Abstract
Background
Patients with implantable cardioverter defibrillator (ICD) use for primary prevention (primary prevention patients) of sudden cardiac death have lower incidence of appropriate ICD therapy (app-Tx) compared with those with ICD use for secondary prevention (secondary prevention patients). However, detail analysis of a second app-Tx after a first app-Tx is still lacking.
Objective
This study aimed to compare the incidence of a second app-Tx in primary vs secondary prevention patients.
Methods
We conducted sub-analysis of the Nippon Storm Study, which was a prospective, observational study involving 985 patients with structural heart disease (left ventricular ejection fraction ≤ 50%). Of these, we selected 251 patients (62 ± 14 years old, 82% men) who experienced at least one appropriate ICD therapy, and compared occurrence of a second app-Tx between primary (n = 116) and secondary (n = 135) prevention patients.
Results
There was no significant difference in the incidence of a second app-Tx between primary and secondary prevention patients (the cumulative incidence for a second app-Tx was 59% at 1 year and 79% at 3 years in primary prevention patients vs the cumulative incidence for the second app-Tx was 59% at 1 year and 75% at 3 years in secondary prevention patients).
Additionally, we evaluated the incidence of a second app-Tx according to basal structural disease (ischemic and non-ischemic cardiomyopathy) and found no significant difference between primary and secondary prevention patients.
Conclusion
Once app-Tx occurs, primary prevention patients acquire the high risk of subsequent ventricular arrhythmias because there is a comparable incidence of a second app-Tx in secondary prevention patients.
Keywords: Implantable cardioverter defibrillator, Ventricular arrhythmia, Primary prevention, Appropriate ICD therapy
1. Introduction
Several large randomized trials have shown an excellent role of implantable cardioverter-defibrillators (ICDs) for reducing mortality in patients with a high risk of sudden cardiac death, regardless of their indication (primary or secondary prevention) and underlying heart disease (ischemic [ICM] or non-ischemic cardiomyopathy [NICM]) [1], [2], [3], [4], [5], [6], [7]. However, patients implanted for primary prevention (primary prevention patients) are thought to have lower risk of ventricular arrhythmias than patients implanted for secondary prevention (secondary prevention patients). This is because previous clinical studies have shown a lower (approximately 50%) incidence of appropriate ICD therapy (app-Tx) in primary prevention patients compared with secondary prevention patients [8], [9], [10]. From another point of view, primary prevention patients in whom app-Tx is performed once are supposed to develop the risk of subsequent ventricular arrhythmias after implantation of the ICD. However, detailed analysis from the perspective of substantial risk development of ventricular arrhythmias after first app-Tx in patients with primary prevention is still lacking. The first app-Tx in primary prevention patients might suggest that their risk of a subsequent app-Tx has risen to a certain level equivalent to secondary prevention patients. We hypothesized that the risk of a second app-Tx for ventricular tachycardia (VT) and/or fibrillation (VF) in primary prevention patients is potentially equivalent to that in secondary prevention patients after first app-Tx.
2. Methods
2.1. Study participants
We conducted sub-analysis of the Nippon Storm Study, which was a prospective, observational study involving 1570 patients who were enrolled from 48 Japanese centers. The details of the overall study design of the Nippon Storm Study have already been published [11], [12]. All patients received a detailed informed consent and the study protocol was approved by the hospital’s institutional review board. The procedures were in accordance with the ‘Declaration of Helsinki’ and the ethical standards of the responsible committee on human experimentation. Data collection, including registration of patients with a new ICD or cardiac resynchronization therapy with ICD capabilities (CRT-D), began in October 2010 and data accumulation for the registry was terminated in July 2012. All patients were prospectively followed for at least 2 years.
To assess the potential risk for VT/VF, we selected 985 patients with structural disease with a left ventricular ejection fraction (LVEF) ≤ 50% from the Nippon Storm Study to standardize the basal condition between primary and secondary prevention patients. Patients with congenital genetic cardiac disease, such as long-QT syndrome, Brugada syndrome, and idiopathic ventricular fibrillation (LVEF > 50%), were excluded. This is because these patients have specific substrates of VT/VF, which are considered to have different mechanisms from patients with other structural heart disease.
