Abstract
Background
Undernutrition and tuberculosis are the major public health problems of people living in middle and low-income countries. Even though single studies have been reported from different areas of Ethiopia, there is no national-level study that estimates the overall burden of undernutrition among tuberculosis patients. Therefore, this study aimed to estimate the overall magnitude of undernutrition among adult tuberculosis patients in Ethiopia.
Methods
We searched out records from databases such as PubMed/MEDLINE, HINARI, EMBASE, Scopus, Google Scholar, and African journals and the online Ethiopian University repositories for published and unpublished articles. The data were extracted using a standardized data extraction format. Meta-analysis was computed using STATA version 11 software. The Cochrane Q-test and I2 with its corresponding p-value were used to assess the heterogeneity of the study. The presence of publication bias was evaluated using Egger’s test and presented with funnel plots. The random-effects meta-analysis model was computed to estimate the pooled prevalence of undernutrition among adult tuberculosis patients.
Results
A total of Twelve observational studies with 4963 study participants were included in this systematic review and meta-analysis. The pooled prevalence of undernutrition among tuberculosis patients in Ethiopia was 50.8% (95% CI 43.97, 57.63). The results of subgroup analysis showed that the highest prevalence of undernutrition among TB patients was observed from studies done in the Amhara region (65.63%). In this meta-analysis, the pooled prevalence of undernutrition among TB-HIV co-infected patients was 45.45% (95%CI 21.85, 56.07).
Conclusion
The prevalence of undernutrition among TB patients in Ethiopia was noticeably high. The result of this study showed that undernutrition is more severe in the Amhara regional state. Additionally, TB-HIV co-infected patients are highly affected by undernutrition. Therefore, the Ministry of Health in collaboration with clinicians should give special attention to provide nutritional care and support for TB patients as part of regular care. Moreover, special nutritional support should be designed for TB-HIV co-infected patients.
Abbreviations: AOR, Adjusted Odds Ratio; BMI, Body Mass Index; CI, Confidence Interval; TB, Tuberculosis; WHO, World Health Organizations
Keywords: Tuberculosis, Undernutrition, Ethiopia, Systematic review, Meta-analysis
1. Introduction
Tuberculosis (TB) is a communicable disease caused by Mycobacterium tuberculosis and is the leading cause of death from a single infectious agent. The bacteria mainly affect the lungs but can also disseminate to other sites of the body. According to the WHO 2019 Global tuberculosis report, there were 10 million infections and 1.2 million deaths among HIV-negative people. HIV infection increases the risk of TB and in 2018 there were 251 thousand TB deaths among HIV-infected people [1]. The burden of TB infection is higher in Sub-Saharan Africa, which is estimated to be 24% of the 10 million global TB cases [1]. In Ethiopia, TB is the leading cause of death from infectious diseases, and there were 30 thousand deaths due to TB every year [2].
Undernutrition is a major public health problem for people living in underdeveloped regions of the world. According to the Food and Agriculture Organization (FAO) 2016 estimate, 815 million people are undernourished with majorly living in developing countries, particularly Sub-Saharan Africa accounts for 22.7% [3]. In Ethiopia, 33% of adult men aged 15–49 and 22% of adult women aged 14–49 exhibited undernourishment (Body Mass Index (BMI) < 18.5 kg/m2) [4]. TB-infected individuals are more vulnerable to undernutrition compared to the general population. Globally, one-quarter of TB infection is due to malnutrition [5]. Undernutrition and TB have a bidirectional relationship. A person having active TB develops undernutrition and in turn, undernutrition increases the person's susceptibility to developing TB [5], [6]. A comparative cross-sectional study conducted in the Amhara region, Ethiopia, indicated that 33.8% of excess undernutrition was observed in TB patients compared with TB-free populations [7]. Undernutrition weakens the individual immune system, which increases the risk of primary TB infection or activation of latent TB. Moreover, undernutrition increases the risk of mortality, poor treatment outcome, and TB relapse [3], [5], [8].
The findings of previous studies showed that the morbidity and mortality of TB are mainly associated with undernutrition, low socio-economic status, and poor immune function [9], [10], [11]. A retrospective cohort study conducted in Taipei, Taiwan, indicated that underweight (BMI < 18.5 kg/m2) was a significant predictor of all causes of TB mortality [12]. Therefore, improving the nutritional status of TB patients is a critical step towards the reduction of mortality due to TB [9]. Moreover, nutritional supplements that contain both macro-and micronutrients could help people to resist TB infection, decrease the relapse of TB, and to increase TB treatment outcome through strengthening their immune system [13], [14]. In Ethiopia, different observational studies were conducted to show the magnitude of undernutrition among TB patients. The results of these studies revealed that the prevalence of undernutrition among TB patients was ranged from 28.5% to 71.35% [15], [16].
