Abstract
Background
Acral lentiginous and mucosal melanoma that represent lesions without cumulative sun-induced damages account for 65% of melanomas among Asians but constitute only 5% in Caucasians. The distinct clinical manifestations might influence the clinical course, response to treatment, and outcomes. Factors associated with the prognosis of high-risk resected melanoma in Asians are still rarely reported.
Methods
Clinical, histological determinants of non-distant metastatic melanoma patients who underwent complete resection in 2014–9 were analyzed.
Results
Mucosal melanoma, nodular melanoma, and acral lentiginous melanoma accounted for 45.1%, 40.2%, and 14.2% of total melanoma cases (N = 82), respectively. Among cutaneous melanomas, all patients were diagnosed with Breslow's depth more than 4 mm (T4), 51% with ulceration, 95.6% with diameter more than 6 mm, 59% with lympho-vascular invasion, and 74% with regional lymph node infiltration. In mucosal melanomas, 78.3% were diagnosed in advanced stages, 14.5% with regional spread to lymph nodes and 77% with regional infiltration beyond mucosa. Lesions with ulceration were associated with higher risk of distant metastasis (OR 3.003, 95%CI:1.01–9.09). Infiltration into regional lymph node was associated with shorter overall survival (median survivals were 17 vs 23.4 months, Mantel-Cox test P = 0.049). Patients diagnosed at Breslow T4 were also associated with poorer overall survival than T1-3 (median survivals were 23 vs 32 months, Mantel-Cox test P = 0.047).
Conclusion
The majority of melanoma patients in our population were diagnosed in advanced stages with a higher risk for recurrence and progression into distant metastasis. Regional lymph node involvement and thicker tumor (T4) were associated with poor prognosis.
Keywords: Melanoma, High-risk, Asians, Prognosis, T4 tumor
Highlights
-
•
Most of melanoma patients in Indonesia are diagnosed with high-risk of progression and worse survival.
-
•
Revealing factors-associated with high-risk of disease progression is important to set up surveillance program.
-
•
Refinement of public education and care delivery are required to advance management of melanoma in Indonesia.
Abbreviations
- AJCC
American Joint Committee on Cancer
- ALM
acral lentiginous melanoma
- CM
cutaneous melanoma
- EC
Ethical Clearance
- NM
nodular melanoma
- MM
mucosal melanoma
- N
Node status
- OR
Odds Ratio
- SD
Standard deviation
- SLNB
sentinel lymph node biopsy
- T
Tumor size
1. Introduction
Melanoma is the most potentially lethal form of skin cancer that develops through uncontrolled melanocyte proliferation [1]. Although melanoma is traditionally considered as a rare cancer with around 270,000 new cases per year worldwide, the incidence has risen much higher than any other cancer [1,2]. Risk factors, incidence, and clinical manifestations of melanoma vary greatly by regions, ethnicity, age, and sex [3]. In Caucasians, 90% of melanoma cases originate from the sun-exposed skin and predominantly present as superficially spreading melanomas. In Asians, cutaneous melanomas (CM) are primarily manifested as acral lentiginous melanoma (ALM) [4] and nodular melanoma (NM) [4,5]. In addition, mucosal melanomas (MM) constitute almost a quarter of all melanomas among Asians [4,6]. Both MM and acral melanomas contribute only 5% of cases in Caucasians [7].
Current evidence has shown that ALM, NM, and MM have specific patterns of underlying genetic mutations, clinical course, and relatively worse prognosis [[8], [9], [10]]. Localized melanomas are considered curable using current treatment approaches of wide local excision with sufficient safety margins and adjuvant treatments. However, disease recurrence and progression are relatively high reaching 13.4% in the first two years and almost 45% in CM as well as 80% in MM during longer follow-up [6,11]. Prognosis of melanoma has been associated with intrinsic tumor characteristics including Breslow thickness, nodal involvement, primary and adjuvant treatments [3]. No universally accepted guidelines of follow-up after complete resection for patients with melanoma are currently available [3].
Most of the literature and clinical recommendations for diagnosis and treatment of melanoma derive from studies among Caucasians [3,8,12]. There is a lack of information regarding patterns of recurrence and risks of disease progression, as well as prognosis of patients with melanoma after complete resection among Asians. Accordingly, this study evaluated patterns of progression into distant metastasis and prognosis with their association with clinicopathological characteristics which might add important information for clinicians to advise patients, design surveillance plan and advanced treatment options particularly for high-risk patients with melanoma.
2. Materials and method
2.1. Study population
All patients with melanoma diagnosed and treated at the Division of Surgical Oncology, Dr. Sardjito Hospital in 2013–2018 with radical resection were retrospectively recruited. Melanoma patients confirmed histologically, with no distant metastasis at diagnosis, underwent wide excision, and had follow-up for minimum of 3 months were included in this study. Exclusion criteria were incomplete surgical resection, received neoadjuvant treatment, and loss to follow-up. Staging or retrospective re-staging was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) system [13]. Sentinel node lymph node biopsy (SLNB) was not routinely performed in our hospital, regional lymph node dissection as an alternative was performed according to results of physical examination and ultrasonography to the regional lymph nodes. Routine lymph node dissection was performed for patients with clinical stage of III. High-risk melanomas are determined as those with a risk of recurrence and disease progression including Breslow depth >4 mm, and positive regional lymph nodes, with ulceration [13].
