Abstract
Pneumatoceles are air filled, thin-walled cystic lesions with in the lung parenchyma that occur infrequently in neonates and infants, often as a complication of positive pressure ventilation, air leak syndrome or ventilator associated pneumonia. Whilst majority of pneumatoceles regress spontaneously over days to weeks, few large pneumatoceles may lead to acute cardiorespiratory insufficiency and may require drainage under computerized tomography or fluoroscopic guidance. We present a case report of an unstable extreme preterm infant with a large pneumatocele and respiratory failure, that was treated successfully by drainage under bedside ultrasound guidance.
Keywords: Extreme preterm, Neonates, Pneumatocele, Ultrasound
Introduction
Pneumatoceles are air filled, thin-walled cystic lesions with in the lung parenchyma that occur infrequently in neonates and infants. Extreme prematurity, positive pressure ventilation, air leak syndromes including pulmonary interstitial emphysema, pneuomediastinum & pneumothorax, ventilator associated pneumonia (VAP) and trauma are known risk factors for pneumatoceles [1]. In the presurfactant era, positive pressure ventilation and associated lung injury and air leak syndromes were commonly associated with pneumatoceles with estimated incidence around 3.2% in preterm infants [2]. With the routine use of surfactant and noninvasive ventilation, the incidence of pneumatoceles have decreased to 1.8% in infants less than 30 weeks gestational age [3]. Infectious causes particularly VAP have been implicated in majority of the preterm infants with pneumatocele in the most recent case series [4]. Various pathogens including Staphylococcus aureus, Coagulase negative Staphylococci, Klebsiella pneumoniae and Escherichia coli can cause