1 µM dihydrorhodamine-123 (DHR-123, Molecular Probes Inc., Eugene, USA) by detecting its fluorescent product rhodamine-123. Utilizing the NETosis-associated release of extracellular DNA, NET formation was visualized with 0.5 µg/mL 4‘,6-diamidin-2-phenylindol (DAPI, Sigma Aldrich, Steinheim, Germany).
Live cell imaging was done using a Leica DMi8 microscope in combination with a motorized adjustable microscope stage, a Leica DFC9000 camera and a pE-4000 light source (CoolLED, NY, USA). Leica Application Suite X software (version 3.4.2.18368, all Leica equipment from Leica Microsystems, Wetzlar, Germany) automatically recorded phase contrast images and both fluorescence images (in total 9 frames per cycle). The turn-around time was 35±2 seconds over a time span of 8 hours. In every assay, stable test conditions were maintained using a stage top incubator (ibidi).
Image Data Analysis
Image sequences obtained consisting of 2700 frames per µ-slide were analyzed using Imaris software (versions 9.0.2 and 9.0.3, bitplane, Zurich, Switzerland). Phase contrast images provided the basis for migratory analyses. Cell migration was analyzed in 30-minute periods and was detected up to 3 hours after cell-gel contact. Cell-gel contact occurred approximately 45 minutes before exposure to local anesthetics and 50 minutes before the beginning of microscopic evaluation. Imaris recognized the cells that match the selected criteria, determined spots and calculated tracks for every moving cell. The received data included the following parameters: Track Displacement (TD, Euclidean track; in total as well as subdivided into x- and y-directed movement), Track Straightness (Str, fraction of Euclidean track length and total track length epitomizing the cell’s directness with a maximum of 1 for linear movement), Mean Track Speed (v), and Track Length (TL, accumulated Track). To exclude passive cells and reduce artefacts due to spots on non-PMN, tracks under 25 µm Track Length and under a duration of 800 s were excluded.
ROS production and NETosis were quantified by detecting the fluorescent area and processing the total surface areas per time point (Figure 1). Both of these PMN functions visualized in Excel (Microsoft Excel 2016) showed characteristic graphs, with a parabolic curve for ROS-production and a sigmoidal curve for NETosis. To analyze ROS production, the time of maximal intracellular ROS was calculated by creating and fitting a third degree polynomial trendline to the graph and extracting the matching equation.
Figure 1.
ROS production (TmaxROS) and NETosis (ET50NET) visualized by time-related total surface areas.
To calculate ET50NET, the data were processed using Phoenix 64 version 8.0.0 (Certara, New York, USA).
Flow Cytometry
In addition to live cell imaging, an aliquot of the cells was observed using flow cytometry (FACSCalibur) in combination with CellQuest Pro software version 5.2 (both from BD Biosciences, San Jose, USA). All analyses were performed using FlowJo software version 10.0.7 (FlowJo LLC, Ashland, OR, USA). The methods used were: an oxidative burst to observe ROS production, and detection of cell-surface antigens CD11b, CD62L and CD66b.21,22
For the oxidative burst, cells were preincubated in 1 mL of Dulbecco’s phosphate-buffered saline (DPBS, Sigma Aldrich, Steinheim, Germany), 10 µL DHR 123 (10 µM), and 10 µL seminaphtharhodafluor (SNARF, 10 µM, Invitrogen, Eugene, USA). The oxidative burst was triggered by adding either 10 µL fMLP (10 µM) and 10 µL tumor necrosis factor alpha (TNFa, 1 µg/mL, Pepro Tech Inc., Rocky Hill, USA), or 10 µL phorbol-12-myristate-13-acetate (PMA; 10 µM, Sigma-Aldrich). A concentration of local anesthetic identical to that of the live cell imaging samples was made up simultaneously. To assess cell vitality, 5 µL Propidium Iodide (PI, 1.5 mM, Invitrogen, Eugene, USA) was added.
Finally, for cell-surface antigen expression detection, the antibodies mentioned above and labelled either phycoerythrin (PE, CD11b ICRF44), fluorescein isothiocyanate (FITC, CD62L DREG-56), or allophycocyanin (APC, CD66b G10F5, all BioLegend) were used.
Statistical Analysis
Data from live cell imaging and flow cytometry were collected in Excel (Microsoft Excel 2016). SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA) was used for further statistical analyses. Initially, normal distribution was tested with the Kolmogorov–Smirnov-test. Based on non-existing normal distribution, a Kruskal–Wallis one-way analysis of variance (ANOVA) was used to compare the groups. Bonferroni correction (method) was used in post hoc analysis and P-values below 0.05 were considered statistically significant. The distribution of results is visualized with boxplots displaying median, lower quartile, and calculated minima and maxima. Statistical outliers are represented as circles, and extreme values are depicted as asterisks.
Results
Migration
The median track count per concentration of bupivacaine with detectable migration was n=208 (range 95–309).
The median track count per concentration of lidocaine with detectable migration was n=376 (range 273–467).