All patients were treated according to national recommendations and local hospital guidelines. After resection and adjuvant treatment, follow-up was performed every month for the first 3 month, every 3 months for a year, and afterward every 6 months. Thorough physical examination, image documentation, biannual chest X-ray, and ultrasonography or further imaging as indicated were performed during the follow-up visits. The protocol of this study was reviewed and approved by Medical and Health Research Ethics Committee (KE/0939/09/2020). The study has been registered in the reserachregistry6129 (ttps://www.researchregistry.com) and has been reported following to the STROCSS guidelines [14].
2.2. Data processing
Clinical, pathological, and follow-up data including age, sex, Breslow thickness, stage, histological subtype, regional lymph node involvement were extracted from the medical records. Surveillance after acute treatment was performed according to national and local hospital guidelines using physical examination, ultrasonography, X-ray, and computerized tomography (CT) scan as indicated. Any evidence of distant metastasis in the lung, liver, brain, and bone was recorded. Any event of mortality was documented, and the overall survival was calculated.
2.3. Statistical analysis
The associated clinical and pathological risk factors for progression into distant metastases were categorically compared using univariable and multivariable logistic regression analyses. Overall survival between different variables was analyzed using Kaplan-Meier curve and Log-rank (Mantel-Cox) tests. All statistical analyses were performed using the SPSS 17.0 software (SPSS Inc., Chicago). Statistically significant result was determined if P-value was less than 0.05.
3. Results
3.1. Patient clinical characteristics
The median age for patients with melanoma in this study (N = 82) was 62 years (range, 15–95 years) consisting of 63.4% (N = 52) females and 36.6% (N = 30) males. Among patients with CM (N = 45), 73.3% (N = 33) were NM and 26.7% (N = 12) were ALM. Patients with MM made up 45.1% (N = 37) of all patients. For CM, the most common locations were acral and extremities (93%, N = 42). All patients with CM (N = 45) were initially diagnosed with Breslow tumor thickness more than 4 mm (T4), 75.6% (N = 34) were positive for regional lymph nodes, 95.6% (N = 43) were with diameter more than 6 mm, 75.6% (N = 34) were at Stage III, and 46.7% (N = 21) were presented with ulceration. In MM, head and neck mucosa were the most prevalent sites (78.3%, N = 29), followed by gynecological mucosa (13.5%) and gastrointestinal mucosa (8.1%). For patients with MM (N = 37), 48.6% (N = 18) were with T4 lesions, 83.8% (N = 31) with diameter more than 6 mm, and 78.3% (N = 29) were diagnosed in the advanced stages. The demographic and clinical characteristics of patients with melanoma are summarized in Table 1.
Table 1.
Clinical characteristics of melanoma patients and the comparison between cutaneous and mucosal melanomas.
| Variables | Variables | Melanoma (N = 82) | Cutaneous Melanoma (N = 45) | Mucosal melanoma (N = 37) |
|---|---|---|---|---|
| Median age | 62 | 64 | 59 | |
| Age category | <65 | 49 (60%) | 23 (51%) | 23 (62.2) |
| 65–75 | 17 (20.5%) | 13 (29%) | 7 (18.9%) | |
| >75 | 16 (19.5%) | 9 (20%) | 7 (18.9) | |
| Sex | Male | 30 (36.6%) | 16 (35.6%) | 14 (37.8%) |
| Female | 52 (63.4%) | 29 (64.4%) | 23 (62.2%) | |
| Histological subtype type | SSM | 0 (0%) | 0 (0%) | 0 (0%) |
| ALM | 12 (14.6%) | 12 (26.7%) | 0 (0%) | |
| NM | 33 (40.2%) | 33 (73.3%) | 0 (0%) | |
| LMM | 0 (0%) | 0 (0%) | 0 (0%) | |
| Mucosal | 37 (45.2%) | 0 (0%) | 37 (100%) | |
| Breslow tumor thickness | T1 | 1 (%) | 0 (%) | 1 (2.7%) |
| T2 | 7 (%) | 0 (%) | 7 (18.9%) | |
| T3 | 11 (%) | 0 (%) | 11 (29.7%) | |
| T4 | 63 (%) | 45 (100%) | 18 (48.6%) | |
| Ulceration | Yes | 24 (29.3%) | 24 (53.3%) | 0 (0%) |
| No | 58 (70.7%) | 21 (46.7%) | 37 (100%) | |
| Diameter | ≤6 mm | 8 (9.8%) | 2 (4.4%) | 6 (16.2%) |
| >6 mm | 74 (90.2%) | 43 (95.6%) | 31 (83.8%) | |
| Node | N0 | 42 (51.2%) | 11 (24.4%) | 31 (83.4%) |
| N1 | 7 (8.5%) | 4 (8.9%) | 3 (8.1%) | |
| N2 | 10 (12.2%) | 9 (20%) | 1 (2.7%) | |
| N3 | 23 (28.1%) | 21 (46.7%) | 2 (5.4%) | |
| AJCC stage | I | 4 (4.9%) | 0 (0%) | 4 (10.8%) |
| II | 15 (18.3%) | 11 (24.4%) | 4 (10.8%) | |
| III | 52 (63.4%) | 34 (75.6%) | 18 (48.6%) | |
| IV | 11 (13.4%) | 0 (0%) | 11 (29.7%) | |
| Location | Acral | 13 (15.8%) | 13 (28.9%) | 0 (0%) |
| Head/neck | 2 (2.4%) | 2 (4.4%) | 0 (0%) | |
| Trunk | 1 (1.2%) | 1 (2.2%) | 0 (0%) | |
| Extremities | 29 (35.3%) | 29 (64.4%) | 0 (0%) | |
| Mucosal | 37 (45.2%) | 0 (0%) | 37 (100%) | |
SSM: superficial spreading melanoma.
