Abstract
Melatonin, secreted in a typical diurnal rhythm pattern, has been reported to prevent osteoporosis; however, its role in osteoclastogenesis remains unclear. In the present study, the ability of melatonin to inhibit receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis and the associated mechanism were investigated. Raw264.7 cells were cultured with RANKL (100 ng/ml) and macrophage colony-stimulating factor (M-CSF; 30 ng/ml) for 7 days, and tartrate-resistant acid phosphatase (TRAP) staining was used to detect osteoclastogenesis following treatment with melatonin. In addition, the effect of melatonin on cathepsin K and microRNA (miR)-882 expression was investigated via western blotting and reverse transcription-quantitative PCR. Melatonin significantly inhibited RANKL-induced osteoclastogenesis in Raw264.7 cells. From bioinformatics analysis, it was inferred that nuclear receptor subfamily 1 group D member 1 (NR1D1/Rev-erbα) may be a target of miR-882. In vitro, melatonin upregulated Rev-erbα expression and downregulated miR-882 expression in the osteoclastogenesis model. Rev-erbα overexpression boosted the anti-osteoclastogenesis effects of melatonin, whereas miR-882 partially diminished these effects. The present results indicated that the miR-882/Rev-erbα axis may serve a vital role in inhibiting osteoclastogenesis following RANKL and M-CSF treatment, indicating that Rev-erbα agonism or miR-882 inhibition may represent mechanisms through which melatonin prevents osteoporosis.
Keywords: melatonin, osteoclasts, microRNA-882, Rev-erbα, osteoclastogenesis
Introduction
Osteoporosis is an increasingly serious life-threatening medical issue (1-4). It often results in fractures, with associated complications such as hypostatic pneumonia, deep-vein thrombosis and bedsores, which can be fatal (5). Osteoporosis occurs due to a decrease in the number/activity of osteoblasts and/or increase in the number/activity of osteoclasts (6). The pathogenesis of osteoporosis involves complex signaling pathway regulation and protein modification, and a lot about this process remains unknown (7-9). In the past years, research has identified key signalling molecules that modulate bone formation and/or bone resorption, including Wnt (10,11), Akt (12,13), MAPK (14,15), AMP-activated protein kinase (AMPK) (16,17), receptor activator of nuclear factor-κB ligand (RANKL) (18,19), osteoprotegerin (OPG) (20,21) and tumor necrosis factor superfamily member 14 (22). Currently, there is no efficacious anti-osteoporosis treatment with minimal side effects; thus, there is an urgent unmet medical need for novel treatment strategies.
Bone homeostasis is maintained through osteogenesis and osteoclastogenesis (23). Osteoclasts originate via a process of differentiation from hematopoietic stem cells or monocytes (24). Osteoclast differentiation (or osteoclastogenesis) involves multiple steps, which include transformation into tartrate-resistant acid phosphatase (TRAP)-positive cells, merging into multinucleated cells, activating bone resorption and finally undergoing spontaneous apoptosis (25). RANK, its cellular ligand RANKL, macrophage colony-stimulating factor (M-CSF) and OPG (a decoy soluble receptor for RANKL) are four critical factors for osteoclastogenesis (26,27). RANKL is highly conserved and is a member of the TNF family (28). RANK (encoded by Tnfrsf11a) is a receptor activator of nuclear factor-κB, which is expressed via M-CSF stimulation on the surface of Raw264.7 cells (29). OPG competitively inhibits the binding of RANKL to RANK, and polymorphisms in the OPGgene are associated with osteoporosis (30,31). Furthermore, the interaction between M-CSF and CSF1 receptor is crucial for proliferation and differentiation in osteoporosis (32).
The existing drugs for osteoporosis are inefficient and produce unsatisfactory results (33); therefore, it is critical to develop safe and effective treatment options. Natural substances provide a new avenue for the treatment of osteoporosis. Melatonin is a methoxyindole that is synthesized in, and secreted predominantly from, the pineal gland (34). Although it is also synthesized in mitochondria, virtually every cell can produce melatonin, including cells of the bone marrow (35). This secretion is performed at night as part of the circadian rhythm; the schedule of the circadian rhythm is orchestrated by the suprachiasmatic nuclei and synchronized with the light/dark cycle (34). Furthermore, light can inhibit melatonin production (36). The biological rhythm of melatonin can be estimated by the melatonin content in the plasma/saliva or by measuring urinary 6-sulfatoxymelatonin, the primary hepatic metabolite (34,37,38). Some studies have determined that melatonin may reinforce the coupling of rhythms, such as the sleep/wake cycle and core temperature, although these findings are based on clinical trial information (34,39,40). Sánchez-Barceló et al (41) summarized the physiological effect of melatonin on the bone. Specifically, low concentrations (in the µM range) of melatonin were found to promote the proliferation and differentiation of osteoblasts and the expression levels of bone differentiation markers (such as type I collagen, osteopontin, bone sialoprotein and osteocalcin) (41). Additionally, melatonin can simultaneously inhibit osteoclast differentiation by promoting OPG secretion and eliminating the free radicals produced by osteoclasts (41). However, the underlying mechanism by which melatonin exerts its effects on individuals afflicted by osteoporosis remains to be identified. Thus, the current study aimed to investigate the effects of melatonin on RANKL-induced osteoclastogenesis in Raw264.7 cells.
Materials and methods
Cell culture
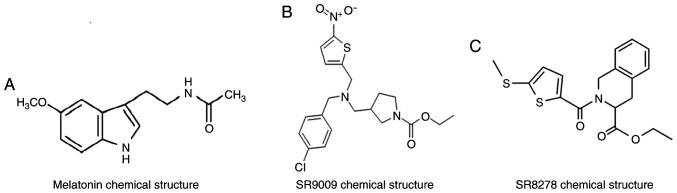
Raw264.7 cells (The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) were cultured in DMEM containing 10% FBS and 1% streptomycin and penicillin (all HyClone; Cytiva) in a 37°C incubator with 5% CO2 and maximum humidity. The culture medium was replenished daily. Cells were incubated in serum-free medium 24 h before treatment. Raw264.7 cells were cultured for 7 days with 100 ng/ml RANKL (R&D Systems, Inc.) and 30 ng/ml M-CSF (R&D Systems, Inc.) in the presence of varying concentrations (0.1 or 1 µmol) of melatonin (Sigma-Aldrich; Merck KGaA; Fig. 1A) for 48 h at 37°C. Raw264.7 cells were cultured in the presence of varying concentrations of SR9009 (5, 10 and 15 µmol; MedChemExpress; Fig. 1B) or SR8278 (5, 10 and 15 µmol; MedChemExpress; Fig. 1C) for 48 h at 37°C.
