Abstract
A 68-year-old man was diagnosed with non-muscle-invasive bladder cancer and underwent transurethral resection of the bladder tumor (TURBT) in June 2014. The pathological diagnosis was urothelial carcinoma (UC), Grade 2, pT1. He was treated with intravesical bacillus Calmette–Guérin (BCG) instillation after TURBT. In February 2016, he received anti-tuberculosis treatment for systemic BCG infection, and tuberculosis treatment was continued. In September 2018, he presented with bilateral scrotum swelling and underwent bilateral orchiectomy following a diagnosis of antituberculotics-resistant epididymitis. The pathological findings were metastatic UC of the bilateral epididymis and testis. One months later, fluorodeoxyglucose-positron emission tomography/computed tomography showed para-aortic lymph node and peritoneal metastases. He was treated with chemotherapy of gemcitabine and cisplatin. We herein report a very rare case of synchronous metastatic UC of the bilateral epididymis and testis after intravesical BCG treatment.
Keywords: Testicular metastases, Bladder cancer, Intravesical bacillus Calmette–Guérin
Introduction
About 70% of patients with bladder cancer are diagnosed with non-muscle-invasive bladder cancer (NMIBC), and the incidence of intravesical recurrence is more than 50% [1]. Intravesical bacillus Calmette–Guérin (BCG) therapy is useful for the adjuvant treatment of patients with high- and intermediate-risk NMIBC [2–4]. However, in high-risk patients, which includes patients with Ta/T1 high grade urothelial carcinoma (UC) and or carcinoma in situ, the disease becomes muscle-invasive bladder cancer (MIBC) in 10–40% of cases [5, 6]. Furthermore, some NMIBC patients treated with intravesical BCG develop distant metastasis [7]. We herein report a very rare case of synchronous bilateral epididymal and testicular metastases of UC after intravesical BCG.
Case report
A 68-year-old man was newly diagnosed with a bladder tumor at another hospital in 2014. He underwent transurethral resection of the bladder tumor (TURBT) in May 2014, and the pathological diagnosis was confirmed to be UC, Grade 2 according to the 1973 WHO system, pT1. He received six courses of weekly intravesical BCG instillation. He continued surveillance with cystoscopy after BCG treatment.
In September 2015, he developed a slight fever, and computed tomography (CT) showed an aortic saccular aneurysm. CT images taken 11 months earlier showed no evidence of the aneurysm. Based on the CT findings, the possibility of an infected aneurysm could not be denied. This was managed by an endovascular aortic stent graft due to the diagnosis of infectious aortic aneurysm. In January 2016, CT showed pneumonia in the bilateral lung fields and a saccular mass around the endovascular aortic stent graft. Mycobacterium tuberculosis complex was isolated from his sputum culture. The isolated identified Mycobacterium tuberculosis complex was examined using a multiplex polymerase chain reaction ordered by the Department of Clinical Infectious Diseases. Based on the results, the investigators identified BCG Tokyo 172. Conclusively, the culture of the sputum grew Mycobacterium bovis. He was diagnosed with systemic BCG infection with both lung and aortic aneurysms including a saccular mass. He was referred to the department of infectious disease in our hospital in February 2016 and started on anti-tuberculosis treatment with rifampicin (RFP), isoniazid (INH) and ethambutol (EB) for the systemic BCG infection. EB was discontinued after two months because of negative culture in the sputum, but RFP and INH are still being administered by the Department of Clinical infectious diseases.
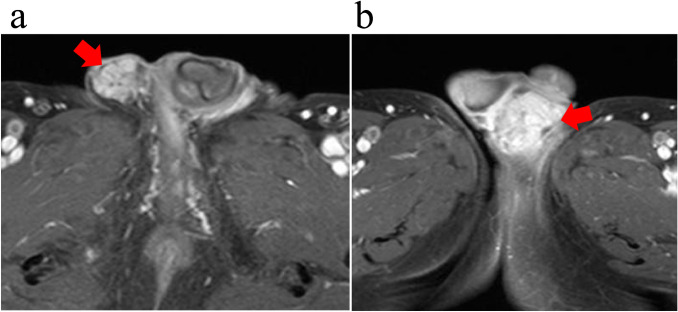
Cystoscopic examinations every three months showed no evidence of intravesical recurrence after 2014. However, he presented with bilateral painless indurations and swelling of the scrotum in August 2018. Although anti-infectious chemotherapy (levofloxacin and cefcapene pivoxil hydrochloride) in addition to INH and EB was administered due to the diagnosis of epididymitis, these treatments resulted in no improvement of the condition, even after four months of anti-infectious chemotherapy. He was referred to our department for surgical treatment in November 2018. Ultrasound revealed swelling of the bilateral epididymides and unclear testicular capsules. On T1-weighted magnetic resonance imaging (MRI), swelling of the bilateral epididymis with abnormal contrast high intensity was noted. Bilateral tuberculous epididymitis was suspected (Fig. 1a, b).