ALM: acral lentiginous melanoma.
NM: nodular melanoma.
LMM: lentigo malign melanoma.
3.2. Associated risks for the progression into distant metastasis
After median follow-up of 25 months, 33 patients (40.2%) had disease progression into distant organ metastasis. Proportions of the patients that developed distant metastasis were 44% (N = 20) with CM and 35.1% (N = 13) with MM. Single organ metastasis was found in 17 patients (51.5%) and multiple organ metastases were found in 16 patients (48.5%). The mean time of progression into distant metastasis was 13.2 months after surgical resection. The lung was the most common internal organ for distant metastasis from melanoma (45%, N = 15). Ulceration was the most significant risk for distant metastasis among all patients with melanoma (OR 6.711, 95% CI: 2.012–50.00, P = 0.05), Table 2. For specific organ metastasis, location of lesions in the acral and extremities was associated with more prevalent risk of lung metastasis (OR 7.181, 95% CI: 1.045–49.35, P = 0.045), nodular melanoma type was significantly associated with bone metastasis (OR 11.557, 95% CI: 1.442–92.61, P = 0.0021), and more female patients were associated with liver metastasis (OR 4.739, 95% CI: 1.00–10.7, P = 0.05), as shown in Table 3. No specific attributable risk factors were associated with distant metastases in the brain and the bone.
Table 2.
Odds ratios and 95% confidence intervals of different clinicopathological variables to the risk of progression into distant metastasis using univariable and multivariable logistic regression.
| Variables |
Category |
Melanoma (total) |
Cutaneous melanoma |
Mucosal melanoma |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) |
P value |
OR (95%CI) |
P-value |
OR (95%CI) |
P value |
OR (95%CI) |
P value |
OR (95%CI) |
P value |
OR (95%CI) |
P value |
|||
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||||
| Age (years) | ≤65 | ref | ref | ref | ref | ref | ref | ref | ||||||
| >65 | 0.762 (0.308–1.888) | 0.463 | 0.708 (0.245–2.047) | 0.524 | 0.497 (0.148–1.664) | 0.257 | 0.304 (0.461–2.017) | 0.304 | 1.250 (0.307–5.085) | 0.755 | 1.504 (0.248–9.130) | 0.658 | ||
| Sex | Male | 1.171 (0.662–2.073) | 0.578 | 0.546 (0.196–1.521) | 0.247 | 2.105 (0.609–7.246) | 0.996 | 0.252 (0.052–1.229) | 0.088 | 0.960 (0.239–3.853) | 0.954 | 0.938 (0.204–4.317) | 0.935 | |
| Female | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ||||
| Histological type | Nodular | 1.155 (0.749–2.798) | 0.938 | 1.089 (0.342–3.468) | 0.886 | 0.504 (0.086–2.964) | 0.448 | 0.512 (0.217–1.712) | 0.448 | – | – | – | – | |
| Other | ref | ref | ref | ref | ||||||||||
| Breslow thickness | >4 mm | 1.067 (0.398–2.858) | 0.503 | 1.305 (0.373–4.567) | 0.677 | 2.557 (0.277–28.747) | 0.447 | 0.857 (0.222–3.315) | 0.690 | 1.238 (0.266–5.767) | 0.758 | |||
| ≤4 mm | ref | ref | ||||||||||||
| Node | Positive | 1.201 (0.496–2.906) | 0.684 | 1.236 (0.288–5.309) | 0.775 | 1.149 (0.190–6.957) | 0.880 | 2.702 (0.602–12.048) | 0.195 | 0.909 (0.143–5.781) | 0.920 | 0.369 (0.032–4.225) | 0.432 | |
| Negative | ref | ref | ref | ref | ref | ref | ref | ref | ||||||
| Ulceration | Yes | 3.003 (1.010–9.090) | 0.05 | 6.711 (1.745–25.6) | 0.06 | 10.00 (2.012–50.00) | 0.005 | 2.294 (0.613–8.587) | 0.218 | – | – | – | – | |
| No | ref | ref | ref | ref | ref | ref | – | – | – | |||||
| Stage (AJCC VIII) | Early (I-II) | ref | ref | ref | ref | ref | ref | ref | – | – | ||||
| Advanced (III) | 0.905 (0.319–2.562) | 0.85 | 0.510 (0.114–2.286) | 0.510 | 0.947 (0.242–3.715) | 0.938 | ref | ref | 1.000 (1.000) | 1.000 | 0.279 (0.025–3.047) | 0.295 | ||
| Location | Acral, extremities | 1.534 (0.630–3.734) | 0.346 | 2.669 (0.625–11.656) | 0.185 | 1.652 (0.139–19.654) | 0.782 | 0.726 (0.032–16.305) | 0.40 | – | – | – | – | |
| Trunk, head and neck | ref | ref | ref | ref | ref | ref | ref | |||||||
| Diameter | ≤6 mm | ref | ref | ref | ref | ref | ref | ref | ||||||
| >6 mm | 2.844 (0.304–26.657) | 0.663 | 5.080 (0.370–69.827) | 0.227 | 0.792 (0.036–13.501) | 0.872 | 2.124 (0.045–100.013) | 0.701 | – | – | – | – | ||
Table 3.