Figure 1.
Chemical structures of melatonin, SR9009, and SR8278. (A) Chemical structure of melatonin. (B) Chemical structure of SR9009. (C) Chemical structure of SR8278.
Synthetic RNA oligonucleotides and transfection
MicroRNA (miR)-882 mimics, miR-882 mimic negative control (NC), miR-882 inhibitors and miR-882 inhibitor NC were obtained from Shanghai GenePharma Co., Ltd. Raw264.7 cells were transfected with Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 6-8 h at 37°C, according to the manufacturer's instructions. The type of NCs used was a non-sense sequence. The concentration of miR-882 mimics/inhibitors used for transfection was 20 µM. RNA was extracted 24 h after transfection, and protein was extracted 48 h after transfection. The sequences of all miR-882 mimics and inhibitors were as follows: miR-882 mimics sense, 5′-AGG AGA GAG UUA GCG CAU UAG U-3′ and antisense, 5′-UAA UGC GCU AAC UCU CUC CUU U-3′; miR-882 mimics NC sense, 5′-UUC UCC GAA CGU GUC ACG UTT -3′ and antisense, 5′-ACG UGA CAC GUU CGG AGA ATT -3′; miR-882 inhibitors, 5′-ACU AAU GCG CUA ACU CUC UCC U-3′; miR-882 inhibitors NC, 5′-CAG UAC UUU UGU GUA GUA CAA -3.
RNA extraction and reverse transcription-quantitative PCR
The TRIzol® Reagent kit (Qiagen Sciences, Inc.) was used to extract total RNA from cells according to the manufacturer's protocol. miRNA and mRNA reverse-transcription PCR was performed using the Mir-X miRNA qRT-PCR TB Green® kit (cat. no. 638314; Clontech Laboratories, Inc.) for miRNA and the PrimeScript™ RT reagent kit with gDNA Eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.) for mRNA according to the manufacturer's protocol. The QuantiTect SYBR-Green PCR kit (cat. no. RR820A; Takara Biotechnology Co., Ltd.) was used to perform real-time quantitative PCR. The thermocycling conditions are as follows: Initial denaturation at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec, one cycle at 95°C for 5 sec, 60°C for 1 min and 95°C, and finally annealing at 50°C for 30 sec. The results were analyzed using a Roche Light Cycler® 480 II system (Roche Diagnostics). The relative expression of miR-882 was standardized to U6 RNA expression, while mRNA relative expression was standardized to GAPDH mRNA expression using the well-accepted 2−ΔΔCq method (42). For detailed information see section Performing a Basic Relative Quantification Experiment. The following primer sequences were used: miR-882 forward, 5′-CGC AGG AGA GAG TTA GCG CAT TAG T-3′ and reverse primer was taken by Universal sequence; U6 forward, 5′-CGC TTC GGC AGC ACA TAT AC-3′ and reverse, 5′-TTC ACG AAT TTG CGT GTC AT-3′; cathepsin K forward, 5′-GAA GAA GAC TCA CCA GAA GCA G-3′ and reverse, 5′-TCC AGG TTA TGG GCA GAG ATT -3′; NR1D1 forward, 5′-TAC ATT GGC TCT AGT GGC TCC -3′ and reverse, 5′-CAG TAG GTG ATG GTG GGA AGT A-3′; and GAPDH forward, 5′-AGG TCG GTG TGA ACG GAT TTG -3′ and reverse, 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′.
Western blot analysis
Raw264.7 cells were lysed in RIPA buffer containing phenylmethanesulfonyl fluoride (both Beyotime Institute of Biotechnology), followed by centrifugation at 4°C at 12,000 × g for 30 min. Quantitative analysis of protein concentration was performed using a BCA assay kit (Beyotime Institute of Biotechnology), and each sample was loaded at a concentration of 3 µg/µl in RIPA and loading buffer. Samples were separated via 10% SDS-PAGE at 80 V and the proteins were transferred to polyvinylidene difluoride membranes at 200 mA for 60 min. The membranes were blocked with 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.) for 2 h at room temperature and incubated with primary antibodies diluted in TBS-Tween (TBST; Beijing Solarbio Science & Technology Co., Ltd.; cat. no. T1081) at concentrations according to the manufacturer's instructions overnight at 4°C. The following primary antibodies were used: Anti-cathepsin K (Abcam; cat. no. ab19027; 1:1,000), anti-nuclear receptor subfamily 1 group D member 1 (NR1D1/Rev-erbα; Abcam; cat. no. ab174309; 1:5,000) and anti-GAPDH (ProteinTech Group, Inc.; cat. no. 10494-1-AP; 1:10,000). After primary antibody incubation, the membranes were washed thrice and incubated with an HRP-conjugated goat anti-rabbit IgG secondary antibody (ProteinTech Group, Inc.; cat. no. SA00001-2; 1:10,000) diluted in TBST for 2 h at room temperature. An Ultrasensitive Enhanced Chemiluminescence Detection kit (ProteinTech Group, Inc.; cat. no. PK10002) was used as the visualization reagent. ImageJ software (v1.52; National Institutes of Health) was used for densitometry.
TRAP staining
Raw264.7 cells were plated in 6-well plates (3,000 cells/cm2) and cultured in medium containing 100 ng/ml RANKL and 30 ng/ml M-CSF for 7 days at 37°C prior to TRAP staining, with or without 0.1 or 1 µM melatonin. Plates were washed with PBS and fixed with 4% paraformaldehyde for 15 min at 37°C. TRAP staining working solution was added to the plates and incubated for 60 min at 37°C in the dark. TRAP staining was performed using an acid phosphatase leukocyte kit (Sigma-Aldrich; Merck KGaA; cat. no. 387) in accordance with the manufacturer's protocol. Representative images were acquired using an Eclipse Ti fluorescence microscope (Nikon Corporation; magnification, ×200 and 400). TRAP-positive cells containing >3 nuclei were recorded as osteoclasts.
Cell Counting Kit-8 (CCK-8) assay for cell viability
Raw264.7 cells were plated in 96-well plates at a concentration of 5×103 cells/well. After 24 h, melatonin (0.1, 1 or 10 µmol), the Rev-erbα agonist SR9009 (Fig. 1B) or antagonist SR8278 (Fig. 1C) was added. After 48 h at 37°C, CCK-8 reagent (Dojindo Molecular Technologies, Inc.) was added for 30-60 min to detect cell activity according to the manufacturer's instructions. The results were analyzed with an automated enzyme-linked immunosorbent assay reader ELx808 (BioTek Instruments, Inc.; Agilent Technologies, Inc.) at 450 nm. Cell activity was expressed as OD value.