In Ethiopia, no national-level studies are showing the burden of undernutrition among TB patients. Estimating the overall pooled magnitude of undernutrition among TB patients is very crucial to improve the quality of care through developing appropriate plans, policies, and implementation programs. Therefore, this study aimed to estimate the overall pooled prevalence of undernutrition among adult TB patients in Ethiopia.
2. Methods and materials
2.1. The study protocol and registration
For reporting the findings, we used the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guideline which is provided as a Supplementary File (Table S1) [17]. The study protocol of this systematic review and meta-analysis has been submitted to the International Prospective Register of Systematic Reviews (PROSPERO) and assigned the submission number (ID 202843).
2.2. Databases and search strategy
We searched out records on PubMed/MEDLINE, HINARI, EMBASE, Scopus, Google Scholar, and African journals and the online Ethiopian University repositories for published and unpublished articles. The search was conducted from May 1st to July 30, 2020, and research articles published until July 30, 2020, were included in the final meta-analysis (Table 1). Articles reporting undernutrition among adult tuberculosis patients and/or factors associated with undernutrition among tuberculosis patients in Ethiopia were included in the analysis. Searching was employed using the following keywords; “prevalence”, “magnitude”, “proportion”, “burden”, “undernutrition”, “malnutrition”, “malnourishment”, “underweight”, “tuberculosis”, “adults”, “Ethiopia”. The search strings were developed using “OR” and “AND” Boolean operators. Moreover, the reference lists of all included studies were further screened to identify more additional articles.
Table 1.
Searches in MEDLINE/PubMed, Google Scholar, and other databases for the prevalence of undernutrition among adult tuberculosis patients in Ethiopia.
| Databases | Searching terms | Number of studies |
|---|---|---|
| MEDLINE/PubMed | “Prevalence” OR “magnitude” OR “proportion” OR “burden” AND “undernutrition” OR “malnutrition” OR “malnourishment” OR “underweight” AND “tuberculosis” AND“adults” AND “Ethiopia”. | 280 |
| Google Scholar | “Undernutrition” OR “malnutrition” OR “malnourishment” AND “Tuberculosis” AND “Adults”. | 248 |
| HINARI, EMBASE, Scopus, African journals and online Ethiopian University repository | 24 | |
| Total retrieved articles | 552 | |
| Number of included studies | 12 | |
2.3. Screening and eligibility of the study
In the beginning, all articles retrieved from those databases were imported into the “EndNote reference software version 8 (Thomson Reuters, Stamford, CT, USA) citation manager”. Then, duplicates were sorted and removed using EndNote software. Two investigators (AW and AD) independently evaluated the individual study title and abstracts using predetermined inclusion criteria. Two investigators (AW and AD) also have independently selected the full texts and assessed the eligibility of the study for final analysis. The first author, year of publication, study area, study design, sample size, region in which the study was conducted, and prevalence of undernutrition were extracted. The discrepancies between the authors during the process were resolved through discussion with the other authors to agree. The data were exported in Excel spreadsheets for further analysis.
2.4. Inclusion and exclusion criteria
2.4.1. Inclusion criteria
Studies only conducted in Ethiopia and meeting the following inclusion criteria were included in this systematic review and meta-analysis.
2.4.1.1. Study design
All published and unpublished observational studies (case-control, cohort, and cross-sectional) conducted among adult tuberculosis patients in Ethiopia were included in the study.
2.4.1.2. Language
Only studies reported in the English language were included.
2.4.1.3. Study period
Articles published from January 1, 2000, to July 30, 2020, were included in this study.
2.4.1.4. Study participants
Adult tuberculosis patients were included.
2.4.1.5. Measurement of outcome
Studies reported the prevalence of undernutrition among tuberculosis patients and/or predictors were considered in this study and reported quality control methods.
2.4.2. Exclusion criteria
Articles reported in non-English language, case reports, trials, systematic review, narrative review, policy and program evaluation, statements, updates, and news were excluded from the study. Furthermore, qualitative studies and studies without full text were also excluded from the final analysis.
2.5. Outcome measurement
In this systematic review and meta-analysis, the prevalence of undernutrition (BMI < 18.5 kg/m2) among adult tuberculosis patients was determined by dividing the number of patients having undernutrition by the total number of study subjects included in the final analysis.
2.6. Quality assessment
Two independent authors (AW and AD) assessed the quality of the studies using the critical quality assessment checklist recommended by the “Joanna Briggs Institute (JBI)” [18]. The disagreement between the authors was resolved through discussion. The parameters of the quality assessment criteria were clear inclusion criteria, details of the study population, and appropriate statistical analysis. At last, study articles with a score of five and above were considered as having the good quality to be included in the study. All authors independently assessed the articles for consideration and inclusion in the study (Table S2).
