Abstract
Mesenchymal stem cell (MSC) migration is promoted by low-intensity pulsed ultrasound (LIPUS), but its mechanism is unclear. Since autophagy is known to regulate cell migration, our study aimed to investigate if LIPUS promotes the migration of MSCs via autophagy regulation. We also aimed to investigate the effects of intra-articular injection of MSCs following LIPUS stimulation on osteoarthritis (OA) cartilage. For the in vitro study, rat bone marrow-derived MSCs were treated with an autophagy inhibitor or agonist, and then they were stimulated by LIPUS. Migration of MSCs was detected by transwell migration assays, and stromal cell-derived factor-1 (SDF-1) and C-X-C chemokine receptor type 4 (CXCR4) protein levels were quantified. For the in vivo study, a rat knee OA model was generated and treated with LIPUS after an intra-articular injection of MSCs with autophagy inhibitor added. The cartilage repair was assessed by histopathological analysis and extracellular matrix protein expression. The in vitro results suggest that LIPUS increased the expression of SDF-1 and CXCR4, and it promoted MSC migration. These effects were inhibited and enhanced by autophagy inhibitor and agonist, respectively. The in vivo results demonstrate that LIPUS significantly enhanced the cartilage repair effects of MSCs on OA, but these effects were blocked by autophagy inhibitor. Our results suggest that the migration of MSCs was enhanced by LIPUS through the activation autophagy, and LIPUS improved the protective effect of MSCs on OA cartilage via autophagy regulation.
Keywords: ultrasound, mesenchymal stem cell, migration, autophagy
Introduction
Osteoarthritis (OA) typically develops from cartilage injury caused by sports trauma, aging, and joint degeneration. After the injury, the ability of articular cartilage to self-repair is very limited, and even a minor injury may induce the progressive destruction and degeneration of the joint1.
Mesenchymal stem cells (MSCs) have the ability to differentiate into a variety of cell types, and they are useful for tissue regeneration, including articular cartilage2. MSC transplantation is a cell-based therapy that has the potential to repair articular cartilage injury. The proliferation capacity of MSCs is very strong, and they can differentiate into chondrocytes3.
However, previous studies have found that the therapeutic effect of MSCs was limited by problems. One problem was that a large number of transplanted MSCs could not reach the site of injury, and another problem was that a few of the transplanted MSCs had fallen off from the site of transplantation4-5. Therefore, measures will need to be taken to enhance the migration capacity of MSCs to accelerate the regeneration and repair of cartilage injury.
Autophagy is a degradation process that improves the intracellular environment, and it provides energy and the macromolecular building blocks that are necessary for cell survival and differentiation6. Several studies have demonstrated that the differentiation and biological effects of MSCs are influenced by autophagy, and Beclin1 and LC3 are key genes in autophagosome formation7–9. However, the effects of autophagy on MSC migration are still unknown.
Low-intensity pulsed ultrasound (LIPUS) is a form of mechanical energy that is transmitted through and into tissues as pulsed acoustic pressure waves, and it was confirmed as an effective treatment for promoting cartilage repair10,11. Additionally, LIPUS was shown to promote the chondrogenic differentiation of MSCs12,13. Recently, reports have found that LIPUS enhanced MSC migration via the stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) signaling pathway from a parabiotic source at the injury site14,15. SDF-1 and CXCR4 are chemokine receptors, and they have been indicated to be associated with the migration of MSCs during injury repair in many tissues16–18.
In this study, we aimed to explore the role of autophagy in MSC migration using autophagy inhibitors and agonists, and we investigated the direct influence of LIPUS on MSC migration. In addition, we evaluated the therapeutic effects of LIPUS stimulation after an intra-articular injection of MSCs on OA cartilage. Our findings suggest that LIPUS promoted MSC migration via autophagy activation, and we confirmed that LIPUS enhanced the protective effect of MSCs on OA cartilage via autophagy regulation.
Materials and Methods
The experimental protocol relating to rats was in accordance with the US National Institutes of Health’s Guidelines of Laboratory Animal Use and approved by the Nanjing Medical University Ethics Committee of Nanjing Hospital (20150829).
The Isolation and Culture of MSCs
The MSCs derived from bone marrow were obtained from 60 male Sprague-Dawley (SD) rats (aged 8 weeks), and they were identified based on their surface phenotypes and multipotency, as previously described13,19. The bone marrow in the rat femur was flushed with low-glucose Dulbecco’s modified Eagle’s medium (DMEM; KeyGEN, Nanjing, Jiangsu, China) containing 10% fetal bovine serum (FBS; KeyGEN). The bone marrow fluid was put into 10 ml centrifuge tubes, and 3 ml phosphate-buffered saline (PBS; KeyGEN) was added. Then, it was centrifuged for 10 min at 1000 r/min. The supernatant was abandoned, and the remaining cells were washed twice with PBS and spun at 1000 r/min for 10 min. After washing, 5 ml DMEM was added to the cell suspension, and then the cells were cultured in culture dishes with 5% carbon dioxide (CO2)/95% air at 37 °C. When the cells reached 80% to 90% confluence, they were resuspended by trypsin. The subculture was plated at about 2 × 106 cells per dish. MSCs from passages 3 to 4 were used for all experiments.
Autophagy Agonist and Inhibitor Treatment
To explore the role of autophagy in MSC migration, MSCs (1 × 105 cells/ml) were seeded on the inner chamber of the transwell plate (Greiner Bio One, Frickenhausen, Germany). Then, they were treated with an autophagy inhibitor 3-Methyladenine (3-MA; Selleck, Houston, TX, USA) (1 μM, 5 μM, and 10 μM) or an autophagy agonist rapamycin (Selleck) (1 μM, 5 μM, and 10 μM) for 24 h. The 3-MA or rapamycin was dissolved with dimethylsulfoxide (DMSO).
MSCs Intra-Articular Injection
In vivo study, the third generation of 1 × 106 MSCs was resuspended with 50 µL normal saline20. 3-MA (10 μM) was added to the MSC suspension in order to inhibit autophagy of MSCs. The MSC suspension was injected through the patellar ligament into the right knee joint after anterior cruciate ligament transection (ACLT) operation for 4 weeks.