Target gene prediction
Potential miRNAs that could target Rev-erbα were first predicted by collecting information from databases including TargetScan (http://www.targetscan.org/), miRDB (http://mirdb.org/) and DIANA (http://diana.imis.athena-innovation.gr/), followed by organizing and consolidating these data.
Dual-luciferase reporter assay
Plasmid transfections for luciferase assays in 293T cells (purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences, cultured with DMEM with 10% FBS in a 37°C incubator with 5% CO2) were performed with 0.1 µg reporter constructs (pMIR-REPORT-wild-type-Rev-erbα or pMIR-REPORT-mutant-Rev-erbα plasmids; Shanghai GeneChem Co., Ltd.) and 0.4 µg miR-882 expression plasmid (Shanghai GeneChem Co., Ltd.), in a 24-well plate using Roche X-tremeGENE HP (Roche Diagnostics; cat. no. 06366236001) according to the manufacturer's instructions. Luciferase activity was measured 48 h post-transfection using the Dual Luciferase Reporter Assay System according to the manufacturer's instructions (Promega Corporation). Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
All data were analyzed using SPSS 22.0 software (IBM Corp.) and GraphPad Prism 6.0 (GraphPad Software, Inc.). Each group of experiments was repeated thrice independently, and the values are expressed as the mean ± SD. In the present study, one-way ANOVA followed by Dunnett's post-hoc test was used for determining whether ≥3 groups were statistically different from each other, while an unpaired t-test was used to determine whether 2 groups were statistically different from each other. P<0.05 was used to indicate a statistically significant difference.
Results
Melatonin inhibits RANKL-induced osteoclastogenesis in Raw264.7 cells
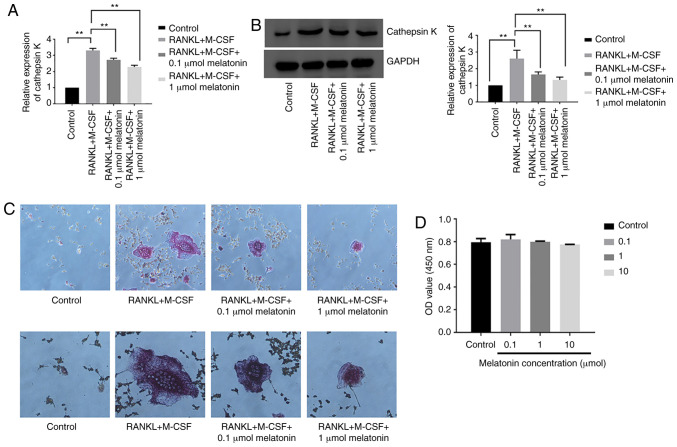
To study the effect of melatonin on osteoclastogenesis, the mRNA and protein expression levels of cathepsin K were examined in Raw264.7 cells cultured for 7 days with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of varying concentrations (0.1 or 1 µmol) of melatonin for 48 h. Cathepsin K was analyzed since its expression represents the level of osteoclastogenesis (43). Melatonin decreased both the mRNA and protein expression levels of cathepsin K following RANKL and M-CSF treatment (Fig. 2A and B). Cell differentiation was further studied using TRAP staining in Raw264.7 cells cultured for 7 days with RANKL and M-CSF in the presence of melatonin for 48 h. Melatonin treatment decreased the number of TRAP-positive cells following RANKL treatment (Fig. 2C). Subsequently, the viability of Raw264.7 cells was analyzed in the presence of varying concentrations (0.1, 1 or 10 µmol) of melatonin for 48 h using the CCK-8 assay. Cell viability was expressed as OD value. The results revealed that melatonin was not cytotoxic to Raw264.7 cells (Fig. 2D). The present findings indicated that melatonin significantly inhibited RANKL-induced osteoclastogenesis in Raw264.7 cells without any observed cytotoxicity.
Figure 2.
Melatonin inhibits RANKL-induced osteoclastogenesis in Raw264.7 cells. Cathepsin K (A) mRNA and (B) protein expression in Raw264.7 cells cultured for 7 days with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of varying concentrations (0.1 or 1 µmol) of melatonin. (C) Tartrate-resistant acid phosphatase activity in Raw264.7 cells cultured for 7 days with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of varying concentrations (0.1 or 1 µmol) of melatonin. Magnification, ×200 (upper panels) and ×400 (bottom panels). (D) Viability of Raw264.7 cells in the presence of varying concentrations (0.1, 1 or 10 µmol) of melatonin. Cell viability was expressed as OD value. Data are represented as the mean ± SD (n=3). **P<0.01. OD, optical density; RANKL, receptor activator of nuclear factor-κB ligand; M-CSF, macrophage colony-stimulating factor.
Melatonin enhances Rev-erbα expression in Raw264.7 cells
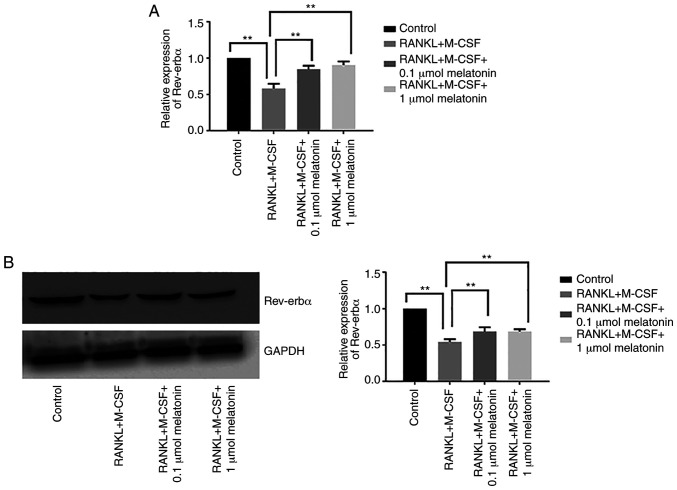
During RANKL-induced osteoclast differentiation, both mRNA and protein expression levels of Rev-erbα were suppressed compared with the control group, which indicated that the inhibitory effect of melatonin on osteoclastogenesis may be associated with Rev-erbα (Fig. 3A and B). By contrast, the addition of melatonin (0.1 or 1 µmol) significantly upregulated Rev-erbα mRNA and protein expression in Raw264.7 cells cultured with RANKL and M-CSF (Fig. 3A and B).
Figure 3.
Rev-erbα expression in Raw264.7 cells is augmented by melatonin. Rev-erbα (A) mRNA and (B) protein expression in Raw264.7 cells cultured for 7 days with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of varying concentrations (0.1 or 1 µmol) of melatonin for 48 h. Data are represented as the mean ± SD (n=3). **P<0.01. RANKL, receptor activator of nuclear factor-κB ligand; M-CSF, macrophage colony-stimulating factor; Rev-erbα/NR1D1, nuclear receptor subfamily 1 group D member 1.
