Abstract
Mycobacterium chelonae is a type of nontuberculous mycobacteria most commonly associated with skin and soft tissue infections. We present a case of recurrent M. chelonae pulmonary infection presenting with severe weight loss. After recurrence, sputum cultures remained positive for 2 years despite appropriate antibiotics. Cultures only became negative after the addition of intravenous imipenem and jejunostomy feeds. The rarity of M. chelonae pulmonary infection means that optimal treatment regimens have not yet been fully established but a regimen of clarithromycin plus an additional antibiotic has been recommended1. The prognosis of such infections also remains unclear but lower rates of macrolide resistance suggest that the prognosis may be better than the closely related species M. abscessus. Although its benefit has not been proven, nutrition supplementation, including percutaneous enteral feeding, can be considered for refractory NTM infection in underweight patients.
Keywords: Mycobacterium chelonae, Nutrition, Non-tuberculous mycobacteria
1. Introduction
Nontuberculous mycobacteria (NTM) include over 170 species [2]. Although not all species are pathogenic in humans, some are capable of causing potentially fatal pulmonary and extrapulmonary disease [1]. Further, the prevalence of NTM infections appears to be increasing across much of the world [3]. NTM are frequently classified into slowly-growing and rapidly-growing groups [1]. There are currently 3 main rapidly-growing mycobacterial (RGM) species of clinical importance: M. abcsessus, M. fortuitum and M. chelonae [4]. M. chelonae most often causes skin and soft tissue infection which can also be a manifestation of disseminated disease [1]. Pulmonary infection has rarely been reported [5].
M. chelonae is commonly found in both natural bodies of water and municipal water supplies and water to is believed to be the usual source of human infection [6]. Risk factors for the development of NTM infection are numerous and include underlying lung disease, immune compromise and a tall slender body habitus especially when associated with pectus excavatum, scoliosis or mitral valve prolapse [7]. In recent years, malnourished status has also been recognized as a risk factor for disease progression in patients with NTM infection [8], [9] although it remains unclear if nutritional supplementation can alter patient outcomes.
We present a case of M. chelonae pulmonary infection successfully treated with a combination of antibiotic therapy and nutritional supplementation.
2. Case presentation
A 61 year-old man initially presented with unexplained weight loss of 9 kg over the preceding 12 months to a weight of 65 kg and a productive cough in June 2005. He had undergone a liver transplant in 2001 for hepatic cirrhosis in the context of hepatitis C infection and prior ethanol abuse. He had undergone a partial gastrectomy in the 1960 s for peptic ulcer disease. He is an ex-smoker with a 20 pack-year history who quit in 1992. During his assessment, he denied any recent dietary changes, night sweats or fevers. Tacrolimus was his only immunosuppressive medication and levels were therapeutic. A CT chest revealed mild bronchiectasis and nodules in the left lower lobe of the lung as well as nodules and patchy groundglass opacities in the right upper lobe (Fig. 1). A CT scan of the abdomen was unremarkable apart from evidence of his prior gastric surgery. A complete blood count, albumin, and bilirubin level were normal. Serum aminotransferases were elevated by approximately 2 times the upper limit of normal on initial assessment but normalized on repeat testing 6 weeks later. Testing for human immunodeficiency virus was negative.
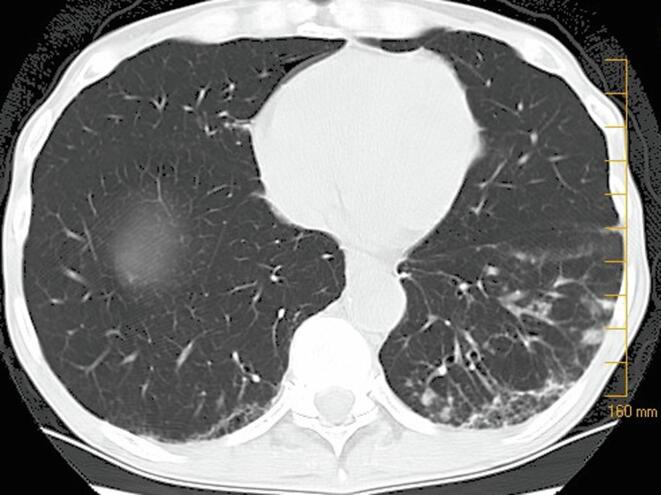
Fig. 1.
