Abstract
Hepatocellular carcinoma (HCC) with extrahepatic metastasis is rare, and its prognosis is extremely poor. There is no standard treatment for HCC with extrahepatic metastasis. We report a case of abscopal effect in HCC with multiple pleural metastases in a patient who was treated with focal radiotherapy to extrahepatic metastasis, and achieved long-term survival. We performed radiotherapy only to the tumor in inferior vena cava and the proximal pleural tumor. The regimen comprised a total dose of 30 Gy administered in ten fractions to these tumors, followed by 12 Gy administered in four fractions (a total of 42 Gy in 14 fractions) as boost irradiation to the remaining tumor, and a complete regression was achieved. There have been some case reports on abscopal effects in HCC, but no reports on patients with multiple pleural metastases. To our knowledge, this is the first case report on the abscopal effect of focal radiotherapy resulting in complete regression of distant multiple pleural metastases.
Keywords: Abscopal effect, Radiotherapy, Hepatocellular carcinoma, Pleural metastasis
Introduction
Hepatocellular carcinoma (HCC) with multiple extrahepatic metastases is rare, with an incidence of 2–18% at the time of surgery or following autopsy [1]. HCC with extrahepatic metastases generally presents with advanced intrahepatic lesions, and there has been no standard treatment for this condition. The prognosis in patients with HCC with extrahepatic metastases is extremely poor; conventional palliative therapy yields an overall survival duration of only 6–14 months [2]. Other authors have reported that the median survival duration is no longer than 6 months in most cases, even with some treatment [3–5]. In general, the currently recommended treatment modality for HCC with extrahepatic metastasis is molecularly targeted therapy using drugs such as sorafenib or lenvatinib [6–8]. However, there are often situations wherein intrahepatic lesions can be controlled; and local treatments, including radiotherapy, can be considered for extrahepatic lesions.
In recent years, the abscopal effect has gained attention in the field of radiation oncology. The abscopal effect is a phenomenon observed in the treatment of metastatic carcinoma whereby shrinkage of untreated tumors occurs concurrently with shrinkage of tumors within the scope of the localized treatment. There have been some case reports of abscopal effects in HCC [9–11], but no such reports among patients with multiple pleural metastases. To our knowledge, this is the first report on the abscopal effect with focal radiotherapy resulting in complete regression of distant multiple pleural metastases.
Case report
A 77-year-old man with chronic hepatitis C was followed up at our hospital. He had comorbidities, including hypertension and type 2 diabetes mellitus. Follow-up abdominal magnetic resonance imaging revealed two masses in the right lobe of the liver (segments S5 and S8). Other detailed examinations revealed that there was neither lymph node involvement nor distant metastases. He was diagnosed with HCC, and hepatic segmentectomy was performed as the initial treatment. The pathological results revealed HCC, and we finally diagnosed a stage II (T2N0M0) tumor according to the 7th edition of the TNM classification system [12]. Two years after the initial surgery, multiple intrahepatic tumors recurred in S1, S3, and S7 of the liver, and radiofrequency ablation of these tumors was performed. No adjuvant treatment such as chemotherapy was performed. Four years after the initial surgery, the intrahepatic tumor also recurred in S2 of the liver and a tumor thrombus was found in the inferior vena cava (IVC). Laparoscopic partial hepatectomy was performed for the metastatic lesion in S2 of the liver, but it was impossible to remove the tumor in the IVC. Therefore, he was referred to our department to undergo focal radiotherapy of the tumor thrombus in the IVC to prevent obstruction.
At the first visit, he was asymptomatic, and his performance status was graded as 0 based on the guidelines of the Eastern Cooperative Oncology Group. His liver function was normal (Child–Pugh class A). A tumor marker test revealed remarkably high levels of alpha-fetoprotein (194 ng/ml [normal < 25 ng/ml]) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) (136 mAU/ml [normal < 40 mAU/ml]). A computed tomography (CT) scan revealed a tumor thrombus in the IVC as well as multiple pleural thickenings in both lungs. We made a diagnosis of HCC with multiple pleural metastases at this time.