2.7. Data processing and analysis
The relevant information/data were extracted and further cleaned using the standard format in Microsoft Excel sheets and the data were exported into STATA version 11.0 (Stata Corporation, College Station, Texas) software for further quantitative analysis. To estimate the pooled national prevalence of undernutrition among adult tuberculosis patients, by considering the variation in true effect sizes across the population, a weighted inverse variance random-effects model was performed for analysis at 95%Cl [19]. The Cochrane Q-test and I2 statistics with its corresponding p-value were used to assess the heterogeneity of the study. The values of I2, 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively [20]. We examined the source of heterogeneity through subgroup analysis based on (sample size, region, and publication year) and sensitivity analysis was executed to investigate the potential source of heterogeneity observed in the pooled prevalence of undernutrition among adult tuberculosis patients. The presence of publication bias was evaluated by using Egger’s test [21] and presented with funnel plots. A statistical test with a P value<0.05 was considered statistically significant.
3. Results
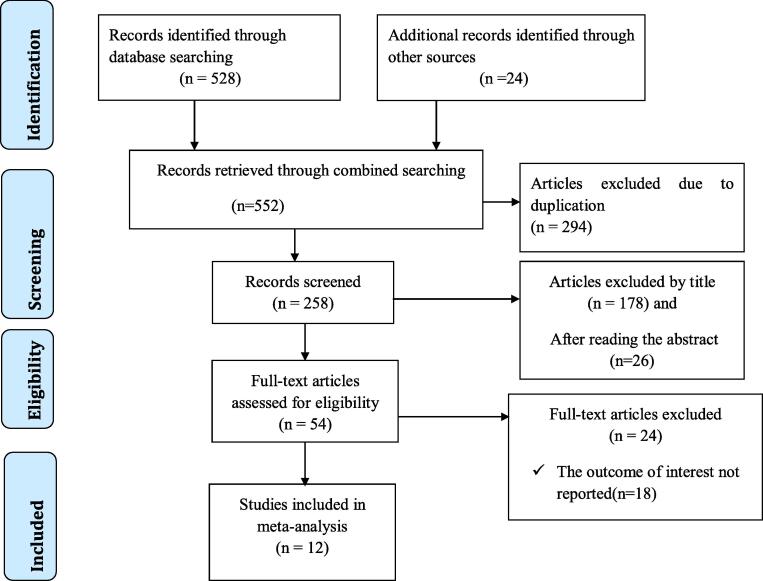
A total of 552 articles were collected from the electronic databases using previously mentioned databases such as PubMed/MEDLINE, Google Scholar, Scopus, EMBASE, CINHAL, HINARI, and Google. After duplicates were removed, 258 articles remained. Then 204 articles were excluded through a reading of their titles and abstracts. Therefore, 54 full-text articles were assessed for eligibility criteria, which results in the further exclusion of 42 irrelevant articles. Finally, 12 studies were fulfilled the inclusion criteria and included in this systematic review and meta-analysis (Fig. 1).
Fig. 1.
Flow chart of study selection for systematic review and meta-analysis for the prevalence of undernutrition among adult tuberculosis patients in Ethiopia.
3.1. Characteristics of included studies
In the current meta-analysis, 12 studies with 4963 study participants were included. All included studies were conducted among adult TB patients in Ethiopia. The mean age of the study participants was 32.73 years old. More than half of the study participants were male (53.2%). More than three-fourths of the study participants have pulmonary tuberculosis (71.38%), and the remaining one-third have extrapulmonary tuberculosis (28.62%). From the included study participants, 22.12% have TB-HIV co-infection.
Regarding study design, six were cross-sectional studies, four were case-control studies and two were cohort studies. Among them, eleven articles were published in peer-reviewed journals between 2006 and 2020, and one unpublished master’s thesis, which was conducted in 2016. The sample size of the included studies ranged from 155 [22] to 1681 [7]. The included studies were reported from four regions and two city administrations of the country. Four studies were conducted in Oromia regional state [15], [23], [24], [25], three studies from Amhara regional state [7], [16], [22], one study from Tigray regional state [26], one study from South Nations Nationalities and Peoples regional state [27], two studies from Addis Ababa city administration [8], [28] and one study from Dire Dawa city administration [29]. Five regions of the country, namely, Somali, Afar, Gambela, Harari, and Benishangul Gumuz, were not included in this study due to the unavailability of studies (Table 2).
Table 2.