Representitive image from a computed tomography of the chest of a patient with Mycobacterium chelonae pulmonary infection from June 2005. Notable findings in this imageare mild bronchiectasis and nodules in the left lower lobe of the lung.
Sputum cultures performed in September 2005 were positive for M. chelonae. The patient was initiated on rifampin 600 mg daily and clarithromycin 500 mg twice daily. With this therapy, his cough and exercise tolerance improved as did his radiographic abnormalities. Liver enzymes remained normal. By March 2006, he had regained 11 kg and his antibiotics were stopped. A repeat sputum culture was negative for mycobacteria in August 2007.
The patient was then lost to follow up until February 2010 when he presented with fever, cough and weight loss. His weigh had dropped 18.5 kg over at least 2 years and his BMI was 17.9. Repeat human immunodeficiency virus testing was negative. Esophagogastroduodenoscopy showed a small stomach with some retained food but he denied early satiety and regurgitation or symptoms of aspiration. He underwent nutritional counselling with the aim of increasing caloric intake. Two sputum cultures were again positive for M. chelonae.
He was started on moxifloxacin 400 mg daily and clarithromycin 500 mg twice daily in March 2010. Unfortunately, he remained febrile and continued to gradually lose weight. A repeat CT scan of chest revealed progression of bronchiectasis in comparison to 2005 as well as new groundglass opacities diffusely (Fig. 2). Despite therapy, repeat sputum cultures in March 2011 remained positive for M chelonae. Susceptibility testing revealed that the organism was sensitive to amikacin, imipenem and clarithromycin but resistant to ciprofloxacin, linezolid, cefoxitin and trimethoprim-sulfamethoxazole.
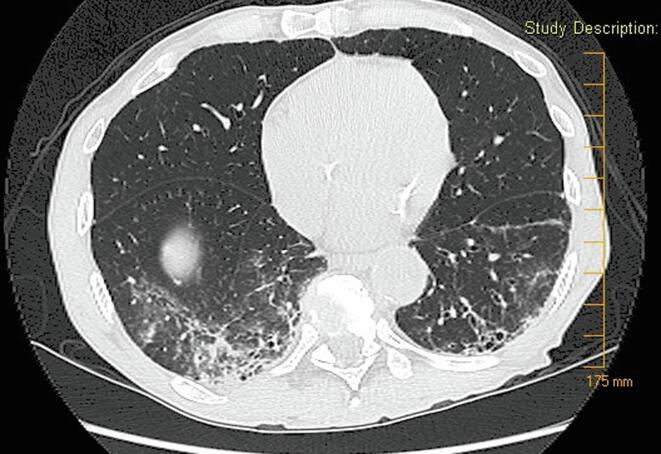
Fig. 2.
Computed tomography of the chest of a patient with recurrence of Mycobacterium chelonae pulmonary infection from June 2010. This image reveals progression of bronchiectasis in comparison to 2005 as well as new groundglass opacities.
He was referred to the mycobacterial disease clinic at our tertiary care centre in March 2012. His weight had fallen to 54.5 kg and sputum cultures were again positive for M. chelonae despite ongoing treatment with clarithromycin and moxifloxacin. His estimated glomerular filtration rate was 35 ml/min/1.73 m2 by diethylenetriaminepentaacetic acid renal scan. His poor renal function was felt to preclude the use of amikacin but imipenem 250 mg twice daily and doxycycline 100 mg twice daily were added to his regimen in April 2012. An elevation in alanine aminotransferase to 5 times the upper limit of normal prompted discontinuation of doxycycline in June 2012. Liver enzymes subsequently returned to baseline. Despite therapy, his weight had continued to decline to 52 kg by August 2012 and a jejunostomy tube was inserted. He received feeds consisting of Resource 2.0 (Nestle Health Science) at 40 cc/hour for 12 h per day in addition to food consumed orally.