Bupivacaine
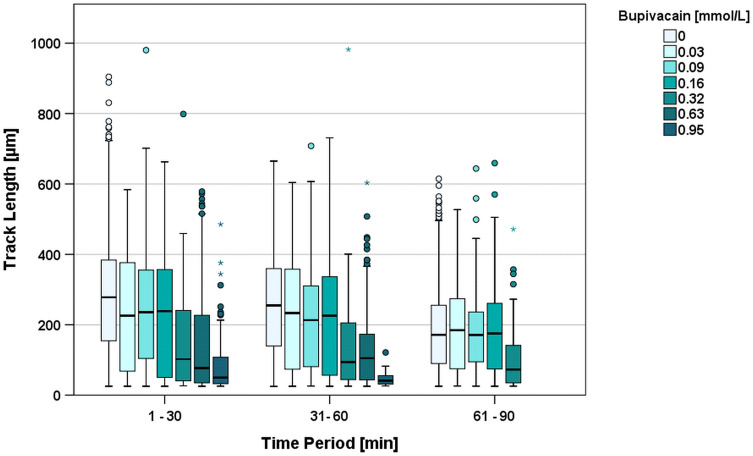
With increasing concentrations of bupivacaine, the TL values decreased (Table 2). In the first 30 minutes the median TL of the control group was 278 µm, whereas cells under 0.03 mmol/L bupivacaine showed a significant (p=0.021) lower median TL of 226 µm (80.6% of control group). For 0.95 mmol/L bupivacaine, the median TL was 49.9 µm (17.8% of control group). Migration was only detectable in concentrations up to 0.95 mmol/L. Higher concentrations resulted in immediate and absolute granulocyte arrest at the beginning of observation (all migration parameters at zero) and were considered significantly different to every other group below that concentration.
Table 2.
Descriptive Statistics of TL in the First 30 Minutes with Either Bupivacaine or Lidocaine Treatment
| Bupivacaine [mmol/L] | Median TL [µm] | IQR | Relative Reduction to Control [%] | Lidocaine [mmol/L] | Median TL [µm] | IQR | Relative Reduction to Control [%] |
|---|---|---|---|---|---|---|---|
| Control | 278 | 225 | – | 0 | 278 | 225 | – |
| 0.03 | 226 | 309 | 81.3 * | 0.007 | 385 | 259 | 138 * |
| 0.09 | 236 | 252 | 84.9 * | 0.14 | 357 | 221 | 128 * |
| 0.16 | 239 | 308 | 86.0 * | 0.71 | 328 | 243 | 118 * |
| 0.32 | 102 | 206 | 36.7 * | 1.42 | 329 | 273 | 118 * |
| 0.63 | 77 | 197 | 27.7 * | 3.55 | 0 | 0 | 0 * |
| 0.95 | 50 | 75 | 18.0 * | 7.1 | 0 | 0 | 0 * |
| 1.58 | 0 | 0 | 0 * | 14.21 | 0 | 0 | 0 * |
| 3.16 | 0 | 0 | 0 * | ||||
Note: Asterisks (*) mark significant changes compared to the control group (see text for further details).
Over the following two time periods, this TL reduction equalized in the groups of 0.03, 0.09, and 0.58 mmol/L compared to the control group (median TL 2nd period = 252 µm; 3rd period = 167 µm). By contrast, the groups of 0.32, 0.63, and 0.95 mmol/L (median TL 2nd period = 41.1 µm; 3rd period = no migration) showed a continuous and significant TL decrease in relation to the control group (Figure 2).
Figure 2.
Track Length [µm] during the first three 30-minute time periods clustered by increasing concentrations of bupivacaine. Higher concentrations resulted in complete migration arrest and are not shown in the figure. *Depicts extreme values and oIndicates outliers.
An analysis of TDX in the first period showed that significant reductions started at 0.32 mmol/L bupivacaine (p<0.001) when compared to the control group. Significant reductions in TL had already occurred at concentrations of 0.03 mmol/L (p=0.025) and 0.16 mmol/L (p<0.001) bupivacaine, compared to the control.
Str was reduced significantly at 0.03 mmol/L (p=0.039) and again at 0.16 mmol/L and above (minimum p=0.033). V was significantly reduced and followed the trend for Str at 0.03 mmol/L (p=0.011), and at 0.16 mmol/L and above (minimum p=0.032).
TDY showed no significant difference between the bupivacaine groups and the control group, and scatter was around 0 µm, confirming with TDX > 0 µm an intact CA-gradient in x-direction and no perpendicular gradient.
