Abstract
Ovarian seromucinous carcinoma (SMC) is an uncommon neoplasia and is composed predominantly of serous and endocervical-type mucinous epithelium. Due to its low frequency and difficult diagnosis, the natural history, characteristic imaging findings, and pathological features of SMC have not been adequately described in the literature thus far. We herein report three cases of ovarian SMC along with magnetic resonance imaging (MRI) findings. The diagnosis of SMC was made after staging laparotomy in all cases, and systemic chemotherapy was performed in two cases. No recurrence was observed in any of the cases. The MRI findings in SMC were so varied that characteristic imaging features useful for diagnosis were not found. In two cases, MRI suggested endometriotic cysts, and endometriosis and seromucinous borderline tumors (SMBTs) were detected concurrently in all cases by histological examination. Thus, it was suggested that SMC develops in multiple stages via endometriosis and SMBT. The cooccurrence of endometriosis and SMBT could also make the diagnosis of SMC more convincing.
Keywords: Endometriosis, Endometrioid carcinoma, Seromucinous borderline tumor, Seromucinous carcinoma
Introduction
Ovarian seromucinous tumors are characterized by various epithelial components mainly composed of uterine endocervical gland like mucinous epithelium, and pilus columnar epithelium resembling oviduct epithelium. These neoplasms were formally categorized in the revised 2014 World Health Organization (WHO) classification of tumors of female reproductive organs [1]. Similar to other epithelial ovarian tumors, this clinical entity includes benign adenomas, borderline tumors, and invasive carcinomas. However, most are borderline tumors (SMBTs), and seromucinous carcinomas (SMCs) are very rare. Thus, little is known about SMC regarding natural history and magnetic resonance imaging (MRI) findings. In addition, there is much debate in terms of the pathological features of SMC.
We herein report three cases of SMC of the ovary arising in endometriotic cysts. Furthermore, we discuss characteristic magnetic resonance imaging (MRI) findings for the diagnosis of SMC and the relationship between seromucinous tumors and endometriosis in the context of the multistage carcinogenesis of SMC.
Case reports
Table 1 lists patient characteristics and pathological findings. Table 2 shows the results of immunohistochemistry.
Table 1.
Clinicopathologic characteristics of three cases
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Age | 45 | 53 | 54 |
| CA19-9 (U/ml) | 49,530 | 59.6 | 24.8 |
| CA125 (U/ml) | 582 | 2,996 | 286.6 |
| Ovarian tumor | Left 8 cm | Right 8 cm | Right 8 cm |
| Surgery | TAH/BSO/PEN/PAN/pOM | TAH/BSO/PEN/PAN/pOM | TAH/BSO/PEN/PAN/pOM |
| Chemotherapy | None | PTX/CBDCA 6 courses | PTX/CBDCA 6 courses |
| Endometriosis | + | + | + |
| SMBT | + | + | + |
| Contralateral ovary | Right 4 cm Endometriotic cyst | Left 10 cm Endometrioid carcinoma G1 | Left 1.7 cm Seromucinous carcinoma |
| Stage | IC1 (pT1c1N0M0) | IIB (pT2bN0M0) | IC3 (pT1c3N0M0) |
| DFS (months) | 32 | 17 | 13 |
TAH total abdominal hysterectomy, BSO bilateral salpingo-oophrectomy, PEN pelvic lymphadenectomy, PAN paraaortic lymphadenectomy, pOM partial omentectomy, PTX pacritaxcel, CBDCA carboplatin, SMBT seromucinous borderline tumor, DFS disease free survival
Table 2.
