Abstract
Background:
Bipartite patella (BPP) is a developmental anomaly that forms when incomplete patellar ossification leaves a residual fibrocartilaginous synchondrosis between ossification centers. Repetitive traction forces across the synchondrosis can cause knee pain, most commonly presenting in adolescence. Symptoms frequently resolve with nonoperative management. Few surgical case series exist to guide treatment approaches for refractory pain.
Purpose:
To investigate the clinical features, surgical techniques, and outcomes associated with operative treatment of symptomatic BPP in pediatric and adolescent athletes and to compare features of the series with a large control group managed nonoperatively.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A retrospective medical record review was conducted of all patients aged ≤20 years who were diagnosed with symptomatic, radiologically confirmed BPP between 2003 and 2018 at a single tertiary-care pediatric hospital (N = 266). Demographic and clinical variables were analyzed, and additional radiologic and perioperative variables were collected for the surgical subcohort.
Results:
Of the 266 patients included, 27 were treated operatively (10.2%). When compared with those treated nonoperatively (with rest, physical therapy, cryotherapy, and anti-inflammatory medications), the operatively managed group experienced a longer duration of symptoms before first presentation (21.5 vs 7.6 months; P < .001) and were more likely to be older (mean age, 15.4 vs 12.4 years; P < .001), female (59.3% vs 35.6%; P = .03), and competitive athletes (100% vs 84.5%; P = .02). In the 27 patients treated operatively, procedures were categorized as isolated fragment excision (n = 9), fragment excision with lateral release (n = 8), isolated lateral release (n = 5), fragment screw fixation (n = 4), and synchondrosis drilling (n = 1). The mean time between surgery and return to sports was 2.2 months. Four patients (14.8%) reported residual symptoms requiring secondary surgery, including lateral release (n = 1), excision of residual fragment (n = 1), and fixation screw removal (n = 2).
Conclusion:
BPP can cause knee pain in adolescent athletes and is generally responsive to nonoperative treatment. Patients undergoing surgical treatment—most commonly female competitive athletes with prolonged symptoms—represented 10% of cases. A variety of surgical techniques may be effective, with a 15% risk of persistent or recurrent symptoms warranting reoperation. Prospective multicenter investigations are needed to identify optimal candidates for earlier interventions and the optimal operative treatment technique.
Keywords: bipartite patella, anterior knee pain, lateral release, fragment excision, pediatric sports medicine
Bipartite patella (BPP) is a developmental anomaly in which a synchondrosis develops between 2 unfused bony patellar ossification centers. The incidence of BPP is 2% to 6% in the general population, with a male-to-female predominance of 9 to 1.5,7,20,25
Developmentally, the patella initially forms as a large cartilaginous sesamoid bone with multiple ossification centers.15,22 These eventually fuse to form a central ossification center in children aged 2 to 6 years, after which accessory ossification centers may form in children aged 8 to 12 years.5,22,26 While these accessory centers typically fuse with the central ossification center to form a continuous subchondral plate, they may remain unfused in some children with a persistent fibrocartilaginous synchondrosis,5,7 constituting BPP.
Three characteristic morphologic variants of BPP were described by Saupe in 1943,23 establishing a classification system.14,23 Type I is an accessory fragment located inferior to the patella, observed in approximately 5% of patients. In type II, the accessory fragment is along the lateral margin of the patella (∼20% of patients). Type III is the most common version of the condition, with an accessory fragment located superolateral to the patella (∼70%).
