Abstract
Objective
To investigate the incidence of thromboembolic events, specifically pulmonary embolism (PE), deep vein thrombosis (DVT), and cerebrovascular accidents (CVA), in patients who tested positive for COVID-19 through RT-PCR in a regional healthcare system in Connecticut.
Materials and methods
All CT angiogram (CTA) and venous duplex extremity ultrasound (US) examinations performed on 192 consecutively documented cases of COVID-19 were retrospectively reviewed at a multi-centered healthcare system. Clinical characteristics and patient outcomes were evaluated and compared between two groups based on the presence or absence of acute thromboembolic events.
Results
Of the 16,264 patients tested for COVID-19, 3727 (23%) were positive. Out of those, 192 patients underwent 245 vascular imaging studies including chest CTA (86), venous duplex ultrasound (134), and CTA head and neck (25). Among those who underwent imaging, 49 (26%) demonstrated acute thromboembolic events which included 13/86 (15%) with PE, 34/134 (25%) with DVT, and 6/25 (24%) with CVA. One patient had positive results on all 3 examinations, and 2 patients had positive results on both chest CTA and venous duplex US. Males were more likely to have a thromboembolic event than females (33/103 (34%) vs. 14/89 (16%), p = 0.009). No significant difference was observed with respect to age, cardiopulmonary comorbidities, malignancy history, diabetes, or dialysis.
Conclusion
Approximately 26% of COVID-19 patients with positive testing who underwent vascular imaging with CTA or venous duplex ultrasound had thromboembolic events including PE, DVT, and CVA. This indicates that COVID-19 patients are at increased risk for thromboembolic complications.
Keywords: Coronavirus, COVID-19, Pulmonary embolism, Deep vein thrombosis, Thromboembolism
Introduction
The novel coronavirus pandemic in the USA has been difficult to manage due to rapid dissemination and ease of transmission. COVID-19 has been causing death primarily due to pneumonia and respiratory failure, which was the initial focus of investigation and treatment. However, as the disease spread and more cases were recognized, it became apparent that the disease affects multiple organs and can cause severe complications resulting in subsequent morbidity and mortality.
While pulmonary embolism was an unexpected finding among preliminary studies in COVID-19 patients [1–4], one of the complications which has since been described in COVID-19 infection is the increased systemic inflammatory response from the virus which leads to a higher risk of hypercoagulability and associated thromboembolic disease [5–8]. Prevention and/or treatment of the thrombo-inflammatory state such as therapeutic or prophylactic anticoagulants can be effective [5, 9]. However, providers must first recognize the increased risk of vascular complications and deploy intense surveillance measures.
Initial recognition of coronavirus-related venous and arterial thromboembolic complications prompted expanded use of vascular imaging studies such as CT angiograms and venous duplex ultrasounds at our institution. This study investigates the incidence of thromboembolic events of COVID-19 patients who underwent dedicated imaging for systemic or pulmonary thrombosis or emboli.
Materials and methods
This study received approval from the Hartford HealthCare Institutional Review Board (HHC-2020-0110).
Patients
This study was conducted as a retrospective review. All patients who underwent COVID-19 testing at the inpatient and outpatient settings of the campuses of a regional healthcare system anchored by a large (> 700 bed) urban teaching hospital in the northeast USA were eligible.
A retrospective review of certain vascular imaging studies performed on patients who tested positive for COVID-19 was performed via electronic medical record (EPIC Systems, Verona, WI, USA) and a system wide PACS (Inteleviewer, Montreal, QC, Canada). Specifically, these are all chest and head/neck CT angiograms (CTA) and venous duplex ultrasound (US) studies performed from March 20, 2020, to May 3, 2020, inclusive, on patients diagnosed with COVID-19.
Images and patient information were collected from the EPIC electronic medical record system as well as the picture archiving and communication system (PACS). All patients were confirmed positive for COVID-19 on reverse transcriptase-polymerase chain reaction (RT-PCR). All patients who underwent a CTA examination, which included CTA pulmonary embolism protocol, CTA chest/abdomen/pelvis, CTA head and neck, and all venous duplex ultrasound examinations of the upper and lower extremities, were included in the study. Abdominal ultrasounds were excluded.