LIPUS Stimulation
In vitro, the LIPUS transducer (HT2009 -1, Ito Corporation, Tokyo, Japan) was placed under the transwell plate and coated with a coupling agent. Then, 4 intensities (20 mW/cm2, 30 mW/cm2, 40 mW/cm2, or 50 mW/cm2) of LIPUS (on-off ratio of 20%, frequency of 3 MHz) waves were transferred through the bottom of the plate, as previously described15. The sham LIPUS stimulation with no ultrasound irradiation was the control group. The cells were stimulated for 20 min once a day for 10 days in 5% CO2/95% air at 37 °C. To demonstrate the impact of autophagy in MSCs stimulated by LIPUS, the MSCs were incubated with 3-MA or rapamycin before the LIPUS irradiation.
In vivo, the knee joint of rats was exposed to LIPUS after MSCs suspension intra-articular injection (once every 10 days for 4 times). The LIPUS parameters were the same as the in vitro study: 20 min/day for 40 days.
Transmission Electron Microscopy
To demonstrate the existence of autophagy, transmission electron microscopy was utilized to observe the cells at the ultrastructural level. MSCs were collected and washed with cold PBS. Then, they were fixed with 2% glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) and washed twice with PBS. The cells were fixed with 1% osmium tetroxide (Sigma-Aldrich) and dehydrated. They were incubated with propylene oxide (Sigma-Aldrich) and embedded in epoxy resin (Sigma-Aldrich). Cells were sliced and stained with lead citrate (Sigma-Aldrich), and they were imaged with transmission electron microscopy (JEM-1011, JEOL, Akishima, Tokyo, Japan).
Immunofluorescence
We detected a specific autophagy marker for LC3-positive MSCs using immunofluorescence staining. MSCs were washed twice with PBS. Then, they were fixed with 4% paraformaldehyde in PBS for 30 min, and they were permeabilized with methanol. The MSCs were treated with 3% hydrogen peroxide in methanol for 15 min and washed with tap water for 15 min. The cells were incubated with 0.5% Triton X-100 (KeyGEN) for 5 min, and then, they were washed and incubated with blocking solution (10% goat serum in PBS) for 30 min. Next, they were treated with anti-LC3B antibody (1:200 dilution; Novus Biological, Littleton, CO, USA) overnight at 4 °C. After washing with blocking solution 3 times, the cells were incubated with secondary antibodies. The cells were stained with diamidine phenylindole (DAPI) (Molecular Probes, Waltham, MA, USA), and they were imaged using a confocal microscope (Dmi 6000-B, Leica, Brunswick, Germany).
Western Blot Analysis
A total protein extraction kit (KeyGEN) was used for protein extraction. The extracted proteins (20 to 25 µg/well) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the gel-separated proteins were electroblotted onto nitrocellulose membranes. Next, the membranes were blocked with 3% bovine serum albumin for 2 h. Then, they were incubated with anti-Beclin1 (1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-LC3 (1:1500; Novus Biological), anti-SDF-1 (1:1000; Cell Signaling Technology), anti-CXCR4 (1:5000; Novus Biological), anti-collagen II (COL2) (1:5000; Abcam, Cambridge, USA), anti-aggrecan (AGG) (1:1000; Novus Biological), and anti-β-actin (1:1000; Cell Signaling Technology) antibodies at 4 °C overnight. After being washed, the blots were treated with a goat antimouse secondary antibody (1:5000; Santa Cruz, Dallas, TX, USA) at room temperature for 2 h. Following 3 washes, the blots were developed with an enhanced chemiluminescence substrate (ECL kit; KeyGEN).
Transwell Migration Assay
The migration assay was performed in a 6-well transwell using 8-µm pore polycarbonate membranes (Greiner Bio One). MSCs were seeded at 1 × 105 cells/ml in the upper chamber of the transwell assembly. Either the autophagy agonist or inhibitor was added into the lower chamber, which contained 1 ml medium. After incubation under hypoxia (1% O2, 5% CO2) for 10 h, we gently scraped off the upper surface of the membrane. Nonmigrating cells were removed and rinsed with PBS. The membrane was then fixed with 4% paraformaldehyde for 15 min, and it was stained with 0.5% crystal violet for 10 min. The number of migrating cells was determined by counting 5 random fields per well.
Animal Experiments
Forty-eight (250 to 300 g) 8-week-old male SD rats were obtained from the Qinglongshan Experimental Animal Center of Jiangsu Province, China. All experimental protocols were approved by the Ethics Committee of Nanjing Medical University Nanjing Hospital. All rats were divided into the following 6 groups: the control group, the OA model group, the OA + MSCs group, the OA + MSCs + 3-MA group, the OA + MSCs + LIPUS group, and the OA + MSCs + 3-MA + LIPUS group. Rats receiving a sham operation served as the control. The rat OA model was produced by ACLT, as described previously21–23. In OA model groups, the rats received vehicle injections of 0.9% normal saline and a sham LIPUS stimulation. In the OA + MSCs group, the OA model rats were treated with an intra-articular injection of MSCs suspension and a sham LIPUS stimulation. In the OA + MSCs + 3-MA group, the OA model rats were treated with an intra-articular injection of MSCs suspension with the addition of 3-MA and a sham LIPUS stimulation. In the OA + MSCs + LIPUS group, the OA model rats were treated with LIPUS stimulation after an intra-articular injection of MSCs suspension. In the OA + MSCs + 3-MA + LIPUS group, the OA model rats were treated with an intra-articular injection of MSCs suspension with the addition of 3-MA and LIPUS stimulation.
Four weeks after the operation, all rats were sacrificed and the femoral condylar cartilages were used for histopathological analysis and molecular analyses. The femoral condylar cartilages from 24 rats (4 rats in each group) were fixed in neutral formalin and decalcified in ethylenediaminetetraacetic acid (EDTA) for 3 weeks. It was embedded in paraffin and divided into 4-µm thick sections using a microtome. The pathological changes in cartilage, including surface irregularities and the formation of cracks, were detected using safranin-O/fast green staining. Fibrosis, matrix distribution, cartilage loss, and chondrocyte colonization were assessed using the Mankin scoring system by 2 independent experts24,25 (Table 1). Moreover, we selected 3 pathological sections from each sample and 5 visual fields in each section for cartilage thickness analysis. In this process, we used Image Pro Plus 6.0 software to measure the thickness of total articular cartilage (TAC) (cartilaginous surface to the bonding line) according to the bone histological metrology method, and the average value was taken26. Finally, we extracted the protein from the femoral condylar articular cartilage of another 24 rats (4 rats in each group). Briefly, the rats were euthanized by overdose injections of anesthetic. Under sterile conditions, the femoral condyle articular cartilage in the knee was pulverized into powder in liquid nitrogen, then the lysis buffer in the protein extraction kit was added, and samples were centrifuged at 16,000×g and 4 °C for 10 min. And then, we detected the expression of COL2 and AGG using Western blot analysis.