Immunohistochemistry of three cases
| Case | 1 | 2 | 3 |
|---|---|---|---|
| ER | + | + | + |
| ARID1A | Loss | + (retained) | + (retained) |
| p53 | Wild type | Wild type | Wild type |
| PTEN | Loss | + | Loss |
ER estrogen receptor. ARID1A AT-rich interactive domain-containing protein 1A. PTEN phosphatase and tensin homolog
Case 1
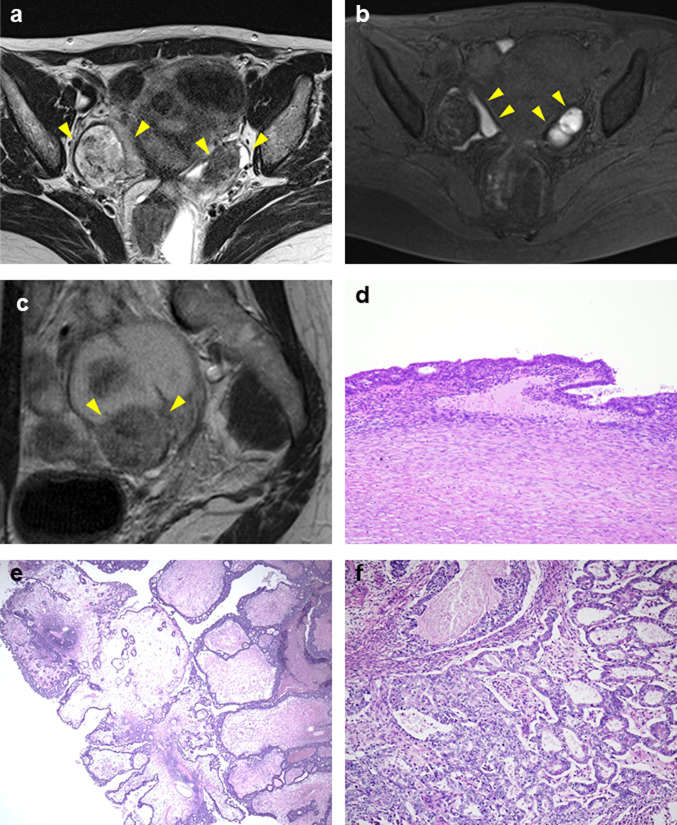
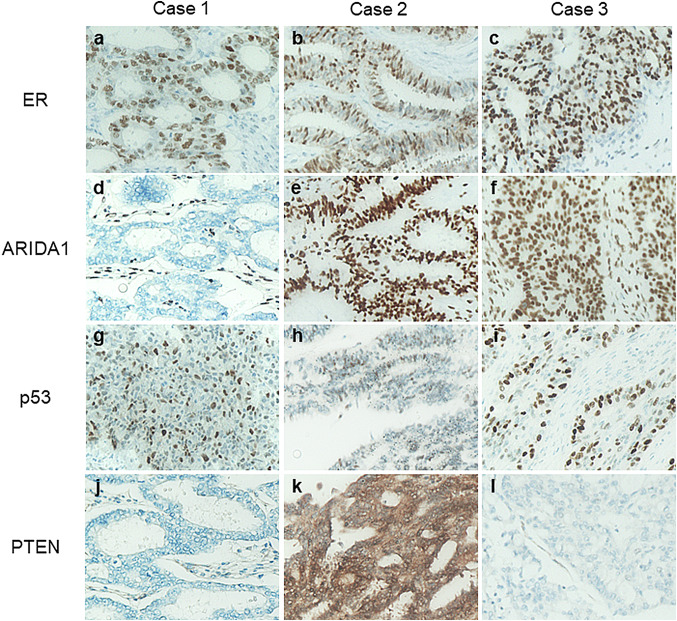
A 45-year-old woman with a history of right ovarian endometriotic cyst presented with lower abdominal pain. Pelvic MRI revealed bilateral ovarian tumors measuring 8 cm on the right and 4 cm on the left (Fig. 1a). Both cysts showed high signal intensity on fat suppressed T1 weighed image (WI) and intermediate intensity on T2WI, indicating endometriotic cysts (Fig. 1a, b). The right ovarian tumor contained a papillary solid portion with heterogeneous intensity on T2WI (Fig. 1c), and this lesion showed restricted water diffusion on diffusion weighed imaging (DWI). Marked fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) was also shown on the right ovarian tumor. These findings suggested malignant ovarian tumor arising in endometriotic cyst. Macroscopically, surgical findings revealed a solid and papillary prominence lesion in the right ovarian tumor. Histologically, endometriotic cyst walls were observed in both right (Fig. 1d) and left ovarian tumors. In the right ovary, tumor cells showed papillary growth from the edematous stroma, indicating SMBT (Fig. 1e). A variety of epithelia, such as monolayer cubic epithelium and mucus-producing epithelium, were observed. Moreover, tumor cells invaded into the stroma with various growth patterns, such as tubular, solid or cribriform (Fig. 1f). The case was diagnosed as SMC with an SMBT background. Immunohistochemically, estrogen receptor (ER) was positive, AT-rich interactive domain-containing protein 1A (ARID1A) was lost, the expression of p53 was wild type, and phosphatase and tensin homolog (PTEN) was negative (Fig. 4a–j).