Odds ratios and 95% confidence intervals of variables associated with specific distant organ metastases.
| Variables |
Pulmonal metastasis |
Pleural metastasis |
Bone metastasis |
Liver metastasis |
Brain metastasis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | OR (95%CI) | P value | OR (95%CI) | P-value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age (years) | ≤65 | ref | ref | ref | ref | ref | ||||||
| >65 | 0.463 (0.117–1.182) | 0.271 | 1.677 (0.442–6.354) | 0.447 | 1.020 (0.165–6.290) | 0.983 | 0.102 (0.010–1.002) | 0.05 | – | – | ||
| Sex | Male | 0.795 (0.218–2.896) | 0.728 | 0.580 (0.159–2.113) | 0.409 | 0.602 (0.129–2.806) | 0.518 | 0.211 (0.044–1.000) | 0.05 | – | – | |
| Female | ref | ref | ref | ref | ref | ref | ref | |||||
| Histological type | Nodular | 0.709 (0.152–3.301) | 0.661 | 0.360 (0.084–1.543) | 0.169 | 11.557 (1.442–92.608) | 0.021 | 5.236 (0.726–37.76) | 0.101 | 1.414 (0.040–50.26) | 0.849 | |
| Other | ref | ref | ref | – | ref | ref | ||||||
| Breslow thickness | >4 mm | 0.505 (0.087–2.916) | 0.503 | 2.119 (0.421–10.670) | 0.363 | 6.806 (0.824–56.206) | 0.075 | 2.531 (0.393–16.292) | 0.328 | 0.267 (0.009–7.600) | 0.440 | |
| ≤4 mm | ref | ref | ||||||||||
| Node | Positive | 3.199 (0.461–22.217) | 0.240 | 0.489 (0.078–3.072) | 0.446 | 0.094 (0.004–2.083) | 0.135 | 2.010 (0.242–16.717) | 0.518 | – | – | |
| Negative | ref | ref | ref | ref | ref | ref | ||||||
| Ulceration | Yes | 3.257 (0.746–14.084) | 0.116 | 0.480 (0.097–2.384) | 0.370 | – | - | 16.95 (1.379–200) | 0.027 | 4.100 (0.204–82.53) | 0.357 | |
| No | ref | ref | ref | ref | ref | ref | ref | – | ||||
| Stage (AJCC VIII) | Early (I-II) | ref | ref | ref | ref | ref | ref | |||||
| Advanced (III) | 0.247 (0.035–1.730) | 0.159 | 0.532 (0.086–3.313) | 0.499 | 5.686 (0.186–173.907) | 0.319 | 0.269 (0.022–3.276) | 0.269 | – | – | ||
| Location | Acral, extremities | 7.181 (1.045–49.35) | 0.045 | 3.855 (0.615–24.186) | 0.150 | 0.527 (0.038–7.248) | 0.632 | 0.349 (0.029–4.121) | 0.349 | – | – | |
| Trunk, head and neck | ref | ref | ref | ref | ref | ref | ref | |||||
3.3. Overall survival according to clinicopathological variables
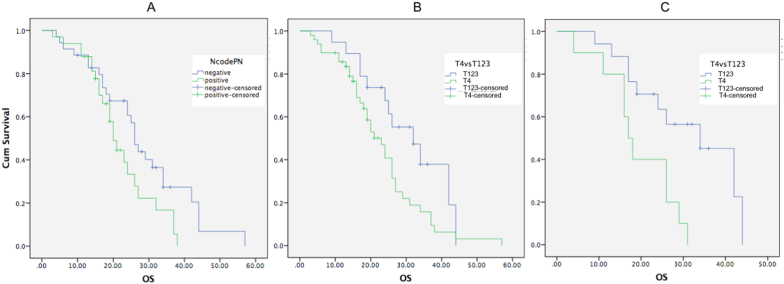
CM had shorter overall survival compared to MM (19.8 months and 23.4 months, respectively and P = 0.792). Positive regional lymph node was associated with shorter overall survival compared to those with negative regional lymph node (means of overall survival were 22.14 months and 28.19 months, respectively and Log-rank/Mantle-Cox test P = 0.05). Breslow T4 was associated with shorter overall survival compared to those with ≤ 4 mm (means of overall survival were 23.04 and 30.59 months, respectively and Log-rank/Mantle-Cox test P = 0.047). In subtype of nodular melanoma, Breslow T4 was also correlated with worse overall survival compared to those with ≤ 4 mm (means of overall survival were 19.4 and 31.2 months, respectively and Log-rank/Mantle-Cox test P = 0.006; Fig. 1). Other clinicopathological variables including clinical stage, age, sex, ulceration, and anatomic location were not significantly associated with overall survival.
Fig. 1.
Association of lymph node status and Breslow depth with worse prognosis in melanoma patients. (A) Tumor infiltration to the regional lymph nodes were associated with shorter overall survival (means were 22.14 and 28.19 months in positive and negative regional lymph nodes, respectively; Log-rank Mantel-Cox test, P = 0.001). (B) Breslow thickness more than 4 mm was associated with worse overall survival (means were 23.04 and 30.59 months in T4 and T1-3, respectively; Log-rank Mantel-Cox test, P = 0.047). (C) In nodular melanoma, Breslow thickness more than 4 mm was associated with worse overall survival (means were 19.4 and 31.2 months in T4 and T1-3, respectively; Log-rank Mantel-Cox test, P = 0.006).