Table 1.
Mankin Scoring Scale.
Subgroup 1: fibrillation
|
Grading was performed separately for the medial femoral condyle, lateral femoral condyle, medial tibial plateau, and lateral tibial plateau. The minimum total score was 4, and the maximum total score is 16.
Statistical Analysis
The data conformance to the normal distribution is described by mean ± SD and analyzed by SPSS 23.0 software (IBM Corp, Armonk, NY, USA). The single-factor analysis of variance (ANOVA) or the Kruskal-Wallis test was used to compare multiple groups. Bonferroni correction was applied to all multiple comparisons. P-values <0.05 were considered statistically significant.
Results
Autophagy Promotes MSC Migration
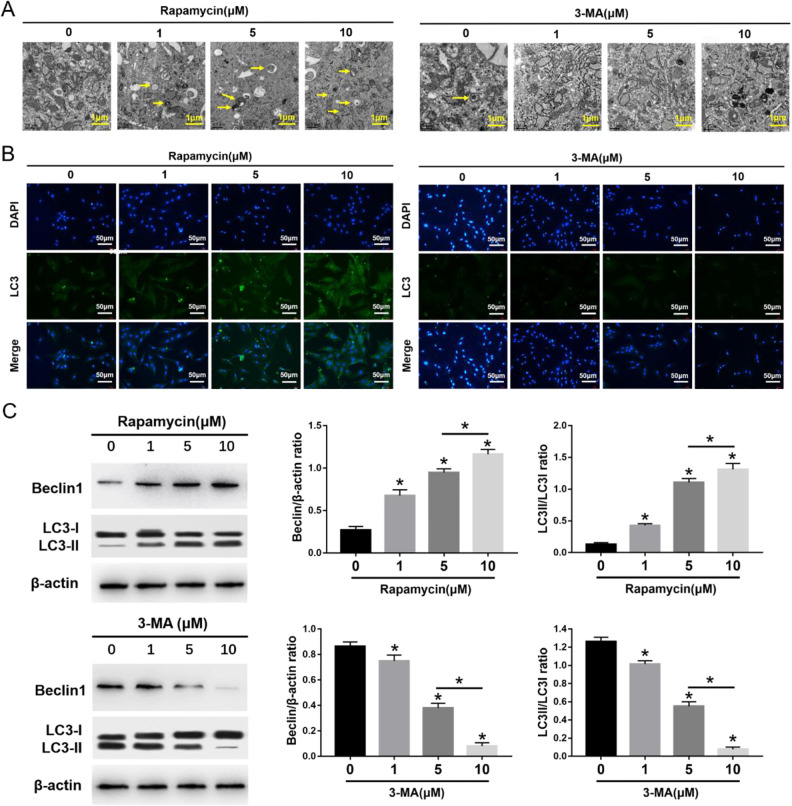
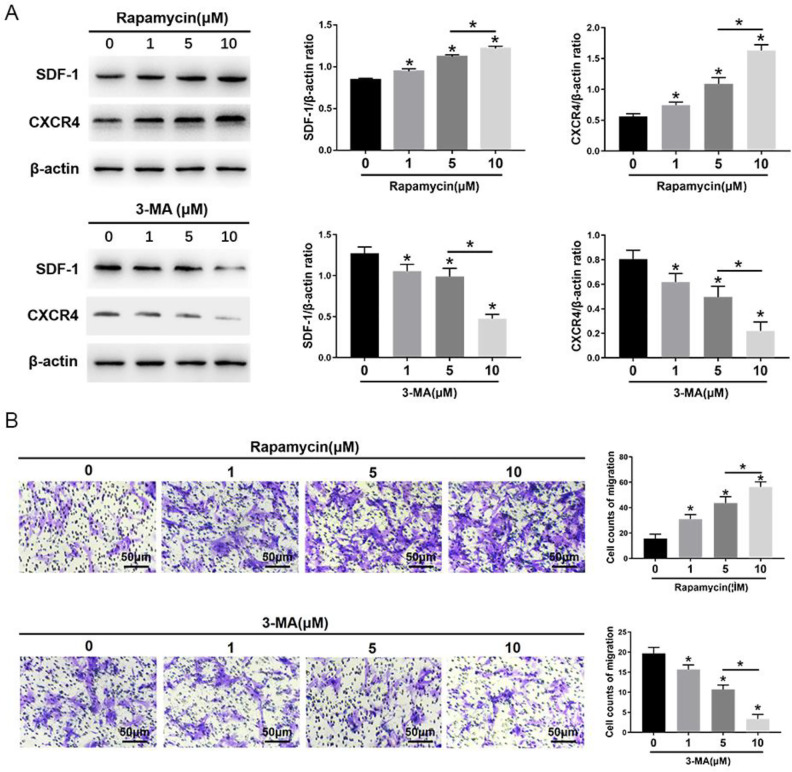
The morphometric ultrastructural analyses indicated that autophagosomes (double or multiple layered-autophagic vacuoles [arrows]) were increased following rapamycin (1, 5, 10 μM) treatment, but they were decreased following 3-MA (1, 5, 10 μM) treatment in MSCs (Fig. 1A). Immunofluorescence staining showed that LC3-positive cells were enhanced following rapamycin treatment, but they were decreased following 3-MA treatment in MSCs (Fig. 1B). The autophagy-specific proteins Beclin1 and LC3II were analyzed by Western blot. The expression of Beclin1 and the ratio of LC3II/LC3I were significantly increased in MSCs after rapamycin treatment, especially at the highest concentration of 10 μM. Conversely, the expression of Beclin1 and the ratio of LC3II/LC3I were significantly decreased in MSCs after 3-MA treatment, especially at the highest concentration of 10 μM (Fig. 1C). The migration assay and Western blot analysis indicated that the number of migrating MSCs and the expression of migrating related proteins, SDF-1 and CXCR4, were significantly increased after rapamycin treatment, especially at the highest concentration of 10 μM rapamycin (Fig. 2). In contrast, the number of migrating MSCs and the expression of SDF-1 and CXCR4 were decreased after 3-MA treatment, especially at the highest concentration of 10 μM 3-MA. These results imply that autophagy promotes the migration of MSCs (Fig. 2).