2.2. Patients’ classification
The patients were classified into two groups as primary prevention patients and secondary prevention patients according to their indication for ICD/CRT-D at baseline. Further, they were divided into two sub-groups (finally four sub-groups) on the basis of their clinical course of app-Tx as follows: group-1A, primary prevention patients without app-Tx; group-1B, primary prevention patients with first app-Tx; group-2A, secondary prevention patients without app-Tx; and group-2B, secondary prevention patients with first app-Tx.
2.3. Definition of app-Tx
Every event was confirmed by the detail analysis of electrograms that were acquired from ICD/CRT-D in each center to discriminate whether each ICD therapy was appropriate or not. App-Tx was defined as appropriate anti-tachycardia pacing and/or shock therapy prior to sustained VT/VF. First app-Tx was defined as the first event of app-Tx from the day of ICD implantation. Second app-Tx was defined as subsequent app-Tx at least 24 h apart from the first app-Tx to discriminate the same episode.
2.4. Analysis of the first app-Tx
Initially, the incidence of the first app-Tx was compared between primary (group-1) and secondary prevention patients (group-2) using event-free survival analysis to show fundamental risk stratification of the enrolled patients. Further, to characterize primary prevention patients with first app-Tx (group-1B), we compared their clinical background with other groups (primary prevention patients without app-Tx [group-1A] and secondary prevention patients with first app-Tx [group-2B]).
2.5. Analysis of the second app-Tx
We compared the risk of subsequent app-Tx once after the first app-Tx event in primary prevention patients with secondary prevention patients. To achieve this, we evaluated the interval from the day when the first app-Tx occurred to the second app-Tx by setting the day of the first app-Tx as restarting at baseline. Event-free survival for the second app-Tx was compared between group-1B and group-2B.
2.6. Analysis of related factors for increasing the risk of a second app-Tx
We evaluated whether underlying heart diseases (i.e., ICM or NICM) and additional therapy affected event-free survival for patients with app-Tx (group-1B and group-2B). Additional therapy was defined as anti-arrhythmic pharmacotherapy for treating VT/VF after the first app-Tx and non-pharmacotherapy (catheter ablation). Modifying the setting of device therapy was not included in additional therapy. Multivariate analysis for associated factors for occurrence of a second app-Tx was evaluated by including gender, age (<75 years, ≥75 years), and the presence of factors, such as classification of heart failure (heart failure with reduced ejection fraction [LVEF < 40%] or heart failure with midrange ejection fraction [LVEF ≥ 40%]), indication for ICD/CRT-D (primary or secondary), type of device (ICD or CRT-D), and additional therapy for treating VT/VF (performed or not).
2.7. Statistical analysis
The results are expressed as frequencies and percentages for categorical variables and median or mean ± SD for numerical variables. Differences at baseline were evaluated with the Student’s t-test for continuous variables and the χ2 test for categorical valuables. Univariate and multivariate logistic regression analyses were performed to examine whether any variables were related to the patients’ classification. A two-tailed p < 0.05 was considered statistically significant. Event-free survival analysis was evaluated using the Kaplan-Meier method, and log-rank tests were used for statistical hypothesis tests. All statistical analysis was performed with JMP version 14 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline characteristic
The baseline characteristics of this analysis are shown in Table 1. Of 985 patients, we focused on the 251 patients who experienced at least one appropriate ICD therapy (group-1B, group-2B). Comparing 116 primary prevention patients who experienced app-Tx (group-1B) with 135 secondary prevention patients who experienced app-Tx (group-2B), the mean age and gender were similar between both groups. As for anti-arrhythmic drugs, amiodarone was prescribed more often in secondary prevention patients with the first app-Tx (group-2B) than primary prevention patients with the first app-Tx (group-1B) (23% in group-1B and 67% in group-2B, p < 0.0001). The prevalence of ischemic etiology was significantly lower and dilated cardiomyopathy was significantly higher in primary prevention patients with the first app-Tx than in secondary prevention patients with the first app-Tx (group-1B vs group-2B: ischemic etiology; 21% [24/116] vs 45% [61/135], p < 0.01; dilated cardiomyopathy; 48% [56/116] vs 24% [33/135], p < 0.01, respectively) (Table 1). Further, primary prevention patients with the first app-Tx (group-1B) had a significantly lower LVEF and more sever New York Heart Association [NYHA] classification compared with secondary prevention patients with the first app-Tx (group-2B) (LVEF: 27.9% vs 32.9%, p < 0.01; NYHA class I: 10% vs 31%, class II: 40% vs 46%, class III: 45% vs 19%, class IV: 6% vs 4%, p < 0.01, respectively) (Table 1). During follow-up periods, 124 patients died (72 primary prevention patients and 52 secondary prevention patients). Of these, 23 patients had experienced second appropriate ICD therapy (11 primary prevention patients and 12 secondary prevention patients). As for the cause of death, 112 patients died of non-sudden cardiac death and 12 patients died of sudden cardiac death (arrhythmic death).
