Abstract
This systematic review of literature and online reports critically appraised incidence and prevalence estimates of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension to identify the most accurate estimates. Medline® and Embase® databases were searched for articles published between 1 January 2003 and 31 August 2020. Studies were grouped according to whether they were registries (population-based estimates), clinical databases (hospital-based estimates) or claims/administrative databases. Registries were classified into systematic and non-systematic registries, according to whether every national centre participated. Of 7309 publications identified, 5414 were screened after removal of duplicates and 33 were included. Inclusion was based on study type, availability of a clear numerator (diagnosed population) and a population- or hospital-based denominator, or all primary data required to calculate estimates. Only the most recent publication from a database was included. Most studies were based on European data and very few included children. In adults, the range of estimates per million was approximately 20-fold for pulmonary arterial hypertension incidence (1.5–32) and prevalence (12.4–268) and of similar magnitude for chronic thromboembolic pulmonary hypertension incidence (0.9–39) and prevalence (14.5–144). Recent (≤5 years) national systematic registry data from centralised healthcare systems provided the following ranges in adult estimates per million: approximately 5.8 for pulmonary arterial hypertension incidence, 47.6–54.7 for pulmonary arterial hypertension prevalence, 3.1–6.0 for chronic thromboembolic pulmonary hypertension incidence and 25.8–38.4 for chronic thromboembolic pulmonary hypertension prevalence. These estimates were considered the most reliable and consistent for the scientific community to plan for resource allocation and improve detection rates.
Keywords: pulmonary hypertension, pulmonary arterial hypertension, epidemiology, registries
Introduction
Pulmonary hypertension (PH) is a progressive disease characterised by increased pulmonary vascular resistance that ultimately leads to right heart failure and death. Patients usually present with non-specific symptoms, such as shortness of breath, fatigue, angina and syncope.1 Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are two groups of PH (Groups 1 and 4, respectively).2,3 As per the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines, PH should be diagnosed by right heart catheterisation (RHC) and imaging techniques are required to differentiate CTEPH from PAH, with ventilation/perfusion (V/Q) scintigraphy being the recommended diagnostic tool for CTEPH.2,3
PAH and CTEPH are rare diseases with low but also wide ranges of published incidence and prevalence estimates.2,3 The variety of design, data sources and observation period used in studies can create discrepancies in the reported epidemiology of the diseases.
While awareness, diagnosis and clinical management of PAH and CTEPH have greatly improved over the past few decades,4–6 there is still a lack of consensus in the scientific community on which estimates could reflect the true incidence and prevalence of PAH and CTEPH.7–9 Identifying the most generalisable estimates would help clinicians and scientists to assess the likelihood of PAH and CTEPH being under-diagnosed in certain countries and subsequently support realistic goal-setting for improving disease detection. This systematic review aimed to first, identify PAH and CTEPH incidence and prevalence estimates available in the literature and online reports and, second, to critically appraise these estimates, in order to better understand their quality, validity and relevance in research and clinical practice.
Materials and methods
The design of this systematic literature review was based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.10 The search of literature and online reports, screening and data extraction was conducted by the first author (L.L.).
Search strategy
Medline® and Embase® databases were searched using OvidSP® for articles published between 1 January 2003 and 31 August 2020. The start of this search period coincides with a major modification made to the clinical classification of PH at the 2003 World Symposium on PH in Venice, where the term ‘primary pulmonary hypertension’ was replaced by ‘idiopathic pulmonary arterial hypertension’.11 The definition of the term ‘primary pulmonary hypertension’ has changed considerably since its first use in 1950 and previously encompassed both PAH and CTEPH, hence the rationale for excluding articles prior to 2003 that may use outdated and ambiguous terminology.12 The following search string was used to identify articles on the population and outcome of interest: pulmonary hypertension [Title/Abstract] OR pulmonary arterial hypertension [Title/Abstract] AND prevalence [Title/Abstract] OR incidence [Title/Abstract]. The searches were filtered for human studies, written in the English language and with abstract available. Duplicate records were removed. Details of this search strategy are available in Appendix 1.
In addition, ClinicalTrials.gov and the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance websites were screened using the keywords ‘pulmonary hypertension’ and ‘registry’ to identify potentially relevant data sources. Publicly available reports of PH registries were extracted from websites of identified registries.
Eligibility criteria
Eligible articles were (i) studying patients diagnosed with PAH or CTEPH, and included a description of the diagnostic or identification method(s), and (ii) reporting primary data on the incidence and/or prevalence of PAH and/or CTEPH. The primary data had to clearly state the numerator of the diagnosed population and include a population or hospital-based denominator that is not specific to a disease associated with PH (e.g. PAH in systemic sclerosis population) or report all primary data and information from which to calculate estimates that satisfy these criteria. In instances where estimates for PAH or CTEPH were available in several publications from the same database, only the most recent publication was selected. Review articles, letters to the editor, case reports, case studies, clinical trials, in vitro or animal studies were excluded, as were original research articles in which the main disease under investigation was not the disease of interest (PH Groups 2, 3 or 5, subgroups of PAH/CTEPH or not PH). Conference abstracts were included.
Article selection
First, the titles and abstracts were manually screened for eligibility. If the article remained potentially relevant, or its eligibility was not clear from the title and abstract, the full text article was screened. The bibliographies of identified articles were then manually searched for other articles potentially of interest to this literature review.