Figure 1.
Effects of 3-MA and rapamycin on MSC autophagy. (A) Electron microscopy depicting autophagosomes (arrows); scale bars = 0.5 μm. (B) Immunofluorescence staining depicting LC3+ cells (green); scale bars = 50 μm. (C) Western blot analysis of Beclin1, LC3I, LC3II, and β-actin expression in MSCs. The values are the mean ± SD; n = 4, *P < 0.05. 3-MA: 3-methyladenine; MSC: mesenchymal stem cell.
Figure 2.
Effects of 3-MA and rapamycin on MSC migration. (A) Western blot analysis of SDF-1, CXCR4, and β-actin expression in MSCs. (B) The transwell migration capacity of MSCs stained with crystal violet, and the bar graph comparing the counts of migrated cells; scale bars = 50μm. The values are the mean ± SD; n = 4, *P < 0.05. 3-MA: 3-methyladenine; CXCR4: C-X-C chemokine receptor type 4; MSC: mesenchymal stem cell; SDF-1: stromal cell-derived factor-1.
LIPUS Activates Autophagy and Promotes MSC Migration
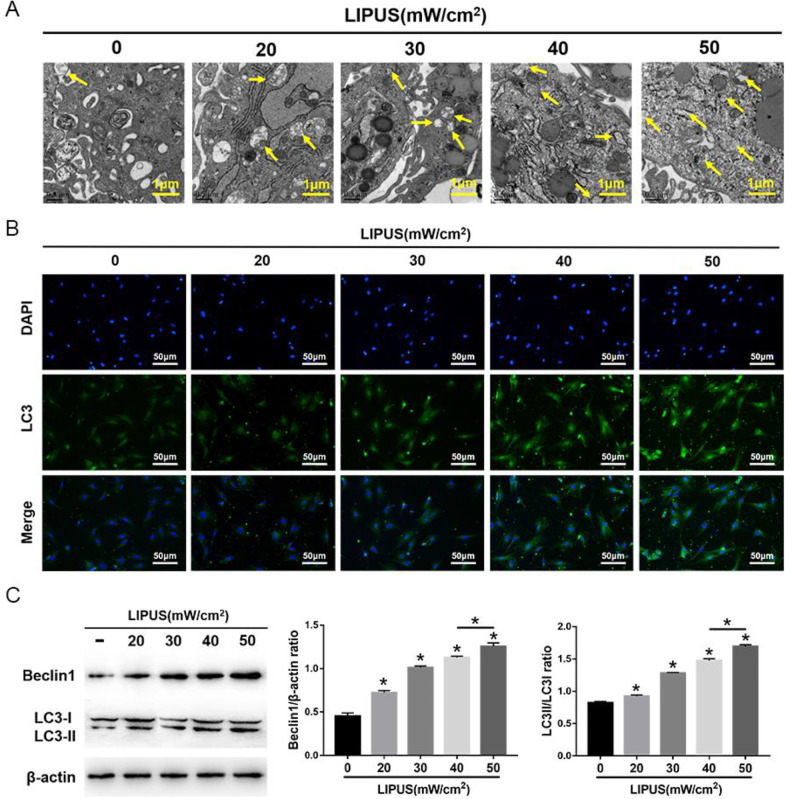
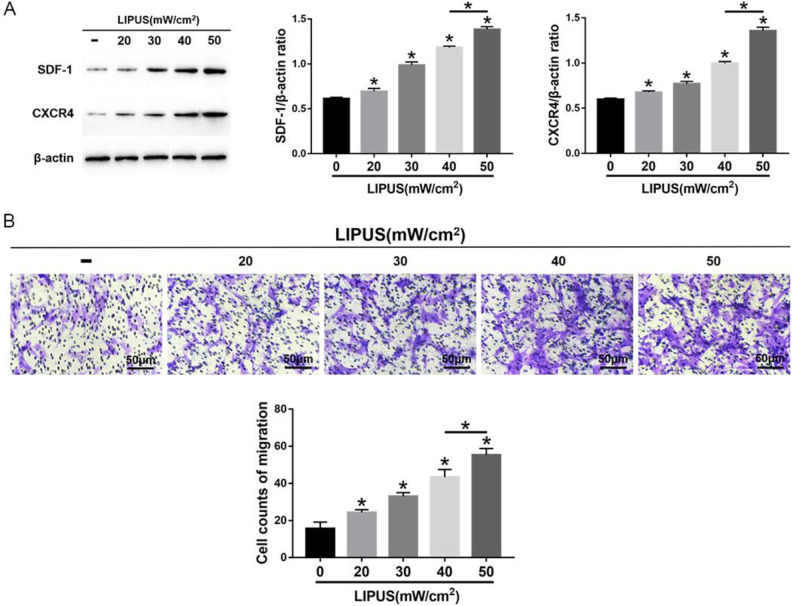
The MSCs were exposed to 4 intensities of LIPUS to demonstrate the effects of LIPUS on MSC autophagy and migration. The transmission electron microscopy indicated an increase in autophagosomes in MSCs after LIPUS stimulation (Fig. 3A). The immunofluorescence staining showed that the LC3-positive cells in MSCs were enhanced after LIPUS stimulation (Fig. 3B). The Western blot analysis revealed that Beclin1 expression and the ratio of LC3II/LC3I were significantly increased in MSCs after LIPUS stimulation, especially at the highest intensity of 50 mW/cm2 (Fig. 3C). The migration assay and Western blot analysis indicated that the number of migrating MSCs and the expression of SDF-1 and CXCR4 were significantly increased after LIPUS stimulation, especially at the highest intensity of 50 mW/cm2 (Fig. 4). These results suggest that LIPUS activated autophagy in MSCs and promoted MSC migration.
Figure 3.