4. Discussion
Our study highlighted that the majority of patients with melanoma were diagnosed in advanced stages with higher risks of relapse, recurrent metastatic disease, as well as poorer prognosis. The relatively low incidence of melanoma in Southeast Asia including Indonesia might contribute to the general lack of public awareness about the disease [15]. We also confirmed that melanoma in Asian patients often presented as MM and ALM and the predominant sites of skin lesions were in the acral of extremities (Table 1). In contrast, 70% of primary melanomas in Caucasians are diagnosed as superficial spreading CM and are located on the trunk and extremities [16]. Different from other Asian countries showing that ALM was the most common melanoma subtype (40–65% of total cases) [4,6], our study found that NM was the most common subtype (73%) followed by ALM (27%) and both subtypes were found in the acral and extremities. NM is commonly found in older patients in the head and neck regions with fast-growing and aggressive clinical course [3]. ALM has often been related with poorer prognosis compared to other types of CM [17] possibly due to the more advanced stages at diagnosis. However, another study found no significant difference in disease progression and overall survival between acral and non-acral cutaneous melanoma [18]. All CM in our study had Breslow T4, diameter more than 6 mm, and more than half were accompanied with ulceration (Table 1). CM among Asians are reported to be significantly thicker and are located in the acral with lesions prone to ulceration [4,15,19]. Although melanoma in children is very rare [20], our study also recorded an unusual nodular melanoma in a 15 years-old child that might need further analysis.
The distinct patterns of clinical presentations and the associated adverse risks of melanomas might partly be due to the cytomorphological pathogenesis and the underlying genetic mutations. ALM and MM show more genetic aberrations in the somatic structural variants while CM demonstrate higher frequencies of small nucleotide variants and indels [8]. Patterns of actionable mutations including BRAF, NRAS, and CDKN2A are significantly different among CM, ALM, and MM although all of them show frequent mutations in the hTERT promoter [8]. MM are significantly associated with lower survival rates [9]. Although several driver mutations including BRAF and NRAS mutations are associated with aggressive behavior, the association of those mutations with worse prognosis are not clear in NM [8,21].
Melanoma is regarded as the most aggressive skin cancer due to its highly metastatic potential as well as its significant poorer prognosis. There were only a few studies that reported variables associated with progression into distant metastasis and the prognosis among melanoma patients from Asians particularly Indonesians. Several studies in melanoma demonstrated that the recurrent diseases were predominantly due to progression into distant metastases rather than locoregional recurrences [6,22]. Our study showed that ulceration was a risk factor of disease progression into distant metastasis (Table 2) confirming previous studies reporting that ulceration was associated with adverse prognosis in melanoma [6,23]. Ulceration indicated extensive skin structural destruction by the cancer cells that cannot be compensated for by the host response of reactive inflammation, fibrin closure, and hyperplasia of the adjacent epidermis. Lymph node infiltration in patients with melanoma has been associated with higher locoregional recurrence and distant metastases, as well as poor prognosis. In our study, however, no significant difference was found in the risk of progression into distant metastasis in patients with and without positive regional lymph node. The potential cause of this finding was the routine sentinel node biopsy was not performed in our hospital; hence occult lymph node infiltration will not always be detected. Sentinel lymph node biopsy is recommended in CM with Breslow thickness more than 1 mm (or more than 0.8 mm with ulceration) [13]. The SLNB is currently used to select patients who might benefit from adjuvant systemic treatments including immunotherapy or targeted BRAF inhibitors [13]. However, the SLNB practice has not been associated statistically with any significant difference in the disease-free survival and overall survival for both intermediate-thickness melanoma (1–4 mm) and thick melanoma (>4 mm), respectively [24]. The benefits and clinicopathological variables predictive of SLNB in acral and extremity melanoma have not been well characterized.
Among patients who progressed into distant metastasis, 45% of them were found in the lung with significant higher risk in ALM (OR 7.181, Table 3). The lung is the second most common internal organ for distant metastasis from melanoma in the first 3-year follow-up [25] because it receives the entire cardiac output from the extremities as well as most of the lymphatic drainage that enters the venous system through the first capillary plexus [26]. Acral melanoma has a propensity to infiltrate the venous system which has direct drainage from the inferior vena cava to the lungs as well as lymphatic drainage to the brachiocephalic vein [26]. The bone is also a common site of distant metastasis from advanced stages of melanoma usually with multiple metastases [27]. However, no study other than this one has previously reported the predilection of bone metastasis from the specific type of NM. The attributed factors for bone metastasis from melanoma are commonly associated with thicker Breslow depth, regional lymph node involvement, and other sites of distant metastases [27]. The liver is a common site of distant metastases in melanoma, although specific predisposition in females has also not been previously reported. The most important associated risk factors of liver metastases from melanoma is advanced stage at diagnosis [28].
MM often show higher rates of recurrences, progression, and local failure [9]. However, our study did not find significant differences of distant metastasis propensity and worse overall survival in comparison to CM (Table 2). In our results, head and neck were the most common sites with tumor thickness more than 2 mm constituting more than 70% of MM. Tumors located in the paranasal sinuses often showed higher mortality due to inaccessibility for early diagnosis and complexity of surgical resection [29]. Recommended surgery procedures to achieve complete resection with/or without regional lymph node clearance in MM is also still debated [30]. MM also often share similar clinical characteristics with ALM such as relative predominance in non-Caucasians and poorer prognosis due to delayed diagnosis [6,30].