Characteristics of included studies to assess the prevalence of undernutrition among adult TB patients in Ethiopia.
| S. No | Author | Publication year | Region | Study Area | Study design | Sample size | Prevalence % |
|---|---|---|---|---|---|---|---|
| 1 | Dargie et al [28] | 2016 | Addis Ababa | Addis Ababa | Cross-sectional | 360 | 39.7 |
| 2 | Feleke et al [7] | 2019 | Amhara | Amhara region | Cross-sectional | 1681 | 57.17 |
| 3 | Hussien et al [23] | 2019 | Oromia | Bale zone | Cross-sectional | 372 | 63.2 |
| 4 | G/meskel et al [27] | 2018 | SNNPR | Hossana | Cross-sectional | 247 | 38.9 |
| 5 | Guadie et al [24] | 2016 | Oromia | Adama | Cross-sectional | 285 | 52.6 |
| 6 | Tesfaye et al [15] | 2016 | Oromia | Shashemene | Cross-sectional | 368 | 28.5 |
| 7 | Seid et al [8] | 2020 | Addis Ababa | Addis Ababa | Cohort | 284 | 46.8 |
| 8 | Hassen et al [29] | 2019 | Dire Dawa | Dire Dawa | Case-control | 285 | 37.9 |
| 9 | Fisseha et al [26] | 2014 | Tigray | Tigray region | Case-control | 230 | 56.08 |
| 10 | Ephrem et al [25] | 2015 | Oromia | Ambo | Case-control | 312 | 49.04 |
| 11 | Kassu et al [22] | 2006 | Amhara | Gondar | Case-control | 155 | 68.38 |
| 12 | Wassie et al [16] | 2014 | Amhara | Gondar | Cohort | 384 | 71.35 |
SNNPR = Southern Nation Nationalities and Peoples Region.
3.2. The pooled prevalence of undernutrition among TB patients in Ethiopia
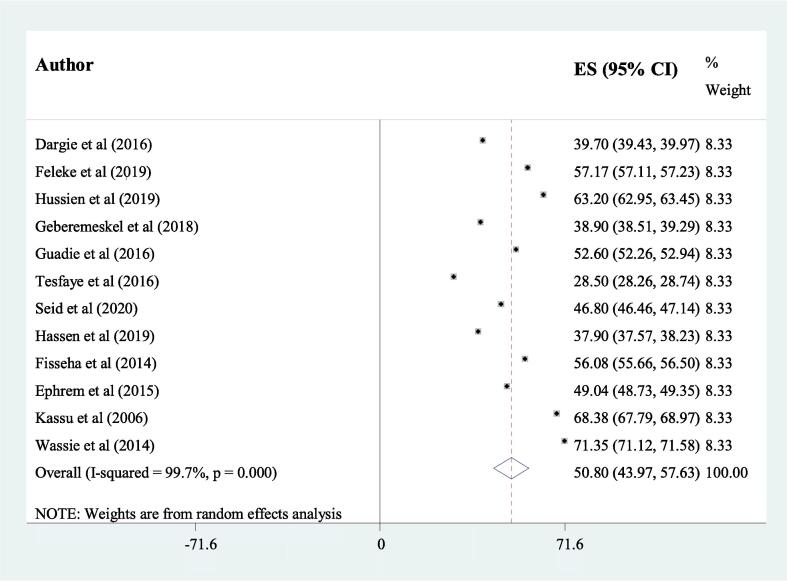
The results of this systematic review and meta-analysis showed that the overall estimated pooled prevalence of undernutrition among adult TB patients in Ethiopia was 50.8% (95% CI 43.97, 57.63) (Fig. 2). The highest prevalence of undernutrition among TB patients (71.35%) was reported from a study conducted in Gondar town, Amhara region, in 2014 [16]. While the lowest prevalence (28.5%) was observed from a study done in Shashemene, Oromia region in 2016 [15]. In this meta-analysis, the pooled prevalence of undernutrition among TB-HIV co-infected patients was 45.45% (95%CI 21.85, 56.07).
Fig. 2.
The pooled prevalence of undernutrition among adult tuberculosis patients in Ethiopia.
3.3. Heterogeneity and publication bias
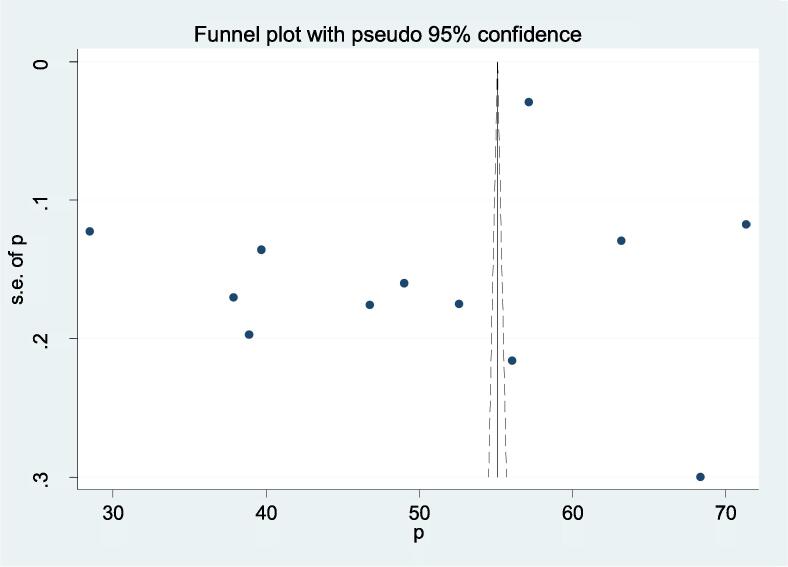
There was evidence of high heterogeneity among the included studies according to Cochrane Q-test (P ≤ 0.001) and I2 test (I2 = 99.7%). Therefore, we used a random-effects meta-analysis model to estimate the overall pooled prevalence of undernutrition among TB patients. Publication bias was assessed using the funnel plot and Egger’s regression test statistics. The funnel plot showed that there was an asymmetrical distribution of the included studies through visual inspection, which indicates there was potential publication bias (Fig. 3). However, The Egger regression test (p = 0.226) and Begg rank correlation statistics (p = 0.9) showed there was no evidence of potential publication bias.