BPP is typically an asymptomatic incidental finding22 and is generally regarded as a developmental or anatomic variant.8 However, it can be a cause of anterior knee pain when repetitive traction forces are imposed across the synchondrosis, most commonly during rigorous athletic activity involving stresses to the extensor mechanism, as in jumping, cutting, and pivoting sports.2,13,26
Pain may also develop after an acute blow or impact injury to a previously asymptomatic accessory ossicle. As a result, awareness of the condition is important to caregivers in the growing superspecialty of pediatric sports medicine. The diagnosis is generally based on radiographs demonstrating a linear lucency within the superolateral, lateral, or inferior portion of the patella, with the clinical correlation of tenderness to palpation over the accessory fragment or synchondrosis. Given the multiple alternative causes of anterior knee pain in adolescents and preadolescents, definitive diagnosis can be challenging. At times, the condition is misdiagnosed as an acute fracture, particularly since minor traumatic events can represent a precipitating symptomatic event on a preexisting BPP, with an acute on chronic–type presentation.24 Generally, however, patellar fracture can be ruled out by the more common insidious onset of symptoms and the absence of a high-energy, discrete, and direct blow to the patella.14 When a direct blow does precipitate the onset of symptoms, magnetic resonance imaging (MRI) may be helpful to differentiate a true fracture from an acute exacerbation of a chronic condition.20
For the more classic overuse presentation, symptoms are frequently alleviated with ≥3 months of nonoperative management, including relative rest, cryotherapy, nonsteroidal anti-inflammatory drugs, and physical therapy exercises designed to stretch and strengthen the quadriceps muscle, thereby offloading the more distal aspect of the extensor mechanism.2,11,16,22 When prolonged nonoperative measures fail, surgical intervention may occasionally be indicated, and various procedures have been employed to improve or eliminate symptoms. However, there is little evidence investigating which surgical approach may be optimal or superior in treating a painful BPP or which procedures may be applied for different presentations of the condition.
Numerous options for surgical management of BPP have been explored. Early reports described isolated excision of the painful accessory fragment,5,12,15 although subsequent series have advocated osteosynthesis,2,11 vastus lateralis release,1 isolated lateral retinacular release,19 or lateral retinacular release plus fragment excision.21 However, no large series have emerged investigating multiple treatment approaches in a comparative fashion.
The purpose of this study was therefore to investigate the presenting features, clinical course, surgical techniques, and outcomes of a series of pediatric and adolescent athletes treated with a variety of surgical approaches for symptomatic BPP, with a comparison with a large control group of patients treated nonoperatively to elucidate potential risk factors for undergoing surgery.
Methods
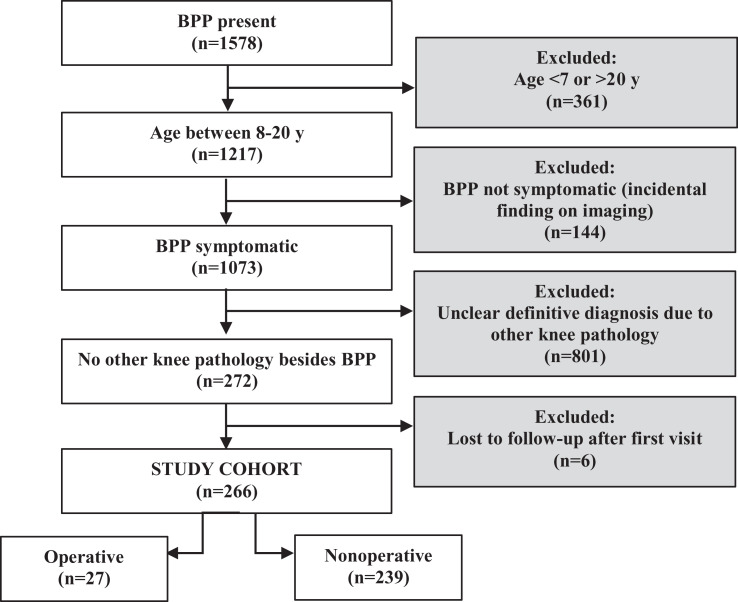
After institutional review board approval, a retrospective electronic medical record review was conducted using a search engine of the records within the orthopaedic department of a tertiary-care pediatric hospital. A preliminary search utilized any mention of “bipartite patella” in clinic notes between January 1, 2003, and July 23, 2018. This returned 1758 notes for 1578 patients, 361 of whom were excluded for age >20 years or <7 years, based on classic descriptions of the condition occurring almost exclusively in preadolescent and adolescent populations. We then excluded 144 patients for absence of knee symptoms, with BPP identified only as an incidental finding on imaging. An additional 801 patients were excluded because the knee pain could not be attributed specifically to the BPP, on the basis of insufficient clinical information in the records or because of the presence of concomitant knee pathology. Six patients were excluded because they were lost to follow-up from the time of the initial visit. The remaining 266 cases included pediatric and adolescent patients diagnosed with symptomatic, radiographically confirmed BPP (Figure 1).
Figure 1.
Flowchart of inclusion/exclusion criteria for bipartite patella (BPP) study cohort.