Table 1.
Patients’ characteristics.
|
Primary revention pts |
Secondary prevention pts |
|||||||
|---|---|---|---|---|---|---|---|---|
| All | Group-1A | Group-1B | Group-2A | Group-2B |
P value |
|||
| Number of patients | 985 | 415 | 116 | 319 | 135 | 1A vs 1B | 1B vs 2B | |
| Clinical parameters | ||||||||
| Age(yo) | 65.7 ± 11.6 | 66.5 ± 12.2 | 65.7 ± 10.7 | 65.2 ± 11.5 | 67.9 ± 10.7 | 0.9 | 0.11 | |
| Male gender | 773 (78%) | 318 (77%) | 96 (83%) | 247 (77%) | 112(83%) | 0.15 | 0.97 | |
| LVEF (%) | 30.3 ± 9.6 | 27.4 ± 8.6 | 27.9 ± 8.7 | 33.6 ± 9.4 | 32.9 ± 9.4 | 0.59 | <0.01 | |
| BNP (pg/ml) | 580 ± 842 | 590 ± 813 | 652 ± 776 | 555 ± 873 | 543 ± 920 | 0.51 | 0.36 | |
| NYHA class | 0.99 | <0.01 | ||||||
| Ⅰ | 189 (9%) | 41 (11%) | 11 (10%) | 95 (30%) | 42 (31%) | |||
| Ⅱ | 391(40%) | 159 (38%) | 46 (40%) | 124 (39%) | 62 (46%) | |||
| III | 354 (36%) | 188 (45%) | 52 (45%) | 88 (28%) | 26 (19%) | |||
| Ⅳ | 51 (5%) | 27 (7%) | 7 (6%) | 12 (4%) | 5 (4%) | |||
| Medication | ||||||||
| βーblocker | 680 (69%) | 292 (70%) | 78 (67%) | 220 (69%) | 88 (65%) | 0.72 | 0.79 | |
| ACE inhibitor/ARB | 670 (68%) | 301 (73%) | 75 (65%) | 213 (67%) | 79 (59%) | 0.11 | 0.36 | |
| Amiodarone | 434 (44%) | 123 (30%) | 27 (23%) | 194 (61%) | 90 (67%) | 0.20 | <0.0001 | |
| Sotarol | 20 (2%) | 3 (1%) | 1 (1%) | 10 (3%) | 6 (4%) | 1.0 | 0.13 | |
| Additional therapy after first app-Tx | ||||||||
| anti-arrhythmic and/or non-pharmacotherapy | 9 (8%) | 32 (24%) | <0.001 | |||||
| anti-arrhythmic pharmacotherapy | 6 (5%) | 22 (16%) | <0.01 | |||||
| non-pharmacotherapy | 3 (3%) | 10 (7%) | 0.09 | |||||
| Underlying heart disease | ||||||||
| IHD or NIHD | <0.01 | <0.01 | ||||||
| IHD | 416 (42%) | 146 (35%) | 24 (21%) | 185 (58%) | 61 (45%) | |||
| DCM | 334 (34%) | 186 (45%) | 56 (48%) | 59 (19%) | 33 (24%) | |||
| HCM | 56 (6%) | 18 (4%) | 11 (10%) | 19 (6%) | 8 (6%) | |||
| CS | 53 (5%) | 19 (5%) | 8 (7%) | 17 (5%) | 9 (7%) | |||
| valvular disease | 25 (3%) | 8 (2%) | 5 (4%) | 9 (3%) | 3 (2%) | |||
| HHD | 16 (2%) | 9 (2%) | 2 (2%) | 5 (2%) | 0 (0%) | |||
| ARVC | 11 (1%) | 2 (1%) | 1 (1%) | 4 (1%) | 4 (3%) | |||
| other | 74 (8%) | 27 (7%) | 9 (8%) | 21 (7%) | 17 (13%) | |||
| Type of device | ||||||||
| ICD | 495 (50%) | 135 (33%) | 41 (35%) | 224 (70%) | 95 (70%) | 0.58 | <0.001 | |
| CRT-D | 490 (50%) | 280 (68%) | 75 (65%) | 95 (30%) | 40 (30%) | 0.58 | <0.001 | |
Data are presented as mean ± SD or n (%).