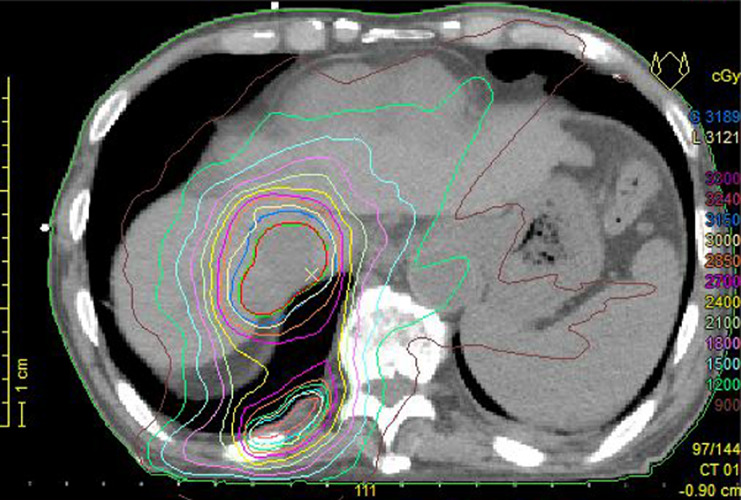
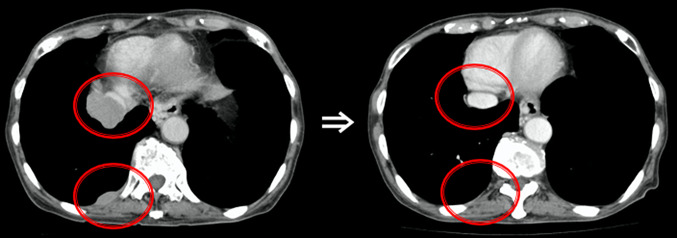
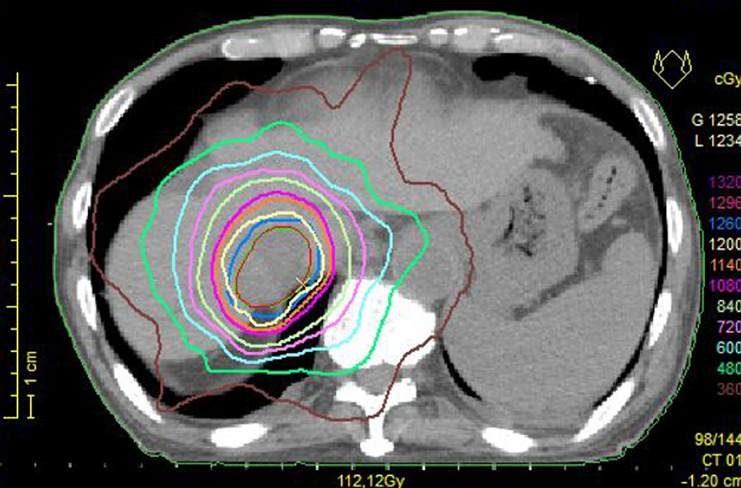
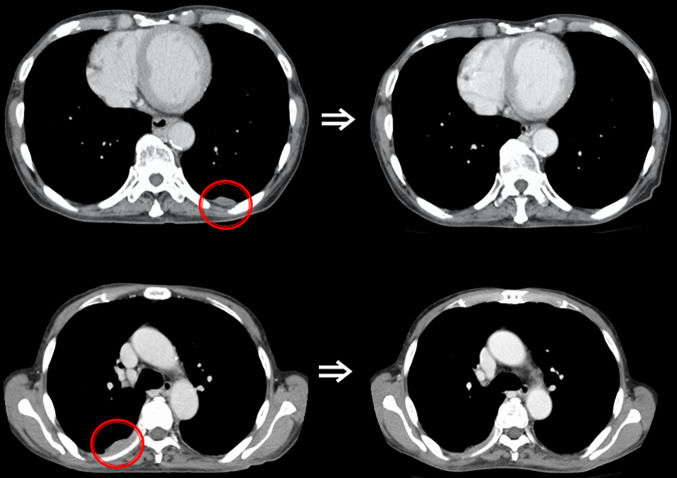
We planned focal radiotherapy of 30 Gy in ten fractions to the tumor thrombus in IVC as palliative treatment. We chose a volumetric modulated arc therapy (VMAT) since it facilitates the delivery of highly conformal radiotherapy compared with conventional radiotherapy. The radiation treatment fields included the tumor thrombus in the IVC and only the right proximal pleural lesions. Other multiple pleural tumors in both lungs were not included in the radiotherapy, and we decided to make a close follow-up of these multiple tumors with no treatment. VMAT was delivered via 10 MV X-rays, with a daily dose of 3 Gy administered three times a week (e.g., Monday, Wednesday, and Friday). We created three radiation volumes: the gross tumor volume, the clinical target volume (CTV), and the planning target volume (PTV). We defined that the CTV equaled the gross tumor volume, and the PTV included the CTV with a margin with the radius of 0.5 cm. We prescribed a minimum dose of at least 95% of the PTV, and inverse optimization was performed. Figure 1 shows the dose distribution of VMAT as the initial radiotherapy. We observed the sizes of the tumors via cone-beam CT as an image-guided radiation therapy during radiotherapy. At the end of planned radiotherapy, we observed complete regression of the right pleural metastasis and a remarkable reduction in the size of the tumor thrombus in the IVC (Fig. 2). We achieved an unexpected early tumor response, and made an adaptive replanning to the remaining tumor in the IVC. An adjunctive dose of 12 Gy in four fractions was given to the remaining tumor in the IVC which finally received a total dose of 42 Gy/14 fractions. Figure 3 shows the dose distribution of VMAT as a second radiotherapy (boost irradiation). There were no acute adverse events, and a late adverse event observed was grade 1 radiation pneumonitis according to the Common Terminology Criteria for Adverse Events version 4.0. One month after the initial radiotherapy, a CT scan showed that all pleural tumors had completely disappeared, although no radiotherapy was applied to them (Fig. 4). We recognized an “abscopal effect” in HCC with multiple pleural metastases.