The study reports and images were examined by one of 5 diagnostic radiologists to assess the presence or absence of acute thromboembolic events. Patients were considered to be positive for vascular events based upon the presence of acute filling defects in a vein or artery on CTA or the presence of echoes in a non-compressible vessel on ultrasound with absence of color signal or Doppler signal. There were no equivocal thromboembolic findings encountered.
Comorbidities such as cardiopulmonary and vascular diseases were recorded for analysis. Cardiovascular diseases included hypertension, coronary artery disease, prior myocardial infarction (MI), and history of cardiac surgery. Chronic respiratory disease included chronic obstructive pulmonary disease (COPD), asthma, current or previous tobacco use, and history of previous pulmonary embolism (PE). The maximum d-dimer, fibrinogen, C-reactive protein (CRP), lactate dehydrogenase (LDH) values, and leukocyte count were also recorded, if available. The use of systemic and mechanical VTE prophylaxis was included.
Imaging studies included were as follows:
Patients with CTA and US up to 7 days prior to positive COVID-19 results on RT-PCR
Patients at least 18 years of age
Studies reviewed until the censoring of data collection on May 3, 2020
Statistical analysis
Comparison of continuous variables was performed with a Student’s t test or Mann-Whitney U test (depending on distribution), and comparison of categorical variables was performed with Pearson’s chi-square test. A p value of < 0.05 is considered statistically significant. SPSS v. 26 (IBM: Armonk, NY 2019) was used for all analyses.
Results
Incidence
A total of 16,264 people were tested for COVID-19 between March 20, 2020, and May 3, 2020 of which 3727 had positive results. Testing sites included drive-up testing centers, urgent care centers, and acute care hospitals at a multi-centered healthcare system in Connecticut. During the study period, 192 patients who tested positive for COVID-19 underwent imaging as depicted in the PRISMA flow diagram (Fig. 1).
Fig. 1.
PRISMA flow diagram
The median age was 62 years old (interquartile range 52–74), and 54% were male. Out of the imaging done in males, 33% were positive for thromboembolism, while 16% of the tests performed in females were positive.
Of the 86 patients who underwent chest CT angiography, 13 (15%) were positive for pulmonary embolism (Table 1). A total of 134 venous duplex ultrasound studies were performed, and 34 (25%) were positive for deep vein thrombosis. Twenty-five patients underwent CTA head and neck, and 6 (24%) were positive for cerebral vascular accident. Of the total 192 COVID-19 patients who had imaging, 26% showed evidence of thromboembolic events. Of note, 42 patients had multiple imaging examinations including a combination of CTAs and lower or upper extremity venous duplex examinations. Two patients had positive results on both chest CTA and venous duplex US, and one patient had positive results on all 3 examinations.
Table 1.
Imaging results
| Imaging modality (n = 245) | No event (n = 192) (%) | Event (n = 53) (%) |
|---|---|---|
| CTA (n = 86) | 73 (85) | 13 (15) |
| Venous duplex US (n = 134) | 100 (75) | 34 (25) |
| CTA head and neck (n = 25) | 19 (76) | 6 (24) |
Patient characteristics
Statistically significant difference was demonstrated between males and females (70% vs. 30%, p = 0.009) in thromboembolic events among all the cardiovascular events that occurred.
The median hospital length of stay was longer in the event group than in the non-event group (10 days [range 8–18.5] vs. 7 days [range 3–14], respectively; p = 0.008). The rate of ICU admission was 70% in the thromboembolic event group compared to 46% in the non-event group (p = 0.007). Patients in the event group were more likely to be intubated (57% vs. 34%, p = 0.004). Nearly all inpatients and those admitted to the ICU received at least systemic and mechanical thromboembolic prophylaxis, although regimens varied. In the event group, 100% of patients were anticoagulated, and 92% were put on sequential compression devices.
Patient characteristic and laboratory values are listed in Table 2. There was no significant difference between event and non-event groups with respect to age, BMI, history of cardiovascular disease, chronic respiratory disease, diabetes, dialysis, malignancy, and death.
Table 2.