Infiltration to regional lymph nodes and Breslow T4 were significantly associated with shorter survival in our study. The delayed detection as also shown in other more common cancer [[31], [32], [33]] might contribute to more advanced disease at presentation as well as poorer survival rates than patients from other Asian countries [6,15]. Therefore, increasing public health education and awareness of potential skin or mucosal lesion as melanoma is very crucial in Indonesia to reduce delayed diagnosis of melanoma. With the relatively low median survival of patients with melanoma in our study and other studies from Indonesia (19 months compared to 3–4 years in China and Japan), improvements of standard of care including diagnosis, referral system, and treatment of melanoma must be effectively performed in Indonesia. It has been also reported that many patients with melanoma from Asia receive only palliative treatment primarily because of too advanced disease and limited options of available treatment [6]. Although surgical resection is the backbone of melanoma treatment, adequate margin-free resection particularly in advanced stages and in MM, this is not always possible. Neoadjuvant therapy in bulky tumors, late stages, and with locoregional metastasis has been currently recommended to aid surgical resection and improve survival [34,35]. Immunotherapy using PD-1, PD-L1, and CTLA-4 inhibitors has been shown clinically to be very effective for high-risk and inoperable melanomas to improve survival and quality of life [12]. However, the immune checkpoint inhibitors are not yet covered by the national insurance in Indonesia. Accordingly, more engagement with the health policy makers, insurance company, and medical industry is also required to raise and improve access to these novel and effective therapies.
Improvement of diagnostic strategies to early detect melanoma is very important to increase the chance of complete resection. In addition to careful physical examination of a suspicious skin lesion and confirmation with histology examination, dermatoscopy has been considered as an effective test to assess skin lesions with an ability of magnification into 6–100 times [36]. Better visualization of raw structures and patterns of skin lesions can be achieved using dermatoscopy [36]. The digital images can be stored and potentially processed using current artificial intelligence to improve diagnosis as well as monitoring of disease progression [36]. In addition to early detection, improvement of surveillance also needs to be performed. There are no universally accepted guidelines for surveillance after complete surgery resection specifically for high-risk patients with mucosal, nodular, and acral melanomas. The generally accepted guidance is thorough and regular skin and lymph node examinations to detect both disease recurrence and new primary tumor. NCCN guidelines have recommended for structured surveillance every 3–6 months for the first 3 years followed with 4–12 months for an additional 2 years [37]. Extension of structured follow-up after 5 years is not specifically recommended [37]. Adjustment of surveillance particularly for patients with high-risk melanomas including those with ulceration, thicker Breslow tumor depth, and positive involvement of lymph nodes might also be required [13].
The prime strength of this study is the elucidation of factors associated with progression into distant metastasis and overall survival from high-risk patients with melanoma of Asian patients predominantly diagnosed as nodular, acral lentiginous and mucosal melanomas. Limitations of this study are those that are naturally due to the retrospective design. In addition, the degree of excessive ulceration and type of infiltrative or attenuative ulceration were not differentiated. Longer follow-up and incorporation of histological and molecular biomarkers including BRAF, NRAS mutations, and PD-L1 [12] expression are required in the future study for planning of further advanced treatment and prognostic determination.
5. Conclusion
Our findings show that although melanoma is not a common cancer in Indonesia, most patients are diagnosed in advanced stages with higher risks of relapse, disease progression, as well as poor prognosis. Easing and facilitating the referral health system for diagnosis and treatment of melanoma is also required to prevent delayed diagnosis and treatment. Future studies should elucidate the clinical course, risk factors for relapse and worse survival, while identifying underlying actionable mutations and gene rearrangements, with more routine follow-up of responses to treatment, in addition to tailoring a more comprehensive surveillance system in Asian/Indonesian patients with melanoma.
6. Provenance and peer review
Not commissioned, externally peer reviewed.
Funding
This study was supported by grants from Universitas Gadjah Mada to TA (DAMAS Nr 133/2020).
Ethical approval
The study has been conducted following ethical principles and the protocol has been approved by the Ethics Committee of the Faculty of Medicine, Public Health, and Nursing - Universitas Gadjah Mada Yogyakarta (KE/0939/09/2020).
Consent
Written informed consent was obtained from the participants. Patient identifying related material was not used in this manuscript.
Author contribution
SLA and TA conceived the study. SLA, RC, HYB collected and analyzed the data. SLA wrote the first draft with critical feedback from WAH and TA. All authors agreed on the final version of the manuscript draft.
Registration of research studies
The study has been registered in the ResearchRegistry with identification number researchregistry6192. Please find in the https://www.researchregistry.com/browse-the-registry#home/registrationdetails/5f9de98dc4597c0015c1a5f8/.
Guarantor
SLA, Universitas Gadjah Mada.
Declaration of competing interest
We declare that no potential conflict of interest exists.