Effects of LIPUS on autophagy in MSCs. (A) Electron microscopy depicting autophagosomes (arrows); scale bars = 0.5 μm. (B) Immunofluorescence staining depicting LC3+ cells (green); scale bars = 50 μm. (C) Western blot analysis of Beclin1, LC3I, LC3II, and β-actin expression in MSCs stimulated with 4 intensities of LIPUS. The values are the mean ± SD; n = 4, *P < 0.05. 3-MA: 3-methyladenine; LIPUS: low-intensity pulsed ultrasound; MSC: mesenchymal stem cell.
Figure 4.
Effects of LIPUS on the migration of MSCs. (A) Western blot analysis of SDF-1, CXCR4, and β-actin expression in MSCs. (B) The transwell migration capacity of MSCs stained with crystal violet and the bar graph comparing the counts of migrated cells; scale bars = 50 μm. The values are the mean ± SD; n = 4, *P < 0.05. CXCR4: C-X-C chemokine receptor type 4; LIPUS: low-intensity pulsed ultrasound; MSC: mesenchymal stem cell; SDF-1: stromal cell-derived factor-1.
LIPUS Promotes the Migration of MSCs by Activating Autophagy
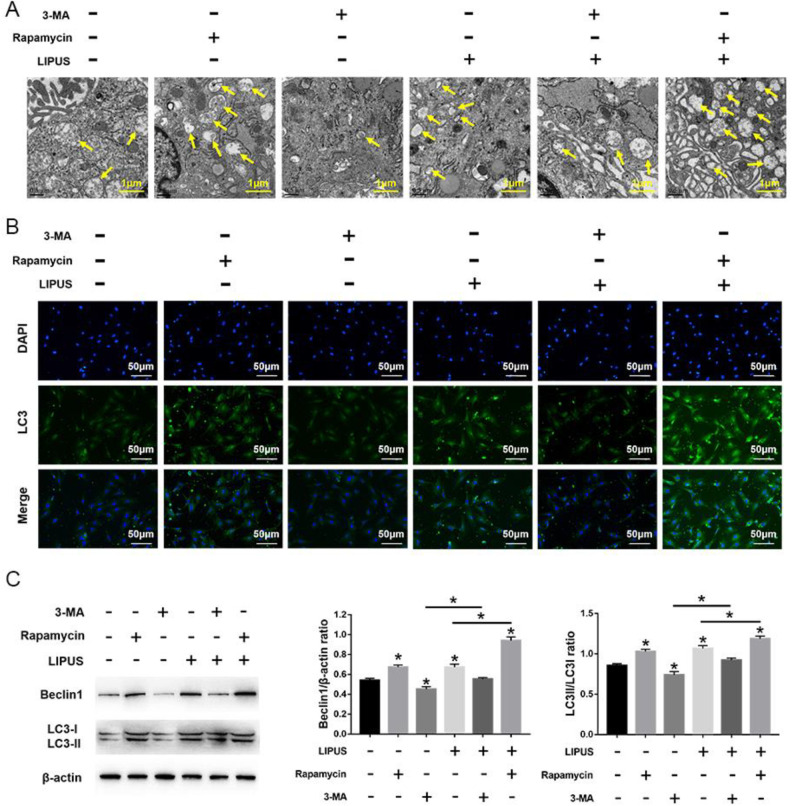
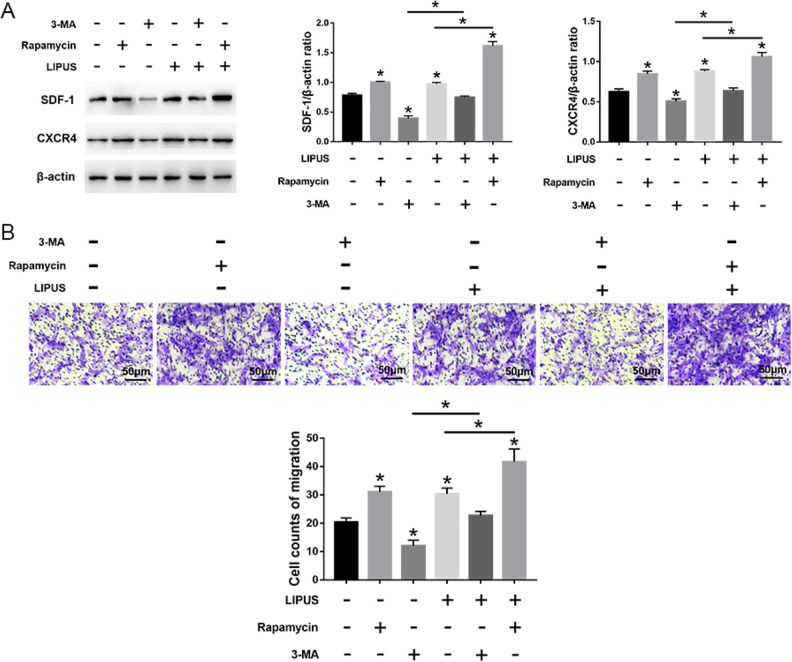
LIPUS significantly increased autophagy in MSCs compared with the untreated cells, but this effect was blocked by 3-MA incubation. Compared with cells treated with 3-MA, the autophagy was significantly increased in cells treated with rapamycin, even after LIPUS stimulation (Fig. 5). Moreover, compared with the unstimulated state, LIPUS stimulation has no effect on the number of migrating cells and the expression of migration-related markers (SDF-1 and CXCR4) in MSCs in the presence of 3-MA. Moreover, the migration stimulated by LIPUS was more noticeable following rapamycin treatment compared with the 3-MA treatment (Fig. 6). Taken together, these results indicate that LIPUS enhances MSC migration by activating autophagy.
Figure 5.
Effects of LIPUS on autophagy in MSCs treated with 3-MA or rapamycin. (A) Electron microscopy depicting autophagosomes (arrows); scale bars = 0.5 μm. (B) Immunofluorescence staining depicting LC3+ cells (green); scale bars = 50 μm. (C) Western blot analysis of Beclin1, LC3I, LC3II, and β-actin expression in MSCs stimulated with 4 intensities of LIPUS. Values are the mean ± SD; n = 4, *P < 0.05. 3-MA: 3-methyladenine; LIPUS: low-intensity pulsed ultrasound; MSC: mesenchymal stem cell.
Figure 6.