Fig. 1.
MRI and microscopic findings of case 1. a Axial T2 weighed-image (WI) shows bilateral ovarian tumors, 8 cm in left and 4 cm in right (arrowheads). The right tumor showed heterogeneous low signal intensity, and the left tumor showed low signal intensity. b Left tumor showed high signal intensity at T1WI with fat saturation, indicating endometriotic cysts (arrowheads). The right tumor showed high signal intensity at the periphery but low signal intensity in the middle, indicating an endometriotic cyst with another component (arrowheads). c On sagittal contrast-enhanced T1WI, the right ovarian tumor contained a solid lesion, indicating a malignant tumor within the endometriotic cyst (arrowheads). d The endometriotic cyst wall is observed in the right ovary (× 200). e In the right ovary, tumor cells show papillary growth from the edematous stroma, indicating a seromucinous borderline tumor (× 100). A variety of epithelia, such as monolayer cubic epithelium and mucus-producing epithelium, can be observed. f Tumor cells invade into the stroma with various growth patterns, such as tubular, solid or cribriform (× 200)
Fig.4.
Immunohistochemistry of three cases (× 200). (a, b and c) ER was positive in three cases. d In case 1, the expression of ARID1A was lost. (e and f) ARID1A was retained in case 2 and 3. (g, h and i) the expression pattern of p53 was wild type in all cases. (j and l) The expression of PTEN was negative in case 1 and 3, and positive in case 2 (k)
Case 2
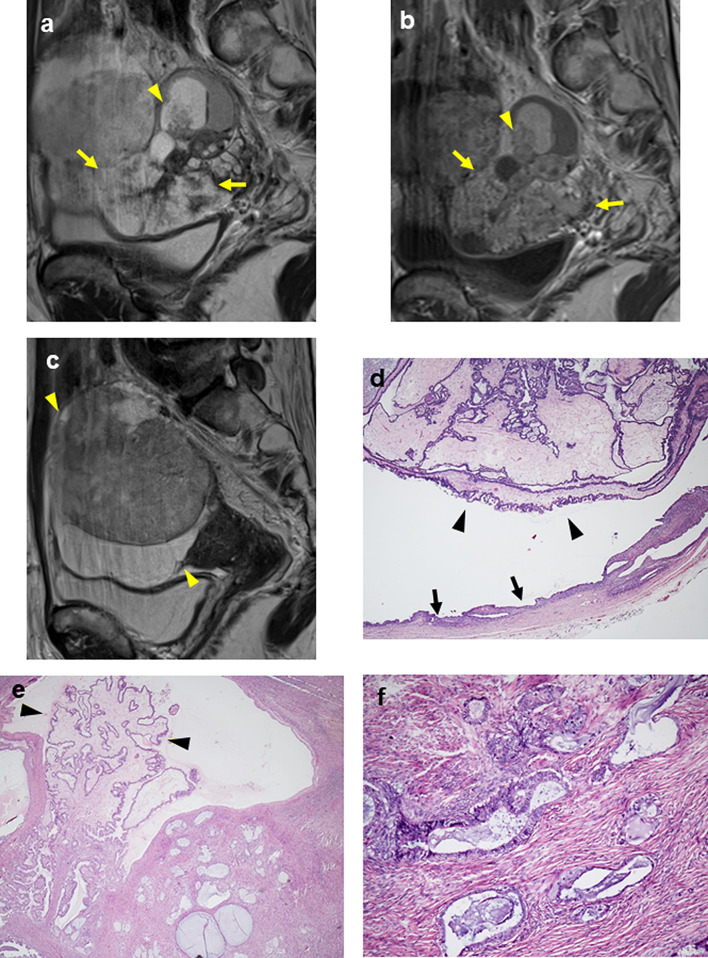
A 53-year-old woman presented with abdominal distension. Pelvic MRI showed bilateral ovarian tumors measuring 8 cm on the right (Fig. 2a, b) and 10 cm on the left (Fig. 2c).