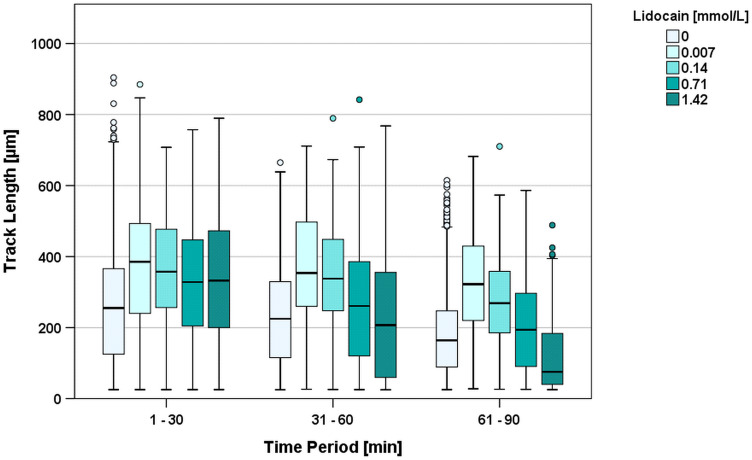
Lidocaine
In the first 30 minutes, the median TL of cells exposed to 0.007 mmol/L lidocaine showed a significantly (p=0.021) increased median track length of 385 µm (138% of control group; median TL 2nd period = 354 µm; 3rd period = 322 µm) (Figure 3). Similarly, the TLs observed with 1.42 mmol/L lidocaine significantly increased compared to the control group: the median TL during the first 30 minutes was 329 µm (118% of the median TL of the control group). However, in contrast to the lower doses of bupivacaine, this effect changed in the second period (TL=207µm; 82.0% in relation to the control group; p=0.004) and in the third period (TL=75.5 µm; 45.3% of control group; p<0.001) without reaching significance. Migration was only detectable with up to 1.42 mmol/L of lidocaine. Higher concentrations resulted in immediate and absolute arrest at the beginning of the observation period. These results were considered significantly different to every other group. (Figure 3)
Figure 3.
Track length [µm] during the first three 30-minute time periods clustered by increasing concentrations of lidocaine. Lidocaine concentrations above 1.42 mmol/L induced an immediate arrest of PMNs and are therefore not shown in the figure. oIndicates outliers.
Compared to the controls, every chosen concentration of lidocaine from 0.007 mmol/L to 14.21 mmol/L showed a significant effect on TL, but this effect was ambivalent. Lower doses (0.007 mmol/L–0.07 mmol/L) resulted in a higher TL, whereas doses above 1.42 mmol/L resulted in decreased migration and far earlier arrests.
TDY showed no significant difference between the lidocaine groups and the control group and scattered at around 0 µm due to perpendicular movement.
ROS Production
The entire time period was analyzed, and the median sample size without control (n=42) was n=3 for every condition. Within the control group, the median TmaxROS was 115 minutes after cell-gel contact.
For bupivacaine, the detected ROS production showed no clear trend with increasing concentrations between 0.03 and 0.63 mmol/L. The median TmaxROS for concentrations between 0.03 and 0.63 mmol/L varied between 71 and 164 minutes. In the cases of 0.95 mmol/L and 1.58 mmol/L, TmaxROS could not be quantified because it reduced significantly in the time frame between PMN preparation and the start of microscopic observation.
For lidocaine, a decrease in TmaxROS values to between 100 and 110 minutes was detectable in concentrations of 0.007, 0.14, 0.71, 0.142, and 7.1 mmol/L. 14.21 mmol/L lidocaine resulted in significantly reduced TmaxROS during pre-observation.
Along with the above-mentioned absolute arrest of all cells, the detected ROS production showed no peak or had ended at the very beginning of microscopy. Therefore, no valid TmaxROS values could be recorded even though the interval between LA contact and microscopy was less than 5 minutes (Table 3).
Table 3.
Summary of TmaxROS and ET50NET Changes Under LA Impact
| LA | LA-Concentration [mmol/L] | TmaxROS [min] Median ± SD |
ET50NET [min] Median ± SD |
|---|---|---|---|
| Bupivacaine | 0 | 115.5 ± 46.0 | 320.4 ± 47.5 |
| 0.03 | 101.6 ± 12.2 | 349.7 ± 31.4 | |
| 0.09 | 81.47 ± 21.64 | 338.6 ± 35.6 | |
| 0.16 | 88.5 ± 34.4 | 296.2 ± 34.0 | |
| 0.32 | 164.4 ± 34.8 | 314.6 ± 12.4 | |
| 0.63 | 70.5 ± 16.9 | 319.3 ± 39.0 | |
| 0.95 | 229.8 ± 37.4 | ||
| 1.58 | 107.3 ± 29.3 | ||
| 3.2 | 83.0 ± 6.11 | ||
| Lidocaine | 0 | 115.5 ± 46.0 | |
| 0.007 | 110.5 ± 24.8 | 355.8 ± 10.2 | |
| 0.14 | 106.6 ± 6.1 | 356.3 ± 5.81 | |
| 0.71 | 102.5 ± 21.5 | 428.9 ± 13.8 | |
| 1.42 | 100.9 ± 17.2 | 392.0 ± 50.7 | |
| 3.55 | 143.7 ± 38.0 | 343.9 ± 26.9 | |
| 7.1 | 101.7 ± 35.7 | 240.7 ± 23.7 | |
| 14.21 | 49.0 ± 18.8 |
Oxidative Burst
The median number of oxidative burst measurements with local anesthetics was n=7 (range 6–12) for each condition. No significant changes between LA-exposure and the control group could be detected. Median fluorescence intensities (MFI) obtained were at 533 for the control, 682 (467–1419) for all concentrations of bupivacaine, and 479 (521–565) for all concentrations of lidocaine after stimulation with PMA. fMLP and TNFa stimulation resulted in MFIs of 10 for the control group, 21 for all concentrations of bupivacaine (range 6–28) and 7 for all concentrations of lidocaine (range 5–9).