Fig. 3.
The funnel plots showing the presence of publication bias in the included studies undernutrition was detected from studies with a sample size<300 (50.11%).
3.4. Subgroup analysis
There was evidence of high heterogeneity across the included studies. Therefore, subgroup analysis was employed to identify the possible source of heterogeneity based on publication year, the region of the study conducted, and sample size. The results of subgroup analysis showed that the highest prevalence of undernutrition among TB patients was observed from studies done in the Amhara region (65.63%), whereas the lowest prevalence of undernutrition was observed from studies conducted in Addis Ababa (41.47%) (Fig. 4). Regarding one publication year, the highest proportion of undernutrition was observed in studies published before 2017 (52.24%) as compared to studies published after 2017 (48.8%). Finally, subgroup analysis was computed based on sample size and the results showed that the highest prevalence of undernutrition was detected in studies with a sample size <300 (50.11%).
Fig. 4.
Subgroup analysis showing the pooled prevalence of undernutrition among adult TB patients from different regions of Ethiopia.
3.5. Sensitivity analysis
We conducted a sensitivity analysis using a random-effects model. Hence, the results showed that no single study influenced the overall pooled proportion of undernutrition among TB patients (Table 3).
Table 3.
Sensitivity analysis of the prevalence of undernutrition among adult TB patients in Ethiopia.
| Study omitted | Prevalence | 95% CI |
|---|---|---|
| Dargie et al. (2016) | 51.81 | 44.72, 58.89 |
| Feleke et al. (2019) | 50.22 | 41.2, 59.24 |
| Hussen et al. (2019) | 49.67 | 42.21, 57.13 |
| G/meskel et al. (2018) | 51.88 | 44.78, 58.97 |
| Guadie et al. ((2016) | 50.63 | 43.26, 58.01 |
| Tesfaye et al. (2016) | 52.83 | 47.12, 58.34 |
| Seid et al. (2020) | 51.16 | 43.86, 58.46 |
| Hassen et al. (2019) | 51.97 | 44.94, 59.01 |
| Fisseha et al. (2014) | 50.32 | 43.02, 57.61 |
| Ephrem et al. (2015) | 50.96 | 43.58, 58.34 |
| Kassu et al. (2006) | 49.2 | 42.05, 56.35 |
| Wassie et al. (2014) | 48.93 | 41.95, 55.91 |
4. Discussion
Undernutrition and tuberculosis are major public health problems among people living in middle and low-income countries. As far as our knowledge, this systematic review and meta-analysis is the first of its kind to estimate the pooled prevalence of undernutrition among tuberculosis patients in Ethiopia. Estimating the overall pooled prevalence of undernutrition among TB patients is very crucial to improve the nutritional status of patients, which in turn increase TB treatment outcome and decrease the morbidity and mortality of TB patients. The findings of this meta-analysis showed that more than half of TB patients in Ethiopia were undernourished 50.8 (95% CI 43.97, 57.63). Even though there was no comparable meta-analysis study similar to this meta-analysis, the result of this study is in line with studies conducted in Kenya (50.15%) [30], Malawi (57%) [31], Ghana (51%) [32], Nepal (50%) [33], and India 55.4% among women and 54.5% among men [34]. This similarity might be due to the proportional socio-economic status of the countries. Additionally, those countries except Ghana and Malawi are listed in WHO TB high burden countries [1].
The finding of this review is higher than a study conducted in Bangladesh (36%) [35] and the United States of America (11.2%) [36]. The possible justification for this variation might be due to differences in the socioeconomic status of the countries. Ethiopia is grouped under a low-income country, whereas the USA is a high-income country and Bangladesh is grouped under a middle-income country. Additionally, this difference might be due to variations in the residence of the study participants. In Ethiopia, 78% of women and 80% of men live in rural areas, and most of the rural population's food intake habit was two times per day [4].
The current study is lower than studies conducted in Brazil (70.6%) [37] and West Bengal, India (61.9%) [38]. The possible explanation for the above variation might be due to variation in the number of study participants. Our study analyses the prevalence of undernutrition among TB patients at the national level with a large sample size, whereas the above-mentioned studies assess the prevalence of undernutrition among TB patients at a specific place of the country with small sample size. Additionally, the Brazil study was conducted among TB-HIV co-infected participants and undernutrition is more severe in TB-HIV co-infected patients [6].
