Abstract
Some subtypes of acute myeloid leukemia (AML) share morphologic, immunophenotypic, and clinical features of acute promyelocytic leukemia (APL), but lack a PML–RARA (promyelocytic leukemia–retinoic acid receptor alpha) fusion gene. Instead, they have the retinoic acid receptor beta (RARB) or retinoic acid receptor gamma (RARG) rearranged. Almost all of these AML subtypes exhibit resistance to all-trans retinoic acid (ATRA); undoubtedly, the prognosis is poor. Here, we present an AML patient resembling APL with a novel cleavage and polyadenylation specific factor 6 (CPSF6)–RARG fusion, showing resistance to ATRA and poor response to chemotherapy with homoharringtonine and cytarabine. Simultaneously, the patient also had extramedullary infiltration.
Keywords: acute myeloid leukemia, CPSF6–RARG fusion, extramedullary infiltration, resemble acute promyelocytic leukemia, resistance to ATRA
Introduction
Acute promyelocytic leukemia (APL) is a special subtype of acute myeloid leukemia (AML), which is characterized by abnormal accumulation of promyelocytes in the bone marrow and peripheral blood. In these patients, the latter symptoms are often accompanied by serious coagulation abnormality. The majority of cases have a PML–RARA (promyelocytic leukemia–retinoic acid receptor alpha) fusion gene caused by a t(15; 17) translocation that leads to a unique sensitivity to therapy with all-trans retinoic acid (ATRA). The current treatment strategy combining with ATRA and arsenic trioxide (ATO) has greatly changed the prognosis of this once uniformly fatal disease.1 Nevertheless, some AMLs resembling APL also revealed a proliferation of hypergranular promyelocytes and exhibited a life-threatening coagulopathy; unexpectedly, the PML–RARA fusion gene was negative. Instead, the retinoic acid receptor beta (RARB) or retinoic acid receptor gamma (RARG) gene was rearranged2–5; all of them were members of the nuclear receptor superfamily sharing high sequence homology (90%).6 Almost all of these subtypes of AML exhibit resistance to ATRA; undoubtedly, the prognosis is poor. Here, we present an AML patient resembling APL with a novel CPSF6–RARG fusion, showing resistance to ATRA and poor response to chemotherapy with homoharringtonine and cytarabine.
Case description
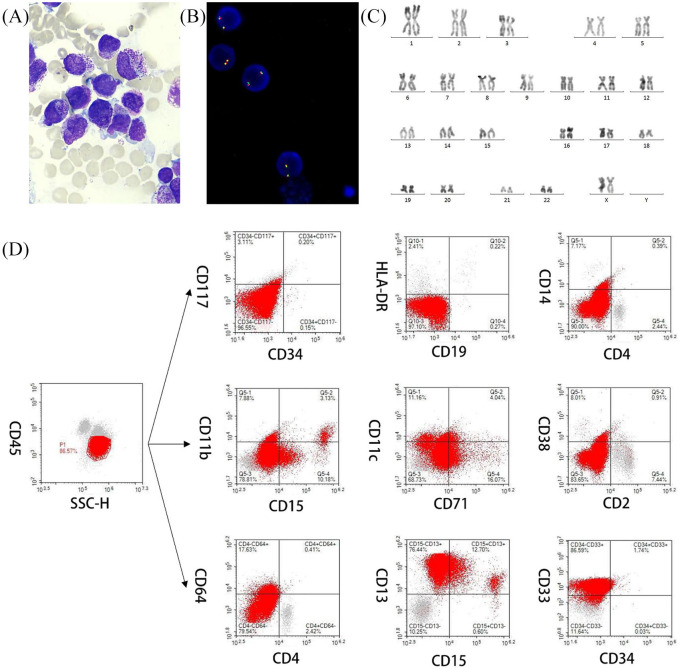
A 67-year-old woman was admitted due to an oral hemorrhage and skin ecchymosis. Her blood test showed a white blood cell count (WBC) of 1.6 × 109/l, hemoglobin level (HGB) of 89 g/l, and platelet count (PLT) of 49 × 109/l. Fibrinogen and D-dimer levels were 0.5 g/l (reference 2.00–4.00) and 45,178 µg/l (reference 0–700). Positron emission tomography-computed tomography (PET-CT) revealed that masses of the right obturator internus and obturator externus had invaded the right pubic bone and affected the right edge of the cervix; multiple enlarged lymph nodes were present in the posterior peritoneum and beside the iliac arteries (Figure 1). A bone marrow (BM) smear revealed hypercellularity, with 72% hypergranular promyelocytes (Figure 2A). The BM histochemistry showed a staining pattern of typical APL, being positive for POX, SB, and SE, and negative for NSE, PAS, and NAF. The leukemia cells expressed myeloperoxidase, CD13, and CD33, and partially expressed CD71, but lacked the expression of CD14, CD19, CD34, CD38, CD64, CD117, CD11b, CD11c, HLA-DR, and other T- or B-lymphoid-related markers (Figure 2D). Combining patient clinical manifestations, BM morphology, and immunophenotype, APL was highly suspected. She received ATRA starting on the second day of admission. However, PML–RARA was negative and fluorescence in situ hybridization (FISH) failed to detect the PML-RARA transcript in the BM (Figure 2B). Karyotype analysis revealed 46, XX [20] (Figure 2C). Meanwhile, we detected WT1 mutation by reverse-transcription (RT)-PCR. On day 9 after admission, blood tests showed a WBC of 2.2 × 109/l, HGB 56 g/l, and PLT 37 × 109/l.