The following descriptive, radiologic, and clinical data were collected for all 266 patients: sex, age, athletic status, Saupe classification, duration of preceding symptoms before initial clinic presentation at the study institution, time from initial clinic presentation at the study institution to time of diagnosis of BPP, type of nonoperative management (eg, bracing, cryotherapy, physical therapy) and duration of nonoperative treatment until symptom resolution or return to sport, and whether surgery was recommended. Additional clinical data were collected for the patients treated operatively, including whether they had stopped sports because of pain; whether symptom onset was insidious or traumatic; the precise location of knee pain on clinical examination; the time from symptom onset to the date of surgery; the size of the accessory fragment (also expressed as a percentage of overall patellar size, or surface area); and whether the accessory fragment contained weightbearing articular cartilage, as described in operative notes or, when not in the operative notes, per MRI.
The fragment shape was approximated as an ellipse, and the areas of the fragment and the patella were calculated as , where α and β correspond to fragment/patellar length and width, respectively. The MRI-based designation of weightbearing articular cartilage was made using axial sequences. If the cartilage of the BPP fragment was found to articulate with, or be proximal to but in line with, the cartilage of the lateral trochlear ridge, it was labeled “containing articular cartilage.” If the BPP fragment contained no cartilage on the deep surface or the cartilage was found to be lateral to the lateral trochlear ridge, it was labeled “not containing articular cartilage.” Surgical data collected included the surgical technique employed, tourniquet time (used in all cases), time to radiographic healing (when applicable), reports of residual or recurrent symptoms, and whether reoperation was performed.
Given the retrospective study design, several basic assumptions were made to achieve comprehensive data regarding the posttreatment clinical course when precise dates were not provided in the records. If a patient presented to 1 visit with symptoms but these symptoms had resolved by the following visit, then the date of symptom improvement was estimated to occur at the temporal midpoint between the 2 visits. Similarly, for return to sport, if a patient had not returned to sport at 1 clinic visit but had returned by the next clinic visit, then the date of return to sport was estimated to occur at the temporal midpoint between the 2 visits. For patients who were lost to follow-up, the date of the missed appointment was used as the date of symptom improvement or return to sport.
Surgical Technique
Given the retrospective nature of the study, in the majority of cases there were no clear, identifiable indications for the choice of one procedure over the other. For the subgroup of patients who underwent lateral retinacular release, release of the lateral retinaculum was performed either arthroscopically (n = 11) or in an open fashion (n = 3). For all cases in which open lateral release was performed, concurrent fragment excision was also performed. The release was done using electrocautery off of the lateral border of the patella. Arthroscopic-aided lateral release was performed in 1 of 2 ways: either (1) with an arthroscopic electrocautery device from 1 cm lateral to the superolateral border of the patella to the inferolateral portal; or (2) with long scissors placed through the lateral portal and pushed proximally to a point 1 cm lateral to the superolateral border of the patella, after which the lateral geniculate artery was immediately cauterized.
The technique used for fragment excision varied by Saupe classification. For Saupe I (inferior) BPP, an incision was made over the inferior pole of the patella. The most proximal fibers of the patellar tendon were split longitudinally down to the periosteum of the inferior pole of the patella; the fragment was identified, freed, and excised, with subsequent side-to-side patellar tendon repair. For Saupe II (lateral) or Saupe III (superolateral) BPP, an incision was made over the lateral border of the patella. For Saupe III BPP, the most distal extent of the vastus lateralis tendon was split obliquely, in line with the fibers, down to the periosteum of the superolateral border of the patella; the fragment was identified, freed, and excised with subsequent side-to-side vastus lateralis tendon repair.
The technique used for fragment screw fixation also differed depending on Saupe classification, although the methods of fragment exposure were identical to those described here. Once the synchondrosis was identified, the fibrous tissue was curetted out, and bone graft was tamped into the site of the curettaged synchondrosis for 2 of the 4 patients who underwent this procedure. Compression screws were applied perpendicularly across the site of the synchondrosis.
One patient with Saupe II lateral BPP underwent synchondrosis drilling. The fragment was first exposed in an open fashion, and the fragment was assessed and determined to be stable. Diagnostic arthroscopy was then performed to confirm the integrity of the articular cartilage. A 0.062-inch (1.6-mm) diameter Kirschner wire was then utilized to perform multiple drillings from lateral to medial, across the synchondrotic margin of the ossicle, to stimulate bony bridging across the synchondrosis.