Ethiopia is one of the TB high burden countries with an annually estimated 219,186 new TB Cases and 48,910 TB deaths in 2016 [39]. In 2017, the Ethiopian ministry of Health updated the guideline for the management of TB, drug resistance, TB, and Leprosy. This guideline incorporated the management strategies of malnutrition in TB patients [40]. According to the guideline, TB patients who develop severe malnutrition (BMI < 16 kg/m2) should be treated with Ready -to-Use Supplementary Foods (RUSF) for 03 months. Additionally, moderate malnutrition (BMI < 17 kg/m2) in patients with TB-HIV co-infected individuals, MDR-TB, and pregnant and lactating mothers are also indicated to take supplementary food [40]. The WHO 2017 guideline-recommended treatment adherence support should include food, financial incentives, and transportation access for patients with tuberculosis. Moreover, psychological support including a home visit is mandatory to increase treatment adherence of tuberculosis patients [41].
Tuberculosis is one of the most common causes of death for people living with HIV; conversely, HIV increases the risk of TB infection and promotes the progression of latent TB into active TB [42]. Undernutrition becomes an additional burden for TB-HIV co-infected patients. In this study, the pooled prevalence of undernutrition among TB-HIV co-infected patients was 45.45% (95% CI 21.85, 56.07). The result of this finding is higher than a study conducted in Brazil (27.3%) [37]. The possible justification for this result might be because TB-HIV co-infection exacerbates undernutrition through increased body metabolic rate, decrease absorption of nutrients, and increased protein and fat breakdowns. Additionally, TB-HIV coinfection decreases the person's appetite, which decreases nutritional intake and increases susceptibility to infection [43], [44]. On the other hand, the result of this study is lower than a study done in Tanzania (58%) [45]. This variation might be due to variation in the number of study participants. The above study was conducted using 43 pregnant participants, but in this study, we used a large number of study participants.
The results of subgroup analysis showed that undernutrition among TB patients varies across regions of the country. A higher proportion of undernutrition was observed in Amhara regional state (65.63%), as compared to other regions of the country. This variation might be due to the topography of the Amhara region, in which the soil is suitable for erosion and the productivity of land decreases from time to time. Additionally, most of the people practice agriculture for a long time through traditional methods, and the farmers in the rural area live in poverty. In the Amhara region, approximately 26.1% of the population lived below the country's poverty line in 2016, and the region is highly affected by poverty compared with other regions of the country [46].
In the current review, the highest prevalence of undernutrition was observed in studies published before 2017 (52.24%) as compared with studies published after 2017 (48.8%). The possible justification for this variation might be due to the Ethiopian Ministry of Health updating 2012 TB treatment guideline in November 2017. The updated 2017 TB treatment guideline incorporates management strategies for malnutrition for tuberculosis patients [40].
Finally, subgroup analysis was computed based on the sample size. The finding showed that the highest magnitude of undernutrition was detected in studies with a sample size <300 compared with studies with a sample size greater than 300. This variation could be explained by the fact that when the sample size increases, the accuracy and precision of the data would be increased.
5. The strength and limitations of the study
The strength of this study is we searched published and unpublished studies from different databases. The limitation of this meta-analysis is this study was conducted based on studies reported from four regions and two city administrations, which may result in underrepresentation of the remaining regions of the country. The other limitation of this review is we did’t include the pediatrics population due to a lack of published studies.
6. Conclusion
The prevalence of undernutrition among TB patients in Ethiopia was noticeably high. The majority of patients with pulmonary tuberculosis develop undernutrition. The results of this study showed that undernutrition is more severe in the Amhara regional state. Additionally, TB-HIV co-infected patients are highly affected by undernutrition. Therefore, the Ministry of Health in collaboration with clinicians gives special attention to provide nutritional care and support for TB patients as part of regular care. Moreover, special nutritional support should be designed for TB-HIV co-infected patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All related data have been presented within the manuscript. The dataset supporting the conclusions of this article is available from the authors on request.
Author contributions
AW and AD designed the study. AW and AD designed and run the literature search. All authors (AW, AG, GG, and AD) acquired data, screened records, extracted data, and assessed the risk of bias. AD and AW did the statistical analysis and wrote the report. All authors provided critical conceptual input, analyzed and interpreted the data, and critically revised the report. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2020.100211.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Annabel B., Anna D., Hannah M. World Health Organization; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- 2.Misganaw A., Haregu T.N., Deribe K., Tessema G.A., Deribew A., Melaku Y.A., Amare A.T., Abera S.F., Gedefaw M., Dessalegn M., Lakew Y., Bekele T., Mohammed M., Yirsaw B.D., Damtew S.A., Krohn K.J., Achoki T., Blore J., Assefa Y., Naghavi M. National mortality burden due to communicable, non-communicable, and other diseases in Ethiopia, 1990–2015: findings from the Global Burden of Disease Study 2015. Popul Health Metrics. 2017;15(1) doi: 10.1186/s12963-017-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAo, I. and UNICEF, WFP and WHO (2018) The state of food security and nutrition in the world 2018. Building climate resilience for food security and nutrition, 2017.