Figure 1.

PET-CT revealed multiple swollen lymph nodes in the posterior peritoneum and beside the iliac arteries, accompanied by a slight increase in FDG metabolism. Masses of the right obturator internus and obturator externus invaded the right pubic bone and affected the right edge of the cervix, which were accompanied by increased FDG metabolism.
FDG, 18F-fluorodeoxyglucose; PET-CT, positron emission tomography-computed tomography.
Figure 2.
Morphology, immunophenotype, FISH analysis, and karyotyping. (A) Several promyelocytes, with hypergranulated cytoplasm and invaginated nuclei, are shown (Wright–Giemsa stained bone marrow smear, × 1000). (B) Interphase FISH revealed the absence of PML–RARA fusion signals in AML using PML–RARA dual-color, dual-fusion translocation probes. (C) G-banded karyotype showing 46, XX[20]. (D) The leukemia cells expressed myeloperoxidase, CD13, and CD33, and partially expressed CD71, but lacked the expression of CD14, CD19, CD34, CD38, CD64, CD117, CD11b, CD11c, and HLA-DR.
AML, acute myeloid leukemia; FISH, fluorescence in situ hybridization; PML–RARA, promyelocytic leukemia–retinoic acid receptor alpha.
Because there was no sign of differentiation and improvement of coagulopathy after 1 week of ATRA treatment, we initiated a homoharringtonine 2 mg d1-5, cytarabine 50 mg d1-7 (HA) regimen as induction therapy. To avoid exacerbating the coagulopathy, the doses of chemotherapy were decreased. On day 3 after chemotherapy, blood tests showed a WBC of 5.2 × 109/l, HGB 56 g/l, and PLT 10 × 109/l. At the same time, the patient progressed to anuria, a progressive increase in serum creatinine and BNP, with a poor diuretic effect. Considering acute renal failure, we discontinued ATRA and began continuous renal replacement therapy (CRRT) at the bedside for 6 days, then changed to three hemodialysis treatments per week. We performed a BM aspiration on day 12, revealed hypercellularity with 83% hypergranular promyelocytes. On day 15 after chemotherapy, a puncture of the right obturator internus mass was performed, and pathological analysis suggested that monocyte-like cells had proliferated abnormally; a diagnosis of a hematopoietic malignant tumor was considered. Puncture flow cytometric analysis showed that AML cells accounted for 50.7%. The patient died due to an intracranial hemorrhage on day 17 after chemotherapy.