Statistical Analysis
Patient and clinical characteristics were summarized by treatment group and compared across treatment groups using a chi-square test, Student t test, or Mann-Whitney U test, as appropriate. The proportion of patients who returned to sports and the proportion of patients who required reoperation were estimated with a 95% CI and compared across treatment groups using a Fisher exact test. Continuous outcomes, including time to return to sports and time to resolution of symptoms, were summarized by median and interquartile range and compared across treatment groups using the Kruskal-Wallis test. All tests were 2-sided, and P values <.05 were considered significant.
Results
Of the 266 patients included in this study (62.0% male; mean age, 12.7 years; range, 7-20 years), 27 (10.2%) were treated operatively (mean age, 15.4 years; range, 10-20 years). The number of operative and nonoperative cases per 3-year increment over the entire study period is reflected in Figure 2. It demonstrates an increase in the overall volume of cases over time, which was proportional to the overall volume growth of the orthopaedic department and sports medicine division of the study institution.
Figure 2.
Trend of increasing volume of cases of symptomatic bipartite patella diagnosed at the study institution. The relative distribution of operative and nonoperative cases is shown in 3-year increments from 2003 to 2017.
When compared with the 239 patients who underwent nonoperative treatment, those treated operatively experienced a longer duration of symptoms before first presentation (21.5 vs 7.6 months; P < .001), and they were more likely to be older (mean age, 15.4 vs 12.4 years; P < .001), female (59.3% vs 35.6%; P = .03), and competitive athletes (100% vs 84.5%; P = .02). Of note, 6 of the 186 patients managed nonoperatively who underwent physical therapy had residual symptoms and were recommended—but they declined—operative intervention.
Between the operative and nonoperative groups, there was no difference in physical examination findings, such as crepitus, popping/clicking, swelling, palpable accessory fragment prominence, and quadriceps weakness. Because the diagnosis of BPP was made at the initial clinic visit in most cases, the time from initial clinic presentation at the study institution to time of diagnosis of BPP was zero for 78% of the operative group and 95% of the nonoperative group. The median was significantly longer in the nonzero remainder of the nonoperative cohort (28 days) when compared with the nonzero remainder of the operative cohort (16 days; P < .001). There was also no significant difference in length of nonoperative management attempted across the 2 groups, with the operative group undergoing a median 2.2 months of nonoperative management before surgery and the nonoperative group undergoing a median 1.9 months (P = .24) before symptom resolution or return to sport. The distribution of Saupe classifications was also not different across the 2 groups (P = .53). In the operative and nonoperative groups, 16 and 164 patients had Saupe III ossicles (59% vs 69%); 8 and 49, Saupe II (30% vs 21%); and 3 and 24, Saupe I (11% vs 10%), respectively. A summary of these findings is presented in Table 1.
Table 1.
Descriptive and Clinical Data Collected for Patients Treated Operatively and Nonoperativelya
| Treatment, No. (%) | |||
|---|---|---|---|
| Operative (n = 27) | Nonoperative (n = 239) | P Value | |
| Descriptive | |||
| Sex | .03 | ||
| Female | 16 (59) | 85 (36) | |
| Male | 11 (41) | 154 (64) | |
| Competitive athlete | 27 (100) | 202 (85) | .02 |
| Age at surgery or presentation, yb | 15.4 (10-20) | 12.4 (7-20) | <.001 |
| Clinical characteristics | |||
| Saupe classification | .53 | ||
| Inferior | 3 (11) | 24 (10) | |
| Lateral | 8 (30) | 49 (21) | |
| Superolateral | 16 (59) | 164 (69) | |
| Fragment area as mean % of patellar area | 9.4 | ||
| Weightbearing cartilage on fragment | 5 (19) | ||
| Laterality | |||
| Right | 9 (33) | ||
| Left | 18 (67) | ||
| Pain | |||
| Superolateral | 14 (52) | ||
| Anterior | 11 (41) | ||
| Stopped sports due to pain? | 23 (85) | ||
| Days from initial clinic presentation to BPP diagnosisc,d | 16 (16-70) | 28 (11-41) | <.001 |
| Months of nonoperative managementc | 2.2 (1.2-9.5) | 1.9 (1.4-3.1) | .24 |
| Attempted physical therapy |
27 (100) | 186 (78) | .002 |
| Time from symptom onset to surgeryc | 2.2 y (1.7 mo to 10.1 y) | —e | |
| Duration of symptoms at presentationc | 21.5 mo (0 d to 10 y) | 7 mo (1 d to 5 y) | .001 |
| Symptom onset | |||
| Insidious | 20 (71) | —e | |
| Traumatic | 7 (29) | ||
| Surgery recommended | 27 (100) | 6 (2.5) | |
aData are reported as No. (%) unless otherwise indicated. Bolded P values indicate statistically significant differences between groups (P < .05). BPP, bipartite patella.
bMean (range).
cMedian (interquartile range).
dAmong patients with nonzero values given that 78% (21/27) and 95% (228/239) of patients in the operative and nonoperative groups had zero values, thereby making median and interquartile range values all zero.
eData not available given the lack of detail/accuracy in medical records of this treatment group, as assessed by retrospective review.