NETosis
For bupivacaine, the significant decrease started at 1.58 mmol/L (Kruskal–Wallis: p=0.033) with a median ET50NET of 107 minutes, compared to the control group with a median ET50NET of 320 minutes. Looking at the highest tested concentration of 3.16 mmol/L (Kruskal–Wallis: p=0.074), a median ET50NET of 83 minutes was observed, equaling 26% of the mean ET50NET within the control group.
For lidocaine, significant and though dose-dependent paradoxical effects were observed. Concentrations of 0.007–1.42 mmol/L resulted in an increase in ET50NET, whereas concentrations of 7.1 mmol/L and above led to a decrease in ET50NET.
Maximal increase was seen with 0.71 mmol/L, resulting in an ET50NET of 429 minutes (Kruskal–Wallis: p=0.175). The decreases observed were more distinctive: with 7.1 mmol/L the ET50NET was 241 minutes (Kruskal–Wallis: p=0.318), and with 14.21 mmol/L – the highest concentration used - the ET50NET was reduced to 49 minutes (Kruskal–Wallis: p=0.227). A summary of all TmaxROS and ET50NET-values is shown in Table 3.
Based on the LA concentrations and their corresponding ET50NET values, an inverse sigmoidal model was used to plot a curve for each LA to the data. The LA concentration at 50% reduction of the initial effect level was defined as EC50. The calculated EC50 value of lidocaine equaled 7.96 mmol/L, while bupivacaine showed a higher potency with an EC50 concentration of 1.1 mmol/L (Figure 4).
Figure 4.
Fitted curves of the LA dependent concentrations – ET50 connection indicating the induction of NETosis of human granulocytes by bupivacaine and lidocaine.
Along with the above-mentioned absolute arrest of all cells, ROS production peaked before the beginning of microscopy. Therefore, no valid TmaxROS values were recorded even though the interval between LA contact and microscopy was less than 5 minutes.
Cell Viability
Cell viability was evaluated by flow cytometry. The percentage of dead cells varied between 1.07% and 7.78% and exposure to LAs did not provoke any significant changes.
Cell-Surface Antigen Expression
The number of samples varied between 4 and 12 samples per condition (mean=6). The cell-surface antigen expression, measured by detecting the fluorescence intensity, showed no significant differences between the MFI of LA-exposed samples and the control group (Table 4).
Table 4.
Results of Flow-Cytometric Detection on Cell-Surface Antigen Expression [Median Fluorescence Intensities. Ranges in Brackets]
| Cell-Surface Antigen | MFI of Control Group | MFI Including All Concentrations of Bupivacaine | MFI Including All Concentrations of Lidocaine |
|---|---|---|---|
| CD11b | 569(3–1244) | 560(126–1147) | 620(386–1020) |
| CD62L | 118(2–328) | 142(62–328) | 118(81–217) |
| CD66b | 107(2–209) | 106(10–337) | 140(65–233) |
An overview of the tendencies of all analyzed aspects is shown in Table 5.
Table 5.
Summary of LA Effects on PMNs in Our in vitro Study
| Local Anesthetic | Bupivacaine | Lidocaine | ||
|---|---|---|---|---|
| Concentration Range | Low (~0.01 mM) |
High (max. 15 mM) |
Low (~0.02 mM) |
High (max. 17 mM) |
| Clinical Location | Intravascular | Extravascular | Intravascular | Extravascular |
| Effect on PMNs | ||||
| Viability | ↔ | ↔ | ↔ | ↔ |
| CD66b (“Activation”) | ↔ | ↔ | ↔ | ↔ |
| CD62L or CD11b (“Adhesion”) | ↔ | ↔ | ↔ | ↔ |
| Chemotactic migration | ↔ | ↓↓↓ | ↑ | ↓↓↓ |
| Induction period of ROS-production | ↔ | ↓↓↓ | ↔ | ↓↓↓ |
| Intensity of ROS-production | ↔ | ↔ | ↔ | ↔ |
| Duration until NETosis-Onset | ↓ | ↓↓↓ | ↑ | ↓↓↓ |
Note: ↔ indicates no change, ↑/↓ indicates slight increase or decrease and ↓↓↓ indicates strong decrease.
Discussion
This study investigated the impact of clinically used doses of amide type local anesthetics bupivacaine and lidocaine on isolated PMNs with regard to granulocyte migration, time dependency of ROS production and NET formation, and viability.