Data extraction
Data extraction was performed using Microsoft Excel. To the best of the authors’ knowledge, there is no data extraction form validated for PH. However, the data extraction form used was very similar to the validated Joanna Briggs Institute data extraction form for prevalence studies.13 Data extracted included details of the publication (first author, year of publication), the study (design, observation period, location), the patient population (age, diagnosis, size of population), the outcomes of interest (denominator, incidence and prevalence estimates) and any relevant information that would contribute to appraisal or interpretation of epidemiology estimates. For publications that did not have estimates directly available, the country’s population at the time of study was taken from the US Census14 or Eurostat15 as appropriate, and was used to derive incidence and prevalence.
Critical appraisal
A narrative synthesis of the evidence is presented herein. Results are grouped according to the following study designs: (i) national systematic registries, defined as those in which all national referral PH expert centres participate and all patients with a confirmed PAH or CTEPH diagnosis are invited to enrol, (ii) non-systematic registries, including national registries, in which the majority but not all PH-treating centres are included, as well as multi-centre registries, (iii) claims/administrative databases, where estimates are based on prescriptive or diagnostic codes, and (iv) clinical (hospital) databases, in which diagnoses are clinically confirmed. Clinical databases and registries are differentiated by whether they present hospital-based estimates (clinical database) or population-based estimates (registries).
The appraisal of PAH and CTEPH incidence and prevalence estimates was based on assessment of: database type, number of centres included, country and healthcare system, observation period, age criteria, diagnosis or identification methods, PH classification used, sample size and finally the numerator and denominator of the estimates. These factors were selected and evaluated by the authors; further details of this process are presented in the discussion. The latest PH guideline recommendations2,3 were followed to assess the validity of the disease diagnosis and classification.
Results
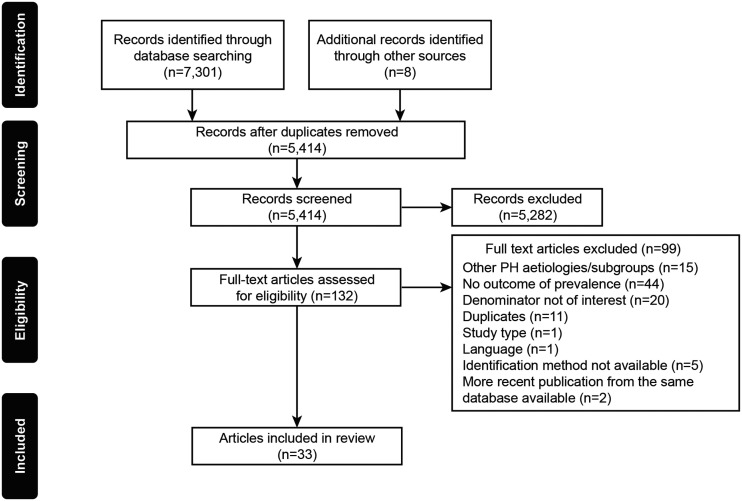
The literature search returned a total of 7309 publications. Following the removal of duplicates, 5414 publications were screened, and 33 were found to meet the eligibility criteria, as summarized by the PRISMA flow diagram in Fig. 1. The selected publications comprised 30 manuscripts, one conference abstract and two online registry reports (the UK PH Audit (2019)16 and the Swedish Pulmonary Arterial Hypertension Registry (SPAHR)),17 from 13 European, 3 Asian, 2 North American countries and 1 South American country. Fifteen publications were on PAH, 7 on CTEPH and 11 included both diseases. PAH and CTEPH epidemiology estimates were mostly reported for adult populations only (n = 12 for PAH, n = 7 for CTEPH and n = 7 for publications studying both diseases).
Fig. 1.
PRISMA flow diagram.
PH: pulmonary hypertension.
All studies were open cohort studies (patients were continually added at diagnosis) and used the 2003 PH classification (Venice) or a later version.11 When the study observation period was partly prior to 2003, the latest classification available at end of the study was systematically used (i.e. 2003 classification or a later version), avoiding misclassification bias by the use of out-dated and ambiguous PH terminology. The included publications are summarised in Tables 1–4 and Supplementary Tables 1–2.
Table 1.
Study details and epidemiology estimates from identified studies investigating PAH epidemiology in adults.