Fig. 2.
MRI and microscopic findings of case 2. a On the sagittal T2-weighted image, the upper portion of the right tumor showed shading and cysts with small papillary lesions (arrowheads), suggesting SMBT. The lower portion consisted mainly of large papillary structures (arrows). b On contrast-enhanced T1WI, both papillary lesions showed strong enhancement. c On the sagittal T2-weighted image, the left ovarian tumor measuring 10 cm consisted of solid and cystic lesions (arrowheads), and the solid lesion showed heterogeneous low signal intensity. d Tumor cells with a papillary growth pattern with edematous stroma (arrowheads) were observed inside the endometriotic cyst wall (arrows) in the right tumor (× 100). e Edematous stroma (arrowheads) was prominent in the right tumor (× 100). f Endocervical-like mucinous cells were observed in the epithelium with stromal invasion in the right ovary (× 200)
The right tumor consisted of endometriotic cysts and large papillary structures. The endometriotic cyst contained small papillary projections similar to large papillary structures at the bottom, and both of the papillary structures showed high intensity on T2WI and enhancement, suggesting SMBT (Fig. 2a, b). The left ovarian tumor was a solid and cystic lesion (Fig. 2c), and the solid lesion showed restricted water diffusion and weak enhancement compared with the uterine body. These features were considerably different from those of the right tumor, and the left tumor was thought to be a different pathological type of tumor. Surgery was performed. Macroscopically, the right tumor had multilocular cysts, papillary growth lesions, and bloody fluid collection. The left tumor was a grayish white papillary and solid tumor. Histologically, in the right ovary, tumor cells with papillary growth patterns were observed inside the endometriotic cyst wall (Fig. 2d). Edematous stroma was prominent, indicating SMBT (Fig. 2e). Endocervical-like mucinous cells were observed in the epithelium with stromal invasion (Fig. 2f), and finally, this case was diagnosed as SMC. Immunohistochemically, ER was positive, ARID1A was retained, the expression of p53 was wild type, and PTEN was positive (Fig. 4b–k). The left ovarian tumor was endometrioid carcinoma, grade 1.
Case 3
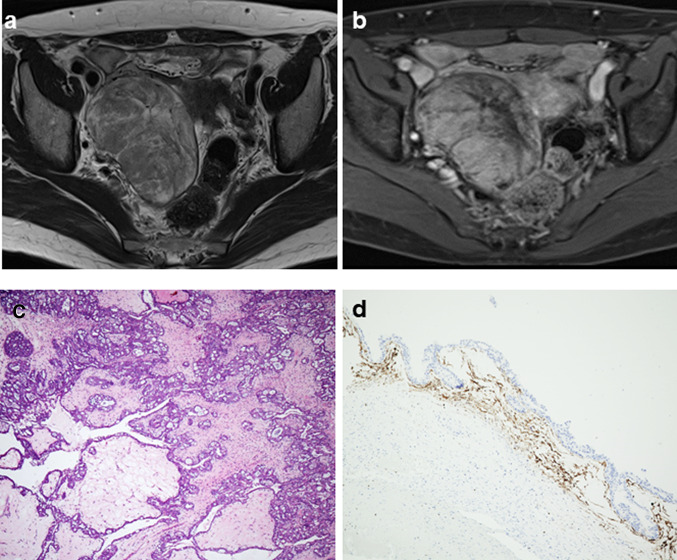
The third case was a 54-year-old woman with a chief complaint of lower abdominal pain. An 8 cm diameter tumor was almost a solid lesion with strong enhancement (Fig. 3a) and restricted diffusion. It showed heterogenous low signal intensity on T2WI (Fig. 3b). The operation was performed. Macroscopically, the right ovarian tumor has a whitish solid appearance with partially cystic lesions. Histologically, tumor cells show papillary or cribriform growth patterns with edematous stroma (Fig. 3c). Partially, necrosis and expansile invasion were detected, and this case was diagnosed as SMC with an SMBT background. In the immunohistochemistry analysis, CD10-positive cells were observed in the cystic wall, indicating the presence of endometriosis (Fig. 3d). In addition, ER was also positive, ARID1A was retained, the expression of p53 was wild type, and PTEN was lost (Fig. 4c–l).