Fig. 1.
Dose distribution during the initial radiotherapy. The radiation fields include the tumor plug in the inferior vena cava and close to the right pleural lesions
Fig. 2.
Axial thoracic computed tomography (CT) scans before and after the 27 Gy radiotherapy. Complete regression of the right pleural metastasis and a remarkable reduction in the size of the tumor plug in the IVC. IVC inferior vena cava
Fig. 3.
Dose distribution during the second radiotherapy. The radiation fields include the remaining tumor plug in the inferior vena cava as boost irradiation
Fig. 4.
Axial thoracic computed tomography (CT) scans before and 3 months after the radiotherapy. The axial CT scan shows the disappearance of the tumor outside the radiation field
Three months after initial radiotherapy, the tumor had disappeared and complete regression was maintained according to a follow-up CT scan results. The level of alpha-fetoprotein had decreased from 194 ng/ml to 10 ng/ml (normal < 25 ng/ml) and that of PIVKA-II had decreased from 136 mAU/ml to 20 mAU/ml (normal < 40 mAU/ml). However, 1 year after the initial radiotherapy, the tumor recurred in the right side of the chest wall. This was treated via VMAT consisting of a total dose of 30 Gy administered in ten fractions. In addition, one-and-a-half years after the initial radiotherapy, the tumor recurred in S3 of the left hepatic lobe. We planned focal radiotherapy again because its previous administration was effective. This was treated via VMAT consisting of a total dose of 30 Gy administered in ten fractions. Then a CT scan revealed multiple lung metastases; thus, we started targeted drug treatment using lenvatinib, and achieved a complete response of multiple lung metastases.
Three years after the initial radiotherapy, the patient is alive without the disease and there are no remarkable adverse events.
The multiple pleural tumors, which showed an “abscopal effect,” continue to maintain complete regression till now.
Discussion
We have discovered two important clinical issues. First, we recognized an abscopal effect in HCC with multiple pleural metastases by focal radiotherapy. Second, the patient achieved long-term survival despite multiple pleural metastases. Hypofractionated radiotherapy might be useful for an abscopal effect in HCC.