Rev-erbα activation boosts the effect of melatonin on the inhibition of osteoclastogenesis, whereas Rev-Erbα inhibition promotes osteoclastogenesis
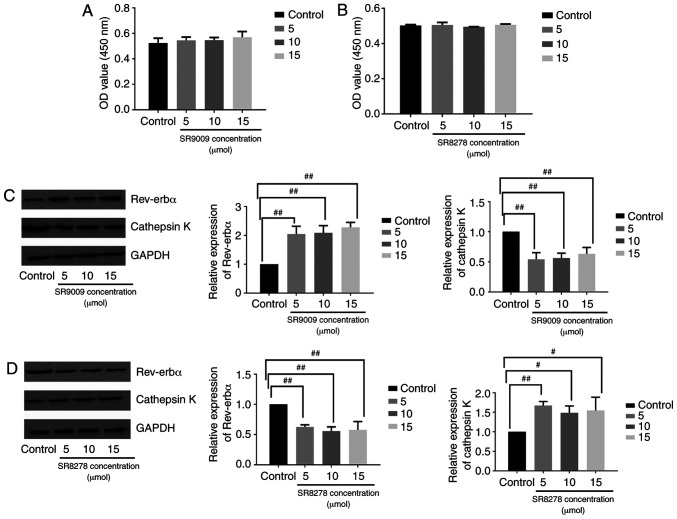
Raw264.7 cells were treated with the Rev-erbα agonist SR9009 or antagonist SR8278 to assess the function of Rev-erbα in osteoclastogenesis. The viability of Raw264.7 cells was analyzed in the presence of varying concentrations of SR9009 (5, 10 and 15 µmol) or SR8278 (5, 10 and 15 µmol) for 48 h via CCK-8 assay. SR9009 and SR8278 were not toxic to Raw264.7 cells at the concentrations tested (Fig. 4A and B). Western blotting revealed that in Raw264.7 cells cultured with RANKL, M-CSF and melatonin (1 µmol), osteoclastogenesis was inhibited by melatonin more significantly in cells treated with the Rev-erbα agonist SR9009 compared with the osteoclastogenesis process in cells cultured without SR9009 (Fig. 4C). The Rev-erbα antagonist SR8278 hampered the ability of melatonin to influence osteoclastogenesis in Raw264.7 cells cultured with RANKL, M-CSF and 1 µmol melatonin (Fig. 4D). Overall, these results indicated that the inhibitory effect of melatonin on osteoclastogenesis may be mediated by Rev-erbα.
Figure 4.
Rev-erbα activation increases the inhibitory effect of melatonin on Raw264.7 cell osteoclastogenesis, whereas the inhibition of Rev-erbα produces the opposite effect. Viability of Raw264.7 cells in the presence of varying concentrations of (A) SR9009 and (B) SR8278 (5, 10 and 15 µmol). Cell viability was expressed as OD value. Rev-erbα and cathepsin K expression in Raw264.7 cells cultured for 7 days with RANKL (100 ng/ml) and M-CSF (30 ng/ml), and for 48 h with 1 µmol melatonin in the presence of varying concentrations of (C) SR9009 and (D) SR8278 (5, 10 and 15 µmol). Data are represented as the mean ± SD (n=3). #P<0.05 and ##P<0.01. OD, optical density; RANKL, receptor activator of nuclear factor-κB ligand; M-CSF, macrophage colony-stimulating factor; Rev-erbα/NR1D1, nuclear receptor subfamily 1 group D member 1.
miR-882 regulates Rev-erbα protein expression
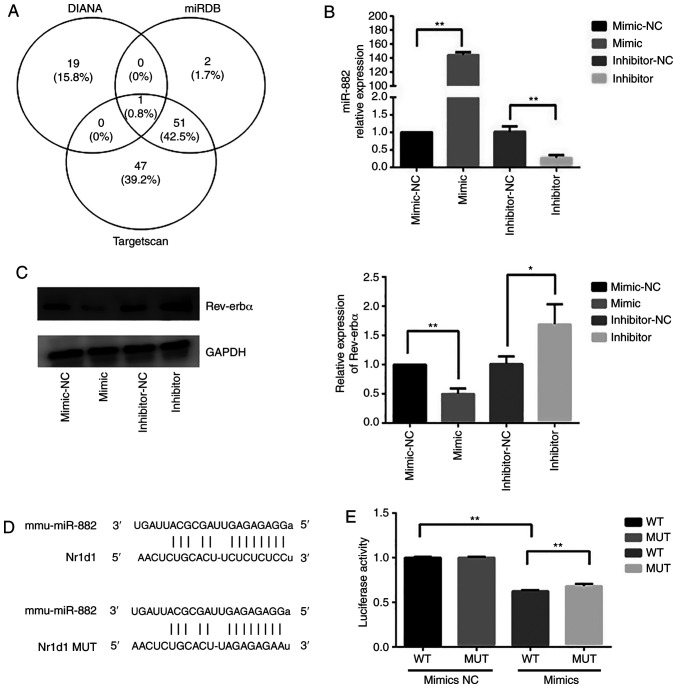
Subsequently, whether Rev-erbα expression was regulated by miRNAs was investigated. Potential miRNAs that could target Rev-erbα were first predicted by collecting information from databases including TargetScan, miRDB and DIANA, followed by organizing and consolidating these data. The overlapping miRNAs across different databases are shown in the Venn diagram in Fig. 5A. Since miR-882 was identified by the intersection of the three databases, it was hypothesized that miR-882 may regulate Rev-erbα mRNA expression. Raw264.7 cells were transfected with miR-882 mimics, inhibitors or corresponding NCs. miR-882 expression in Raw264.7 cells transfected with miR-882 mimics was significantly higher than that with the mimic-NC; additionally, Raw264.7 cells transfected with miR-882 inhibitors exhibited the opposite effect (Fig. 5B). Subsequently, whether Rev-erbα expression may be modulated by miR-882 was examined. Transfection with miR-882 mimics significantly decreased Rev-erbα protein expression in Raw264.7 cells, whereas the inhibition of miR-882 produced the opposite effect (Fig. 5C). Next, the wild-type 3′-untrans-lated region (UTR) of Rev-erbα mRNA was cloned with the presumed miR-882-binding sites, along with the mutant 3′-UTR located upstream of the luciferase-coding sequence (Fig. 5D). Luciferase activity was decreased in cells co-transfected with miR-882 mimics and Rev-erbα mRNA wild-type 3′-UTR fragments compared with in cells co-transfected with miR-882 mimics NC and Rev-erbα mRNA wild-type 3′-UTR fragments, and compared with in cells co-transfected with miR-882 mimics and Rev-erbα mRNA mutant 3′-UTR fragments (Fig. 5E). These results indicated that Rev-erbα may be a direct target of miR-882 and implied that miR-882 may exert its influence on osteoclastogenesis by targeting Rev-erbα.