Abbreviations: app-Tx, appropriate ICD therapy; Group-1A, primary prevention patients without app-Tx; group-1B, primary prevention patients with the first app-Tx; group-2A, secondary prevention patients without app-Tx; group-2B, secondary prevention patients with the first app-Tx; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide; NYHA, New York Heart Association; ACE inhibitors, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; IHD, ischemic heart disease; NIHD, non-ischemic heart disease; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; CS, cardiac sarcoidosis; HHD, hypertensive heart disease; ARVC, arrhythmogenic right ventricular cardiomyopathy; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy defibrillator.
3.2. Analysis of the first app-Tx
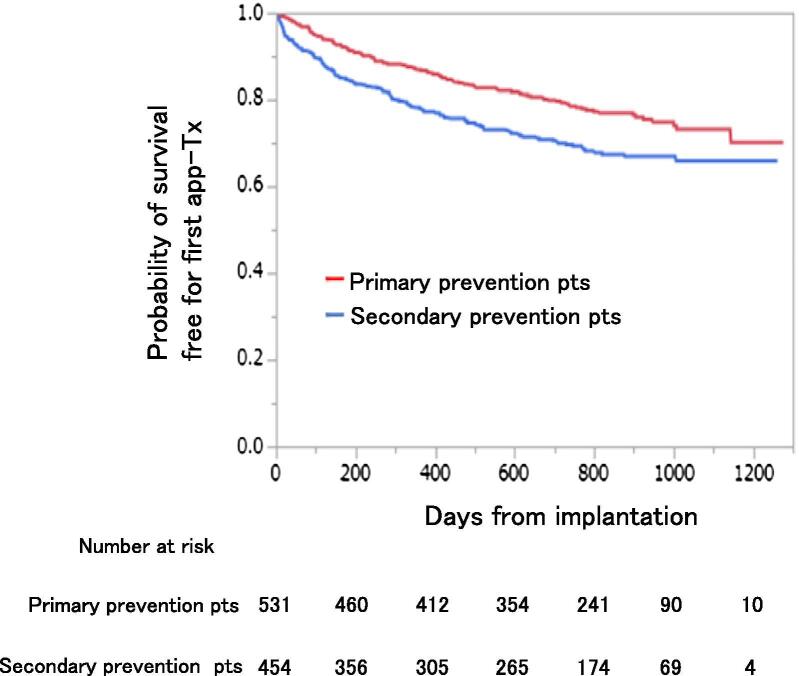
As shown in Fig. 1, 116 of 531 primary prevention patients (22%) experienced a first app-Tx (group-1B) and 135 of 454 secondary prevention patients (30%) experienced a first app-Tx (group-2B). Event-free survival analysis for the first app-Tx showed that secondary prevention patients had a significantly higher cumulative incidence for the first app-Tx than primary prevention patients (log-rank test: p = 0.001, Fig. 2). Cox regression analysis showed that secondary prevention patients had an increasing risk of the cumulative incidence for the first app-Tx compared with primary prevention patients (p = 0.001, hazard ratio [HR]: 1.5, 95% confidence interval [CI]: 1.18–1.94). In primary prevention patients, the cumulative incidence for the first app-Tx was 13% (95% CI: 0.10–0.16) at 1 year and 27% (95% CI: 0.23–0.32) at 3 years. In secondary prevention patients, the cumulative incidence for the first app-Tx was 21% (95% CI: 0.18–0.24) at 1 year and 36% (95% CI: 0.31–0.41%) at 3 years.