To clarify the molecular pathology, next-generation sequencing (NGS) was used on day 1 after chemotherapy. Total RNA from liquid bone marrow samples was extracted using the PureLink RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA). The library was constructed using a KAPA Stranded RNA-seq Kit with RiboErase (HMR) (KAPA Biosystems, Wilmington, MA, USA) according to manufacturer’s instructions. Paired-end sequencing with a read length of 150 bp were performed on an Illumina HiSeq™4000 sequencer (Illumina Inc. San Diego, CA, USA). Raw data were mapped to human reference genome hg19 using FusionCatcher (version 0.99.4e). The NGS was based on genome GRCh37/hg19, which includes DNA sequencing and whole transcriptome mRNA sequencing. Mutational hotspots or whole coding regions of 88 genes that are known to be mutated frequently in hematologic malignancies were sequenced using a targeted gene panel (Nanjing Geneseeq Technology Inc., Nanjing, China). As expected, the NGS detected a fusion transcript within chromosome 12, in which CPSF6 exon 4 (NM_001300497) with RARG exon 4 (NM_000966) were fused in-frame (Figure S1, Supplemental file S1). Furthermore, we identified co-mutations with KRAS exon 2 [NM_004985.3: c.35G>C (p.G12A)] (Figure S2, Supplemental file S1), EZH2 exon 20 (NM_004456.4: c.2196_2201delATACAG [p.R732_Y733del)] (Figure S3, Supplemental file S1), and EZH2 exon 20 [NM_004456.4: c.2204_2211delAGGCTGAT (p.Q735Rfs*25)] (Figure S4, Supplemental file S1). To confirm the CPSF6–RARG fusion, we performed RT-PCR using the bone marrow sample and the following primers: forward (at CPSF6 exon 2), 5′-TGGTGGACAACAGATGAAGACT-3′ and reverse (at RARG exon 4), 5′-CTTGCAGCCTTCACAAGAGC-3′. Sanger sequencing revealed that the fusion gene was the in-frame fusion of CPSF6 exon 4 with RARG exon 4 (Figure 3B).
Figure 3.
Molecular analysis of the CPSF6–RARG fusion. (A) Schematic diagram of CPSF6, RARG, CPSF6–RARG fusion transcript, and the fusion protein of the patient. The breakpoint is indicated by a red line. (B) Sanger sequencing of the PCR product analysis of the CPSF6–RARG fusion transcript junctions revealed a fusion between exon 4 of the CPSF6 gene and exon 4 of the RARG gene.
CPSF6, cleavage and polyadenylation specific factor 6; DBD, DNA binding domain; LBD, ligand-binding domain; PRD, proline-rich domain; RARG, retinoic acid receptor gamma; RRM, RNA recognition motif; RSLD, arginine/serine-like domain.
Discussion
APL is a special subtype of AML. The majority of the patients express the PML–RARA fusion gene, which is the target for treatment with ATRA. Indeed, ATRA must be initiated immediately after the diagnosis is suspected due to the life-threatening coagulopathy. The application of the current standard therapy makes APL the most curable subtype of AML. AMLs with RARG or RARB rearrangements share morphologic, immunophenotypic, and clinical features of APL, but lack the PML–RARA fusion gene. These patients have high mortality on account of lack of response to ATRA. CPSF6–RARG is the most common fusion in these subtypes of AML; early identification can help change treatment strategies in time and improve prognosis. Cleavage and polyadenylation specific factor 6 (CPSF6) is a subunit of cleavage factor I, which is involved in messenger RNA (mRNA) precursor 3′-end processing.7 RARG has a unique function in maintaining the self-renewal of hematopoietic stem cells (HSCs).8 Four cases of CPSF6–RARG fusion and one case of RARG–CPSF6 fusion have previously been reported (Table 1)5,9–11; in our patient, clinical manifestations, cell morphology and immunophenotype were similar to APL, but they did not respond to ATRA. As listed in Table 1, Patient #1, DNMT3A mutation and a tetraploidy karyotype of 92, XXXX[2] were detected. NGS revealed that the 3′-region of the RARG gene (from exon 4 to exon 9) was reversed and fused in-frame with the 5′-region of the CPSF6 gene (from exon 1 to exon 4). The patient received ATRA, ATO (arsenic trioxide), and IDA (idarubicin), the IAG regimen [IDA, cytosine arabinoside, and granulocyte-colony stimulating factor (G-CSF)], and decitabine treatments. Unfortunately, all treatments were ineffective.5 Like patient #1, patient #2 was resistant to ATRA, ATO, and the MA regimen (mitoxantrone and cytarabine). A CPSF6–RARG rearrangement within chromosome 12 was detected, accompanied by a WT1 mutation; the main transcript was the fusion of CPSF6 exon 4 with RARG exon 2.9 For patient #3, the 3′-region of the RARG gene (from exon 1 to exon 9) was reversed and fused in-frame with the 5′-region of the CPSF6 gene (from exon 1 to exon 4), co-mutations with WT1, KRAS. In this patient, ATRA and DNR (daunorubicin) were not effective, but she was sensitive to the DA (daunorubicin and cytarabine) regimen, followed by two cycles of high dose Ara-C. She remained in complete remission (CR) until last follow up5; these results are consistent with the report of Zhang in that RARG-rearranged APL-like AMLs are sensitive to a 3 + 7 regimen; in their report, this regimen benefited NUP98–RARG-positive AML patients.12 However, the IA (idarubicin and cytarabine) regimen failed to work in patient #4. In contrast, after one course of standard HA regimen, the patient achieved CR and remained in CR until the last follow up; this patient’s CPSF6–RARG fusion transcript was the longest, and was generated by the fusion of exon 5 of CPSF6 and exon 1 of RARG.10 The author of that report claimed that homoharringtonine is an effective drug for the novel subtype AML with CPSF6–RARG fusion. Nevertheless, in our case, considering the patient’s severe coagulation dysfunction and thrombocytopenia, we adopted a dose-adjusted HA regimen (Hom 2 mg d1-5, Ara - C 50 mg d1-7). Unfortunately, the treatments were ineffective for our patient and she eventually died due to an intracranial hemorrhage.