Figure 5.
miR-882 targets Rev-erbα. (A) Venn diagram of the miRNAs overlapping with Rev-erbα from three different databases (TargetScan, miRDB and DIANA). miR-882 was the only miRNA identified in all three databases that potentially regulated Rev-erbα expression. (B) Transfection efficiency of miR-882 mimic and inhibitors. (C) Rev-erbα protein expression after transfection with miR-882 mimics, inhibitors and corresponding NCs. (D) Binding sites for miR-882 and Rev-erbα. (E) Luciferase activity in 293T cells co-transfected with miR-882 mimic and Rev-erbα WT or MUT 3′-untranslated region. Data are represented as the mean ± SD (n=3). *P<0.05 and **P<0.01. miRNA/miR, microRNA; NC, negative control; WT, wild-type; MUT, mutant; Rev-erbα/NR1D1, nuclear receptor subfamily 1 group D member 1.
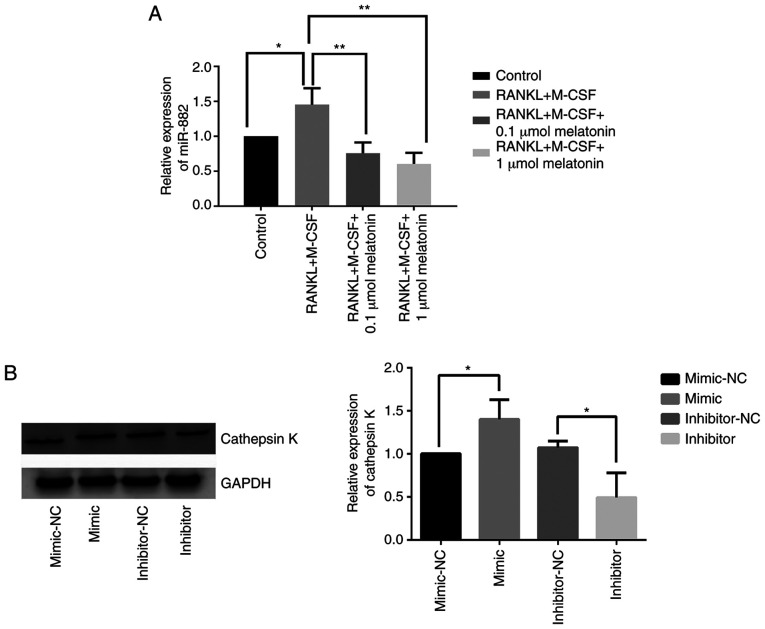
Melatonin downregulates miR-882 expression and the inhibition of miR-882 hinders RANKL-induced osteoclastogenesis
After demonstrating that Rev-erbα is a target gene of miR-882, whether miR-882 could regulate osteoclastogenesis was further explored. To determine the role of miR-882 in the inhibition of RANKL-induced osteoclastogenesis by melatonin, miR-882 expression was examined in Raw264.7 cells cultured with RANKL and M-CSF in the presence of varying concentrations of melatonin via reverse transcription-quantitative PCR. The results indicated that melatonin augmented Rev-erbα expression by decreasing miR-882 expression, resulting in decreased miR-882 expression compared with RANKL and M-CSF treatment (Fig. 6A). To further explore the function of miR-882 upon melatonin treatment, miR-882 mimics, inhibitors or corresponding NCs were transfected into Raw264.7 cells, and cathepsin K expression was examined. The overexpression of miR-882 upregulated cathepsin K expression compared with the mimic-NC; additionally, transfection with inhibitors decreased cathepsin K expression compared with the inhibitor-NC (Fig. 6B). The current results indicated that miR-882 inhibition may inhibit osteoclastogenesis to prevent osteoporosis, whereas the overexpression of miR-882 may promote osteoclastogenesis.
Figure 6.
Melatonin downregulates miR-882 expression in RANKL-induced Raw264.7 cells, whereas the overexpression of miR-882 promotes osteoclasto-genesis in Raw264.7 cells. (A) miR-882 expression in Raw264.7 cells cultured for 7 days with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of varying concentrations (0.1 or 1 µmol) of melatonin. (B) Cathepsin K expression after transfection with miR-882 mimics, inhibitors and corresponding NCs. Data are represented as the mean ± SD (n=3). *P<0.05 and **P<0.01. RANKL, receptor activator of nuclear factor-κB ligand; M-CSF, macrophage colony-stimulating factor; miR, microRNA; NC, negative control.
Discussion
Previous research has demonstrated that melatonin impacts osteoclastogenesis (44-47). Melatonin results in the concentration-dependent inhibition of osteoclastogenesis at pharmacological concentrations (44), which is consistent with the present findings. Notably, the inhibitory effect of melatonin may not be associated with the melatonin receptor, as demonstrated in a previous study (45). In an in vitro experiment with Transwell or layered mesenchymal stem cells and peripheral blood monocytes, melatonin inhibited osteoclastogenesis in the layered culture, but not the Transwell culture (46). Moreover, in vivo, melatonin can inhibit titanium particle-induced osteolysis (47). Thus, the present study examined the effect of melatonin on osteoclastogenesis, and the current data demonstrated that melatonin inhibited RANKL-induced osteoclastogenesis by promoting Rev-erbα expression via miR-882. The present results highlight the potential for melatonin in the treatment of osteoporosis. Osteoporosis is associated with clock genes, such as Rev-erbα (48), Bmal1 (49) and cryptochrome circadian clocks 2 (50). Osteoporosis intervention such as oral salmon calcitonin, administration of teriparatide and pulsed electromagnetic field therapy at different time points in one day can provide different levels of bone protection, demonstrating the role of the circadian rhythm in the mechanisms of osteoporosis (51). The parathyroid hormone-responsive circadian clock serves a crucial role in the process of mouse femur fracture healing (52).