The dosages of each of the LAs under investigation were chosen according to their use in the clinical setting. An incubation period of 30 minutes was chosen to prevent PMN activation due to contact with artificial surfaces at longer periods. Both drugs have been used in clinical routine for decades.9,10 As bupivacaine is not suitable for intravenous (iv) administration due to its adverse effects on both the cardiovascular and central nervous systems,9 the high concentrations in our study correspond to the tissue levels yielded locally after directly injecting 0.5% bupivacaine (corresponding to approximately 15.8 mmol/L).9 Bupivacaine is adsorbed into the bloodstream from the injection site and distributed throughout the body, after which detectable plasma levels can be measured.9 Berrisford et al, for example, observed a plasma concentration of 1.45 µg/mL (equivalent to 0.005 mmol/L) after administration of a 20 mL bolus of 0.5% bupivacaine for an intercostal nerve block. After a 48-hour continuous bupivacaine infusion of 2 mg/kg bodyweight (BW) via this intercostal block, the plasma concentration rose to 4.92 µg/mL (equivalent to 0.017 mmol/L).23
Lidocaine can be administered intravenously or locally.10,24 After a local injection of 2% lidocaine, tissue concentration is around 20.5 mg/mL (equivalent to 71.1 mmol/L).10 For iv analgesia, Greenwood et al measured a plasma level of 4.6 µg/mL (equal to 0.016 mmol/L) after injecting an initial bolus of 1.5 mg/kg BW lidocaine followed by a continuous infusion of 120 mg/hour lidocaine for a bodyweight of 70 kg or more.25 In general, lidocaine plasma concentrations of up to 6 µg/mL (0.021 mmol/L) are considered safe during surgery and for anti-arrhythmic therapy.24,26
Granulocyte migration and migration efficacy decreased with increasing concentrations of bupivacaine. This result is in line with several other studies which have investigated the influence of local anesthetics on PMNs.4,5,27 Migration ceased at concentrations of 1.58 mmol/L and above. Surprisingly in this study, lidocaine at lower concentrations of up to 1.42 mmol/L increased PMN migration. This result correlates with a study by Cook et al. They incubated equine neutrophils in vitro with therapeutic dosages of lidocaine, detecting increased migration with comparable concentrations of lidocaine.28 Similar findings were observed by Erskine et al. In their study, granulocytes obtained from patients who had received spinal anesthesia for hip surgery showed greater movement than granulocytes from patients under general anesthesia.29 Furthermore, higher concentrations of lidocaine led to reduced migration, matching several other studies.4–6,16 Migration stopped at concentrations beyond 1.42 mmol/L. This occurrence may be due to an overwhelming inactivation of the NaV 1.3 channel and the non-canonical effects exerted by local anesthetics,4,5,7,16 or due to an increase in intracellular calcium concentration, as has been observed in investigations into the effect of LAs on tumor cell lines and muscle cells,30–32 though these investigations require further elucidation. Another study by Baptista-Hon et al revealed reduced rates of colon carcinoma cell invasion.33 Other studies investigating the effect of lidocaine administration during breast cancer surgery found reduced metastasis rates in patients treated with lidocaine.34,35
In a concentration-dependent manner, both LAs shifted ROS production towards an earlier onset. No ROS production was observed at high concentrations of both LAs, as cells were activated at the beginning of the experiments and these cells stopped their metabolism before microscopic observation began. These results are in line with several other studies.27,36,37 This observation is also likely to be attributed to the canonical and non-canonical effects of LAs as mentioned above.
Oxidative burst was unaffected for both substances investigated here. This result is in line with studies by Ploppa et al and by Mikawa et al, who did not observe a relevant change in oxidative burst for clinically used doses of local anesthetics.38,39 Contrary results were found by Billert et al36. In their study using PMNs obtained from cord blood of term newborns, a reduced oxidative burst at low concentrations of bupivacaine was observed, whereas, surprisingly, high concentrations did not result in decreased oxidative burst levels. However, high concentrations of lidocaine caused decreased oxidative burst levels in their study.
NETosis, depicted by ET50NET in this study, was reduced in a concentration-dependent manner for both substances, with a low concentration of lidocaine resulting in a slight increase of NET formation as well as in migration. Bupivacaine resulted in a significantly earlier onset of NET formation when compared to lidocaine. This attributed to its greater lipophilic structure.
Other aspects of LA-induced granulocyte arrest and altered defense mechanisms lie beyond direct antimicrobial or anti-tissue effects. Recently, investigations into the interactions between NETs and cancer growth/metastasis have revealed a distressing prospect. Circulating tumor cells are “invited” by NETs to settle and proliferate via CCDC25, a NET-DNA-receptor found on tumor cells.40,41 Bearing in mind that approximately 50% of all intravascular granulocytes are located in the so-called “marginated pools”42 – meaning they are temporarily immobilized, mainly in the venules of the lungs, liver and kidneys – an LA-induced NET-DNA presenting a landing strip for released cancer cells may intensify metastases in these organs. Galos et al reported on reduced NETosis levels after i.v. administration of lidocaine during breast cancer surgery, which might lead to reduces recurrence rates.14 Similar results were observed by Tohme et al for oncologic liver surgery.13
Cell viability was unaffected in our study. Other studies have shown a reduced survival of leucocytes when exposed to comparable concentrations of local anesthetics, but incubation was 24 hours, according to their protocols.43,44 Different cell types may have different sensitivities to local anesthetic mediated toxicity. Breu et al, for example, observed significant damage to chondrocytes after a one-hour incubation period.45
Cell-surface antigen expression was unaffected by both substances investigated here. This is contrary to other studies showing reduced levels of CD11b, CD62L (Selectin) and CD66b with increasing concentrations of LAs.5,46–49 It is speculated that these findings are due to different cell separation methods and handling regimens.