Fig. 3.
MRI and microscopic findings of case 3. a On T2WI, the right ovarian tumor showed heterogeneous high signal intensity as a whole. b Gadolinium-enhanced T1WI with fat saturation showed a right solid ovarian tumor with relatively strong enhancement compared with the uterine body. c Tumor cells showed papillary or cribriform growth patterns with edematous stroma (× 100). d Immunohistochemically, CD10-positive cells were observed in the cystic wall, indicating endometriosis (× 200)
Discussion
We described three cases of ovarian SMC with the following three points to be emphasized. First, the MRI findings of the 3 cases differed greatly, and there was no robust common finding. Second, all our patients had a background of endometriosis. Third, SMBT was also present in these cases. These points have profound implications when considering the development and differentiation of SMC of the ovary.
At present, there are no known characteristic MRI findings available to diagnose SMC. Han et al. reported that the solid portion of SMCs showed intermediate signal intensity on T2WI, in contrast to the high signal intensity of SMBT, but it was not easy to differentiate them [2]. In reviewing our cases, in case 1, although the coexistence of endometriotic cysts and solid portions with restricted water diffusion suggested endometriosis-associated ovarian carcinoma, it was difficult to estimate the histological type. In case 2, as distinctive papillary structures, which consisted of a peripheral high signal intensity portion and central low signal intensity portion on T2WI [3], were prominently observed, SMBT was strongly suggested, but SMC was unexpected. In case 3, carcinoma, such as clear cell carcinoma or endometrioid carcinoma, was strongly suspected since the tumor was almost solid with strong contrast enhancement. Accordingly, the MRI findings of SMC were diverse from case to case, as if the findings reflected the coexistence of endometriosis, SMBT and carcinoma, so this might be a feature of the MRI findings of SMC. In other words, the fact that MRI findings in SMC did not reveal any characteristic findings that are useful for diagnosis might reflect the fact that SMC presents with an extreme pathological diversity of findings.
The background of endometriosis is a distinctive clinical feature of SMC based on our observation. In both case 1 and case 2, MRI findings strongly suggested endometriosis, and histopathologically, endometriosis was confirmed. In case 3, although there were no obvious findings suggesting endometriosis on MRI, immunohistochemistry of CD10 revealed the presence of endometriosis. There are currently no exact data on the proportion of SMCs associated with endometriosis. According to the largest case series including 19 cases of SMC, endometriosis was identified in 10 cases (53%) [4], and another study reported that endometriosis was found in 5 of 8 cases (63%) of SMC [2]. Presumably, a closer examination, such as with immunostaining of CD10, may lead to a higher complication rate for endometriosis.
The coexistence of SMBT is also one of the notable findings for the diagnosis of SMC, even though it is not always essential. Taylor et al. detected the SMBT component in 10 of 18 SMC cases (53%) [4]. Generally, the pathological diagnosis of SMBT is much easier than that of SMC, because SMBT has distinctive features, such as papillary structure with broad fibrous and edematous stalk. Therefore, concurrent SMBT aids in the diagnosis of SMC and makes it more convincing; it can at least indicate that the carcinoma is classified as seromucinous.
SMC is an extremely unique ovarian neoplasia due to its checkered destiny and tortuous history. Morphologically, SMC is defined as a carcinoma composed predominantly of serous and endocervical-type mucinous epithelium, and foci containing clear cells and areas of endometrioid and squamous differentiation are not uncommon in SMC [1, 5]. However, it must be said that the identity and diagnostic reproducibility of SMC are vulnerable, because there is no immunohistochemical target or genetic mutation that can clearly distinguish this disease [6]. Particularly, the distinction of SMC from endometrioid carcinoma with mucinous differentiation would be arbitrary in some cases. Indeed, in the forthcoming revision of the World Health Organization (WHO) classification of tumors of female reproductive organs, the SMC classification will likely disappear; it will be included in endometrioid carcinoma. Nevertheless, we insist on the presence of SMC. Our MRI and pathological observations and the evidence that one-third of seromucinous tumors have ARID1A mutations [7], which are seen in endometrioid carcinoma and clear cell carcinoma but not in other types of ovarian carcinoma [8], strongly indicate that SMC is an endometriosis-related neoplasia. Clear cell carcinoma can be easily distinguished from endometrioid carcinoma and SMC in that estrogen receptor is negative. Meanwhile, distinguishing between endometrioid carcinoma and SMC is difficult. However, that does not mean they should be considered the same; it is just, because the molecular genetic findings that can distinguish the two have not yet been obtained. Intriguingly, endometrioid borderline ovarian tumors are much rarer than SMBT, even though endometrioid carcinoma of the ovary is much more common than SMC. This evidence suggests that endometrioid borderline tumors are not always precancerous conditions of ovarian endometrioid cancer. In contrast, in addition to this epidemiological observation, the fact that endometriosis and SMBT coexist with SMC implies that SMC develops in multiple stages via endometriosis and SMBT. This might be a unique feature of SMC that cannot be seen in ovarian endometrioid carcinomas. Taken together, we speculate that ovarian SMC does exist and should be distinguished from endometrioid carcinoma with mucinous differentiation in terms of carcinogenic mechanisms.