A previous study has demonstrated that certain proteins encoded by clock genes, such as Rev-erbα, a member of the NR1D1, are expressed rhythmically in Raw264.7 cells (53). Rev-erbα, which is present in abundant levels in adipose cells, macrophages and muscle cells, was reported to govern the circadian rhythm, as well as lipid and glucose metabolism (54). Additionally, it serves a crucial role in inflammatory reactions and diseases, including diabetes and atherosclerosis, by suppressing the transcription and translation of down-stream genes (55,56). Rev-erbα is a critical component of the biological clock and one of the important participants in regulating biological rhythms (57). Furthermore, other studies have demonstrated that the abnormal expression levels of Rev-erbα are closely associated with diseases, such as osteoporosis (48), acute myocardial infarction (58), Alzheimer's disease (59) and skeletal muscle myopathies (60). SR9009, the biological effect of which is caused by an interaction with Rev-erbα, is a Rev-erbα agonist (61). SR9009 can enhance basal metabolism by raising oxygen consumption, enriching mitochondrial content and accelerating glucose and fatty acid metabolism in skeletal muscle (62). In addition, SR9009 decreases the synthesis of lipids, cholesterol and bile acid in the liver, and downregulates fat reserves in white adipose tissue based on in vitro and in vivo experiments (63). SR8278 is structurally similar to SR9009, but functionally different (64). SR8278 can promote microglia polarization toward a phagocytic M2-like phenotype during which purinergic receptor P2Y12R expression is upregulated (65). Previous studies corroborate the present finding that Rev-erbα impacts osteoclastogenesis (48,66). For example, the Rev-erbα agonist SR9009 inhibits osteoclastogenesis in postmenopausal mice by upregulating fatty acid binding protein 4 (66). This demonstrates that Rev-erbα is a crucial component in the inhibition of osteoclast differentiation. Furthermore, it may be closely associated with the occurrence and development of osteoporosis.
miRNAs are non-coding RNAs that contain 21-23 nucleotides and exert their influence by binding to the 3′-UTR of target mRNAs to inhibit their translation (67). The present study identified a new interaction between miRNAs and Rev-erbα during osteoclastogenesis using online databases. The current results indicated that Rev-erbα could be regulated by miRNAs binding to the 3′-UTR of Rev-erbα mRNA. miR-882 is localized to chromosome 12 on GRCm38.p6, and, to the best of our knowledge, an association between miR-882 and osteoporosis has not yet been reported. The present results indicated that miR-882 promoted osteoporosis by binding to the Rev-erbα 3′-UTR, inhibiting Rev-erbα translation, and thereby negatively regulating Rev-erbα expression. In the present study, miR-882 expression was decreased upon melatonin treatment. Additionally, the inhibition of miR-882 hampered osteoclastogenesis, whereas the miR-882 overexpression promoted osteoclastogenesis. Thus, the down-regulation of miR-882 may represent a potential strategy to treat osteoporosis by stalling osteoclastogenesis. However, further studies on the role of miR-882 in protein signalling pathways to regulate diverse biological behaviours are required.
Calcium and vitamin D can be used to treat osteoporosis, but they result in severe side effects (68). A prior meta-analysis reported that the small risk of significant adverse effects, such as kidney stones, myocardial infarction, hypercalcemia and hospitalization with acute gastrointestinal symptoms, together with the moderate risk of minor side effects, including constipation, probably outweighs any benefits of calcium supplements used for the treatment of fractures (68).
In conclusion, the present study demonstrated that the miR-882/Rev-erbα axis may serve a vital role in osteoporosis, suggesting that melatonin may first decrease miR-882 expression, followed by elevating Rev-erbα expression and then lowering cathepsin K expression, and finally inhibiting osteoclastogenesis (Fig. 7). Thus, miR-882 and Rev-erbα comprise a potential novel therapeutic dual-target mechanism through which melatonin may impact osteoporosis. The role of non-coding RNAs and circadian rhythms in the progression of osteoporosis was explored to provide a basis for the application of melatonin to sensitize osteoporotic cells, and potentially patients, to drug treatment in the future.
Figure 7.
Potential mechanism by which melatonin may affect RANKL-induced osteoclastogenesis. Melatonin may first decrease miR-882 expression, then augment Rev-erbα expression and decrease cathepsin K expression, ultimately inhibiting osteoclastogenesis. RANKL, receptor activator of nuclear factor-κB ligand; miR, microRNA; Rev-erbα/NR1D1, nuclear receptor subfamily 1 group D member 1.
Acknowledgments
Not applicable.
Abbreviations
- AMPK
AMP-activated protein kinase
- RANKL
receptor activator of nuclear factor-κB ligand
- OPG
osteoprotegerin
- TRAP
tartrate-resistant acid phosphatase
- M-CSF
macrophage colony-stimulating factor
- UTR
untranslated region
- Rev-erbα/NR1D1
nuclear receptor subfamily 1 group D member 1
Funding
The present study was supported by the Natural Science Foundation of Liao Ning (grant no. 2019-BS-294) and the Construction of Clinical Medical Research Center of Orthopaedics and Sports Rehabilitation Diseases in Liaoning Province (grant no. 2019416030).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the authors on reasonable request.
Authors' contributions
YT and YZ contributed to the conception and design. YT, ZG and RZ performed cell cultures. YT, ZG, RZ and YZ contributed to acquisition and analysis of data, revising the manuscript critically for important intellectual content, and approved the manuscript for publication. YT and ZG performed the statistical analysis and manuscript preparation. YZ obtained funding for the study and approved the final version of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
References
- 1.Gosch M, Kammerlander C, Nicholas JA. Treatment of osteoporosis in older adults. Panminerva Med. 2014;56:133–143. [PubMed] [Google Scholar]
- 2.Kurra S, Fink DA, Siris ES. Osteoporosis-associated fracture and diabetes. Endocrinol Metab Clin North Am. 2014;43:233–243. doi: 10.1016/j.ecl.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Maeda SS, Lazaretti-Castro M. An overview on the treatment of postmenopausal osteoporosis. Arq Bras Endocrinol Metabol. 2014;58:162–171. doi: 10.1590/0004-2730000003039. [DOI] [PubMed] [Google Scholar]
- 4.Yun H, Delzell E, Saag KG, Kilgore ML, Morrisey MA, Muntner P, Matthews R, Guo L, Wright N, Smith W, et al. Fractures and mortality in relation to different osteoporosis treatments. Clin Exp Rheumatol. 2015;33:302–309. [PMC free article] [PubMed] [Google Scholar]