Limitations
As we performed an in vitro study with a collagen-I-matrix we can simulate extravascular PMN migration after extravasation. Nevertheless, this in vivo situation might be different due to interferences. It also could be argued that we did not perform assays on phagocytosis, but ROS production is quantified by standard flow cytometry and by time resolved fluorescence analysis. ROS production is the indispensable requirement for an effective killing of bacterial intruders and we already could show that the contact time between bacteria and the phagosome is also of extraordinary importance. If the migration period is shortened by NETosis induction and NETosis occurs too early, already phagocyted, but still living bacteria are released gain. The antibacterial function (eg, trapping and MPO-effects) of NETs is undermined by an incomplete destruction of already ingested monads. A LA-induced premature NETosis is to be assumed to hinder antibacterial effectivity of PMNs (unpublished data from our laboratory). Furthermore, we used incubation periods of 30 minutes. Other studies used different incubation times. To have a short time until start of PMN observation we purified the PMNs prior to incubation and did not incubate whole blood with the LAs. We have chosen this setup with a short time in contact with artificial surfaces and hypotonic lyses to prevent PMN activation due to other reasons then the LAs before microscopy. Furthermore, in our experimental setup life-cell-imaging requires observation periods of 8 hours. With longer incubation periods of up to 3 or 4 hours, PMNs would have been out of the body for >12 hours probably generating activation and cell death during the subsequent observation period.
Conclusion
In conclusion, our experimental setup with in vitro examinations of a collagen-I-matrix proves that PMN activity sequence starts with migration before the cells undergo conformational change, start ROS production, and finally undergo NETosis.50 Our data provide evidence that LAs in higher clinically applied concentrations have an inhibitory effect on PMN´s ability to migrate, the extent of this migration, and the ability to migrate efficiently. The time required until onset of ROS production and NETosis was reduced in a dose-dependent manner for both drugs, whereas the intensity and overall amount of ROS production and NETosis were unaffected. Bupivacaine generated a greater impact at lower concentrations compared to lidocaine. Taken together, we hypothesize that a perioperative application of LAs is able to modulate surgical trauma (Table 5).
Acknowledgments
We would like to thank the laboratory staff of the Department of Anesthesiology of the University Medical Center Regensburg for their outstanding technical assistance throughout this research project. We would also like to thank the volunteer donors. Without their participation and flexibility, this study would not have been possible. We also would like to thank Rosemary Simpson and Arlyn Bradley from “Bradley & Simpson – English Language Specialists” for language editing.
Funding Statement
This study was funded in-house by the Department of Anesthesiology.
Data Sharing Statement
The datasets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. These authors contributed equally to this study and are co-first authors: Gesche Kolle and Thomas Metterlein.
Disclosure
The authors declare no conflicts of interest. Parts of this work were presented as an abstract at the Deutscher Anaesthesiecongress 2019 (German Anesthesiology Congress 2019) in Leipzig, Germany.
References
- 1.Wagemans MF, Scholten WK, Hollmann MW, Kuipers AH. Epidural anesthesia is no longer the standard of care in abdominal surgery with ERAS. What are the alternatives? Minerva Anestesiol. 2020;86. doi: 10.23736/S0375-9393.20.14324-4. [DOI] [PubMed] [Google Scholar]
- 2.Razi SS, Stephens-McDonnough JA, Haq S, et al. Significant reduction of postoperative pain and opioid analgesics requirement with an enhanced recovery after thoracic surgery protocol. J Thorac Cardiovasc Surg. 2020. doi: 10.1016/j.jtcvs.2019.12.137. [DOI] [PubMed] [Google Scholar]
- 3.Ates İ, Aydin ME, Ahiskalioglu A, Ahiskalioglu EO, Kaya Z, Gozeler MS. Postoperative analgesic efficacy of perioperative intravenous lidocaine infusion in patients undergoing septorhinoplasty: a prospective, randomized, double-blind study. Eur Arch Otorhinolaryngol. 2020;277(4):1095–1100. doi: 10.1007/s00405-020-05801-6. [DOI] [PubMed] [Google Scholar]
- 4.Poffers M, Bühne N, Herzog C, et al. Sodium channel Nav1.3 is expressed by polymorphonuclear neutrophils during mouse heart and kidney ischemia in vivo and regulates adhesion, transmigration, and chemotaxis of human and mouse neutrophils in vitro. Anesthesiology. 2018;128(6):1151–1166. doi: 10.1097/ALN.0000000000002135. [DOI] [PubMed] [Google Scholar]
- 5.Berger C, Rossaint J, van Aken H, Westphal M, Hahnenkamp K, Zarbock A. Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients. J Immunol. 2014;192(1):367–376. doi: 10.4049/jimmunol.1301363. [DOI] [PubMed] [Google Scholar]