Clinical characteristics and outcomes
| Characteristics (n = 192) | No event (n = 145) (%) | Event (n = 47) (%) | p |
|---|---|---|---|
| Sex | 0.009 | ||
| Male, n = 103 | 70 (48) | 33 (70) | |
| Female, n = 89 | 75 (52) | 14 (30) | |
| Age (years) | |||
| Mean ± SD | 63.0 ± 15.7 | 59.5 ± 13.7 | 0.171 |
| Range | 24–91 | 18–89 | |
| BMI (kg/m2), mean ± SD | 31.1 ± 8.70 | 30.3 ± 8.8 | 0.580 |
| Cardiovascular disease | 95 (66) | 31 (66) | 0.956 |
| Chronic respiratory disease | 47 (32) | 13 (28) | 0.541 |
| Diabetes | 64 (44) | 25 (53) | 0.427 |
| Dialysis | 13 (9) | 3 (6) | 0.578 |
| Malignancy | 29 (20) | 8 (17) | 0.653 |
| Anticoagulation | 131 (90) | 47 (100) | 0.027 |
| Mechanical VTE prophylaxis | 131 (90) | 43 (92) | 0.815 |
| Length of stay (days), median (IQR) | 7 (3–14) | 10 (8–18.5) | 0.008 |
| Care status | 0.007 | ||
| ICU | 66 (46) | 33 (70) | |
| Inpatient | 70 (48) | 14 (30) | |
| ED | 9 (6) | 0 (0) | |
| Requiring intubation | 49 (34) | 27 (57) | 0.004 |
| Death | 16 (11) | 8 (17) | 0.281 |
| d-dimer ng/mL (< 250), n = 176 | 121 (83) | 42 (89) | 0.144 |
| Fibrinogen ng/mL, n = 67 | 651.0 ± 209.0 | 596.9 ± 244.9 | 0.337 |
| CRP mg/dL, n = 164 | 14.5 ± 11.5 | 19.3 ± 12.1 | 0.024 |
| LDH IU/L, n = 163 | 455.6 ± 235.9 | 525.3 ± 259.6 | 0.103 |
| Lymphocyte count, n = 192 | 13.0 ± 7.3 | 18.2 ± 8.7 | < 0.001 |
Discussion
Until recently, hypercoagulability associated with COVID-19 infections and thromboembolic complications was unexpected and likely underestimated. Early radiologic investigations understandably focused primarily on lung parenchymal findings in chest CT. However, since then several papers ranging from case reports and small cohort studies reported elevated incidence of pulmonary thromboembolic events. There is now growing awareness of COVID-19 related to multi-system complications secondary to thromboembolism leading to increasing numbers of angiographic imaging studies ordered by providers. In our review, out of 245 angiographic imaging studies that were obtained on COVID-19 patients, we found 26% of patients with thromboembolic events. These include pulmonary embolism, cerebral vascular accidents, and deep vein thrombosis from CT angiography of the chest or head/neck or venous duplex ultrasounds of the upper or lower extremities. This is significantly higher than expected. Although there are no literature references reporting the incidence of thromboembolism in patients with viral illnesses, a frame of reference by comparing to White’s estimates in 2003 reported that venous thromboembolism occurs for the first time in approximately 100 persons per 100,000 each year in the USA and rises exponentially from < 5 cases per 100,000 persons for < 15 years old to approximately 500 cases (0.5%) per 100,000 persons at age 80 years [10]. Approximately one-third of patients with symptomatic VTE manifest as pulmonary embolism, whereas two-thirds manifest as deep vein thrombosis alone [10]. This indicates that our rate of VTE during COVID-19 is significantly higher compared to pre-COVID. Moreover, the Spanish registry for both DVT and PE reports incidence of VTE at 1.6 per 1000 ED visits [11]. Numbers published by Singer et al. estimated a 0.17% incidence for DVT and PE over a 5-year period in the ED in the USA [12].