| Study classification | Study description | Country | Time period range | Number of participants | Publication (study acronym) | Annual incidence (ppm) | Prevalence (ppm) |
|---|---|---|---|---|---|---|---|
| National systematic registry | National systematic registry | UK | 2018–2019 | NR | NHS Digital, 201916,a | – | 54.7b |
| Sweden | 2008–2019 | 1034 | Kjellström et al., 2020 (SPAHR)17,a | 5.8b | 47.6b | ||
| UK (Scotland) | 1997–2006 | NR | Peacock et al., 200725,a | 7.6 | 26 | ||
| Czech Republic | 2000–2007 | 191 | Jansa et al., 201441,a | 10.7 | 22.4 | ||
| Latvia | 2007–2016 | 130 | Skride et al., 201820,a | 13.7 | 45.7 | ||
| Non-systematic | National, non-systematic | Portugal | 2008–2010 | 46 | Baptista et al., 201344,a | 1.5 | – |
| South Korea | 2008–2011 | 297 | Chung et al., 2015 (KORPAH)26 | 1.9 | – | ||
| France | 2002–2003 | 674 | Humbert et al., 200622,a | 2.4 | 15 | ||
| Switzerland | 1999–2004 | 152 | Tueller et al., 200823 | 3.5 | 15.5 | ||
| Spain | 2007–2008 | 866 | Escribano-Subias et al., 2012 (REHAP)33,a | 3.7 | 16 | ||
| Germany | 2007–2014 | 1752 | Hoeper et al., 2016 (COMPERA)21,a | 3.9 | 25.9 | ||
| Poland | 2018 | 970 | Kopeć et al., 2020 (BNP-PL)19,a | 5.2 | 30.8 | ||
| Non-national (multi-centre) | USA | 2006–2007 | 2967 | Frost et al., 2011 (REVEAL)5,a,c | 2.3 | 12.4 | |
| Claims/administrative databases | Claims (Medicare) | USA | 1999–2007 | 215 | Kirson et al., 201128 | – | 30.4b |
| Claims (PHIC) | Colombia | 2015 | 18 | Miranda-Machado et al., 201947,a | – | 28 | |
| Claims (HIRA) | South Korea | 2008–2016 | 1307 | Song et al., 201827 | 4.8 | 20.2 | |
| National hospitalization database (SMR) | UK (Scotland) | 1986–2001 | 374 | Peacock et al., 200725 | 7.1 | 52 | |
| Administrative database (ICES) | Canada | 1993–2012 | 6705 | Wijeratne et al., 201824 | 32 | 268 | |
| Clinical databases | Single-centre study | UK | 2001–2010 | 598 | Hurdman et al., 2012 (ASPIRE)48,a | 6.1 | – |
| Single-centre study | Israel | 1998–2005 | 84 | Fruchter and Yigla, 200849 | 7.1b | – | |
| Single-centre study | USA | 2016 | 154 | Dubroff et al., 202050,a | 14 | 93 |
Notes: Studies are ordered by study design and then in ascending order of incidence estimate. Estimates are rounded to one decimal place, except where only integers were published.
aPAH definition included mPAP >/≥ 25 mmHg at rest (± or >30 mmHg on exercise) and PAWP </≤ 15 mmHg, as assessed by RHC.
bEstimates are derived from the publication using the method outlined in Table 5.
cREVEAL registry included an expanded criterion for patients with PAWP ≤18 mmHg.
ASPIRE: Assessing the spectrum of pulmonary hypertension identified at a REferral centre; COMPERA: Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; HIRA: Health Insurance Review and Assessment Service; ICES: Institute for Clinical Evaluative Sciences; KORPAH: Korean Registry of Pulmonary Arterial Hypertension; NHS: National Health Service; NR: not reported; PAWP: pulmonary arterial wedge pressure; ppm: patients per million; REHAP: Spanish Registry of Pulmonary Arterial Hypertension; REVEAL: The Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management; RHC: right heart catheterisation; SMR: Scottish Morbidity Record scheme; SPAHR: Swedish Pulmonary Arterial Hypertension Registry; BNP-PL: Polish Registry of Pulmonary Hypertension.
Table 2.
Study details and epidemiology estimates from identified studies investigating PAH epidemiology in children.
| Study classification | Study description | Country | Time period | Number of participants | Publication (study acronym) | Annual incidence (ppm) | Prevalence (ppm) |
|---|---|---|---|---|---|---|---|
| National, non-systematic registry | National, non-systematic registry | France | 2005–2006 | 50 | Fraisse et al., 201051 | – | 3.7 |
| Poland | 2018 | 80 | Kwiatkowska et al., 2020 (BNP-PL)52 | 2.4 | 11.6 | ||
| Spain | 2009–2012 | 142 | Del Cerro Marin et al., 2014 (REHIPED)53 | 2.6 | 14 | ||
| Netherlands | 1991–2005 | 154 | Van Loon et al., 201154 | 3 | 20 | ||
| Claims/administrative database | Claims (MarketScan) | USA | 2010–2013 | 695 | Li et al., 201718 | 4.8 | 28.2 |
| Administrative database | Turkey | 2009–2013 | 2079 | Pektas et al., 201634 | 16.7a | – | |
| Administrative database (ICES) | Canada | 1993–2012 | 1198 | Wijeratne et al., 201824 | 12a | 397 |
Notes: Studies are ordered by study design and then in ascending order of incidence estimate. Estimates are rounded to one decimal place, except where only integers were published.
aEstimates are derived from the publication using the method outlined in Table 5.
ICES: Institute for Clinical Evaluative Sciences; ppm: patients per million; REHIPED: The Spanish Registry for Paediatric Pulmonary Hypertension; BNP-PL: Polish Registry of Pulmonary Hypertension.
Table 3.