Acknowledgment
The authors specifically thank all patients and their families for supporting this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amsu.2020.12.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Guy G.P., Thomas C.C., Thompson T., Watson M., Massetti G.M., Richardson L.C. Centers for Disease Control and Prevention (CDC), Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030. MMWR Morb. Mortal. Wkly. Rep. 2015;64(21):591–596. [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Ossio R., Roldán-Marín R., Martínez-Said H., Adams D.J., Robles-Espinoza C.D. Melanoma: a global perspective. Nat. Rev. Canc. 2017 doi: 10.1038/nrc.2017.43. [DOI] [PubMed] [Google Scholar]
- 4.Chi Z., Li S., Sheng X., Si L., Cui C., Han M., Guo J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Canc. 2011 doi: 10.1186/1471-2407-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinonce H.T., Aji R.P.M., Hayati N., Pudjohartono M.F., Kameswari B., Anwar S.L., Irianiwati BRAF V600 mutation profiling in primary skin nodular melanoma in Indonesia: an analysis using high resolution pyrosequencing. BMC Res. Notes. 2020 doi: 10.1186/s13104-020-05000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen X., Li D., Zhao J., Li J., Yang T., Ding Y., Peng R., Zhu B., Huang F., Zhang X. Time-varying pattern of recurrence risk for localized melanoma in China. World J. Surg. Oncol. 2020 doi: 10.1186/s12957-019-1775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin C.C., Wu X.C., Jemal A., Martin H.J., Roche L.M., Chen V.W. U.S; Cancer: 2005. Incidence of Noncutaneous Melanomas in the. [DOI] [PubMed] [Google Scholar]
- 8.Hayward N.K., Wilmott J.S., Waddell N., Johansson P.A., Field M.A., Nones K., Patch A.M., Kakavand H., Alexandrov L.B., Burke H., Jakrot V., Kazakoff S., Holmes O., Leonard C., Sabarinathan R., Mularoni L., Wood S., Xu Q., Waddell N., Tembe V., Pupo G.M., De Paoli-Iseppi R., Vilain R.E., Shang P., Lau L.M.S., Dagg R.A., Schramm S.J., Pritchard A., Dutton-Regester K., Newell F., Fitzgerald A., Shang C.A., Grimmond S.M., Pickett H.A., Yang J.Y., Stretch J.R., Behren A., Kefford R.F., Hersey P., Long G.V., Cebon J., Shackleton M., Spillane A.J., Saw R.P.M., López-Bigas N., Pearson J.V., Thompson J.F., Scolyer R.A., Mann G.J. Whole-genome landscapes of major melanoma subtypes. Nature. 2017 doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 9.Yde S.S., Sjoegren P., Heje M., Stolle L.B. Mucosal melanoma: a literature review. Curr. Oncol. Rep. 2018 doi: 10.1007/s11912-018-0675-0. [DOI] [PubMed] [Google Scholar]
- 10.Jung H.J., Kweon S.S., Lee J.B., Lee S.C., Yun S.J. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatology. 2013 doi: 10.1001/jamadermatol.2013.5853. [DOI] [PubMed] [Google Scholar]
- 11.Von Schuckmann L.A., Hughes M.C.B., Ghiasvand R., Malt M., Van Der Pols J.C., Beesley V.L., Khosrotehrani K., Smithers B.M., Green A.C. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatology. 2019 doi: 10.1001/jamadermatol.2019.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michielin O., Van Akkooi A.C.J., Ascierto P.A., Dummer R., Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019 doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 13.Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., Haydu L.E., Eggermont A.M.M., Flaherty K.T., Balch C.M., Thompson J.F. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual, CA. Cancer J. Clin. 2017 doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G. STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019 doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Chang J.W.C., Guo J., Hung C.Y., Lu S., Shin S.J., Quek R., Ying A., Ho G.F., Nguyen H.S., Dhabhar B., Sriuranpong V., Tiambeng M.L., Prayogo N., Yamazaki N. Sunrise in melanoma management: time to focus on melanoma burden in Asia, Asia. Pac. J. Clin. Oncol. 2017 doi: 10.1111/ajco.12670. [DOI] [PubMed] [Google Scholar]
- 16.Singh P., Kim H.J., Schwartz R.A. Superficial spreading melanoma: an analysis of 97 702 cases using the SEER database. Melanoma Res. 2016 doi: 10.1097/CMR.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 17.Bello D.M., Chou J.F., Panageas K.S., Brady M.S., Coit D.G., Carvajal R.D., Ariyan C.E. Prognosis of acral melanoma: a series of 281 patients. Ann. Surg Oncol. 2013 doi: 10.1245/s10434-013-3089-0. [DOI] [PubMed] [Google Scholar]
- 18.Wada M., Ito T., Tsuji G., Nakahara T., Hagihara A., Furue M., Uchi H. Acral lentiginous melanoma versus other melanoma: a single-center analysis in Japan. J. Dermatol. 2017 doi: 10.1111/1346-8138.13834. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.Y., Chay W.Y., Tang M.B., Chio M.T., Tan S.H. Melanoma: differences between asian and caucasian patients. Ann. Acad. Med. Singapore. 2012 [PubMed] [Google Scholar]
- 20.Tariq S., Shallwani H., Waqas M., Bari M.E. Congenital and infantile malignant melanoma of the scalp: a systematic review. Ann. Med. Surg. 2017 doi: 10.1016/j.amsu.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkhoran S., Milne O., Zalaudek I., Puig S., Malvehy J., Kelly J.W., Marghoob A.A. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch. Dermatol. 2010 doi: 10.1001/archdermatol.2009.369. [DOI] [PubMed] [Google Scholar]