necrotizing pneumonia and cystic lung parenchymal lesions. Check valve communication with bronchial tree can lead to unidirectional inflow of air into these cysts leading to progressive hyperinflation [1,4]. Whilst majority of pneumatoceles resolve spontaneously over days to weeks, few pneumatoceles increase in size and may cause or contribute to respiratory failure and long term ventilatory dependance. Rapidly enlarging pneumatoceles may cause acute cardiorespiratory compromise and is associated with increased mortality. Currently, there is no consensus on management of pneumatoceles in preterm infants. Conservative treatment includes use of high frequency ventilation, decreasing mean airway pressure, positioning affected side down and unilateral ventilation of unaffected lung. If conservative measures fail, invasive interventions including percutaneous decompression via needle or chest tube is undertaken, often guided with imaging including computerized tomography (CT) or fluoroscopy which may not be feasible in an unstable infant. We present a case report of successful decompression of pneumatocele under guidance of a bedside ultrasound (US) in an unstable extremely preterm infant [1,[4], [5], [6], [7]].
Case report
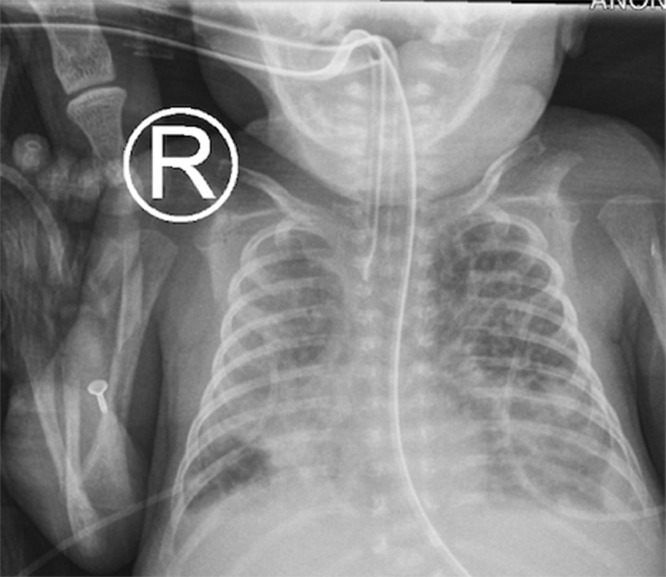
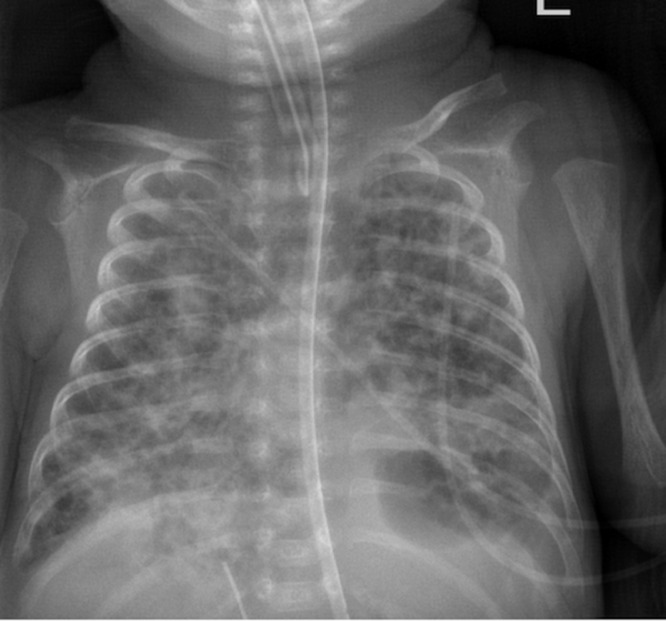
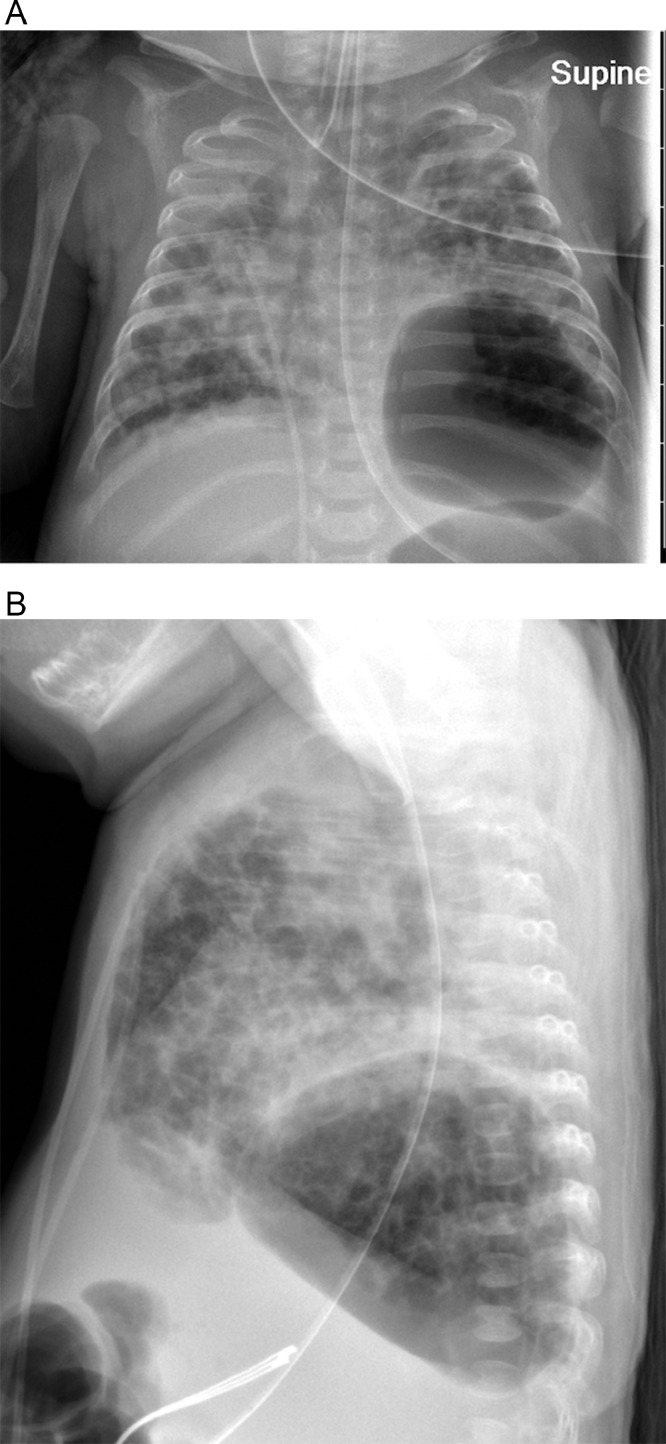
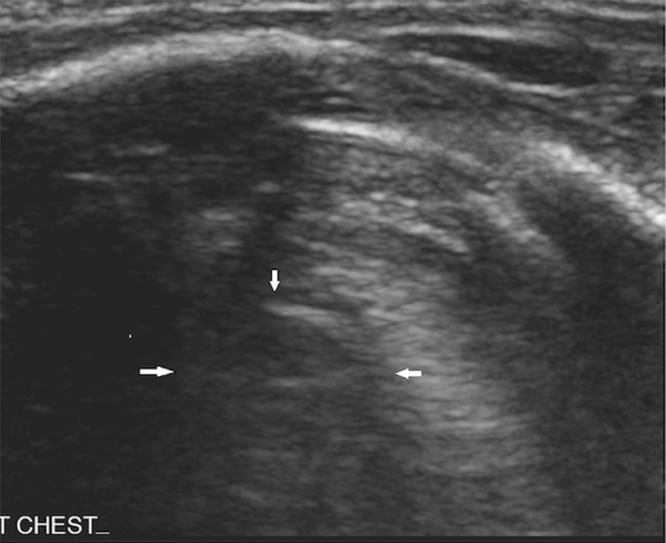
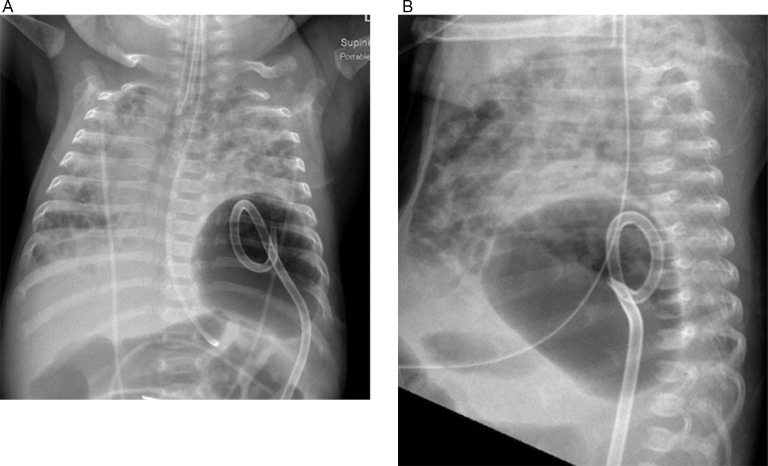
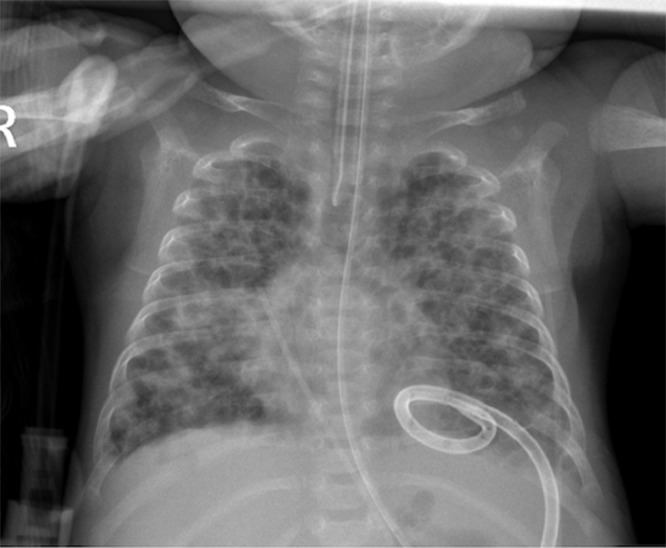
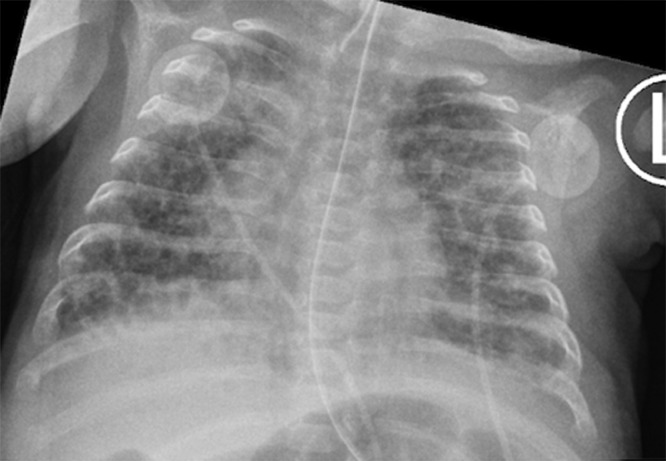
A baby girl was born at 25 weeks and 6 days gestation with a birth weight of 810 grams by vaginal delivery to a 31-year-old mother whose pregnancy was complicated by gestational diabetes, preterm prolonged rupture of membranes since 23 weeks gestation, oligohydramnios and