- 6.Piegeler T, Votta-Velis EG, Bakhshi FR, et al. Endothelial barrier protection by local anesthetics: ropivacaine and lidocaine block tumor necrosis factor-α-induced endothelial cell Src activation. Anesthesiology. 2014;120(6):1414–1428. doi: 10.1097/ALN.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50(3):265–282. doi: 10.1111/j.1399-6576.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaczmarek DJ, Herzog C, Larmann J, et al. Lidocaine protects from myocardial damage due to ischemia and reperfusion in mice by its antiapoptotic effects. Anesthesiology. 2009;110(5):1041–1049. doi: 10.1097/ALN.0b013e31819dabda. [DOI] [PubMed] [Google Scholar]
- 9.Heppolette CAA, Brunnen D, Bampoe S, Odor PM. Clinical pharmacokinetics and pharmacodynamics of levobupivacaine. Clin Pharmacokinet. 2020;59:715–745. doi: 10.1007/s40262-020-00868-0. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg L. Pharmacokinetics and pharmacodynamics of lignocaine: a review. WJA. 2015;4(2):17. doi: 10.5313/wja.v4.i2.17. [DOI] [Google Scholar]
- 11.Zhu G, Zhang L, Dan J, Zhu Q. Differential effects and mechanisms of local anesthetics on esophageal carcinoma cell migration, growth, survival and chemosensitivity. BMC Anesthesiol. 2020;20(1):126. doi: 10.1186/s12871-020-01039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Yang L, Zhu X, et al. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: a retrospective study. Br J Anaesth. 2020;125:141–148. doi: 10.1016/j.bja.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Tohme S, Yazdani HO, Al-Khafaji AB, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galoș EV, Tat T-F, Popa R, et al. Neutrophil extracellular trapping and angiogenesis biomarkers after intravenous or inhalation anaesthesia with or without intravenous lidocaine for breast cancer surgery: a prospective, randomised trial. Br J Anaesth. 2020;125:712–721. doi: 10.1016/j.bja.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Dobson GP. Addressing the global burden of trauma in major surgery. Front Surg. 2015;2:43. doi: 10.3389/fsurg.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossaint J, Zarbock A. Perioperative inflammation and its modulation by anesthetics. Anesth Analg. 2018;126(3):1058–1067. doi: 10.1213/ANE.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 17.Shankar Hari M, Summers C. Major surgery and the immune system: from pathophysiology to treatment. Curr Opin Crit Care. 2018;24(6):588–593. doi: 10.1097/MCC.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 18.Bredthauer A, Kopfmueller M, Gruber M, et al. Therapeutic anticoagulation with argatroban and heparins reduces granulocyte migration: possible impact on ECLS-therapy? Cardiovasc Ther. 2020;2020:9783630. doi: 10.1155/2020/9783630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doblinger N, Bredthauer A, Mohrez M, et al. Impact of hydroxyethyl starch and modified fluid gelatin on granulocyte phenotype and function. Transfusion. 2019;59(6):2121–2130. doi: 10.1111/trf.15279. [DOI] [PubMed] [Google Scholar]
- 20.Hattenkofer M, Gruber M, Metz S, Pfaehler S-M, Lehle K, Trabold B. Time course of chemotaxis and chemokinesis of neutrophils following stimulation with IL-8 or FMLP. Eur J Inflamm. 2018;16:205873921881917. doi: 10.1177/2058739218819171. [DOI] [Google Scholar]
- 21.Bitzinger DI, Zausig YA, Paech C, et al. Modulation of immune functions in polymorphonuclear neutrophils induced by physostigmine, but not neostigmine, independent of cholinergic neurons. Immunobiology. 2013;218(8):1049–1054. doi: 10.1016/j.imbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Trabold B, Gruber M, Fröhlich D. Functional and phenotypic changes in polymorphonuclear neutrophils induced by catecholamines. Scand Cardiovasc J. 2007;41(1):59–64. doi: 10.1080/14017430601085948. [DOI] [PubMed] [Google Scholar]
- 23.Berrisford RG, Sabanathan S, Mearns AJ, Clarke BJ, Hamdi A. Plasma concentrations of bupivacaine and its enantiomers during continuous extrapleural intercostal nerve block. Br J Anaesth. 1993;70(2):201–204. doi: 10.1093/bja/70.2.201. [DOI] [PubMed] [Google Scholar]
- 24.Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78(12):1229–1246. doi: 10.1007/s40265-018-0955-x. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood E, Nimmo S, Paterson H, Homer N, Foo I. Intravenous lidocaine infusion as a component of multimodal analgesia for colorectal surgery-measurement of plasma levels. Perioper Med (Lond). 2019;8:1. doi: 10.1186/s13741-019-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collinsworth KA, Kalman SM, Harrison DC. The clinical pharmacology of lidocaine as an antiarrhythymic drug. Circulation. 1974;50(6):1217–1230. doi: 10.1161/01.cir.50.6.1217. [DOI] [PubMed] [Google Scholar]
- 27.Welters ID, Menzebach A, Langefeld TW, Menzebach M, Hempelmann G. Inhibitory effects of S-(-) and R-(+) bupivacaine on neutrophil function. Acta Anaesthesiol Scand. 2001;45(5):570–575. doi: 10.1034/j.1399-6576.2001.045005570.x [DOI] [PubMed] [Google Scholar]