Among the 27 patients in the operative group (9 right, 18 left), 20 (71%) reported insidious onset of pain; the remaining 7 patients (29%) experienced a discrete minor trauma in the form of a low-energy direct blow, which precipitated symptom onset. BPP symptom duration before surgery was 2.2 years (range, 1.7 months–10.1 years), with 14 patients having superolateral knee pain (52%) and 11 having anterior knee pain (41%). In the operative group, 23 patients (85%) had been forced to discontinue sports because of pain. On average, the accessory fragment constituted 9.4% of the total patellar area in this operative cohort. The accessory ossicle contained weightbearing articular cartilage in 5 of 27 cases (18.5%). Table 2 summarizes the operative details and basic clinical outcomes.
Table 2.
Operative Details and Outcomes for Patients Who Underwent Surgerya
| Operative Technique | ||||||
|---|---|---|---|---|---|---|
| Isolated Lateral Release | Isolated Fragment Excision | Synchondrosis Drilling | Fragment Excision + Lateral Release | Screw Fixation | All Operations | |
| No. (%) | 5 (19) | 9 (33) | 1 (4) | 8 (30) | 4 (15) | 27 |
| Mean fragment area, mm2 | 71.3 | 137.8 | 64 | 82.2 | 151.5 | 108 |
| Mean fragment %b | 9.10 | 11.20 | 6.70 | 7.20 | 11.60 | 9.40 |
| Articular cartilage involved, No. (%) | 1 (20) | 1 (11) | 0 (0) | 2 (25) | 1 (25) | 5 (19) |
| Mean OR time,c min | 45 | 52 | 34 | 63 | 50 | 48 |
| Mean time to healing on radiograph, mo | — | — | 3.2 | — | 4.8 | — |
| Mean time to RTS, mo | 1.8 | 2 | 3 | 2.6 | 3 | 2.2 |
| Reoperation needed, No. (%) | 0d | 1e (11) | 0 (0) | 1 (13) | 2 (50) | 4 (15) |
| Reoperation technique (years after surgery) | — | Arthroscopic lateral release (4) | — | Excision of residual fragment (4) | ROH (2), ROH, second fixation (1) | — |
aDashes indicate not applicable. OR, operating room; ROH, removal of hardware; RTS, return to sport.
bFragment % was calculated as the surface area of the bipartite fragment divided by the surface area of the overall patella, with each measured by their maximum length and width on AP radiographs or coronal plane MRI sequences.
cOR time: tourniquet time documented in the operative records.
dPercentage of total patellar area. One patient was recommended reoperation for residual tenderness over the bipartite fragment but was lost to follow-up.
eThis patient experienced symptom resolution until a direct blow of the knee to a dashboard in a motor vehicle accident.
Procedures were categorized as isolated fragment excision (n = 9), fragment excision with lateral release (n = 8), isolated lateral release (n = 5), fragment screw fixation (n = 4), and arthroscopic drilling (n = 1). Six surgeons performed these operations. Nineteen patients reported full resolution of symptoms and ability to return to sports postoperatively; the mean time between surgery and return to sports for these patients was 2.2 months. Four patients had incomplete follow-up, precluding definitive documentation of degree of symptom resolution and timing of return to sport.
Four patients (14.8%) reported persistent or recurrent symptoms requiring secondary surgery, which occurred at a mean 2 years after the initial surgery. One patient who had undergone isolated fragment excision had experienced postoperative symptom resolution until a direct blow to the knee on a dashboard during a motor vehicle accident at the 4-year postoperative time point. After failed subsequent nonoperative management, a lateral release was performed, which yielded subsequent resolution of symptoms. Two patients who had received fragment screw fixation reported hardware-related symptoms and underwent screw removal at 8 months and 2 years postoperatively. The fourth patient who presented to the study institution with persistent pain had previously undergone fragment excision with lateral release at another institution. Imaging demonstrated a small residual fragment, and the patient underwent revision fragment excision 12 months after the initial procedure. The patient reported symptom resolution 4 months after the revision procedure.