- 5.Hegyi L. The risk of immobility in geriatrics. Eurorehab. 2001;3:151–154. [Google Scholar]
- 6.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malluche HH, Koszewski N, Monier-Faugere MC, Williams JP, Mawad H. Influence of the parathyroid glands on bone metabolism. Eur J Clin Invest. 2006;36(Suppl 2):S23–S33. doi: 10.1111/j.1365-2362.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Wang Y, Liu Y, Ma J, Li Y. Altered gene expression involved in insulin signaling pathway in type II diabetic osteoporosis rat model. Endocrine. 2013;43:136–146. doi: 10.1007/s12020-012-9757-1. [DOI] [PubMed] [Google Scholar]
- 9.Kameda Y, Takahata M, Mikuni S, Shimizu T, Hamano H, Angata T, Hatakeyama S, Kinjo M, Iwasaki N. Siglec-15 is a potential therapeutic target for postmenopausal osteoporosis. Bone. 2015;71:217–226. doi: 10.1016/j.bone.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Canalis E. Wnt signalling in osteoporosis: Mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 11.Manolagas SC. Wnt signaling and osteoporosis. Maturitas. 2014;78:233–237. doi: 10.1016/j.maturitas.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Mao Z, He S, Zhan Y, Ning R, Liu W, Yan B, Yang J. Icariin protects against glucocorticoid induced osteoporosis, increases the expression of the bone enhancer DEC1 and modulates the PI3K/Akt/GSK3β/β-catenin integrated signaling pathway. Biochem Pharmacol. 2017;136:109–121. doi: 10.1016/j.bcp.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Wang X, Chang H, Gao X, Dong C, Li Z, Hao J, Wang J, Fan Q. Mongolian Medicine echinops prevented postmenopausal osteoporosis and induced ER/AKT/ERK pathway in BMSCs. Biosci Trends. 2018;12:275–281. doi: 10.5582/bst.2018.01046. [DOI] [PubMed] [Google Scholar]
- 14.Cong Q, Jia H, Li P, Qiu S, Yeh J, Wang Y, Zhang ZL, Ao J, Li B, Liu H. p38alpha MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Sci Rep. 2017;7:45964. doi: 10.1038/srep45964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan BL, Tong ZW, Li SD, Wu L, Liao JL, Yang YX, Li HH, Dai YJ, Li JE, Pan L. Decreased microRNA-182-5p helps alendronate promote osteoblast proliferation and differentiation in osteoporosis via the Rap1/MAPK pathway. Biosci Rep. 2018;38:BSR20180696. doi: 10.1042/BSR20180696. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Dong W, Qi M, Wang Y, Feng X, Liu H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem Biophys Res Commun. 2018;505:1195–1202. doi: 10.1016/j.bbrc.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 17.de Pablos RM, Espinosa-Oliva AM, Hornedo-Ortega R, Cano M, Arguelles S. Hydroxytyrosol protects from aging process via AMPK and autophagy, a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol Res. 2019;143:58–72. doi: 10.1016/j.phrs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Buchwald ZS, Yang C, Nellore S, Shashkova EV, Davis JL, Cline A, Ko J, Novack DV, DiPaolo R, Aurora R. A bone anabolic effect of RANKL in a murine model of osteoporosis mediated through FoxP3+ CD8 T cells. J Bone Miner Res. 2015;30:1508–1522. doi: 10.1002/jbmr.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi J, Hu KS, Yang HL. Roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis. Int J Clin Exp Pathol. 2015;8:11995–2004. [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L, Tang K, Quan Z, Zhao Z, Jiang D. Association between seven common OPG genetic polymorphisms and osteoporosis risk: A meta-analysis. DNA Cell Biol. 2014;33:29–39. doi: 10.1089/dna.2013.2206. [DOI] [PubMed] [Google Scholar]
- 21.Lin H, Zhang G, Chen X, Wu X, Wu C, Ca H, Hu Z. The relationship between the g.27450A>T genetic variant of OPG gene and osteoporosis in Chinese postmenopausal women. Int Immunopharmacol. 2014;21:464–467. doi: 10.1016/j.intimp.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Brunetti G, Storlino G, Oranger A, Colaianni G, Faienza MF, Ingravallo G, Di Comite M, Reseland JE, Celi M, Tarantino U, et al. LIGHT/TNFSF14 regulates estrogen deficiency-induced bone loss. J Pathol. 2020;250:440–451. doi: 10.1002/path.5385. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Liang T, Zhu Y, Qiu J, Qiu X, Lian C, Gao B, Peng Y, Liang A, Zhou H, et al. Melatonin prevents bone destruction in mice with retinoic acid-induced osteoporosis. Mol Med. 2019;25:43. doi: 10.1186/s10020-019-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 25.An J, Hao D, Zhang Q, Chen B, Zhang R, Wang Y, Yang H. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int Immunopharmacol. 2016;36:118–131. doi: 10.1016/j.intimp.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Ledesma-Colunga MG, Adán N, Ortiz G, Solís-Gutiérrez M, López-Barrera F, Martínez de la Escalera G, Clapp C. Prolactin blocks the expression of receptor activator of nuclear factor κB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Res Ther. 2017;19:93. doi: 10.1186/s13075-017-1290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai L, Zhou K, Wang S, Zhang H, Fan N, Li J, Tan X, Hu L, Fan X. Psoralen and bakuchiol ameliorate M-CSF plus RANKL-induced osteoclast differentiation and bone resorption via inhibition of AKT and AP-1 pathways in vitro. Cell Physiol Biochem. 2018;48:2123–2133. doi: 10.1159/000492554. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review) Mol Med Rep. 2015;11:3212–3218. doi: 10.3892/mmr.2015.3152. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40:706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naidu VG, Dinesh Babu KR, Thwin MM, Satish RL, Kumar V, Gopalakrishnakone P. RANKL targeted peptides inhibit osteoclastogenesis and attenuate adjuvant induced arthritis by inhibiting NF-κB activation and down regulating inflammatory cytokines. Chem Biol Interact. 2013;203:467–479. doi: 10.1016/j.cbi.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Qin S, Zhang Q, Zhang L. Effect of OPG gene mutation on protein expression and biological activity in osteoporosis. Exp Ther Med. 2017;14:1475–1480. doi: 10.3892/etm.2017.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse CSF-1 receptor gene results in osteopetrosis, mono-nuclear phagocyte deficiency, increased primitive progenitor cell frequencies and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.V99.1.111. [DOI] [PubMed] [Google Scholar]
- 33.Cheng C, Wentworth K, Shoback DM. New frontiers in osteoporosis therapy. Annu Rev Med. 2020;71:277–288. doi: 10.1146/annurev-med-052218-020620. [DOI] [PubMed] [Google Scholar]
- 34.Claustrat B, Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Tan DX, Reiter RJ. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melat Res. 2019;2:44–66. doi: 10.32794/mr11250011. [DOI] [Google Scholar]