Study details and epidemiology estimates from identified studies investigating CTEPH epidemiology in adults.
| Study classification | Study description | Country | Time period | Number of participants | Publication (study acronym) | Annual incidence (ppm) | Prevalence (ppm) |
|---|---|---|---|---|---|---|---|
| National systematic registry | National systematic registry | Sweden | 2008–2019 | 417 | Kjellström et al., 2020 (SPAHR)17 | 3.1a | 25.8a |
| Latvia | 2007–2016 | 44 | Skride et al., 2018 20 | 5.1 | 15.7 | ||
| UK | 2018–2019 | 2492 | NHS Digital, 201916 | 6.0a | 38.4a | ||
| Non-systematicregistry | National, non-systematic | Slovakia | 1998–2014 | 81 | Bohacekova et al., 201655 | – | 18 |
| Portugal | 2008–2010 | 33 | Baptista et al., 201344 | 1.1 | – | ||
| Spain | 2007–2018 | 1019 | Martínez-Santos et al., 2019 (REHAP)56 | 1.7 | 22.5 | ||
| Germany | 2016 | 392 | Kramm et al., 2018 (COMPERA)57 | 5.7 | – | ||
| Claims/administrative database | Claims | USA | 1999–2007 | 431 | Kirson et al., 201128 | – | 39.4a |
| Administrative database (PMSI) | France | 2009-2015 | 3138 | Cottin et al., 201958 | – | 47 | |
| Administrative database (ICES) | Canada | 1993–2012 | 4360 | Wijeratne et al., 201824 | 39a | 144 | |
| Clinical database | Multi-centre | Spain | 1998–2017 | 42 | Llanos-González et al., 201959 | 0.9 | 14.5 |
| Single-centre study | Israel | 1998–2005 | 16 | Fruchter et al., 200849 | 1.3 | – | |
| Single centre study | UK | 2001–2006 | 105 | Condliffe et al., 200860 | 1.8 | – | |
| Single-centre study | UK | 2001–2010 | 242 | Hurdman et al., 2012 (ASPIRE)48 | 3.7 | – | |
| Single-centre study | Thailand | 2012–2016 | 20 | Puengpapat and Pirompanich, 201861 | 37.8 | – |
Notes: Studies are ordered by study design and then in ascending order of incidence estimate. Estimates are rounded to one decimal place, except where only integers were published.
aEstimates are derived from the publication using the method outlined in Table 5.
ASPIRE: assessing the spectrum of pulmonary hypertension identified at a REferral centre; COMPERA: Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; ICES: Institute for Clinical Evaluative Sciences; NHS: National Health Service; PMSI: French exhaustive hospital discharge database; ppm: patients per million; REHAP: Spanish Registry of Pulmonary Arterial Hypertension; SPAHR: Swedish Pulmonary Arterial Hypertension Registry.
Table 4.
Study details and epidemiology estimates from identified studies investigating CTEPH epidemiology in children.
| Study classification | Study description | Country | Time period | Number of participants | Publication (study acronym) | Annual incidence (ppm) | Prevalence (ppm) |
|---|---|---|---|---|---|---|---|
| Non-systematic registry | National, non-systematic | Spain | 2009–2012 | 2 | Del Cerro Marin et al., 2014 (REHIPED)53 | 0.076 | 0.22 |
| Netherlands | 1991–2005 | 5 | Van Loon et al., 201154 | 0.1a | – | ||
| Claims/administrative database | Administrative database | Turkey | 2009–2013 | 22 | Pektas et al., 201634 | 0.2 | – |
| Administrative database (ICES) | Canada | 1993–2012 | 65 | Wijeratne et al., 201824 | 2a | 19 |
Notes: Studies are ordered by study design and then in ascending order of incidence estimate.
aEstimates are derived from the publication using the method outlined in Table 5.
ICES: Institute for Clinical Evaluative Sciences; ppm: patients per million; REHIPED: The Spanish Registry for Paediatric Pulmonary Hypertension.
Table 5.
Method of deriving estimates from articles not stating the incidence/prevalence of PAH/CTEPH per million individuals.
| Estimate(s) | Publication | Method of derivation |
|---|---|---|
| PAH and CTEPH prevalence | Kirson et al., 201128 | Numerator derived by summing the numerators for patients aged <65 years (based on administrative claims data for a privately-insured population) and those aged ≥65 years (based on administrative claims data for a random sample of the Medicare population). US population in 2007 according to US census used as denominator: Overall population: 301,231,297. <65 years: 263,405,496. ≥65 years: 37,825,711. RHC-confirmed PAH prevalence was: <65 years: 25 ppm, ≥65 years: 68 ppm. Total calculated by: (263,405,496 × 25) + (37,825,711 × 68)/301,231,297 = 30.4 ppm (derived) RHC-confirmed CTEPH prevalence was: <65 years: 16≥65 years: 202Total calculated by : (263,405,496 × 16) + (37,825,711 × 202)/301,231,297 =39.4 ppm (derived) |
| PAH and CTEPH incidence | Wijeratne et al., 201824 | Estimates at 2012 approximated from graphsPAH: 12 for childrenCTEPH: 39 for adults, 2 for children |
| PAH prevalence, CTEPH incidence and prevalence | NHS Digital, 201916 | Numerators (number of active PAH patients on 31 March 2019: 3551, number of patients newly diagnosed with CTEPH in 2018–2019: 387 and number of CTEPH patients alive on 31 March 2019 in Great Britain: 2492) taken directly from publication. Great Britain population in June 2019 according to Office for National Statistics (64,903,100). |
| PAH and CTEPH incidence and prevalence | Kjellström et al., 202017 | Numerators for PAH estimates (number of PAH patients alive in 2019: 487 and number of PAH patients newly diagnosed in 2019: 59) calculated as 59% and 56% of respectively 825 PH patients alive in 2019 and 106 PH patients newly diagnosed in 2019. Numerators for CTEPH estimates (number of CTEPH patients alive in 2019: 264, and number of CTEPH patients newly diagnosed in 2019: 32) calculated as 32% and 30% of number of PH patients alive in 2019 and number of PH patients newly diagnosed in 2019, respectively. Swedish population in 2019 according to Eurostat used as denominator (10,230,185). |
| PAH incidence | Fruchter and Yigla, 200849 | Estimate (7.09) derived by summing incidence of idiopathic PH (1.92), and PH associated with (i) collagen vascular disease (3.08), (ii) haematological disease (1.17) and (iii) liver disease (0.92). |
| CTEPH incidence | Van Loon et al., 201154 | CTEPH patients comprised 0.15% of the total PH study population. Therefore, estimate (0.1) was calculated as 0.15% of PH incidence (63.7). |
| PAH incidence | Pektas et al., 201634 | Estimate (16.7) derived by summing the incidence of iPAH (11.7), PAH-CHD (4.5) and CTD-PAH (0.5). |
CTD-PAH: connective tissue disease-associated pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; iPAH: idiopathic pulmonary arterial hypertension; NHS: National Health Service; PAH: pulmonary arterial hypertension; PAH-CHD: pulmonary arterial hypertension associated with congenital heart disease; PH: pulmonary hypertension; ppm: patients per million; RHC: right heart catheterisation.