Comparing results of this study with other reports of thromboembolic events in COVID-19 patients, our data is comparable to the study by Grillet et al. from France, in which 23% of thromboembolic events were found [13]. However, that paper only had contrast-enhanced CT exams and did not include venous duplex studies. Cavagna et al. and Helms et al. reported an even higher level of pulmonary thromboembolic events—41% and 43%, respectively [6, 14]. Cavagna et al. also looked at DVT and reported a 12% prevalence of DVT compared to 25% from our study [14]. Spiezia et al. from Italy, closer to our estimate, found 5 out of 22 (23%) ICU patients with COVID-19 developed in-hospital deep vein thrombosis despite anticoagulant prophylaxis [8]. Lodigiani et al., on the other hand, found 16/44 (36%) patients who underwent imaging tests had venous thromboembolism, and 63% were PE, compared to 15% from our study [15]. Lastly, Grandmaison et al. reported 59% venous thromboembolism in ICU patients in a single day, most of which were DVTs [16]. These reports indicate a higher rate of thromboembolism due to coronavirus infection, and providers caring for patients with or under investigation for COVID-19 need to be hypervigilant about thromboembolic complications and may want to have a low threshold for performing imaging tests to look for thrombus and emboli. There should be considerations for prophylaxis using mechanical and medical anticoagulation in COVID-19 patients early in their management. Moreover, although most of the reported thromboembolic events were discovered in the ICU or general ward setting, there may be more cases where patients present to the emergency department with thromboembolism during their quarantine as COVID cases increase (Figs. 2 and 3). In which case, emergency radiologists should be on higher alert to detect thromboembolic complications on a CT or ultrasound study.
Fig. 2.
Seventy-four-year-old female presented to the emergency department with acute stroke symptoms and respiratory distress. Images a, b, c, and d were obtained for evaluation of stroke and pulmonary embolism. (a) Axial MIP CTA at the level of the circle of Willis showed occlusive thrombus filling the M1 segment of the right middle cerebral artery (solid arrow) with no substantial filling of the distal branches of the right anterior and middle cerebral arteries. Incidental 1-cm bilobed saccular aneurysm of the right internal carotid artery near the origin of the right posterior communicating artery (dotted arrow). (b) Axial CT at the level of the body of the lateral ventricles 2 days after demonstrated evolution of large right anterior and middle cerebral artery territorial infarcts with cytotoxic edema (asterisk) and effacement of the right lateral ventricle. (c) Axial CTA at the level of the hila displayed right upper and lower lobe peripheral subpleural ground-glass opacities (box). (d) Axial CTA at the level of the hila also showed pulmonary emboli in the right and left main pulmonary arteries (black arrow)
Fig. 3.
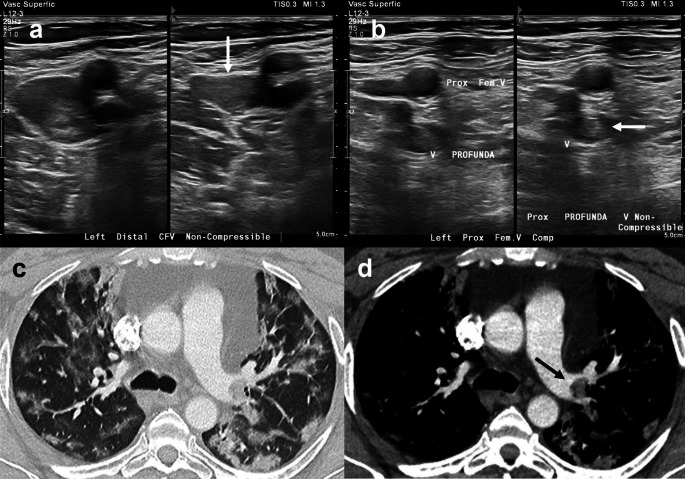
Images a and b represent a 56-year-old male with respiratory failure positive for COVID-19. Left lower extremity duplex US demonstrated low echogenicity within distended non-compressible veins (white arrows) reflecting acute thrombus involving the (a) distal common femoral and (b) proximal profunda veins. Images c and d represent a 48-year-old male admitted with respiratory failure due to COVID-19 pneumonia. Axial CTA at the level of the left hilum demonstrated (c) multifocal ground-glass opacities and consolidation predominantly in the periphery of both lungs and (d) pulmonary embolus in the left main pulmonary artery (black arrow)
One issue to consider with regard to CT angiography exams is the administration of intravenous contrast. Some COVID-19 patients have multi-organ failure including acute kidney injury predisposing them to contrast nephropathy [17]. Consequently, the need for angiographic studies to rule out PE and CVA is balanced by preserving kidney function. However, with a lower threshold to obtain venous duplex ultrasound studies, physicians can detect DVTs earlier, ideally before the evolution to PE.