Effects of LIPUS on the migration of MSCs treated with 3-MA or rapamycin. (A) Western blot analysis of SDF-1, CXCR4, and β-actin expression in MSCs. (B) The transwell migration capacity of MSCs stained with crystal violet and the bar graph comparing the counts of migrated cells; scale bars = 50 μm. The values are the mean ± SD; n = 4, *P < 0.05. 3-MA, 3-methyladenine; CXCR4: C-X-C chemokine receptor type 4; LIPUS: low-intensity pulsed ultrasound; MSC: mesenchymal stem cell; SDF-1: stromal cell-derived factor-1.
LIPUS Enhances the Ability of MSCs to Repair Cartilage in OA via Autophagy Regulation
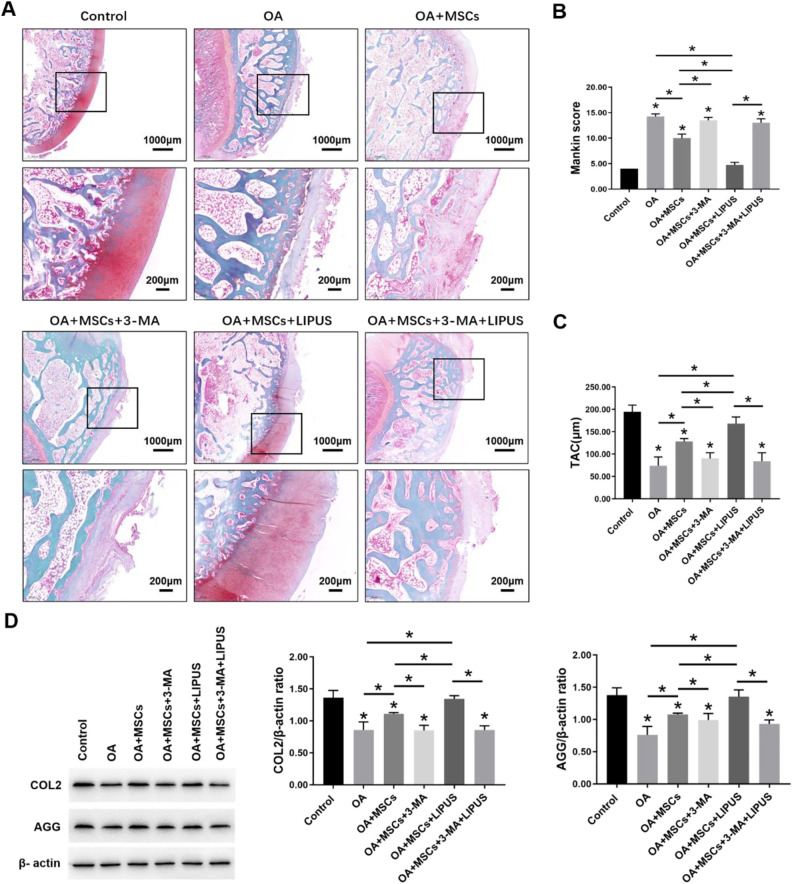
We evaluated the effects of LIPUS stimulation on the therapeutic effects of MSCs during OA cartilage repair. Pathological staining showed that the surface of the OA group was slightly uneven and irregular, and the cartilage fibrosis was slight. The staining was slight to moderately lost, and the chondrocytes were disordered. Also, the number of chondrocytes was lower than the control group. Compared with the OA group, a slightly uneven cartilage surface and an increase in chondrocytes were observed in the OA + MSCs group. Moreover, in the OA + MSCs + LIPUS group, the cartilage surface was more regular and even than in the OA + MSCs group. No difference in the cartilage tissue was observed among the OA group, the OA + MSCs + 3-MA group, and the OA + MSCs + 3-MA + LIPUS group (Fig 7A).
Figure 7.
Effects of LIPUS stimulation after intra-articular injection of MSCs on OA cartilage. (A) Morphological changes of cartilage revealed by safranin-O/fast green staining and observed under a microscope; scale bars = 1000 μm, 200 μm. (B) Bar graph comparing the Mankin scores. (C) Bar graph comparing the TAC thickness (μm). (D) Western blot analysis of COL2, AGG, and β-actin expression in cartilage. The values are the mean ± SD; n = 4, *P < 0.05. LIPUS: low-intensity pulsed ultrasound; MSC: mesenchymal stem cell; OA: osteoarthritis; TAC: total articular cartilage.
The Mankin scores of the OA group were much higher than the control group. Compared with the OA group, there was a significant decrease in the scores for the OA + MSCs group and the OA + MSCs + LIPUS group, particularly in the OA + MSCs + LIPUS group. There was no significant difference in the scores among the OA group, the OA + MSCs + 3-MA group, and the OA + MSCs + 3-MA + LIPUS group (Fig. 7B).
The TAC thickness of the OA group was much lower than the control group. Compared with the OA group, there was a significant increase in the thickness for the OA + MSCs group and the OA + MSCs + LIPUS group, particularly in the OA + MSCs + LIPUS group. There was no significant difference in the thickness among the OA group, the OA + MSCs + 3-MA group, and the OA + MSCs + 3-MA + LIPUS group (Fig. 7C).
The Western blot analysis results indicated that the protein expression of COL2 and AGG were significantly decreased in the OA group compared with the control group. Compared with the OA group, the protein expression of COL2 and AGG were significantly increased in the OA + MSCs group and the OA + MSCs + LIPUS group, particularly in the OA + MSCs + LIPUS group. There was no significant difference in the protein expression of COL2 and AGG among the OA group, the OA + MSCs + 3-MA group, and the OA + MSCs + 3-MA + LIPUS group (Fig. 7D). These results suggest that MSCs have a protective effect on OA cartilage, and this effect can be enhanced by LIPUS. These effects were significantly blocked by autophagy inhibitor 3-MA.
Discussion
Our study aimed to investigate the impact of autophagy on MSC migration, and it aimed to determine if LIPUS promotes MSC migration by regulating autophagy. In addition, we attempted to confirm the effects of MSCs on OA cartilage repair after LIPUS stimulation. We propose that the activation of autophagy promoted MSC migration, and LIPUS promoted MSC migration by activating autophagy. Moreover, we demonstrated that LIPUS enhanced the cartilage repair effects of MSCs on OA via autophagy regulation.