- 4.Central Statistical Agency (CSA) [Ethiopia] and ICF. 2016. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF. Avilable at: https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf (Accessed on November 10, 2020).
- 5.Guideline, W., nutritional care and support for patients with tuberculosis. 2013. 2016. [PubMed]
- 6.Semba R.D., Darnton-Hill I., de Pee S. Addressing Tuberculosis in the Context of Malnutrition and HIV Coinfection. Food Nutr Bull. 2010;31(4_suppl4):S345–S364. doi: 10.1177/15648265100314S404. [DOI] [PubMed] [Google Scholar]
- 7.Feleke B.E., Feleke T.E., Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. 2019;19(1) doi: 10.1186/s12890-019-0953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seid G., Ayele M. Undernutrition and mortality among adult tuberculosis patients in addis Ababa, Ethiopia. Adv Prevent Med. 2020;2020:1–9. doi: 10.1155/2020/5238010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen Y.-F., Chuang P.-H., Yen M.-Y., Lin S.-Y., Chuang P., Yuan M.-J., Ho B.-L., Chou P., Deng C.-Y. Association of body mass index with tuberculosis mortality: a population-based follow-up study. Medicine. 2016;95(1):e2300. doi: 10.1097/MD.0000000000002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A. Nutritional status in multi-drug resistance-pulmonary tuberculosis patients. Indian J Commun Health. 2014;26(Supp 2):204–208. [Google Scholar]
- 11.Bhargava A., Bhargava M. Tuberculosis deaths are predictable and preventable: Comprehensive assessment and clinical care is the key. J Clin Tubercul Other Mycobact Dis. 2020;19:100155. doi: 10.1016/j.jctube.2020.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai, H.-H., Y.-J. Lai, and Y.-F. Yen, Association of body mass index with timing of death during tuberculosis treatment. PloS one, 2017. 12(1): p. e0170104. [DOI] [PMC free article] [PubMed]
- 13.Papathakis, P. and E. Piwoz, Nutrition and Tuberculosis: A review of the literature and considerations for TB control programs. United States Agency for International Development, Africa's Health 2010 Project, 2008: p. 1.
- 14.Grobler, L., et al., Nutritional supplements for people being treated for active tuberculosis. Cochrane Database of Systematic Reviews, 2016(6). [DOI] [PMC free article] [PubMed]
- 15.Tesfaye, A., Magnitiude of Undernutrition and Associated Factors among Adult Tuberculosis patients attending Government Health care Facilities of Shashemane , Ethiopia [MPH Thesis]. 2016.
- 16.Mesele M. Weight gain and associated factors among adult tuberculosis patients on treatment in northwest ethiopia: a longitudinal study. J Nutr Disorders Ther. 2014;04(02) doi: 10.4172/2161-0509-4.1000143. [DOI] [Google Scholar]
- 17.Moher, D., et al., Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med, 2009. 6(7): p. e1000097. [DOI] [PMC free article] [PubMed]
- 18.Institute J.B. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews: checklist for prevalence studies. Crit Apprais Checkl Preval Stud. 2017;7 [Google Scholar]
- 19.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 20.Rücker G., Schwarzer G., Carpenter J.R., Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8(1) doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassu A., Yabutani T., Mahmud Z.H., Mohammad A., Nguyen N., Huong B.T.M., Hailemariam G., Diro E., Ayele B., Wondmikun Y., Motonaka J., Ota F. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur J Clin Nutr. 2006;60(5):580–586. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 23.Hussien B., Hussen M.M., Seid A., Hussen A. Nutritional deficiency and associated factors among new pulmonary tuberculosis patients of Bale Zone Hospitals, southeast Ethiopia. BMC Res Notes. 2019;12(1) doi: 10.1186/s13104-019-4786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guadie, F.F., Assessment of Nutritional Status and Associated Factors Among Adult TB Patients on Directly Observed Treatment of Short Course in Health Facilities at Adama Town, East Shewa Zone, Ethiopia. 2016.
- 25.Ephrem, T., et al., Determinants of active pulmonary tuberculosis in Ambo Hospital, West Ethiopia. Afr J Prim Health Care Fam Med, 2015. 7(1): p. e1-e8. [DOI] [PMC free article] [PubMed]
- 26.Fisseha G. Determinant factors of treatment failure among tuberculosis patients under directly observed therapy in Tigray regional state public hospitals, north Ethiopia: a case–control study. Global J Med Res F Dis. 2014;2104(14):5. [Google Scholar]
- 27.Geberemeskel, T., et al., Undernutrition and Associated Factors among Adult Tuberculosis Patients in Hossana Town Public Health Facilities, Southern Ethiopia. Journal of Tropical Diseases, 2018. 06(01).