All but one18 incidence estimates were incidence proportions (incidence based on person at risk) rather than incidence rate (incidence based on person-time at risk). Studies calculated incidence using the last year of observation (n = 19), an average of each annual incidence of the period (n = 4) and an average over the whole observation period (n = 4). Point prevalence using the last year of observation was reported in 12 studies. Period prevalence using the last year of observation was reported in 11 studies, and two used the whole observation period. For simplicity, the terminology ‘incidence’ and ‘prevalence’ are used consistently in this review. Supplementary Table 3 contains full details on how incidence and prevalence were calculated and reported. Estimates for incidence are presented in patient per million (ppm) per year and estimates for prevalence are presented in ppm at a given time.
Incidence and prevalence of PAH in adults
The published estimates of PAH epidemiology in adults are summarised in Table 1. The publications include five national systematic registries, eight non-systematic registries, five claims/administrative databases and three clinical databases (Table 1). One publication used two different study designs and is therefore counted twice, resulting in a total of 21 studies. For six of the 13 registries, the observation period was prior to 2010, and the most recent registries include the SPAHR (2019 data cut-off),17 the UK National Health Service (NHS) Audit (2019),16 Polish Registry of Pulmonary Hypertension (BNP-PL, 2018),19 Latvian registry (2016)20 and the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA, 2014).21 Mean age ranged between 43 and 67 years, and female gender predominated in all data sources (55–81%, Supplementary Table 1). The French and Swiss registries included information on the number of patients who withdrew: none of the 121 incident cases included in the French registry were lost to follow-up,22 24 patients were lost to follow-up in the Swiss registry and these withdrawals occurred uniformly throughout the follow-up period.23
The ranges of estimates for PAH incidence and prevalence were 1.5–32 and 12.4–268 ppm, respectively. National systematic registries reported PAH adult incidence to be between 5.8 and 13.7 ppm (four studies), while estimates from all non-systematic registries except one (BNP-PL; 5.2 ppm) were below 4 ppm (1.5–3.9 ppm; seven studies). A similar trend was observed for PAH prevalence, with estimates from national systematic registries being generally higher than those from non-systematic registries (Table 1).
The estimates from claims/administrative databases overlapped with those from registries, with the exception of a Canadian (Ontario) administrative database study reporting an incidence of 32 ppm and a prevalence of 268 ppm.24 PAH estimates from all databases other than the Canadian study, including clinical databases, ranged from 4.8 to 14 ppm for incidence and from 20.2 to 93 ppm for prevalence.
In Scotland, the USA and South Korea, estimates were available from registries as well as claims/administrative databases. For Scotland, the registry-based estimate was similar to that reported in a claims/administrative database study for PAH incidence, with estimates of 7.6 and 7.1 ppm, respectively, while prevalence estimates differed more substantially (26 and 52 ppm, respectively).25 The registry-based estimate used RHC-confirmed diagnoses, whilst the administrative database included patients with an International Classification of Diseases, ninth revision (ICD-9) or ICD-10 code for a discharge diagnosis of primary PH/PAH (ICD-9 code 416.0; ICD-10 code I27.0).25 For the USA and South Korea, national estimates from claims/administrative databases were more than double compared with the respective multi-centre registries (Table 1).5,26–28
Incidence and prevalence of PAH in children
Paediatric epidemiology was reported among four national non-systematic registries and three claims/administrative database studies (Table 2). PAH incidence and prevalence ranged from 2.4 to 16.7 ppm and 3.7 to 397 ppm, respectively. Considering only registry-based estimates, incidence was approximately 2–3 ppm and prevalence ranged from 3.7 to 20 ppm, while estimates from claims/administrative databases were higher (Table 2).
Incidence and prevalence of CTEPH in adults
The systematic review identified 15 publications (Table 3). Mean age ranged between 58 and 73 years, and female gender represented 37–70% of CTEPH patients (Supplementary Table 2). The ranges of CTEPH incidence and prevalence in adults were 0.9–39 ppm and 14.5–144 ppm, respectively (Table 3).
According to national systematic registries (three studies), the incidence of CTEPH was between 3.1 and 6.0 ppm and prevalence ranged from 15.7 to 38.4 ppm. Estimates from non-systematic registries (four studies) were similar or lower than those from systematic registries.
Estimates were also reported in three claims/administrative databases, including the Canadian administrative database study reporting high incidence (39 ppm) and prevalence (144 ppm),24 and five clinical databases.