Unfortunately, however, the majority of mechanisms of multistage carcinogenesis of SMC is unclear. As previously reported, immunostaining for p53 generally shows wild type pattern in endometriosis associated ovarian neoplasia [9], and our cases had similar results. Inactivating mutations of PTEN is also an important molecular carcinogenic mechanism, and it occurs in 15–20% of ovarian endometrioid carcinoma and 10% of clear cell carcinomas [10]. In the present report, it is unique in that two-thirds of the cases were found to lose this expression. Another important mutation is Kirsten rat sarcoma two viral oncogene homolog (KRAS), although we did not investigate it. Kim et al. demonstrated that KRAS mutation was detected 69% of SMBT, whereas less than 7% of ovarian endometrioid carcinoma have mutation of KRAS [11]. Finding molecular genetic evidence suggesting that SMC develops in multiple stages is an important challenge for the future.
In summary, we reported three cases of SMC. The MRI findings in SMC are varied, and characteristic imaging features useful for diagnosis were not found. Importantly, all cases had coexisting endometriosis and SMBT, and thus, it is suggested that SMC develops in multiple stages via endometriosis and SMBT. Concurrent endometriosis and SMBT could also make the diagnosis of SMC more convincing.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for the publication of this case report and its accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kurman RJ, International Agency for Research on Cancer., World Health Organization . WHO classification of tumours of female reproductive organs. 4. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 2.Han JW, Kim KA, Chang HY, et al. Newly categorized seromucinous tumor of the ovary: magnetic resonance imaging findings. J Comput Assist Tomogr. 2019;43:119–127. doi: 10.1097/RCT.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 3.Kurata Y, Kido A, Moribata Y, et al. Diagnostic performance of MR imaging findings and quantitative values in the differentiation of seromucinous borderline tumour from endometriosis-related malignant ovarian tumour. Eur Radiol. 2017;27:1695–1703. doi: 10.1007/s00330-016-4533-x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor J, McCluggage WG. Ovarian seromucinous carcinoma: report of a series of a newly categorized and uncommon neoplasm. Am J Surg Pathol. 2015;39:983–992. doi: 10.1097/PAS.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 5.Nagamine M, Mikami Y. Ovarian seromucinous tumors: pathogenesis, morphologic spectrum, and clinical issues. Diagnostics (Basel) 2020 doi: 10.3390/diagnostics10020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambau PF, McIntyre JB, Taylor J, et al. Morphologic reproducibility, genotyping, and immunohistochemical profiling do not support a category of seromucinous carcinoma of the ovary. Am J Surg Pathol. 2017;41:685–695. doi: 10.1097/PAS.0000000000000812. [DOI] [PubMed] [Google Scholar]
- 7.Wu CH, Mao TL, Vang R, et al. Endocervical-type mucinous borderline tumors are related to endometrioid tumors based on mutation and loss of expression of ARID1A. Int J Gynecol Pathol. 2012;31:297–303. doi: 10.1097/PGP.0b013e31823f8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matias-Guiu X, Stewart CJR. Endometriosis-associated ovarian neoplasia. Pathology. 2018;50:190–204. doi: 10.1016/j.pathol.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Catasus L, Bussaglia E, Rodrguez I, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–1368. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Kurman RJ, Shih Ie M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]