Fig. 1.
Magnetic resonance imaging (MRI) findings. Swelling of the bilateral epididymis with abnormal contrast high intensity (a, b)
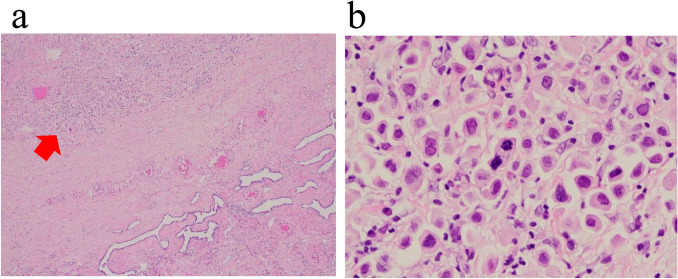
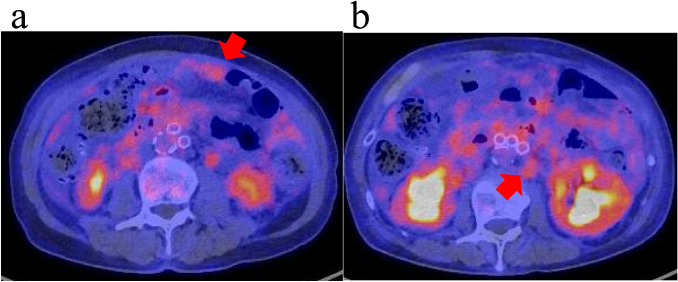
We decided to treat him with surgical treatment due to his diagnosis of antituberculotics-resistant bilateral tuberculous epididymitis. We performed bilateral inguinal orchiectomy in February 2019. The pathological specimens showed pleomorphic cells with a high N/C ratio in the epididymis diffused to the tunica vaginalis (Fig. 2a, b). An immunostaining panel revealed that tumor cells were positive for cytokeratin 7 (CK7), cytokeratin 20 (CK20) and Trans-acting T-cell Specific Transcription Factor Gata-3 (GATA3). These characteristics were consistent with bilateral epididymal and testicular metastases of UC. One months later, fluorodeoxyglucose-positron emission tomography/computed tomography showed para-aortic lymph node and peritoneal metastases and no other malignant disease (Fig. 3a, b). Although we did not perform a lymph node biopsy, based on the pathological and image examinations, he was diagnosed with metastatic bladder cancer.
Fig. 2.
Representative histology of urothelial carcinoma based on the epididymis metastases and diffuse invasion of the tunica vaginalis (a, b) (H-E)
Fig. 3.
Fluorodeoxyglucose-positron emission tomography/computed tomography showed para-aortic lymph node and peritoneal metastases (a, b)
He then started gemcitabine and cisplatin treatment. After four cycles of gemcitabine and cisplatin treatment, CT showed that he had achieved a complete response and had stable disease after 1.3 years’ follow-up.
Discussion
BCG therapy is the most successfully employed adjuvant treatment for patients with high-risk NMIBC. Several guidelines recommend that patients with high-risk NMIBC be offered BCG therapy. However, some go on to develop progressive disease [8, 9].
Oddens et al. reported on 1355 patients treated with intravesical BCG treatment for NMIBC in the randomized phase 3 study (30,962) conducted by European Organization for Research and Treatment of Cancer (EORTC). In that study, 5.3% of the patients who received full-dose maintenance BCG showed distant metastasis during 3-year follow-up.[7]. The most common metastatic site from UC of the bladder cancer is the lymph nodes, peritoneum, bone, kidney, lungs and liver [10]; testicular metastasis of UC is very rare, and only 10 patients with testicular metastasis of UC have been reported. Furthermore, only one report showed asynchronous UC metastasis to the bilateral testes [11]. To our knowledge, this is the first report of synchronous bilateral epididymal and testicular metastases of UC after intravesical BCG treatment.
Ulbright et al. reported 26 cases of metastatic carcinoma to the testes. The most common primary site was the prostate, followed by the kidney and colon [12]. The mechanism underlying the pathway to the testes of metastatic UC may involve direct overgrowth, implantation, hematogenous and lymphatic pathways. Half of the patients with UC testicular metastasis had confirmed prostatic invasion [13]. The mechanism underlying the pathway to the testes of metastatic UC might therefore involve the prostatic ducts and vas deferens. In the present case, however, we were unable to confirm prostatic invasion in the TURBT specimens.