In our series, it was interesting to observe that COVID-19 patients with more comorbidities did not have a statistically higher risk of thromboembolism. Despite this finding, Wu et al. reported that risk factors associated with severe infection and death include male sex and comorbidities such as hypertension and diabetes [18]. Furthermore, our study revealed that men were more likely than women to have thromboembolic events. Perhaps physicians may also want to have a higher level of suspicion for thromboembolism in men.
The limitations to our study include a relatively small sample size in which we collected data for a limited time period. Moreover, we only collected data on patients with confirmed positive COVID-19 diagnoses. Another limitation was that there were inconsistencies in reporting lab values; not every patient had data for all of the lab collections. It would be helpful to know if the patients who tested positive for COVID-19 later developed thromboembolism outside of our study’s date range for inclusion. There is limited data on those who tested negative for COVID-19 during the time period, and it may be valuable to collect data on them to determine their rate of thromboembolism as a point of comparison. As a future direction, it would also be interesting to see if there is a higher rate of thromboembolism during the traditional influenza season compared to the beginning of the COVID-19 pandemic.
Conclusion
This study reports the incidence of arterial and venous thromboembolic events detected by CTA and venous duplex ultrasound occurring in patients who tested positive for COVID-19 in a multi-centered healthcare system, including a large regional tertiary care urban teaching hospital, in the northeast during the peak period of the novel coronavirus pandemic. Similar to other recent studies, our results showed that COVID-19 patients are at increased risk of thromboembolic complications. Specifically, we report that 26% of COVID-19 patients had thromboembolism. Increased hypercoagulability in patients with COVID-19 may decrease the threshold for clinicians to order angiographic imaging studies when there is clinical suspicion for PE, stroke, or DVT.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Edison Lee, Email: Edison.lee@hhchealth.org.
Adam Krajewski, Email: Adam.krajewski@hhchealth.org.
Cynthia Clarke, Email: Cynthia.clarke@hhchealth.org.
David O’Sullivan, Email: David.OSullivan@hhchealth.org.
Timothy Herbst, Email: Timothy.Herbst@hhchealth.org.
Steven Lee, Email: Steven.Lee@hhchealth.org.
References
- 1.Casey K, Iteen A, Nicolini R, Auten J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am J Emerg Med. 2020;38:1544. doi: 10.1016/j.ajem.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cellina M, Oliva G. Acute pulmonary embolism in a patient with COVID-19 pneumonia. Diagn Interv Imaging. 2020;101:325–326. doi: 10.1016/j.diii.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danzi GB, Loffi M, Galezaai G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? European Society of Cardiology. 2020;19:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiology: Cardiothoracic Imaging. 2020;2:2. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH, Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1714350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oudkerk M, Buller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297:1. doi: 10.1148/radiol.2020209016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez S, Ruiz-Artacho P, Merlo M, Suero C, Antolin A, Casal JR, Sanchez M, Ortega-Duarte A, Genis M, Piñera P, investigators of the ESPHERIA registry and the Venous Thromboembolic Disease Group of the Spanish Society of Emergency Medicine (ETV-SEMES) Risk profile, management, and outcomes of patients with venous thromboembolism attended in Spanish Emergency Departments. Medicine. 2017;96:e8796. doi: 10.1097/MD.0000000000008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer AJ, Thode HC, Peacock WF. Admission rates for emergency department patients with venous thromboembolism and estimation of the proportion of low risk pulmonary embolism patients: a US perspective. Clin Exp Emerg Med. 2016;3:126–131. doi: 10.15441/ceem.15.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavagna E, Muratore F, Ferrari F. Pulmonary thromboembolism in COVID-19: venous thromboembolism or arterial thrombosis? Radiology: Cardiothoracic Imaging. 2020;2:4. doi: 10.1148/ryct.2020200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S, Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandmaison G, Andrey A, Périard D, Engelberger RP, Carrel G, Doll S, Dexpert JB, Krieger C, Ksouri H, Hayoz D, Sridharan G. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open. 2020;4:e113–e115. doi: 10.1055/s-0040-1713167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamietzky M, Moore W, Fansiwala K, et al. Pulmonary embolism on CTPA in COVID-19 patients. Radiology: Cardiothoracic Imaging. 2020;2:4. doi: 10.1148/ryct.2020200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Chen X, Cai Y, Xia J’, Zhou X, Xu S, Huang H, Zhang L, Zhou X, du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]