Incidence and prevalence of CTEPH in children
CTEPH epidemiology among children was identified in two non-systematic registries and two claims/administrative database studies. The Canadian administrative database study reported an incidence of 2 ppm and a prevalence of 19 ppm,24 while the others estimated the incidence and prevalence to be lower than 1 ppm (Table 4).
Discussion
This systematic literature review reports a wide variation in the published estimates of PAH and CTEPH epidemiology. In adults, the range of estimates was approximately 20-fold for PAH incidence and prevalence (1.5–32 and 12.4–268 ppm, respectively) and a similarly large range was observed for CTEPH incidence and prevalence (0.9–39 and 14.5–144 ppm, respectively). The critical appraisal of the most robust estimates, outlined in this discussion, focuses on the adult population, since so few paediatric studies were identified.
The variation in estimates across the included studies could be indicative of geographic differences in PAH and CTEPH populations. Recent publications have suggested racial and ethnic differences in prevalence, presentation and outcomes of PH sub-groups.29–31 However, most estimates identified in this research originated from Western European countries with comparable ethnicity and socioeconomic status. No major differences were noted for demographic distribution of the populations observed in the selected studies.
The geographic variation of disease estimates may be the consequence of differences in healthcare systems and patient tracking. In the UK, PH care is centralised: patients must be referred to one of the eight designated PH centres to receive diagnosis and disease-targeted treatment. These centres are governed by NHS England and are audited annually,32 making the PH audit an authoritative data source for PH epidemiology in the UK. Similarly, in Sweden, the seven nationally designated specialist centres automatically invite every patient with a confirmed PAH or CTEPH diagnosis to enrol into the SPAHR registry.17 As a result, 88% of the national PH population and 91% of the national PAH population, registered by the National Board of Health and Welfare, were estimated to be covered by the Swedish registry in 2017.17 Interestingly, the most recent nationwide estimates from the Swedish and UK registries are similar for PAH prevalence (47.6 and 54.7 ppm) and are in the same range for CTEPH prevalence (25.8 and 38.4 ppm).16,17 In countries where these referral pathways and/or registry upkeep are not mandatory, national registries may not be representative and their estimates are likely an underestimate. Indeed, in the present review, estimates from non-systematic national registries and/or countries with decentralised healthcare systems, such as the national registries from Switzerland, France or Spain, were lower than those from the UK and Sweden (PAH prevalence from 15 to 16 ppm).22,23,33 Despite using non-systematic patient registration, in which centres were invited – rather than mandated – to participate, the Polish registry reported a higher PAH prevalence estimate (30.8 ppm). This likely reflects the fact that all expert PH centres in the country agreed to participate.19
Routine clinical practice also differs between countries and may also influence the reported estimates. Confirmation of PH/PAH diagnosis by RHC is the gold standard as per the current ESC/ERS guidelines.2,3 While being routinely carried out in France, the UK and Sweden, only 40% and 72% of registrants in the South Korean and Swiss registries underwent RHC.23,26 In countries where RHC or V/Q scanning are either unavailable or not systematically used, estimates of epidemiology can be less accurate due to misdiagnosis.
The type of data source used to derive incidence or prevalence estimates could also cause variability in the epidemiology estimates. Large claims/administrative databases were used by several authors, despite the limitation of unconfirmed PAH or CTEPH diagnosis with this study type.18,24,27,28,34 PH ICD codes can be assigned to patients with suspected PH to ensure reimbursement for the diagnostic procedure, regardless of the final diagnosis. These coding practices likely contribute to an overestimation of patient numbers in claims/administrative databases. The coding status of PAH and CTEPH is also problematic. Although the ICD-10 codes released in October 2017 allow the identification of idiopathic or hereditable PAH (primary PH; I27.0), PAH associated with other conditions (secondary PAH; I27.21) and CTEPH (I27.24),35 no study identified in this systematic literature review included the five digit ICD-10 code implemented in 2017 and thus, may have misclassified patients. The highest PAH and CTEPH estimates were reported from the Canadian (Ontario) administrative database study, in which only 40.9% of the incidence cohort had a record of RHC. Furthermore, the study algorithm allowed all PH patients, except those in Group 1, to belong to multiple PH Groups and, as a result, 35.4% of patients were assigned to more than one PH diagnosis. This study may therefore be overestimating the true epidemiology of the diseases in Ontario.24
Several studies using claim/administrative databases implemented algorithms combining ICD codes with medication and procedure codes to improve the specificity of patient identification. There is no standardisation across studies and only a few code-based algorithms have been evaluated against clinical data, albeit with a limited success.36 The risk of including false-positive patients (those who were not diagnosed with the disease of interest) remains a limitation of this data source and may partly explain the higher PAH estimates reported in the US and South Korean claims/administrative databases compared with the respective country’s national registries.5,26–28
Registries have the advantage of being designed with the purpose of supporting scientific research for a specific disease and, as such, only patients with a confirmed diagnosis should be included. The same advantage is true for clinical databases (i.e. hospital-based estimates as per the definitions used in this systematic review); however, their national coverage is less clear and, therefore, their estimates cannot be extrapolated to the whole country’s population.