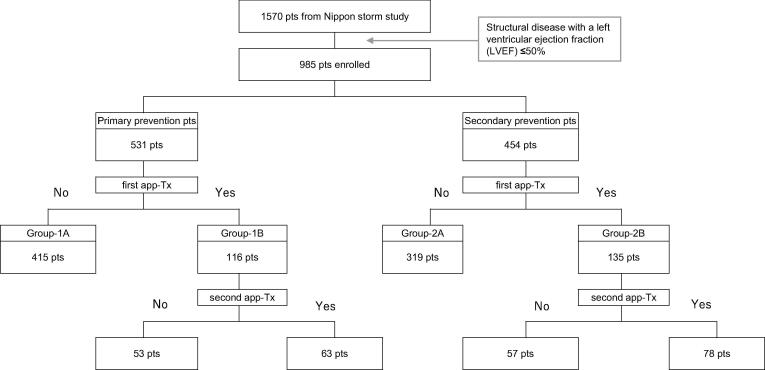
Fig. 1.
Clinical course of appropriate therapy for primary and secondary prevention patients. Abbreviations: app-Tx, appropriate ICD therapy. Group-1A, primary prevention patients without app-Tx; group-1B, primary prevention patients with the first app-Tx; group-2A, secondary prevention patients without app-Tx; group-2B, secondary prevention patients with the first app-Tx.
Fig. 2.
Survival curve for a first app-Tx between primary and secondary prevention patients. Abbreviations: app-Tx, appropriate ICD therapy; pts, patients.
Table 1 shows the baseline characteristics of the patients in the four sub-groups. The clinical background was not significantly different between primary prevention patients with and without app-Tx (group-1A vs group-1B), except for the prevalence of ischemic heart disease and hypertrophic cardiomyopathy (21% [24/116] vs 35% [146/415], p < 0.01; 10% [11/116] vs 4% [18/415], p = 0.038, respectively).
3.3. Analysis of the second app-Tx
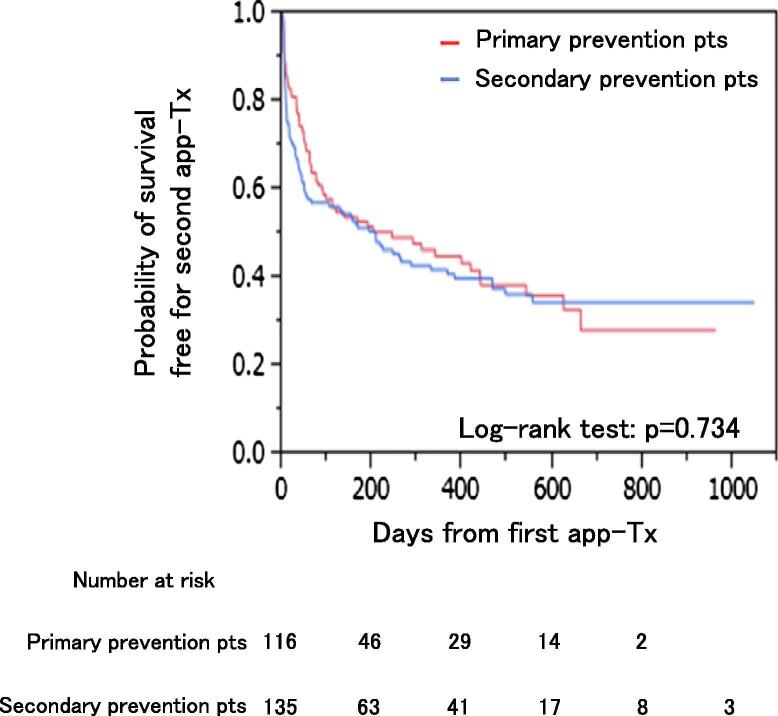
As shown in Fig. 1, a second app-Tx was performed in 63 of 116 (54%) primary prevention patients (group-1B) and in 78 of 135 (58%) secondary prevention patients (group-2B). Event-free survival analysis for the second app-Tx showed that there was no significant difference between group-1B and group-2B (Fig. 3). Among the patients in group-1B, the cumulative incidence for the second app-Tx was 59% (95% CI: 0.49–0.67) at 1 year and 79% (95% CI: 0.67–0.86) at 3 years. For group-2B, the cumulative incidence for the second app-Tx was 59% (95% CI: 0.50–0.66) at 1 year and 75% (95% CI: 0.66–0.83) at 3 years.