chorioamnionits. Mother received steroids and antibiotics prior to the delivery. Infant was noted to have respiratory depression necessitating intubation in the delivery room. In the neonatal intensive care unit, the infant had severe respiratory distress syndrome (RDS) and hypoxemic respiratory failure requiring increased ventilatory support over the first few days of life. On day 15, she was switched to a high frequency jet ventilation for increased FiO2 requirements. Endotracheal aspirate sent for suspected VAP due to increased respiratory secretions and distress, showed growth of methicillin resistant Staphylococcus aureus (MRSA) with more than 25 white blood cells/low power field on microscopy and subsequently treated with intravenous vancomycin for 10 days. Blood culture sent on day 25 for unexplained metabolic acidosis also grew MRSA and intravenous vancomycin was continued for further 14 days. She had serial chest radiographs to monitor her respiratory status during her admission. On day 31, a small left basilar pneumatocele, measuring about 1.5 cm in diameter was noted on chest radiograph for the first time when compared to her previous radiograph on day 28 (Figs. 1 and 2). Over the next few weeks, the pneumatocele gradually increased in size. On day 64 at 35 weeks post menstrual age, her respiratory status worsened with desaturations to 70% on 100% FiO2. Chest radiograph revealed a large pneumatocele encompassing half of left hemithorax, which was thought to impair left lung expansion, therefore decision was made to decompress the pneumatocele (Fig. 3A). Lateral chest radiograph showed pneumatocele measuring 4.5cm in diameter in posterior lateral aspect of left hemithorax (Fig. 3B). Infant was deemed unstable for transfer to the radiology department for