HCC with multiple extrahepatic metastases has a very poor prognosis and has no standard treatment. The abscopal effect, which involves a reduction of lesions outside the irradiation site after radiotherapy, has been known since a long time ago. In 1953, R.H. Mole first proposed the term “abscopal” (‘ab’—away from, ‘scopus’—target) to refer to the effects of ionizing radiation “at a distance from the irradiated volume but within the same organism” [13]. The abscopal effect is extremely rare, and the mediators of the abscopal effect of radiotherapy were unknown for decades. In 2004, it was postulated for the first time that the immune system might be responsible for these “off-target” anti-tumor effects [14]. Furthermore, immune-mediated abscopal effects were also described in patients with metastatic cancer [15].
Hypofractionated radiotherapy might be useful for an abscopal effect in HCC. It is believed that hypofractionated radiotherapy using fewer and larger fractional doses could facilitate the transmission of antigen information from cancer cells to immune cells. In this case, we performed moderately hypofractionated VMAT scheduled three times per week, which may have been advantageous to any immune response. We carefully observed the tumor size using image-guided radiation therapy; it was possible to administer the minimum necessary dose to the tumor, and there were no remarkable adverse events. It was unclear whether boost irradiation (12 Gy/4 fractions) in this patient contributed to the induction of “abscopal effect.” Moreover, it was unknown that the patient's clinical background as well as tumor biological characteristics are responsible for the abscopal effect.
In recent years, hypofractionated radiotherapy such as stereotactic irradiation has been performed, and there have been more reports on the abscopal effect. The effect of radiotherapy is usually the apoptosis in the presence of fractionated radiation, mainly due to the DNA damage. However, with stereotactic irradiation, in addition to the necrosis in tumor cells, there are also effects on the microcirculatory system. It also affects the immune circulation, and it is said that activating anti-tumor immunity causes indirect cell death. Anti-tumor immunity also affects tumors in the radiation field, corresponding to the rare occurrence of the abscopal effect with stereotactic irradiation. Furthermore, the use of immune checkpoint blocking antibodies such as ipilimumab or pembrolizumab has greatly increased the proportion of abscopal responding patients among selected groups of patients such as those with metastatic melanoma [16, 17].
We can conclude that it may be valuable to perform radiotherapy, especially the hypofractionated schedule, of the focal tumor in patients with HCC with multiple pleural metastases. We believe that even for HCC with distant metastases, it might be worthwhile to perform focal radiotherapy of the tumor. More data are required to determine the appropriate indication for radiotherapy in such cases.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animal performed by any of the authors.
Informed consent
This patient provided written informed consent for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhagwandin SB, Salti GI. Hepatocellular carcinoma with peritoneal metastasis treated with cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Tumori. 2015;101:e1–3. doi: 10.5301/tj.5000197. [DOI] [PubMed] [Google Scholar]
- 2.Mehta S, Schwarz L, Spiliotis J, et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur J Surg Oncol. 2018;44:1786–1792. doi: 10.1016/j.ejso.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 5.Park KW, Park JW, Choi JI, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008;23:900–907. doi: 10.1111/j.1440-1746.2007.05112.x. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Finn RS, Qin S, et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC) J Clin Oncol. 2017;35:4001. doi: 10.1200/JCO.2017.35.15_suppl.4001. [DOI] [Google Scholar]
- 9.Nakanishi M, Chuma M, Hige S, et al. Abscopal effect on hepatocellular carcinoma. Am J Gastroenterol. 2008;103:1320–1321. doi: 10.1111/j.1572-0241.2007.01782_13.x. [DOI] [PubMed] [Google Scholar]
- 10.Lock M, Muinuddin A, Kocha WI, et al. Abscopal effects: case report and emerging opportunities. Cureus. 2015;7:e344. doi: 10.7759/cureus.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuma K, Yamashita H, Niibe Y, et al. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7. New York: Wiley-Blackwell; 2009. pp. 110–113. [Google Scholar]
- 13.Mole RH. Whole body irradiation—radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 14.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Brix N, Tiefenthaller A, Anders H, et al. Abscopal, immunological effects of radiotherapy: narrowing the gap between clinical and preclinical experiences. Immunol Rev. 2017;280:249–279. doi: 10.1111/imr.12573. [DOI] [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiniker SM, Reddy SA, Maecker HT, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016;96:578–988. doi: 10.1016/j.ijrobp.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]