Fig. 3.
Event-free survival for a second app-Tx between primary and secondary prevention patients. Abbreviations: app-Tx, appropriate ICD therapy; pts, patients.
3.4. Analysis of related factors an increased risk of the second app-Tx for primary prevention patients
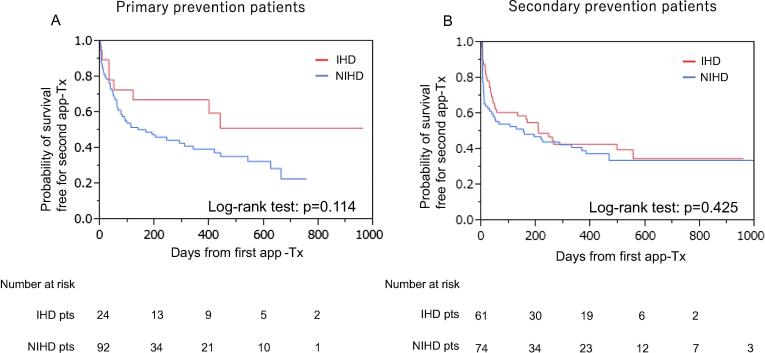
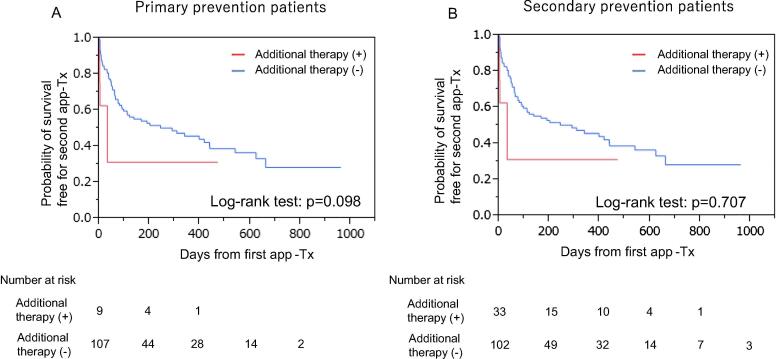
We determined the incidence of a second app-Tx by different etiologies (i.e., ICM and NICM). There was no significant difference in event-free survival between patients with ICM and NICM in group-1B and group-2B (group-1B; 85 patients: ICM/NICM = 24/61, group-2B; 166 patients: ICM/NICM = 92/74, log-rank test: p = 0.114 and p = 0.425, respectively, Fig. 4). Further, the effect of additional therapy just after the first app-Tx was investigated. Additional therapy was applied significantly more often in secondary prevention patients with app-Tx compared with primary prevention patients with app-Tx (32/135 [24%; anti-arrhythmic pharmacotherapy in 22, non-pharmacotherapy in 10] vs 9/116 [8%; anti-arrhythmic pharmacotherapy in 6, and non-pharmacotherapy in 3], p < 0.001). However, the event-free survival curve showed that the incidence of the second app-Tx was not affected by additional therapy for treating VT/VF after the first app-Tx in group-1B and group-2B (log-rank: p = 0.098 and p = 0.707, respectively, Fig. 5). In addition, we accessed the incidence of ICD programing modification after the first appropriate therapy because it might have affected the second appropriate therapy. However, there was no significant difference between primary and secondary prevention patients for ICD programing modification after the first appropriate therapy in our study (group1-B vs group2-B = 21/116(18%) VS 37/135(27%), p = 0.081).
Fig. 4.
Event-free survival for a second app-Tx between IHD and NIHD in primary and secondary prevention patients. Abbreviations: app-Tx, appropriate ICD therapy; pts, patients; IHD, ischemic heart disease; NIHD, non-ischemic heart disease.
Fig. 5.
Event-free survival for a second app-Tx in primary and secondary prevention patients with and without additional antiarrhythmic therapy. Abbreviations: app-Tx, appropriate ICD therapy.