Another key factor in assessing the validity of epidemiology estimates is the observation period for data collection. The number of PAH-targeted treatments available has increased since the first treatment – epoprostenol – was licensed in 199537: more than 10 drugs and formulations are currently available.38–40 In the Czech Republic registry, the increase in PAH-specific therapy options after 2006 is thought to explain why the number of incident cases in 2006 sharply increased (n = 91) given that only 100 prevalent cases were identified in the previous six-year period.41 Moreover, when the aetiology is multifactorial or unclear, there may be an incentive to classify patients as PH Group 1 or 4, which have procedures and drugs available. Given the burden of RHC, in the absence of treatment options, performing this diagnostic procedure to reach a definitive PAH or CTEPH diagnostic may be perceived as unethical, thus it may have been used less frequently in the past.
The observation period is also important to consider when interpreting CTEPH estimates. In Latvia, for example, routine use of V/Q scanning to differentiate CTEPH from PAH was not available across the total observation period (2007–2016) of the national registry and, as a result, the number of CTEPH cases may have been underestimated.20 Treatment options for CTEPH are also evolving rapidly8,40,42,43 and it is therefore important to identify studies using data collected during a recent time period. The most recent publications from the Swedish and UK systematic registries may therefore be the most accurate, as their observation periods are within the previous five years and diagnosis was based on the latest PH guideline’s recommendations.16,17
The length of observation period also determines the extent to which the study’s estimate represents the true epidemiology. For example, epidemiology data provided by the French and US registries were derived from enrolled consecutive PAH patients over a time period of one year,5,22 with the assumption that patients have an annual clinic visit as a minimum. In cases where this assumption does not reflect the reality, patients with less frequent visits captured in the database will be missed, resulting in underestimation of prevalence. Of note, six of the 12 registries reporting PAH incidence estimates had an observation period of ≤4 years, and all but the BNP-PL registry reported incidence to be below 4 ppm.5,19,22,26,33,44 The remaining six registries had an observation period of ≥5 years and incidence for all but the two non-systematic registries conducted in countries with de-centralised healthcare (Germany and Switzerland) was higher.21,23
The present study represents the authors’ recommendation for which estimates are considered most valid, based on objective criteria. The incidence/prevalence estimates were critically appraised according to the criteria outlined herein. The following factors were considered, shown in order of importance, as key for selecting the most valid estimates: (i) study design, with national systematic registries being the most likely to yield accurate estimates, (ii) structure of the healthcare system, with centralised systems in countries with access to the latest treatments and procedures being selected, and (iii) the recency of the observation period (ideally from the previous five years). As such, the estimates from the Swedish registry SPAHR and the UK PH Audit are considered to be the most generalisable.
Critically appraising epidemiology estimates is important for several reasons. These estimates could be used as a guide or threshold level to assess if a country is likely to be under-diagnosing PAH and CTEPH. This would help to set detection rate goals and guide allocation of research grants and healthcare resources by decision-makers. Furthermore, by providing an assessment of the published incidence and prevalence estimates, the authors hope to guide readers in their own critical appraisal of such upcoming data. This is particularly important given that the highest estimates identified in this systematic review create confusion on whether PAH and CTEPH are rare diseases. The critical appraisal presented herein highlighted the possible reasons for overestimation by these studies and is in agreement with the classification of PAH and CTEPH as rare diseases.
This study has several potential limitations. There was the potential for reviewer bias, however, given the sparsity of PH literature, the authors’ specialisation in PH and as the systematic screening of bibliographies of the identified articles was conducted to capture any additional eligible publications, a single reviewer was believed to be sufficient. It remains possible that non-indexed publications may have been missed, particularly publications in non-English language.
The methods used to calculate incidence and prevalence were not consistent across the publications, thus hindering comparison of their estimates.45 While incidence rate is a more accurate estimate of the rate at which the outcome develops, its denominator is more challenging to calculate in open populations that are, per definition, dynamic rather than fixed in time. Incidence rate can only be calculated if periodic follow-up information is available for each patient, including if they developed the disease and when they developed it. Checking every citizen at the beginning and end of the year, to calculate the incidence of PAH or CTEPH in a country over a calendar year is not feasible. However, the population at risk, i.e. the general population of the country, is so large compared with the number of new patients with the disease, that the impact of these patients on the size of the population at risk is negligible. As incidence rate is similar to annual incidence proportion for a rare disease, the only incidence rate identified18 was interpreted in the same way as for incidence proportions in this review. Point prevalence refers to prevalence measured at a particular point in time, while period prevalence refers to prevalence measured over an interval of time. As PAH and CTEPH prevalence estimates have increased over the past few decades, period prevalence calculated over a long period would not be representative of the contemporaneous epidemiological status of the diseases. Thus, this critical appraisal only selected estimates from recent observation periods.
Populations from Asia, Africa and South America were under-represented in the literature. Data from these regions will be required to establish whether generalising estimates from European and North American countries to other regions is appropriate.
This literature review identified few studies in paediatric populations: seven studies reported epidemiology estimates for paediatric cases while the remaining 26 studies only included adult estimates. Due to their pathophysiology, PAH and CTEPH are predominantly diagnosed in older patients, with mean age at diagnosis often being between 50 and 65 years old.2,17 Paediatric PAH and CTEPH are rare forms of rare conditions and the relative lack of research into paediatric cases of rare diseases is still being addressed.46 It is then unsurprising that this systematic literature review highlights the scarcity of paediatric epidemiology studies in PAH and CTEPH, and the need for further study.