Discussion
In this large cohort of pediatric and adolescent patients, comparative analysis of operatively versus nonoperatively treated patients with BPP demonstrated that older adolescents, competitive athletes, girls, and patients with a delayed initial presentation after symptom onset were most likely to require operative intervention.
One explanation for delayed presentation emerging as a risk factor for surgery may lie in the breakdown of sex and age across the groups. Prior studies have demonstrated that female patients in general tend to present to clinic for medical conditions later than their male counterparts,4,6 although these studies were conducted in adult patient populations and female sex was a risk factor for undergoing surgery in the current overall series. Bruins et al6 also demonstrated that older adolescents tend to present to clinic less frequently than pediatric patients, likely owing to relatively greater parental concern over pain reported by younger patients.
The fact that competitive athletes were more likely to go on to surgery may relate to their behavioral tendencies to play through pain as compared with their recreational athlete or nonathlete counterparts—a finding also demonstrated in prior literature.27 Thus, the importance of prolonged periods of adequate rest and activity modification—particularly in the older adolescent subpopulation, which may be most resistant to such interventions—is underscored by these study findings. However, while previous authors have suggested that at least 6 months of rest, immobilization, and/or physical therapy be attempted before operative intervention,2,11 we found that the nonoperative cohort in the current study improved to achieve symptom resolution or return to sports more quickly than this, with a median duration of nonoperative measures just under 2 months.
For those rare patients requiring operative intervention for refractory pain, various surgical approaches were employed in prior studies. However, as outlined in a 2018 editorial commentary, the literature has lacked studies comparing indications or outcomes of the different surgical approaches.9 Notably, in our current investigation, there was no significant difference in time between surgery and return to sports among the operative techniques. This correlates with conclusions drawn in prior systematic reviews of multiple small case series, in which clinical results suggested comparable overall outcomes across different surgical approaches.17,18 As stated previously, given the retrospective nature of the study, it was often difficult to identify clear indications for the choice of one procedure over the other. However, all patients had endured prolonged symptoms refractory to a variety of nonoperative treatment measures.
In the current series, reoperation rates were too low overall to identify meaningful differences among technique-based subcohorts. In one of the earliest studies on the subject, Weaver26 investigated surgical excision of the accessory fragment. He noted favorable outcomes in the majority of patients, although 1 of the 21 patients in the series required subsequent patellectomy for residual symptoms. Many authors have since agreed that accessory fragment excision provides reliable clinical outcomes, although Bourne and Bianco5 recommended long-term follow-up to evaluate development of patellofemoral degenerative changes after excision. Of the 9 patients in the current study who received isolated fragment excision, 1 (11%) underwent reoperation in the form of an arthroscopic lateral retinacular release. The other 8 patients reported symptom improvement.
As an alternative to isolated fragment excision, Ogata21 hypothesized that vastus lateralis release might be useful to treat Saupe II and III BPP. In that study, 10 patients underwent isolated open vastus lateralis release; the vastus lateralis insertion to the painful patellar fragment was detached subperiosteally, while the continuity of the tendon-periosteum complex to the main portion of the patella was preserved. Interestingly, the authors demonstrated high rates of spontaneous bony union after separation of the vastus lateralis tendon from the superolateral patellar fragment in Saupe III cases (6 of 7 patients; 86%). For this reason, excision of the fragment was thought to be unnecessary. However, none of the 3 patients in Ogata’s series with Saupe II BPP underwent bony fusion postoperatively, and the relief of pain was not as prompt or complete as in Saupe III cases. None of the patients in the current study underwent vastus lateralis release.
Mori et al19 published a different study on the efficacy of lateral retinacular release for the painful BPP, performed distal to the vastus lateralis tendon in the retinacular tissue itself. Mori et al suggested that patients with symptomatic BPP may have had pain from not only the bipartite synchondrosis but also concurrent lateral pressure syndrome or patellofemoral syndrome, with lateral retinacular release providing value in improving all knee symptoms from any of the 3 conditions. Felli et al10 subsequently reported that arthroscopic lateral retinacular release facilitated return to sports and symptom improvement in a series of 11 patients. Among the current study cohort, none of the 5 patients who received isolated lateral retinacular release required reoperation. Of 8 patients who underwent fragment excision with concomitant lateral retinacular release, 1 (13%) experienced recurrent pain and required revision surgery. However, the revision consisted of excision of a small portion of unresected bipartite fragment, which provided lasting symptom relief, suggesting a potential technical failure in the initial surgery and underscoring the importance of an adequate excision if such an approach is utilized.