fluoroscopy or CT guided intervention, therefore after discussion with interventional radiologists, bedside US guided chest tube insertion was attempted. A cystic appearing lesion was identified on ultrasound after placing the infant in right decubitus position and a size 8 fr pig tail catheter was placed with US guidance (Fig. 4). The position of catheter was confirmed on chest radiograph and connected to continuous suction with active air leak (Figs. 5A and B). Chest radiograph, performed 9 hours later showed resolution of the pneumatocele and the infant was able to be transitioned back to conventional ventilator 2 days later (Fig. 6). Follow up chest radiograph over next few days showed no recurrence of the pneumatocele. Suction was discontinued on day 69 and chest tube connected to water seal. Follow up chest radiograph showed no re-accumulation and chest tube was removed. Chest radiograph on day 72, following chest tube removal, showed no recurrence of the pneumatocele (Fig. 7).
Fig. 1.
Chest radiograph on day 28 with no pneumatocele.
Fig. 2.
Chest radiograph on day 31 with a small left basilar pneumatocele.
Fig. 3.
(A) Chest radiograph on day 64 with large pneumatocele in anteroposterior view. (B) Chest radiograph on day 64 with large pneumatocele in lateral view.
Fig. 4.
Chest ultrasound view of pneumatocele (white pointed arrows).
Fig. 5.
(A) Chest radiograph on day 64 with pigtail catheter positioned in pneumatocele as seen in the anteroposterior view. (B) Chest radiograph on day 64 with pigtail catheter positioned in pneumatocele as seen in the lateral view.
Fig. 6.
Chest radiograph on day 65 showing decompression of pneumatocele with pigtail catheter in situ.
Fig. 7.
Chest radiograph on day 72 post pigtail catheter removal with no recurrence of pneumatocele.