In general, BCG treatment-related adverse effects (AEs) are tolerable, with only 5% of patients stopping BCG treatments because of AEs. Tuberculous epididymitis was noted in 0.4% of patients following antituberculotic treatment for systemic BCG infection [14, 15]. We initially considered our patient to have tuberculous epididymitis based on the radiographic findings and the history of systemic BCG infection. However, it was difficult to diagnose synchronous bilateral epididymal and testicular UC metastases because of the very low frequency of these metastases.
We encountered a rare case of synchronous bilateral epididymal and testicular metastases of UC after intravesical BCG.
Abbreviations
- NMIBC
Non-muscle-invasive bladder cancer
- BCG
Bacillus Calmette–Guérin
- UC
Urothelial carcinoma
- MIBC
Muscle invasive bladder cancer
- TURBT
Transurethral resection of the bladder tumor
- CT
Computed tomography
- RFP
Rifampicine
- INH
Isoniazid
- EB
Ethambutol
- MRI
Magnetic resonance imaging
- EORTC
European Organization for Research and Treatment of Cancer
Funding
None.
Compliance with ethical standards
Conflict of interest
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naotaka Nishiyama, Email: nishiyan@med.u-toyama.ac.jp.
Yoshinori Ikehata, Email: ikehatay@med.u-toyama.ac.jp.
Noriko Okuno, Email: nsshr25@med.u-toyama.ac.jp.
Masakiyo Sasahara, Email: sasahara@med.u-toyama.ac.jp.
Ippei Sakamaki, Email: sakamaki@med.u-toyama.ac.jp.
Yoshihiro Yamamoto, Email: yamamoto@med.u-toyama.ac.jp.
Hiroshi Kitamura, Email: hkitamur@med.u-toyama.ac.jp.
References
- 1.Kitamura H, Tsukamoto T. Early bladder cancer: concept, diagnosis, and management. Int J Clin Oncol. 2006;11:28–37. doi: 10.1007/s10147-006-0552-y. [DOI] [PubMed] [Google Scholar]
- 2.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette–Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 3.Pan J, Liu M, Zhou X. Can intravesical bacillus Calmette–Guérin reduce recurrence in patients with non-muscle invasive bladder cancer? An update and cumulative meta-analysis. Front Med. 2014;8:241–249. doi: 10.1007/s11684-014-0328-0. [DOI] [PubMed] [Google Scholar]
- 4.Hinotsu S, Akaza H, Naito S, et al. Maintenance therapy with bacillus Calmette–Guérin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU Int. 2011;108:187–195. doi: 10.1111/j.1464-410X.2010.09891.x. [DOI] [PubMed] [Google Scholar]
- 5.Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002;167:1573–1583. doi: 10.1016/S0022-5347(05)65157-9. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ. Bacillus Calmette–Guérin treatment of non-muscle invasive bladder cancer. Int J Urol. 2011;18:113–120. doi: 10.1111/j.1442-2042.2010.02678.x. [DOI] [PubMed] [Google Scholar]
- 7.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette–Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Gontero P, Bohle A, Malmstrom PU, et al. The role of bacillus Calmette–Guerin in the treatment of non-muscle-invasive bladder cancer. Eur Urol. 2010;57:410–429. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology. 2005;66:108–125. doi: 10.1016/j.urology.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Zaffuto E, Bandini M, Moschini M, et al. Location of metastatic bladder cancer as a determinant of in-hospital mortality after radical cystectomy. Eur Urol Oncol. 2018;1:169–175. doi: 10.1016/j.euo.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Kiely G, Kavanagh L, Bolton D, et al. Urothelial carcinoma of the bladder with asynchronous metastases to both testes. Urol Ann. 2013;5:218–219. doi: 10.4103/0974-7796.115743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulbright TM, Young RH. Metastatic carcinoma to the testis: a clinicopathologic analysis of 26 nonincidental cases with emphasis on deceptive features. Am J Surg Pathol. 2008;32:1683–1693. doi: 10.1097/PAS.0b013e3181788516. [DOI] [PubMed] [Google Scholar]
- 13.Saemundsson Y, Simoulis A, Liedberg F. Sanctuary testicular bladder cancer metastasis 10 years after radical cystectomy and adjuvant chemotherapy. Clin Genitourin Cancer. 2018;16:e1097–e1099. doi: 10.1016/j.clgc.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, et al. Bacillus Calmette–Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine (Baltimore) 2014;93:236–254. doi: 10.1097/MD.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamm DL. Efficacy and safety of bacille Calmette–Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31:S86–90. doi: 10.1086/314064. [DOI] [PubMed] [Google Scholar]