Literature regarding the efficacy of screw fixation in treating painful BPP is sparse. It has been suggested that fixation can be useful in cases in which the fragment is very large or involves the articular surface of the joint.2 Other authors contend that fixation may represent too invasive a procedure for this condition.3 In the current cohort, 2 of the 4 patients who underwent fragment fixation (50%) required reoperation to remove fixation implants, a common secondary surgical procedure after many orthopaedic procedures but one that should be discussed with patients for whom this technique is considered. Other authors have postulated that fixation might be superior to fragment excision for larger fragments involving articular cartilage.2 There was, however, no difference in fragment percentage of total patellar area between open reduction and internal fixation (ORIF) and fragment excision in the current cohort. Yet, given the low overall sample size of the operative group, no definitive conclusions can be derived regarding outcome as it pertains to technique. However, all 5 described techniques appear to be acceptable for patients with prolonged symptoms not responsive to nonoperative measures. At our institution, given the findings in the current series, we favor fragment excision unless the fragment is large and contains a significant portion of weightbearing articular cartilage. In this case, fixation with or without bone grafting is preferred for large fragments, depending on the width or extent of the synchondrosis, with wider synchondroses being considered more amenable to bone grafting. If the lateral retinaculum feels abnormally tight and there is clear lateral patellar tilt, with symptoms of lateral patellar hypercompression syndrome or patellofemoral pain, then we often consider arthroscopic lateral retinacular release as an alternative or adjunctive procedure to the earlier described techniques.
There are several limitations to this study. The retrospective nature of the analysis limited the scope and comprehensiveness of preoperative clinical details investigated and necessitated several assumptions related to the chart review, which were designed to avoid gross over- or underestimates for the time of symptom resolution and return to sports. A future aim is to reach out to the operative cohort to collect long-term patient-reported outcome measures via validated knee questionnaires. In addition, because the patients included in the study underwent surgery by 1 of 6 surgeons, the level of detail of operative data recorded was inconsistent, and indications for different surgical techniques varied. Finally, the study sample size was relatively small, with 27 patients in the operative group. Given the relative rarity of the condition meeting surgical indications, prospective multicenter studies are warranted to better understand operative indications and comparative outcomes. Nevertheless, the current study represents the largest series comparing operative versus nonoperative interventions for the painful BPP.
Conclusion
BPP is a rare cause of knee pain in adolescent athletes and is generally responsive to nonoperative treatment. Patients needing surgical treatment—most commonly, adolescent competitive female athletes with a delayed first presentation to clinic—represented approximately 10% of cases and were most likely to have superolateral bipartite fragments. A variety of surgical techniques may be effective, with an overall 15% risk of persistent or recurrent symptoms warranting reoperation. Prospective multicenter investigations are needed to identify optimal candidates for earlier interventions and optimal operative treatment techniques.
Acknowledgment
The authors thank Elizabeth Liotta, MBBS, for her thoughtful research support in the completion of this study and the preparation of this manuscript.
Footnotes
Final revision submitted June 10, 2020; accepted June 19, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: D.E.K. has received educational support from Kairos Surgical. M.S.K. has received consulting fees from OrthoPediatrics and Ossur; speaking fees from Smith & Nephew; and royalties from OrthoPediatrics, Ossur, Elsevier, and Wolters Kluwer. B.E.H. has received educational support from Kairos Surgical; consulting fees from Imagen Technologies; educational consulting fees from Arthrex; and royalties from Springer Science & Business Media; has stock/stock options in Imagen Technologies; and is a member of the Research in OsteoChondritis of the Knee (ROCK) Study Group, which receives unrestricted educational grant funding from Allosource and Vericel. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Boston Children’s Hospital (protocol IRB-P00029408).