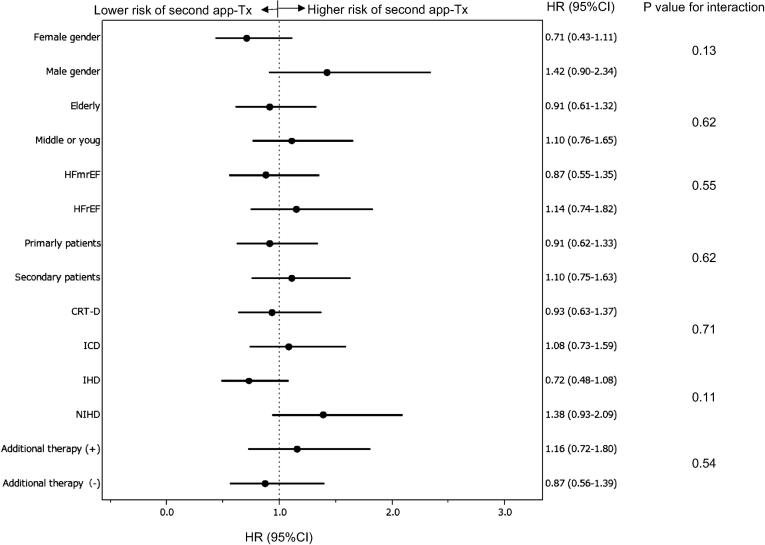
Multivariate analysis with the Cox proportional hazard model showed that no factors were associated with the second app-Tx, including male gender (HR: 1.42 [0.92–2.34]), older patients (HR: 0.91 [0.61–1.32]), heart failure with reduced ejection fraction (HR: 1.14 [0.74–1.82]), primary indication (HR: 0.91 [0.62–1.33]), CRT-D implantation (HR: 0.93 [0.63–1.37], ischemic heart disease (HR: 0.72 [0.48–1.08]), and additional therapy (HR: 1.16 [0.72–1.39]) (Fig. 6).
Fig. 6.
Multivariate model of HRs for the risk of a second app-Tx. Abbreviations: app-Tx, appropriate ICD therapy; HR, hazard ratio; CI, confidence interval; HFmrEF, heart failure with midrange ejection fraction; HFrEF, heart failure with reduced ejection fraction; IHD, ischemic heart disease; NIHD, non-ischemic heart disease; CRT-D, cardiac resynchronization therapy defibrillator, ICD, implantable cardioverter defibrillator.
4. Discussion
In concordance with previous studies, primary prevention patients showed lower incidence of first app-Tx than secondary prevention patients in our study [8], [9], [10]. On the other hand, once app-Tx occurred, primary prevention patients acquired higher risk of subsequent ventricular arrhythmias as well as in secondary prevention patients because the incidence of the second app-Tx was not significantly different between the two groups.
There are some reasons to explain why primary prevention patients showed an equivalent incidence of a second app-Tx as secondary prevention patients. First, NICM was the most prevalent heart disease in primary prevention patients in our study. Because NICM is thought to be a progressive disease, primary prevention patients in our cohort might have acquired potential risk of ventricular arrhythmias to a larger extent compared with patients with ischemic cardiomyopathy. Even though there was no significant difference between the rates of ICM and NICM in primary prevention patients, the incidence of the second app-Tx in primary prevention patients with NICM was relatively higher compared with the other groups (Fig. 4). Second, optimal drug therapy, such as β-blockers and angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) might have affected the incidence of app-Tx. These drugs prevent expansion of infarction into adjacent non-infarcted areas and hypertrophy of myocytes at the remote phase of post-myocardial infarction (i.e., left ventricular remodeling), especially in patients with ICM, which might reduce the risk of ventricular arrhythmias. Previous studies have reported that use of β-blockers, ACE inhibitors, and ARBs significantly reduce the risk of sudden cardiac death in patients with chronic heart failure [13], [14], [15]. However, in the present study, these drugs were prescribed at a similar rate. Despite these drugs, risk of subsequent app-Tx could not be completely inhibited in all patients with heart failure.
Satake et al. reported that prophylactic use of ICD is still low in Japan (CHART-2 study) [16]. Our sub-analysis showed that primary prevention patients had a potential risk for ventricular arrhythmias. Primary prevention patients tend to have a smaller number of ventricular arrhythmias, which require app-Tx to terminate them, than secondary prevention patients. However, once app-Tx is performed, primary prevention patients have an equivalent risk for subsequent ventricular arrhythmia as secondary prevention patients. We should not underestimate the risk of occurrence of life-threatening ventricular tachyarrhythmia in primary prevention patients. Notably, once primary prevention patients experience app-Tx, additional therapy should be considered to the same extent as secondary prevention patients.