Table 1.
AML with CPSF6–RARG and RARG–CPSF6 fusion in literature.
| No | Age/sex | PB | Morphology | Immunophenotype | Cytogenetics | Gene fusion site | Other related gene mutations | Therapy | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/F | WBC: 0.81 × 109/l HB: 4.2 g/dl PLT: 92 × 109/l | 89% hypergranular promyelocytes with auer rods | CD13+ CD33+ MPO+ CD9partial+ CD64partial+ CD117– CD34– HLA– DR– CD11b–CD14– | 92,XXXX[2] | CPSF6 exon4 was fused with RARG exon4 | DNMT3A mutation | ATRA+ATO+IDA, IAG+ATRA+ATO, Decitabine as induction therapy |
No response and die of cerebral hemorrhage |
Liu et al.5 |
| 2 | 38/M | WBC: 1.68 × 109/l HB: 8.0 g/dl PLT: 79 × 109/l | 65% promyelocytes | CD13+ CD33+ CD34+ MPO+ CD117+ CD9partial+ CD64partial+ CD11b– HLA– DR–CD38– CD14– | 46XY[20] | CPSF6 exon4 was fused with RARG exon2 | WT1 mutation |
ATRA+RIF d1–25, MA started at d26 |
Died of diffuse alveolar hemorrhage | Qin et al.9 |
| 3 | 51/F | WBC:20.15 × 109/l HB: 6.5 g/dl PLT: 45 × 109/l | 87.5% hypergranular promyelocytes | CD13+ CD33+ cMPO+ CD9+ CD34partial+ HLA–DR– CD11c– CD38– CD14– | 46,XX,del(12) (p12)[2]/ 46,XX[18] |
CPSF6 exon4 was fused with RARG exon1 | WT1, KRAS mutation |
ATRA+DNR, DA as induction therapy, two cycle HD–Ara–C followed by two cycle 3+7 regimens |
Resistant to ATRA+DNR, but sensitive to DA. CR at last followed |
Liu et al.5 |
| 4 | 55/M | WBC: 1.23 × 109/l HB: 7.6 g/dl PLT: 60 × 109/l | 93% hypergranular promyelocytes with auer rods | CD13+CD33+CD117+CD6+CD34–CD38–HLA–DR– | 46,XY | CPSF6 exon 5 was fused with RARG exon 1 | NA | ATRA+ATO, IA, HA as induction and consolidation therapy | Resistant to ATRA, ATO, IA. But sensitive to HA and achieved CR | Zhang et al.10 |
| 5 | 26/M | WBC: 16.4 × 109/l HB: 10.5 g/dl PLT: 120 × 109/l | 60% blasts, 15% promyelocytes | CD13+CD33+CD64+CD117partial+ CD34–HLA–DR ± CD14– CD11b– | 45,X,-Y[10]/45,idem, add (6) (q?13)[2]/46,XY[8] | RARG intron 9 was fused with CPSF6 exon 6 | BMPR1A, NEAT1, WT1 mutation | ATRA was started on d1 added IA on d2 | NA | Miller et al.11 |
AML, acute myeloid leukemia; ATO, arsenic trioxide; ATRA, all-trans acid; CPSF6, cleavage and polyadenylation specific factor 6; CR, complete remission; DA, daunorubicin and cytarabine; DNR, daunorubicin; F, female; G-CSF, granulocyte-colony stimulating factor; HA, homoharringtonine and cytarabine; HB, hemoglobin; IA, idarubicin and cytarabine; IAG, idarubicin, cytosine arabinoside and G-CSF; IDA, idarubin; M, male; MA, mitoxantrone and cytarabine; NA, not available; No., number; PB, peripheral blood; PLT, platelet; RARG, retinoic acid receptor gamma; Ref, reference; RIF, Realgar-Indigo naturalis formula; WBC, white blood cell.