OA is a chronic degenerative disease characterized by cartilage degeneration and by sclerosis and hyperplasia of the subchondral bone, which leads to stenosis of the articular space27. The MSC-based cell therapy is a potential and ideal treatment for OA28. MSCs are widely used in research for OA because of their capacity in target homing, multipotent differentiation, self-renewal, and paracrine productions29–31. A key process for OA treatment is the migration of MSCs to damaged cartilage. Due to the low migration rate of MSCs, the drawbacks of intra-articular injection of MSCs include cell dispersion, a lack of convergence of the injected contents into an injury site, and an insufficient number of seeded cells for regeneration32,33. Therefore, improving the migration capacity of MSCs will promote cartilage injury repair.
The migration of MSCs is affected by many factors. It appears that chemokine receptors are critical for the migration of MSCs. There is growing evidence that the enhanced expression of SDF-1 and CXCR4 could lead to increased MSC migration. SDF-1/CXCR4 has been found to direct the stem cell migration associated with injury repair in a variety of tissues16,17,34. Autophagy plays a key role in maintaining cell function, resisting hunger, controlling growth, and eliminating abnormal cellular components accumulated during cell senescence35. The mechanism of autophagy was demonstrated to have an essential function in the migration of cancer cells36, pulmonary arterial smooth muscle cells, and macrophages37,38. Yang et al. also indicated that autophagy is involved in SDF-1-mediated dental pulp stem cell (DPSC) migration39. In our previous study, we also demonstrated that autophagy regulates the chondrogenesis of MSCs40. However, the impact of autophagy on regulating MSC migration is unclear. In the current study, we found that the autophagy agonist rapamycin significantly increased the migration of cells and the expression of SDF-1 and CXCR4 in MSCs. However, treatment with 3-MA decreased migration. This suggests a role as an activator of autophagy in MSC migration.
Mechanical stress is an important regulatory factor during MSCs activities41–43. LIPUS is mechanical stress, and it is currently used as a clinical application to promote cartilage repair10,11,44,45. In recent years, studies have confirmed that LIPUS affects cell migration. Takao et al. investigated the effects of LIPUS on the migration of osteoblast-like cells (MC3T3-E1 cells) using a wound healing assay. They found that LIPUS significantly increased MC3T3-E1 osteoblastic cell migration46. Additionally, Wei et al. found that LIPUS increased the SDF-1/CXCR4 signaling pathway, promoted MSC migration to the injury site, and promoted fracture healing15.
In our previous study, we found that LIPUS inhibited autophagy and promoted chondrogenesis of MSCs. But in this study, our results indicated that LIPUS activated autophagy, augmented the SDF-1/CXCR4 signaling pathway, and promoted the migration of MSCs. These effects can be suppressed by inhibiting autophagy in vitro. The effects of LIPUS on MSCs autophagy is opposite in our 2 studies may be due to the use of the chondrogenic medium in the previous study. We also found that intra-articular injection of MSCs decreased the Mankin scores, and it increased the expression of COL2 and AGG in OA. Additionally, we found that LIPUS stimulation after treatment with MSCs significantly enhanced these effects in vivo, but these effects were blocked by inhibiting autophagy. These results indicate that LIPUS promoted MSC migration by activating autophagy, and LIPUS enhanced the therapeutic effects of MSCs on OA cartilage via autophagy regulation.
In summary, we propose that autophagy plays a key role in MSC migration. Autophagy activation promoted MSC migration, and autophagy inhibition demonstrated the opposite effects. Moreover, we propose that MSC migration is promoted by LIPUS by activating autophagy, and LIPUS enhances the cartilage repair effects of MSCs on OA via autophagy regulation. Our results further reveal the mechanism of the effects of LIPUS on MSC migration and provide evidence for using MSCs and LIPUS for cartilage injury repair in the future.
Footnotes
Authors' Note: All the authors are researchers at Department of Rehabilitation Medicine, Nanjing First Hospital, Nanjing Medical University, China. PX and XL are the leaders of our research group.
Authors' Contribution: PX and XL conceived and designed the study. XW, DW, QW, and Xiaoju Wang performed the experiments. PX wrote the manuscript. KC and XL reviewed and edited the manuscript. All authors read and approved the manuscript.
Availability of Data and Materials: All data generated and/or analyzed during this study are included in this published article.
Ethical Approval: The study was approved by the Nanjing Medical University Ethics Committee of Nanjing Hospital (20150829).
Statement of Human and Animal Rights: The experimental protocol relating to rats was in accordance with the US National Institutes of Health’s Guidelines of Laboratory Animal Use and approved by the Nanjing Medical University Ethics Committee of Nanjing Hospital (20150829).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant No. 81501941, 81772437).