With this therapy, his weight increased by 20 kg over 10 months and cough nearly resolved. Three mycobacterial sputum cultures were negative in November 2012 and again in April 2013. By October 2013, he was able to maintain a weight of 69.5 kg with jejunostomy tube feeds held so the tube was removed. Antibiotics were stopped in February 2014. Three mycobacterial sputum cultures were again negative in December 2014. Unfortunately, in May 2016, 1 of 3 sputum cultures was positive for M. chelonae and his weight had dropped to 60 kg. He felt well with no dyspnea and only minimal cough. He has had no progression of radiographic findings or symptoms. As such, he is being followed closely but therapy has not been re-initiated.
3. Discussion
Pulmonary infection with M. chelonae is rare. In a series from a Texas reference laboratory, 154 patients with pulmonary infection from RGM were identified over a 15-year period and only 1 patient was identified as having M. chelonae [10]. Evaluation of previously published reports regarding M. chelonae is complicated by the evolving taxonomy of the species. Prior to 1992, M. chelonae was not recognized as a separate species from M. abscessus and even some studies published after this date do not differentiate between them [11]. However, differentiation between the two species is important due to differences in treatment and, most likely, prognosis [1], [10].
Given the rarity of the condition, optimal treatment for M. chelonae infection is not yet fully established. Recent guidelines for the treatment of NTM pulmonary disease do not offer specific recommendations for the treatment of this organism [12], [13]. However, previous guidelines suggest a combination of clarithromycin plus one of tobramycin, amikacin linezolid, imipenem, clofazimine, or doxycycline with selection of the additional antibiotic based on in vitro sensitivities [1]. Treatment is recommended to continue until 12 months of negative sputum cultures have been obtained [1].
It should be noted that initial management of the patient was not in keeping with these guidelines. Firstly, antibiotic susceptibility testing does was not performed initially and secondly rifampin is not typically part of recommended treatment [1]. Further, only 20% of M. chelonae isolates are susceptible to ciprofloxacin 20%, which likely translates to a low likelihood of susceptibility to moxifloxacin [1]. The rationale for these treatment decisions is unclear since they occurred prior to referral to our clinic.
In the present case, concerns regarding medication side effects (renal dysfunction and hepatotoxicity) also limited antibiotic options. This is common in the treatment of NTM infections with antibiotic discontinuation or dose reduction reported to occur 18–50% of the time in NTM infection [14].
However, even with optimal treatment, relapse of infection followed by persistent culture positivity despite prolonged antibiotics is common in the treatment of NTM and especially RGM [4]. Among RGM, treatment outcomes are best established for M. abscessus. Patients infected with macrolide-resistant strains of this organism are significantly less likely to achieve sputum culture conversion and relapse is nearly universal among those who do [15]. Macrolide resistance is less common and appears less likely to be induced in vitro by macrolide exposure in M. chelonae compared to M. abscessus [16]. These findings suggest that M. chelonae pulmonary infection may carry a better prognosis than M. abscessus although this remains somewhat speculative.
In the case we present, weight loss was the predominant feature although cough and dyspnea developed shortly after his initial presentation. The substantial and recurrent weight loss experienced by the patient we present highlights the importance of nutrition in NTM infections. Low body mass index (BMI) is a well established risk factor for NTM infection [17], [18]. Further, nutritional markers including low body weight, body fat and muscle mass have been correlated with an increased risk of progression of radiographic findings in M. avium complex infection [8], [9], [19]. Nonetheless, it is unclear whether weight loss represents a cause or effect of disease progression or indeed whether both are true. Further, to our knowledge the potential benefit of nutritional supplementation has not been evaluated.
In this case, the role played by nutritional supplementation cannot be definitively separated by that played by antibiotic therapy. The patient’s initial regimen of clarithromycin and moxifloxacin was insufficient as evidenced by his ongoing symptoms and positive sputum cultures despite 2 years of continuous therapy. However, the patient’s clinical status deteriorated even after escalation of antibiotic therapy and the initiation of percutaneous enteral feeding was co-incident with both weight gain and subsequent sputum conversion which suggests a probable benefit.