Other than CPSF6–RARG fusion, in our patient, WT1 mutation was detected by RT-PCR before chemotherapy. It was not, however, found in the NGS of the genes submitted after chemotherapy. WT1 mutation occurs in 5.5% of AML cases and is expressed recurrently in APL, reaching 11%.13 Similar to typical APL, the mutation frequency of WT1 in CPSF6–RARG fusion AML is much higher than in other subtypes of AML.14 It is very clear that patients #2, #3, and #5 (Table 1) all have WT1 mutations. It is speculated that WT1 mutation may influence ATRA sensitivity.15 we also found other co-mutations of this patient, including a KRAS gene mutation, which can cause continuous activation of Ras protein and participate in tumorigenesis.14 In addition, EZH2 is a member of the PRC2 family, coding for a histone methylation enzyme. It has been shown that the EZH2 mutations themselves have no effect on overall survival (OS) or progression-free survival (PFS) in AML.16,17 However, the co-existence of EZH2 mutations with FLT3 or IDH2 mutations seem to be connected with poor OS and PFS.18,19 Only EZH2 mutations were present in our patient, which may have little prognostic significance.
An important clinical characteristic of our patient was extramedullary infiltration, which has never been reported in published studies.5,9–11 In our patient, the right obturator internus, obturator externus, and some lymph nodes confirmed the extramedullary involvement of AML. Indeed, typical APL rarely has extramedullary involvement.20 Research showed that the factors associated with extramedullary infiltration in APL including youth, WBC count >10 × 109/l, and bcr3 PML–RARA breakpoint.21 Obviously, our patient did not have the above characteristics. It has never been reported previously that five patients had extramedullary infiltration, presumably because no systemic examination such as a PET-CT was conducted. Whether AML with CPSF6–RARG fusion tends to extramedullary infiltration remain uncertain, but it is certain that identification of extramedullary infiltration has an important impact on treatment. The appearance of extramedullary infiltration indicates a poor prognosis and may need radiotherapy, more intensive chemotherapy, or even allogeneic hematopoietic stem cell transplantation (allo-HSCT).22 PET-CT may play a critical role in the diagnosis and treatment of this subtype of AML.
In summary, in our APL-like patient, CPSF6–RARG fusion was simultaneously accompanied by extramedullary infiltration. She showed a poor response to ATRA and HA regimen. The available evidence suggests that the prognosis is poor, active treatments such as allo-HSCT in CR deserve further research.
Supplemental Material
Supplemental material, sj-pdf-1-tah-10.1177_2040620720976984 for Acute myeloid leukemia with CPSF6–RARG fusion resembling acute promyelocytic leukemia with extramedullary infiltration by Xiaoyan Han, Chunxiang Jin, Gaofeng Zheng, Yi Li, Yungui Wang, Enfan Zhang, Honghu Zhu and Zhen Cai in Therapeutic Advances in Hematology
Acknowledgments
The authors would like to thank Nanjing Geneseeq Technology Inc. for providing help with NGS analysis.
Footnotes
Author contributions: Xiaoyan Han: wrote part of the manuscript; reviewed literature; manage the patient; provided clinical data.
Chunxiang Jin: wrote part of the manuscript and reviewed literature.
Gaofeng Zheng and Yi Li: reviewed and wrote part of the manuscript.
Yungui Wang and Enfan Zhang: performed molecular studies and provided images.
Honghu Zhu: reviewed and wrote part of the manuscript.
Zhen Cai: supervised and formulated case report; wrote the manuscript; review of the literature.
All authors reviewed the manuscript and contributed to the final draft.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement: The authors declare no competing financial interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (grant number 81800201), and the Natural Science Foundation of Zhejiang Province (grant number LY17H080001).
Ethics approval and consent to participate: Our study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, and informed consent was obtained from this patient. The approval number is (2020) IIT (338).
ORCID iD: Zhen Cai  https://orcid.org/0000-0001-6026-3804
https://orcid.org/0000-0001-6026-3804
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiaoyan Han, Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Chunxiang Jin, Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Gaofeng Zheng, Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Yi Li, Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Yungui Wang, Institute of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China; Key Laboratory of Hematopoietic Malignancies Zhejiang Province, Hangzhou, Zhejiang, China.