ORCID iD: Xueping Li  https://orcid.org/0000-0003-3891-3385
https://orcid.org/0000-0003-3891-3385
References
- 1. Wang Y, Yuan M, Guo Q, Lu S, Peng J. Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Cell Transplant. 2015;24(9):1661–1678. [DOI] [PubMed] [Google Scholar]
- 2. Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003;34(6):565–571. [DOI] [PubMed] [Google Scholar]
- 3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 4. Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O’Byrne E, Pellas TC. Fluorescently labeled mesenchymal stem cells (mscs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23(1):109–119. [DOI] [PubMed] [Google Scholar]
- 5. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. [DOI] [PubMed] [Google Scholar]
- 6. Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15(3):344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12(9):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Y, Huang Q, Yang J, Lou M, Wang A, Dong J, Qin Z, Zhang T. Autophagy impairment inhibits differentiation of glioma stem/progenitor cells. Brain Res. 2010;1313:250–258. [DOI] [PubMed] [Google Scholar]
- 9. Dang S, Xu H, Xu C, Cai W, Li Q, Cheng Y, Jin M, Wang RX, Peng Y, Zhang Y, Wu C, et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy. 2014;10(7):1301–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Li J, Cheng K, Lin Q, Wang D, Zhang H, An H, Gao M, Chen A. Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Biophy. 2011;61(2):427–434. [DOI] [PubMed] [Google Scholar]
- 11. Xia P, Shen S, Lin Q, Cheng K, Ren S, Gao M, Li X. Low-intensity pulsed ultrasound treatment at an early osteoarthritis stage protects rabbit cartilage from damage via the integrin/focal adhesion kinase/mitogen-activated protein kinase signaling pathway. J Ultrasound Med. 2015;34(11):1991–1999. [DOI] [PubMed] [Google Scholar]
- 12. Ebisawa K, Hata K, Okada K, Kimata K, Ueda M, Torii S, Watanabe H. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10(5–6):921–929. [DOI] [PubMed] [Google Scholar]
- 13. Xia P, Wang X, Qu Y, Lin Q, Cheng K, Gao M, Ren S, Zhang T, Li X. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res Ther. 2017;8(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumagai K, Takeuchi R, Ishikawa H, Yamaguchi Y, Fujisawa T, Kuniya T, Takagawa S, Muschler GF, Saito T. Low-intensity pulsed ultrasound accelerates fracture healing by stimulation of recruitment of both local and circulating osteogenic progenitors. J Orthop Res. 2012;30(9):1516–1521. [DOI] [PubMed] [Google Scholar]
- 15. Wei FY, Leung KS, Li G, Qin J, Chow SK, Huang S, Sun MH, Qin L, Cheung WH. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS One. 2014;9(9):e106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22(3):415–427. [DOI] [PubMed] [Google Scholar]
- 17. Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60(3):813–823. [DOI] [PubMed] [Google Scholar]
- 18. Liu N, Tian J, Cheng J, Zhang J. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem. 2013;114(12):2677–2689. [DOI] [PubMed] [Google Scholar]
- 19. Xu L, Song C, Ni M, Meng F, Xie H, Li G. Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRalpha-induced beta-catenin degradation. Int J Biochem Cell Biol. 2012;44(4):612–619. [DOI] [PubMed] [Google Scholar]
- 20. Neybecker P, Henrionnet C, Pape E, Mainard D, Galois L, Loeuille D, Gillet P, Pinzano A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stoop R, Buma P, van der Kraan PM, Hollander AP, Billinghurst RC, Meijers TH, Poole AR, van den Berg WB. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthritis Cartilage. 2001;9(4):308–315. [DOI] [PubMed] [Google Scholar]
- 22. Murata K, Kanemura N, Kokubun T, Fujino T, Morishita Y, Onitsuka K, Fujiwara S, Nakajima A, Shimizu D, Takayanagi K. Controlling joint instability delays the degeneration of articular cartilage in a rat model. Osteoarthritis Cartilage. 2017;25(2):297–308. [DOI] [PubMed] [Google Scholar]
- 23. Ma T, Zhang Z, Song X, Bai H, Li Y, Li X, Zhao J, Ma Y, Gao L. Combined detection of COMP and CS846 biomarkers in experimental rat osteoarthritis: a potential approach for assessment and diagnosis of osteoarthritis. J Orthop Surg Res. 2018;13(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–537. [PubMed] [Google Scholar]
- 25. van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10(1):58–61. [DOI] [PubMed] [Google Scholar]
- 26. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13(4):436–448. [DOI] [PubMed] [Google Scholar]
- 29. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1(1):74–79. [DOI] [PubMed] [Google Scholar]
- 30. Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, Kuroda R, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5(2):146–150. [DOI] [PubMed] [Google Scholar]
- 31. Davatchi F, Sadeghi AB, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19(3):219–225. [DOI] [PubMed] [Google Scholar]
- 32. Hideyuki K, Masayuki S, Takeshi M, Akimoto N, Toshiyuki M, Masaya H, Shiro S, Young-Jin J, Tomoyuki M, Ichiro S. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goki K, Takaaki K, Shingo O, Wirat K, Nobuo A, Kobun T, Hayatoshi S, Masataka D, Koji H, Goldberg JL. Articular cartilage repair with magnetic mesenchymal stem cells. Am J Sports Med. 2013;41(6):1255–1264. [DOI] [PubMed] [Google Scholar]
- 34. Liu N, Tian J, Cheng J, Zhang J. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem. 2013;114(12):2677–2689. [DOI] [PubMed] [Google Scholar]
- 35. Ellen W, Tom VB, Saskia L, Patrizia A, Peter V. Autophagy: for better or for worse. Cell Res. 2012;22(1):43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhan Z, Xie X, Cao H, Zhou X, Zhang XD, Fan H, Liu Z. Autophagy facilitates TLR4- and TLR3-triggered migration and invasion of lung cancer cells through the promotion of TRAF6 ubiquitination. Autophagy. 2014;10(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liao CC, Ho MY, Liang SM, Liang CM. Recombinant protein rVP1 upregulates BECN1-independent autophagy, MAPK1/3 phosphorylation and MMP9 activity via WIPI1/WIPI2 to promote macrophage migration. Autophagy. 2013;(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang H, Gong Y, Wang Z, Jiang L, Chen R, Fan X, Zhu H, Han L, Li X, Xiao J, Kong X. Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia. J Cell Mol Med. 2014;18(3):542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang JW, Zhang YF, Wan CY, Sun ZY, Nie S, Jian SJ, Zhang L, Song GT, Chen Z. Autophagy in SDF-1alpha-mediated DPSC migration and pulp regeneration. Biomaterials. 2015;44:11–23. [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Lin Q, Zhang T, Wang X, Cheng K, Gao M, Xia P, Li X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res Therapy. 2019;10(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Z, Yao SJ, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2010;16(2):575–584. [DOI] [PubMed] [Google Scholar]
- 43. Alves DSM, Martins A, Costa-Pinto AR, Correlo VM, Sol P, Bhattacharya M, Faria S, Reis RL, Neves NM. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5(9):722–732. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Z, Huckle J, Francomano CA, Spencer RG. The influence of pulsed low-intensity ultrasound on matrix production of chondrocytes at different stages of differentiation: an explant study. Ultrasound Med Biol. 2002;28(11-12):1547–1553. [DOI] [PubMed] [Google Scholar]
- 45. Xia P, Ren S, Lin Q, Cheng K, Shen S, Gao M, Li X. Low-intensity pulsed ultrasound affects chondrocyte extracellular matrix production via an integrin-mediated p38 MAPK Signaling Pathway. Ultrasound Med Biology. 2015; 41(6): 1690–1700. [DOI] [PubMed] [Google Scholar]
- 46. Iwai T, Harada Y, Imura K, Iwabuchi S, Murai J, Hiramatsu K, Myoui A, Yoshikawa H, Tsumaki N. Low-intensity pulsed ultrasound increases bone ingrowth into porous hydroxyapatite ceramic. J Bone Miner Metab. 2007;25(6):392–399. [DOI] [PubMed] [Google Scholar]