Discussion
To our best knowledge, this is one of the first case reports of successful drainage of a pneumatocele in an unstable extreme preterm infant under the guidance of bedside ultrasound machine. Our infant was born extremely preterm at 25 weeks gestation with severe RDS requiring positive pressure ventilation and her hospital course was complicated with MRSA pneumonia and bacteremia, all of which are risk factors for pneumatocele. Whilst majority of pneumatoceles regress spontaneously, the pneumatocele in our infant continued to enlarge and contributed to worsening respiratory failure despite use of high frequency ventilation and selective positioning of affected side down. Case reports have described successful decompression of pneumatoceles under CT or fluoroscopic image guidance, unfortunately our infant was too unstable to be transferred to the radiology department. As the pneumatocele was noted to be in postero-lateral position, we consulted interventional radiology for plausibility of bedside US guided drainage. Lung US are being increasingly used to diagnose and manage neonatal lung diseases including RDS, surfactant administration, pneumothorax and pleural effusion [8], [9]. Whilst bedside US guided aspiration and drainage of pleural effusion is well documented, role of US in decompressing pneumatoceles are not well reported [8,9]. Interventional radiologist in our report was able to locate the lesion with US and successfully place a pig tail catheter into the pneumatocele facilitating drainage. We were able to transition the infant to a conventional ventilator, nearly 1 month after the pneumatocele was initially detected and just 2 days after the pneumatocele was decompressed. Our case report shows the feasibility of bedside US in decompression of pneumatocele, particularly when the infant is unstable for transfer for fluoroscopy and CT guided aspiration.
Parent consent statement
Informed consent: A written consent was obtained from parent for publication of the case report. No patient identifying information is included in the report or images.
References
- 1.Rocha G. Pulmonary pneumatoceles in neonates. Pediatr Pulmonol. 2020 doi: 10.1002/ppul.24969. PMID: 32691976. [DOI] [PubMed] [Google Scholar]
- 2.Clarke TA, Edwards DK. Pulmonary pseudocysts in newborn infants with respiratory distress syndrome. AJR Am J Roentgenol. 1979;133(3) doi: 10.2214/ajr.133.3.417. 417-21PMID: 111499. [DOI] [PubMed] [Google Scholar]
- 3.Hussain N, Noce T, Sharma P, Jagjivan B, Hegde P, Pappagallo M. Pneumatoceles in preterm infants-incidence and outcome in the post-surfactant era. J Perinatol. 2010;30(5):330–336. doi: 10.1038/jp.2009.162. Epub 2009 Oct 8. PMID: 19812584. [DOI] [PubMed] [Google Scholar]
- 4.Arora P, Kalra VK, Natarajan G. Pneumatoceles in infants in the neonatal intensive care unit: clinical characteristics and outcomes. Am J Perinatol. 2013;30(8) doi: 10.1055/s-0032-1331028. 689-94PMID: 23283803. [DOI] [PubMed] [Google Scholar]
- 5.de Bie HM, van Toledo-Eppinga L, Verbeke JI, van Elburg RM. Neonatal pneumatocele as a complication of nasal continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2002;86(3):F202–F203. doi: 10.1136/fn.86.3.f202. PMID: 11978755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar J, Mukhopadhyay K, Bhatia A. Successful percutaneous drainage of pneumatoceles in an extremely low-birthweight infant. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222630. bcr2017222630PMID: 29374641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz A, Moores D, Khan F, Baerg J, Radulescu A. Successful treatment of post-infectious pneumatocele via percutaneous drainage in a premature infant. J Pediatr Surg Case Rep. 2019 doi: 10.1016/j.epsc.2019.101235. [DOI] [Google Scholar]
- 8.Chen SW, Zhang MY, Liu J. Application of lung ultrasonography in the diagnosis of childhood lung diseases. Chin Med J (Engl) 2015;128(19):2672–2678. doi: 10.4103/0366-6999.166035. PMID: 26415808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein DA, Mauriat P. Lung ultrasound in the critically ill neonate. Curr Pediatr Rev. 2012;8(3):217–223. doi: 10.2174/157339612802139389. PMID: 23255876. [DOI] [PMC free article] [PubMed] [Google Scholar]