- 28.Cook VL, Neuder LE, Blikslager AT, Jones SL. The effect of lidocaine on in vitro adhesion and migration of equine neutrophils. Vet Immunol Immunopathol. 2009;129(1–2):137–142. doi: 10.1016/j.vetimm.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Erskine R, Janicki PK, Ellis P, James MF. Neutrophils from patients undergoing hip surgery exhibit enhanced movement under spinal anaesthesia compared with general anaesthesia. Can J Anaesth. 1992;39(9):905–910. doi: 10.1007/BF03008337. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann P, Metterlein T, Bollwein G, et al. The myotoxic effect of bupivacaine and ropivacaine on myotubes in primary mouse cell culture and an immortalized cell line. Anesth Analg. 2013;117(3):634–640. doi: 10.1213/ANE.0b013e31829e4197. [DOI] [PubMed] [Google Scholar]
- 31.Metterlein T, Hoffmann P, Späth R, Gruber M, Graf BM, Zink W. In vitro myotoxic effects of bupivacaine on rhabdomyosarcoma cells, immortalized and primary muscle cells. Cancer Cell Int. 2015;15:75. doi: 10.1186/s12935-015-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bundscherer A, Malsy M, Gebhardt K, et al. Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol Res. 2015;95–96:126–131. doi: 10.1016/j.phrs.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Baptista-Hon DT, Robertson FM, Robertson GB, et al. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014;113(Suppl 1):i39–i48. doi: 10.1093/bja/aeu104. [DOI] [PubMed] [Google Scholar]
- 34.Freeman J, Crowley PD, Foley AG, et al. Effect of perioperative lidocaine, propofol and steroids on pulmonary metastasis in a murine model of breast cancer surgery. Cancers (Basel). 2019;11(5):613. doi: 10.3390/cancers11050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson MZ, Crowley PD, Foley AG, et al. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br J Anaesth. 2018;121(1):76–85. doi: 10.1016/j.bja.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 36.Billert H, Czerniak K, Bednarek E, Kulińska K. Effects of local anesthetics on the respiratory burst of cord blood neutrophils in vitro. Pediatr Res. 2016;80(2):258–266. doi: 10.1038/pr.2016.68. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Xu SY, Zhang QG, Lei HY. Bupivacaine induces reactive oxygen species production via activation of the AMP-activated protein kinase-dependent pathway. Pharmacology. 2011;87(3–4):121–129. doi: 10.1159/000323402. [DOI] [PubMed] [Google Scholar]
- 38.Ploppa A, Kiefer R-T, Haverstick DM, Groves DS, Unertl KE, Durieux ME. Local anesthetic effects on human neutrophil priming and activation. Reg Anesth Pain Med. 2010;35(1):45–50. doi: 10.1097/AAP.0b013e3181c75199. [DOI] [PubMed] [Google Scholar]
- 39.Mikawa K, Akamarsu H, Nishina K, Shiga M, Obara H, Niwa Y. Effects of ropivacaine on human neutrophil function: comparison with bupivacaine and lidocaine. Eur J Anaesthesiol. 2003;20(2):104–110. doi: 10.1017/s026502150300019x. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 41.Nolan E, Malanchi I. Neutrophil ‘safety net’ causes cancer cells to metastasize and proliferate. Nature. 2020;583(7814):32–33. doi: 10.1038/d41586-020-01672-3. [DOI] [PubMed] [Google Scholar]
- 42.Tak T, Tesselaar K, Pillay J, Borghans JAM, Koenderman L. What’s your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol. 2013;94(4):595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 43.Werdehausen R, Braun S, Fazeli S, et al. Lipophilicity but not stereospecificity is a major determinant of local anaesthetic-induced cytotoxicity in human T-lymphoma cells. Eur J Anaesthesiol. 2012;29(1):35–41. doi: 10.1097/EJA.0b013e32834cd6c4. [DOI] [PubMed] [Google Scholar]
- 44.Werdehausen R, Braun S, Essmann F, et al. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107(1):136–143. doi: 10.1097/01.anes.0000268389.39436.66. [DOI] [PubMed] [Google Scholar]
- 45.Breu A, Rosenmeier K, Kujat R, Angele P, Zink W. The cytotoxicity of bupivacaine, ropivacaine, and mepivacaine on human chondrocytes and cartilage. Anesth Analg. 2013;117(2):514–522. doi: 10.1213/ANE.0b013e31829481ed. [DOI] [PubMed] [Google Scholar]
- 46.Lan W, Harmon D, Wang JH, Shorten G, Redmond P. The effect of lidocaine on neutrophil CD11b/CD18 and endothelial ICAM-1 expression and IL-1beta concentrations induced by hypoxia-reoxygenation. Eur J Anaesthesiol. 2004;21(12):967–972. doi: 10.1017/s0265021504000353. [DOI] [PubMed] [Google Scholar]
- 47.Ploppa A, Kiefer R-T, Krueger WA, Unertl KE, Durieux ME. Local anesthetics time-dependently inhibit staphylococcus aureus phagocytosis, oxidative burst and CD11b expression by human neutrophils. Reg Anesth Pain Med. 2008;33(4):297–303. doi: 10.1016/j.rapm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Birle A, Nebe CT, Hill S, Hartmann K, Poeschl J, Koch L. Neutrophil chemotaxis in cord blood of term and preterm neonates is reduced in preterm neonates and influenced by the mode of delivery and anaesthesia. PLoS One. 2015;10(4):e0120341. doi: 10.1371/journal.pone.0120341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiefer R-T, Ploppa A, Krueger WA, et al. Local anesthetics impair human granulocyte phagocytosis activity, oxidative burst, and CD11b expression in response to Staphylococcus aureus. Anesthesiology. 2003;98(4):842–848. doi: 10.1097/00000542-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Pai D, Gruber M, Pfaehler S-M, Bredthauer A, Lehle K, Trabold B. Polymorphonuclear cell chemotaxis and suicidal NETosis: simultaneous observation using fMLP, PMA, H7, and live cell imaging. J Immunol Res. 2020;2020:1–10. doi: 10.1155/2020/1415947. [DOI] [PMC free article] [PubMed] [Google Scholar]