4.1. Study limitations
This study has several limitations. First, the prospective, observational design and multicenter registry resulted in a lack of randomization, and there might have been hidden bias. Second, we did not collect data on subsequent changes in NYHA class, LVEF, and brain natriuretic peptide levels during the follow-up period. Patients with recurrent VT/VF attacks might have had further development of anatomical or functional remodeling. Third, we did not differentiate ventricular arrhythmias between VT and VF on the basis of electrograms during ICD therapy. Even in analysis of EGMs, discrimination of VT and VF is sometimes difficult because some VTs have QRS irregularity and variability in rate. Fourth, we could not standardize ICD model and programing for primary and secondary prevention patients because of the study protocol of Nippon Storm Study. Lastly, we could not compare the probability of a second appropriate therapy occurrence after the first therapy in primary prevention patients vs the probability of first appropriate therapy occurrence in secondary prevention patients. This comparison might be the direct way to compare the occurrence of truly second VT/VF event for each group. However, as main study did not collect the date of first VT/VF occurrence in secondary prevention patients, it was not possible to compare progression of VT/VF risk between primary and secondary prevention patients from this viewpoint.
5. Clinical implications and conclusion
In patients with ICD use for primary prevention, once app-Tx is performed, their risk of subsequent app-Tx has risen to certain level similar to secondary prevention patients because there is a comparable incidence of subsequent app-Tx for primary and secondary prevention. Additional therapies to prevent further arrhythmic events should be considered to the same extent in primary prevention patients after the first app-Tx as in secondary prevention patients because of the possibility of requiring a second app-Tx, even in primary prevention patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Moss A.J., Zareba W., Hall W.J. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N. Engl. J. Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 2.Moss A.J., Zareba W., Hall W.J. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Kadish A., Dyer A., Daubert J.P. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 4.Bardy G.H., Lee K.L., Mark D.B. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Bristow M.R., Saxon L.A., Boehmer J. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 6.Bänsch D., Antz M., Boczor S. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–1458. doi: 10.1161/01.cir.0000012350.99718.ad. [DOI] [PubMed] [Google Scholar]
- 7.Strickberger S.A., Hummel J.D., Bartlett T.G. AMIOVIRT Investigators. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia– AMIOVIRT. J. Am. Coll. Cardiol. 2003;41:1707–1712. doi: 10.1016/s0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 8.AVi Sabbag, Mahmoud Suleiman, Avishag Laish-Farkash, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting. Heart rhythm 2015;12:2426-2433 [DOI] [PubMed]
- 9.Rahmawati A., Chishaki A., Ohkusa T. Influence of primary and secondary prevention indications on anxiety about the implantable cardioverter-defibrillator. J. Arrhythmia. 2016;32:102–107. doi: 10.1016/j.joa.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.G.H. van Welsense, J.B. van Rees, C.J. Willem Borleffs, et al. Long-term follow-up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace(2011) 13, 389-394 [DOI] [PubMed]
- 11.Kurita T., Noda T., Nitta T. Nippon storm study design. J. Arrhythmia. 2012;28:277–279. [Google Scholar]
- 12.Noda T., Kurita T., Nitta T. Appropriate duration of driving restrictions after inappropriate therapy from implantable cardiac shock devices-interim analysis of the nippon storm study. Circ. J. 2014;78:1989–1991. doi: 10.1253/circj.cj-14-0589. [DOI] [PubMed] [Google Scholar]
- 13.CIBIS-II Investigators and Committees. The Cardiac Insufficiency BISoprolol study II (CIBIS-II): A randomised trial. Lancet 1999; 352: 9–13 [PubMed]
- 14.Kober L., Torp-Pedersen C., Carlsen J.E. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 1995;80:257–266. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B., Segal R., Martinez F.A. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 16.Satake H., Fukuda K., Sakata Y. Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure. Circ J. 2015;79:381–390. doi: 10.1253/circj.CJ-14-0925. [DOI] [PubMed] [Google Scholar]