- 36.Claustrat B, Geoffriau M, Brun J, Chazot G. Melatonin in humans: A biochemical marker of the circadian clock and an endogenous synchronizer. Neurophysiol Clin. 1995;25:351–359. doi: 10.1016/0987-7053(96)84908-2. In French. [DOI] [PubMed] [Google Scholar]
- 37.Vural EM, van Munster BC, de Rooij SE. Optimal dosages for melatonin supplementation therapy in older adults: A systematic review of current literature. Drugs Aging. 2014;31:441–451. doi: 10.1007/s40266-014-0178-0. [DOI] [PubMed] [Google Scholar]
- 38.van Faassen M, Bischoff R, Kema IP. Relationship between plasma and salivary melatonin and cortisol investigated by LC-MS/MS. Clin Chem Lab Med. 2017;55:1340–1348. doi: 10.1515/cclm-2016-0817. [DOI] [PubMed] [Google Scholar]
- 39.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Amaral FGD, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62:472–479. doi: 10.20945/2359-3997000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez-Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. Scientific basis for the potential use of melatonin in bone diseases: Osteoporosis and adolescent idiopathic scoliosis. J Osteoporos. 2010;2010:830231. doi: 10.4061/2010/830231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, Rauner M, Yadav VK, Crozet L, Bohm M, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568:541–545. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Chen X, Yan J, Li M, Liu T, Zhu C, Pan G, Guo Q, Yang H, Pei M, He F. Melatonin at pharmacological concentrations suppresses osteoclastogenesis via the attenuation of intracellular ROS. Osteoporos Int. 2017;28:3325–3337. doi: 10.1007/s00198-017-4127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Kim HJ, Bae MK, Kim YD. Suppression of osteoclastogenesis by melatonin: A melatonin receptor-independent action. Int J Mol Sci. 2017;18:1142. doi: 10.3390/ijms18061142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sifat M, Samsonraj RM, Munmun F, Glas J, Silvestros M, Kotlarczyk MP, Rylands R, Dudakovic A, van Wijnen AJ, Enderby LT, et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res. 2018;64 doi: 10.1111/jpi.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ping Z, Wang Z, Shi J, Wang L, Guo X, Zhou W, Hu X, Wu X, Liu Y, Zhang W, et al. Inhibitory effects of melatonin on titanium particle-induced inflammatory bone resorption and osteoclastogenesis via suppression of NF-κB signaling. Acta Biomater. 2017;62:362–371. doi: 10.1016/j.actbio.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 48.Song C, Tan P, Zhang Z, Wu W, Dong Y, Zhao L, Liu H, Guan H, Li F. REV-ERB agonism suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss partially via FABP4 upregulation. FASEB J. 2018;32:3215–3228. doi: 10.1096/fj.201600825RRR. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Yu R, Long Y, Zhao J, Yu S, Tang Q, Chen L. BMAL1 deficiency promotes skeletal mandibular hypoplasia via OPG downregulation. Cell Prolif. 2018;51:e12470. doi: 10.1111/cpr.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Z, Xu T, Li Y, Fei W, Yang G, Hong Y. Inhibition of CRY2 by STAT3/miRNA-7-5p promotes osteoblast differentiation through upregulation of CLOCK/BMAL1/P300 expression. Mol Ther Nucleic Acids. 2020;19:865–876. doi: 10.1016/j.omtn.2019.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song C, Wang J, Kim B, Lu C, Zhang Z, Liu H, Kang H, Sun Y, Guan H, Fang Z, Li F. Insights into the role of circadian rhythms in bone metabolism: A promising intervention target? BioMed Res Int. 2018;2018:9156478. doi: 10.1155/2018/9156478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunimoto T, Okubo N, Minami Y, Fujiwara H, Hosokawa T, Asada M, Oda R, Kubo T, Yagita K. A PTH-responsive circadian clock operates in ex vivo mouse femur fracture healing site. Sci Rep. 2016;6:22409. doi: 10.1038/srep22409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira E, Merino B, Quesada I. Role of the clock gene Rev-erbalpha in metabolism and in the endocrine pancreas. Diabetes Obes Metab. 2015;17(Suppl 1):S106–S114. doi: 10.1111/dom.12522. [DOI] [PubMed] [Google Scholar]
- 55.Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 57.Mazzoccoli G, Cai Y, Liu S, Francavilla M, Giuliani F, Piepoli A, Pazienza V, Vinciguerra M, Tamamoto T, Takumi T. REV-ERBalpha and the clock gene machinery in mouse peripheral tissues: A possible role as a synchronizing hinge. J Biol Regul Homeost Agents. 2012;26:265–276. [PubMed] [Google Scholar]
- 58.Wang S, Gu X, Zhang Q, Zhang X, Li Y, Yao Y, Yu B, Zhang Y. Angiotensin II suppresses Rev-erbα expression in THP-1 macrophages via the Ang II type 1 receptor/liver X receptor α pathway. Cell Physiol Biochem. 2018;46:303–313. doi: 10.1159/000488431. [DOI] [PubMed] [Google Scholar]
- 59.Roby DA, Ruiz F, Kermath BA, Voorhees JR, Niehoff M, Zhang J, Morley JE, Musiek ES, Farr SA, Burris TP. Pharmacological activation of the nuclear receptor REV-ERB reverses cognitive deficits and reduces amyloid-β burden in a mouse model of Alzheimer's disease. PloS One. 2019;14:e0215004. doi: 10.1371/journal.pone.0215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welch RD, Flaveny CA. REV-ERB and ROR: Therapeutic targets for treating myopathies. Phys Biol. 2017;14:045002. doi: 10.1088/1478-3975/14/4/045002. [DOI] [PubMed] [Google Scholar]
- 61.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, et al. Regulation of circadian behavior and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thevis M, Schänzer W. Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis - Potential implications for sports drug testing programs. Rapid Commun Mass Spectrom. 2016;30:635–651. doi: 10.1002/rcm.7470. [DOI] [PubMed] [Google Scholar]
- 63.Mazzarino M, Rizzato N, Stacchini C, de la Torre X, Botrè F. A further insight into the metabolic profile of the nuclear receptor Rev-erb agonist, SR9009. Drug Test Anal. 2018;10:1670–1681. doi: 10.1002/dta.2538. [DOI] [PubMed] [Google Scholar]
- 64.Kojetin D, Wang Y, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol. 2011;6:131–134. doi: 10.1021/cb1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J, Kim DE, Griffin P, Sheehan PW, Kim DH, Musiek ES, Yoon SY. Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer's disease. Aging Cell. 2020;19:e13078. doi: 10.1111/acel.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim K, Kim JH, Kim I, Seong S, Kim N. Rev-erbα Negatively Regulates Osteoclast and Osteoblast Differentiation through p38 MAPK Signaling Pathway. Mol Cells. 2020;43:34–47. doi: 10.14348/molcells.2019.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolland MJ, Grey A, Reid IR. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis. Climacteric. 2015;18(Suppl 2):S22–S31. doi: 10.3109/13697137.2015.1098266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the authors on reasonable request.