- 28.Dargie B., Tesfaye G., Worku A. Prevalence and associated factors of undernutrition among adult tuberculosis patients in some selected public health facilities of Addis Ababa, Ethiopia: a cross-sectional study. BMC Nutr. 2016;2(1) doi: 10.1186/s40795-016-0046-x. [DOI] [Google Scholar]
- 29.Hassen J.K., Gizaw A., Mohamed S. Determinants of pulmonary tuberculosis in public health facilities of dire dawa city, eastern Ethiopia: unmatched case-control study. Int J Mycobacteriol. 2019;8(2):118–123. doi: 10.4103/ijmy.ijmy_50_19. [DOI] [PubMed] [Google Scholar]
- 30.Nthiga, I., et al., The nutritional status of pulmonary tuberculosis patients aged 25-44 years attending tuberculosis clinic at Lodwar County and Referral Hospital, Turkana County, Kenya. 2017.
- 31.Zachariah R., Spielmann M.P., Harries A.D., Salaniponi F.M.L. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96(3):291–294. doi: 10.1016/S0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 32.Dodor E. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J. 2008;42(1):22. [PMC free article] [PubMed] [Google Scholar]
- 33.SanchitaSubedi R.S.M., PushpaParajuli GayanandMandal, Yadav D.K. Nutritional status of patients with pulmonary tuberculosis receiving anti-tuberculosis treatment at bp koirala institute of health sciences, Nepal. J Nurs Health Sci. 2019;8(6) [Google Scholar]
- 34.Bhargava A. Undernutrition and the incidence of tuberculosis in India: National and subnational estimates of the population attributable fraction related to undernutrition. Natl Med J India. 2014;27(3):128–133. [PubMed] [Google Scholar]
- 35.Rahaman, M.F.U., et al., Nutritional status of patients with tuberculosis attending at tertiary medical center in Bangladesh. Bangladesh Journal of Medicine, 2019. 30(2): p. 53-57.
- 36.Phan M.N., Guy E.S., Nickson R.N., Kao C.C. Predictors and patterns of weight gain during treatment for tuberculosis in the United States of America. Int J Infect Dis. 2016;53:1–5. doi: 10.1016/j.ijid.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacelo, A.C., et al., Nutritional supplementation is a necessary complement to dietary counseling among tuberculosis and tuberculosis-HIV patients. PLoS One, 2015. 10(8): p. e0134785. [DOI] [PMC free article] [PubMed]
- 38.Mollah, A., et al., Nutritional status of adult Tuberculosis patients in Burdwan municipality area of West Bengal. Indian Journal of Community Health, 2020. 32(2).
- 39.Deribew A. Tuberculosis burden in Ethiopia from 1990 to 2016: evidence from the global burden of diseases 2016 study. Ethiop J Health Sci. 2018;28(5) doi: 10.4314/ejhs.v28i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MOH, Federal democratic republic of Ethiopia ministry of health 2017 Guidelines for clinical and programmatic management of TB, Leprosy and TB/HIV in Ethiopia. sixth edition. Addis Ababa. Avilable at: https://www.afro.who.int/sites/default/files/2019-04/Ethiopia-National%20guidelines%20for%20TB%2C%20DR-TB%20and%20Leprosy%20in%20Ethiopia%20-%20Sixth%20Edition.pdf. 2017.
- 41.Organization, W.H., Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. avilable at: https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf. 2017.
- 42.Bruchfeld J., Correia-Neves M., Källenius G. Tuberculosis and HIV coinfection. Cold Spring Harb Perspect Med. 2015;5(7) doi: 10.1101/cshperspect.a017871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niyongabo T., Henzel D., Idi M., Nimubona S., Gikoro E., Melchior J.C., Matheron S., Kamanfu G., Samb B., Messing B., Begue J., Aubry P., Larouze B. Tuberculosis, human immunodeficiency virus infection, and malnutrition in Burundi. Nutrition. 1999;15(4):289–293. doi: 10.1016/S0899-9007(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 44.Van Lettow, M., et al., Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutrition Reviews, 2003. 61(3): p. 81-90. [DOI] [PubMed]
- 45.Bakari, M., et al., Nutritional status of HIV-infected women with tuberculosis in Dar es Salaam, Tanzania. Public health action, 2013. 3(3): p. 224-229. [DOI] [PMC free article] [PubMed]
- 46.UNICEF-Ethiopia Amhara Regional State 2007/08 – 2015/16 Budget-Brief. 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All related data have been presented within the manuscript. The dataset supporting the conclusions of this article is available from the authors on request.