References
- 1. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404–411. [DOI] [PubMed] [Google Scholar]
- 2. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455–461. [DOI] [PubMed] [Google Scholar]
- 3. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006. [DOI] [PubMed] [Google Scholar]
- 4. Banks I, Baker P. Men and primary care: improving access and outcomes. Trends in Urology & Men’s Health. 2013;4(5):39–41. [Google Scholar]
- 5. Bourne MH, Bianco AJ, Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69–73. [PubMed] [Google Scholar]
- 6. Bruins Slot MH, Rutten FH, van der Heijden GJ, et al. Gender differences in pre-hospital time delay and symptom presentation in patients suspected of acute coronary syndrome in primary care. Fam Pract. 2012;29(3):332–337. [DOI] [PubMed] [Google Scholar]
- 7. Canizares GH, Selesnick FH. Bipartite patella fracture. Arthroscopy. 2003;19(2):215–217. [DOI] [PubMed] [Google Scholar]
- 8. Echeverria TS, Bersani FA. Acute fracture simulating a symptomatic bipartite patella: report of a case. Am J Sports Med. 1980;8(1):48–50. [DOI] [PubMed] [Google Scholar]
- 9. Fithian DC. Editorial commentary: got evidence? What we really need is an algorithm for treating symptomatic bipartite patella. Arthroscopy. 2018;34(5):1559–1560. [DOI] [PubMed] [Google Scholar]
- 10. Felli L, Formica M, Lovisolo S, Capello AG, Alessio-Mazzola M. Clinical outcome of arthroscopic lateral retinacular release for symptomatic bipartite patella in athletes. Arthroscopy. 2018;34(5):1550–1558. [DOI] [PubMed] [Google Scholar]
- 11. Gaheer RS, Kapoor S, Rysavy M. Contemporary management of symptomatic bipartite patella. Orthopedics. 2009;32(11):843–849. [DOI] [PubMed] [Google Scholar]
- 12. Green WT, Jr. Painful bipartite patellae: a report of three cases. Clin Orthop Relat Res. 1975;110:197–200. [PubMed] [Google Scholar]
- 13. Iossifidis A, Brueton RN. Painful bipartite patella following injury. Injury. 1995;26(3):175–176. [DOI] [PubMed] [Google Scholar]
- 14. Ireland ML, Chang JL. Acute fracture bipartite patella: case report and literature review. Med Sci Sports Exerc. 1995;27(3):299–302. [PubMed] [Google Scholar]
- 15. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes: the diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;305:223–228. [PubMed] [Google Scholar]
- 16. Marya KM, Yadav V, Devagan A, Kundu ZS. Painful bilateral bipartite patellae—case report. Indian J Med Sci. 2003;57(2):66–67. [PubMed] [Google Scholar]
- 17. Matic GT, Flanigan DC. Efficacy of surgical interventions for a bipartite patella. Orthopedics. 2014;37(9):623–628. [DOI] [PubMed] [Google Scholar]
- 18. McMahon SE, LeRoux JA, Smith TO, Hing CB. The management of the painful bipartite patella: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2798–2805. [DOI] [PubMed] [Google Scholar]
- 19. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13–18. [DOI] [PubMed] [Google Scholar]
- 20. O’Brien J, Murphy C, Halpenny D, McNeill G, Torreggiani WC. Magnetic resonance imaging features of asymptomatic bipartite patella. Eur J Radiol. 2011;78(3):425–429. [DOI] [PubMed] [Google Scholar]
- 21. Ogata K. Painful bipartite patella: a new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573–578. [DOI] [PubMed] [Google Scholar]
- 22. Ogden JA, McCarthy SM, Jokl P. The painful bipartite patella. J Pediatr Orthop. 1982;2(3):263–269. [DOI] [PubMed] [Google Scholar]
- 23. Saupe H. PrimÄre Knochenmarkseiterung der Kniescheibe. Deutsche Zeitschrift für Chirurgie. 1943;258(6):386–392. [Google Scholar]
- 24. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75–78. [DOI] [PubMed] [Google Scholar]
- 25. Suzue N, Matsuura T, Iwame T, et al. Prevalence of childhood and adolescent soccer-related overuse injuries. J Med Invest. 2014;61(3-4):369–373. [DOI] [PubMed] [Google Scholar]
- 26. Weaver JK. Bipartite patellae as a cause of disability in the athlete. Am J Sports Med. 1977;5(4):137–143. [DOI] [PubMed] [Google Scholar]
- 27. Weinberg R, Vernau D, Horn T. Playing through pain and injury: psychosocial considerations. J Clin Sport Psychol. 2013;7(1):41–59. [Google Scholar]