Enfan Zhang, Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Honghu Zhu, Institute of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China; Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Zhen Cai, Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine, Zhejiang University, No. 79, Qingchun Road, Hangzhou, Zhejiang Province 310003, China; Institute of Hematology, Zhejiang University, Hangzhou, China.
References
- 1. Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J 2015; 5: e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osumi T, Tsujimoto SI, Tamura M, et al. Recurrent RARB translocations in acute promyelocytic leukemia lacking RARA translocation. Cancer Res 2018; 78: 4452–4458. [DOI] [PubMed] [Google Scholar]
- 3. Such E, Cervera J, Valencia A, et al. A novel NUP98/RARG gene fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood 2011; 117: 242–245. [DOI] [PubMed] [Google Scholar]
- 4. Ha JS, Do YR, Ki CS, et al. Identification of a novel PML-RARG fusion in acute promyelocytic leukemia. Leukemia 2017; 31: 1992–1995. [DOI] [PubMed] [Google Scholar]
- 5. Liu T, Wen L, Yuan H, et al. Identification of novel recurrent CPSF6-RARG fusions in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood 2018; 131: 1870–1873. [DOI] [PubMed] [Google Scholar]
- 6. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 1996; 10: 940–954. [PubMed] [Google Scholar]
- 7. Martin G, Gruber AR, Keller W, et al. Genome-wide analysis of pre-mRNA 3' end processing reveals a decisive role of human cleavage factor I in the regulation of 3' UTR length. Cell Rep 2012; 1: 753–763. [DOI] [PubMed] [Google Scholar]
- 8. Purton LE. Roles of retinoids and retinoic acid receptors in the regulation of hematopoietic stem cell self-renewal and differentiation. PPAR Res 2007; 2007: 87934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin YZ, Huang XJ, Zhu HH. Identification of a novel CPSF6-RARG fusion transcript in acute myeloid leukemia resembling acute promyelocytic leukemia. Leukemia 2018; 32: 2285–2287. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Z, Jiang M, Borthakur G, et al. Acute myeloid leukemia with a novel CPSF6-RARG variant is sensitive to homoharringtonine and cytarabine chemotherapy. Am J Hematol 2020; 95: E48–E51. [DOI] [PubMed] [Google Scholar]
- 11. Miller CA, Tricarico C, Skidmore ZL, et al. A case of acute myeloid leukemia with promyelocytic features characterized by expression of a novel RARG-CPSF6 fusion. Blood Adv 2018; 2: 1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Li F, Wang J, et al. RARγ-rearrangements resemble acute promyelocytic leukemia and benefit from 3 + 7 regimen. Leuk Lymphoma 2019; 60: 1831–1834. [DOI] [PubMed] [Google Scholar]
- 13. Krauth M-T, Alpermann T, Bacher U, et al. WT1 mutations are secondary events in AML, show varying frequencies and impact on prognosis between genetic subgroups. Leukemia 2015; 29: 660–667. [DOI] [PubMed] [Google Scholar]
- 14. Kim E, Ilic N, Shrestha Y, et al. Systematic functional interrogation of rare cancer variants identifies oncogenic alleles. Cancer Discov 2016; 6: 714–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conserva MR, Redavid I, Anelli L, et al. RARG gene dysregulation in acute myeloid leukemia. Front Mol Biosci 2019; 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Dai H, Wang Q, et al. EZH2 mutations are related to low blast percentage in bone marrow and -7/del(7q) in de novo acute myeloid leukemia. PLoS One 2013; 8: e61341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mechaal A, Menif S, Abbes S, et al. EZH2, new diagnosis and prognosis marker in acute myeloid leukemia patients. Adv Med Sci 2019; 64: 395–401. [DOI] [PubMed] [Google Scholar]
- 18. Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010; 18: 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129: 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009; 113: 1875–1891. [DOI] [PubMed] [Google Scholar]
- 21. de Botton S, Sanz MA, Chevret S, et al. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia 2006; 20: 35–41. [DOI] [PubMed] [Google Scholar]
- 22. Zhou WL, Wu HB, Wang LJ, et al. Usefulness and pitfalls of F-18-FDG PET/CT for diagnosing extramedullary acute leukemia. Eur J Radiol 2016; 85: 205–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tah-10.1177_2040620720976984 for Acute myeloid leukemia with CPSF6–RARG fusion resembling acute promyelocytic leukemia with extramedullary infiltration by Xiaoyan Han, Chunxiang Jin, Gaofeng Zheng, Yi Li, Yungui Wang, Enfan Zhang, Honghu Zhu and Zhen Cai in Therapeutic Advances in Hematology