4. Conclusions
M. chelonae is a rare cause of pulmonary infection. Although optimal treatment regimens have not yet been fully established but a regimen of clarithromycin plus an additional antibiotic has been recommended. The prognosis of M. chelonae pulmonary infection also remains unclear but lower rates of macrolide resistance suggest that the prognosis may be better than the closely related species M. abscessus. Although its benefit has not been proven, nutrition supplementation, including percutaneous enteral feeding, can be considered for refractory NTM infection in underweight patients.
5. Ethics Statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Griffith D.E. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Forbes B.A., Kraft C.S. Mycobacterial Taxonomy. J. Clin. Microbiol. 2017;55(2):380–383. doi: 10.1128/JCM.01287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevots D.R., Marras T.K. Epidemiology of Human Pulmonary Infection with Nontuberculous Mycobacteria: A Review. Clin. Chest Med. 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasperbauer S.H., De Groote M.A. The Treatment of Rapidly Growing Mycobacterial Infections. Clin. Chest Med. 2015;36:67–78. doi: 10.1016/j.ccm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Ko Y. Nontuberculous Mycobacterial Lung Disease Caused by Mycobacterium chelonae: A Case Report. Tuberc. Respir. Dis. 2013;74:191–194. doi: 10.4046/trd.2013.74.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones R.S., Shier K.L., Master R.N., Bao J.R., Clark R.B. Current significance of the Mycobacterium chelonae-abscessus group. Diagn Microbiol Infect Dis. 2019;94(3):248–254. doi: 10.1016/j.diagmicrobio.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Honda J.R., Knight V., Chan E.D. Pathogenesis and Risk Factors for Nontuberculous Mycobacterial Lung Disease. Clin. Chest Med. 2015;36:1–11. doi: 10.1016/j.ccm.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Ikegame S. Nutritional assessment in patients with pulmonary nontuberculous mycobacteriosis. Intern. Med. Tokyo Jpn. 2011;50:2541–2546. doi: 10.2169/internalmedicine.50.5853. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto K. Nutritional indicators are correlated with the radiological severity score in patients with Mycobacterium avium complex pulmonary disease: a cross-sectional study. Intern. Med. Tokyo Jpn. 2014;53:397–401. doi: 10.2169/internalmedicine.53.1277. [DOI] [PubMed] [Google Scholar]
- 10.Griffith D.E., Girard W.M., Wallace R.J. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am. Rev. Respir. Dis. 1993;147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 11.Brown-Elliott B.A., Wallace R.J. Clinical and Taxonomic Status of Pathogenic Nonpigmented or Late-Pigmenting Rapidly Growing Mycobacteria. Clin. Microbiol. Rev. 2002;15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haworth C.S., Banks J., Capstick T., Fisher A.J., Gorsuch T., Laurenson I.F., Leitch A., Loebinger M.R., Milburn H.J., Nightingale M., Ormerod P., Shingadia D., Smith D., Whitehead N., Wilson R., Floto R.A. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72(Suppl 2):ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927.supp2. [DOI] [PubMed] [Google Scholar]
- 13.Daley C.L. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline: Executive Summary. Clin. Infect. Dis. 2020;71:e1–e36. doi: 10.1093/cid/ciaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leber A., Marras T.K. The cost of medical management of pulmonary nontuberculous mycobacterial disease in Ontario. Canada. Eur. Respir. J. 2011;37:1158–1165. doi: 10.1183/09031936.00055010. [DOI] [PubMed] [Google Scholar]
- 15.Jeon K. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am. J. Respir. Crit. Care Med. 2009;180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 16.Brown B.A., Wallace R.J., Onyi G.O., De Rosas V., Wallace R.J. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob. Agents Chemother. 1992;36:180–184. doi: 10.1128/aac.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim R.D. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am. J. Respir. Crit. Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirac M.A. Environment or host?: A case-control study of risk factors for Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2012;186:684–691. doi: 10.1164/rccm.201205-0825OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.J. Risk factors for deterioration of nodular bronchiectatic Mycobacterium avium complex lung disease. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2014;18:730–736. doi: 10.5588/ijtld.13.0792. [DOI] [PubMed] [Google Scholar]