- 22.Romano E., Scordo M., Dusza S.W., Coit D.G., Chapman P.B. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J. Clin. Oncol. 2010 doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss S.A., Hanniford D., Hernando E., Osman I. 2015. Revisiting Determinants of Prognosis in Cutaneous Melanoma, Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyrgidis A., Tzellos T., Mocellin S., Apalla Z., Lallas A., Pilati P., Stratigos A. Sentinel lymph node biopsy followed by lymph node dissection for localised primary cutaneous melanoma. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD010307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soliman M., Petrella T., Tyrrell P., Wright F., Look Hong N.J., Lu H., Zezos P., Jimenez-Juan L., Oikonomou A. The clinical significance of indeterminate pulmonary nodules in melanoma patients at baseline and during follow-up chest CT. Eur. J. Radiol. Open. 2019 doi: 10.1016/j.ejro.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott C.D., Harpole D.H. The biology of pulmonary metastasis. Thorac. Surg. Clin. 2016 doi: 10.1016/j.thorsurg.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Gómez-León N., Pacheco-Barcia V., Ballesteros A.I., Fraga J., Colomer R., Friera A. Skeletal muscle and solitary bone metastases from malignant melanoma: multimodality imaging and oncological outcome. Melanoma Res. 2018 doi: 10.1097/CMR.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damsky W.E., Rosenbaum L.E., Bosenberg M. Decoding melanoma metastasis. Cancers. 2011 doi: 10.3390/cancers3010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus D.M., Marcus R.P., Prabhu R.S., Owonikoko T.K., Lawson D.H., Switchenko J., Beitler J.J. Rising incidence of mucosal melanoma of the head and neck in the United States. J. Skin Cancer. 2012 doi: 10.1155/2012/231693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latteri S., Teodoro M., Malaguarnera M., Mannino M., Currò G., La Greca G. Abdominal perineal resection or wilde local excision in primary anorectal malignant melanoma. Case report and review. Ann. Med. Surg. 2017 doi: 10.1016/j.amsu.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anwar S.L., Avanti W.S., Nugroho A.C., Choridah L., Dwianingsih E.K. Risk factors of distant metastasis after surgery among different breast cancer subtypes : a hospital-based study in Indonesia. 2020;18(1):117–133. doi: 10.1186/s12957-020-01893-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anwar S.L., Raharjo C.A., Herviastuti R., Dwianingsih E.K., Setyoheriyanto D., Avanti W.S., Choridah L., Harahap W.A., Darwito, Aryandono T., Wulaningsih W. Pathological profiles and clinical management challenges of breast cancer emerging in young women in Indonesia: a hospital-based study. BMC Wom. Health. 2019;19 doi: 10.1186/s12905-019-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anwar S.L., Prabowo D., Avanti W.S., Dwianingsih E.K., Harahap W.A., Aryandono T. Clinical characteristics and the associated risk factors of the development of bilateral breast cancers: a case-control study. Ann. Med. Surg. 2020;60:285–292. doi: 10.1016/j.amsu.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaria R.N., Menzies A.M., Burton E.M., Scolyer R.A., Tetzlaff M.T., Antdbacka R., Ariyan C., Bassett R., Carter B., Daud A., Faries M., Fecher L.A., Flaherty K.T., Gershenwald J.E., Hamid O., Hong A., Kirkwood J.M., Lo S., Margolin K., Messina J., Postow M.A., Rizos H., Ross M.I., Rozeman E.A., Saw R.P.M., Sondak V., Sullivan R.J., Taube J.M., Thompson J.F., van de Wiel B.A., Eggermont A.M., Davies M.A., Andrews M.C., Berry D.A., Block M.S., Boland G.M., Bollin K.B., Carlino M.S., Carvajal R.D., Cohen J., Davar D., Delman K.A., Dummer R., Farwell M.D., Fisher D.E., Fusi A., Glitza I.C., de Gruijl T.D., Gyorki D.E., Hauschild A., Hieken T.J., Larkin J., Lawson D.H., Lebbe C., Lee J.E., Lowe M.C., Luke J.J., McArthur G.A., McDermott D.F., McQuade J.L., Mitchell T.C., Petrella T.M., Prieto P.A., Puzanov I., Robert C., Salama A.K., Sandhu S., Schadendorf D., Shoushtari A.N., Sosman J.A., Swetter S.M., Tanabe K.K., Turajlic S., Tyler D.S., Woodman S.E., Wright F.C., Zager J.S., Ascierto P.A., Spillane A.J., van Akkooi A.C.J., Wargo J.A., Blank C.U., Tawbi H.A., Long G.V. Neoadjuvant systemic therapy in melanoma: recommendations of the international neoadjuvant melanoma consortium. Lancet Oncol. 2019 doi: 10.1016/S1470-2045(19)30332-8. [DOI] [PubMed] [Google Scholar]
- 35.Gabuniya N., Khajuria A., Geh J.L.C., Alsaadi D. Case report: neoadjuvant systemic therapy for melanoma. Ann. Med. Surg. 2020 doi: 10.1016/j.amsu.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolner Z.J., Yélamos O., Liopyris K., Rogers T., Marchetti M.A., Marghoob A.A. Enhancing skin cancer diagnosis with dermoscopy. Dermatol. Clin. 2017 doi: 10.1016/j.det.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coit D.G., Thompson J.A., Algazi A., Andtbacka R., Bichakjian C.K., Carson W.E., Daniels G.A., DiMaio D., Fields R.C., Fleming M.D., Gastman B., Gonzalez R., Guild V., Johnson D., Joseph R.W., Lange J.R., Martini M.C., Materin M.A., Olszanski A.J., Ott P., Gupta A.P., Ross M.I., Salama A.K., Skitzki J., Swetter S.M., Tanabe K.K., Torres-Roca J.F., Trisal V., Urist M.M., McMillian N., Engh A. NCCN guidelines insights: melanoma, version 3.2016. J. Natl. Compr. Canc. Netw. 2016 doi: 10.6004/jnccn.2016.0101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.