In conclusion, the appraisal of available evidence identified through a systematic literature review suggests that the following adult estimates are the most reliable for the scientific community to use as a guide for resource allocation and improvement of detection rates: approximately 5.8 ppm for PAH incidence, 47.6–54.7 ppm for PAH prevalence, 3.1–6.0 ppm for CTEPH incidence and 25.8–38.4 ppm for CTEPH prevalence.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020977300 for Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review by Laurence Leber, Amélie Beaudet and Audrey Muller in Pulmonary Circulation
Acknowledgements
Medical writing and editorial support were provided by Victoria Atess, PhD, and Richard McDonald (Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc), funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson. The authors would like to thank Viviane Patricia Sprecher (Actelion Pharmaceuticals Ltd, at the time of study) for her support on the systematic literature search.
Footnotes
Author contributions: All authors contributed to the conception and design of the study, analysis and interpretation of the data, and critical revision of the manuscript.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.B. and A.M. are employees of Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson and L.L. was an employee of Actelion Pharmaceuticals Ltd at the time of study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson, Allschwil, Switzerland.
Guarantor: Audrey Muller will act as guarantor for integrity of data and its reporting in this manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Rose-Jones LJ, McLaughlin VV. Pulmonary hypertension: types and treatments. Curr Cardiol Rev 2015; 11: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 4.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 5.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest 2011; 139: 128–137. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Okumura N, Adachi S, et al. < Editors’ choice> Pulmonary hypertension: diagnosis, management, and treatment. Nagoya J Med Sci 2019; 81: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delcroix M, Kerr K, Fedullo P. Chronic thromboembolic pulmonary hypertension. Epidemiology and risk factors. Ann Am Thorac Soc 2016; 13 Suppl 3: S201–S206. [DOI] [PubMed] [Google Scholar]
- 8.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swinnen K, Quarck R, Godinas L, et al. Learning from registries in pulmonary arterial hypertension: pitfalls and recommendations. Eur Respir Rev 2019; 28: 190050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statemen t. 2009; 339: b2535. [DOI] [PMC free article] [PubMed]
- 11.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43: 5s–12s. [DOI] [PubMed] [Google Scholar]
- 12.Foshat M, Boroumand N. The evolving classification of pulmonary hypertension. Arch Pathol Lab Med 2017; 141: 696–703. [DOI] [PubMed] [Google Scholar]
- 13.Munn Z, Moola S, Lisy K, et al. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E and Munn Z (eds) JBI manual for evidence synthesis JBI, https://synthesismanual.jbi.global (2020, accessed 19 November 2020).
- 14.US Census, www.census.gov/programs-surveys/popest/data/tables.html (2018, accessed 1 January 2020).
- 15.Eurostat, https://ec.europa.eu/eurostat/data/database (2018, accessed 1 January 2020).
- 16.NHS Digital. National Audit of Pulmonary Hypertension Great Britain, 2018–19, tenth annual report, https://files.digital.nhs.uk/BA/4EF20E/NAPH%2010AR%20-%20Main%20Report.pdf (2019, accessed 1 October 2020).
- 17.Kjellström BWM, Dahlerup H, Hesselstrand R, et al. Swedish Pulmonary Arterial Hypertension Registry annual report 2019, www.ucr.uu.se/spahr/arsrapporter (2019, accessed 1 October 2020).
- 18.Li L, Jick S, Breitenstein S, et al. Pulmonary arterial hypertension in the USA: an epidemiological study in a large insured pediatric population. Pulm Circ 2017; 7: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopeć G, Kurzyna M, Mroczek E, et al. Characterization of patients with pulmonary arterial hypertension: data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J Clin Med 2020; 9: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skride A, Sablinskis K, Lejnieks A, et al. Characteristics and survival data from Latvian pulmonary hypertension registry: comparison of prospective pulmonary hypertension registries in Europe. Pulm Circ 2018; 8. DOI: 10.1177/2045894018780521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeper MM, Huscher D, Pittrow D. Incidence and prevalence of pulmonary arterial hypertension in Germany. Int J Cardiol 2016; 203: 612–613. [DOI] [PubMed] [Google Scholar]
- 22.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 23.Tueller C, Stricker H, Soccal P, et al. Epidemiology of pulmonary hypertension: new data from the Swiss registry. Swiss Med Wkly 2008; 138: 379–384. [DOI] [PubMed] [Google Scholar]
- 24.Wijeratne DT, Lajkosz K, Brogly SB, et al. Increasing incidence and prevalence of World Health Organization Groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada . Circ Cardiovasc Qual Outcomes 2018; 11: e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007; 30: 104–109. [DOI] [PubMed] [Google Scholar]
- 26.Chung WJ, Park YB, Jeon CH, et al. Baseline characteristics of the Korean Registry of Pulmonary Arterial Hypertension. J Korean Med Sci 2015; 30: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song S, Lee SE, Oh SK, et al. Demographics, treatment trends, and survival rate in incident pulmonary artery hypertension in Korea: a nationwide study based on the health insurance review and assessment service database. PloS One 2018; 13: e0209148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirson NY, Birnbaum HG, Ivanova JI, et al. Prevalence of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension in the United States. Curr Med Res Opin 2011; 27: 1763–1768. [DOI] [PubMed] [Google Scholar]
- 29.Medrek SK, Sahay S. Ethnicity in pulmonary arterial hypertension: possibilities for novel phenotypes in the age of personalized medicine. Chest 2018; 153: 310–320. [DOI] [PubMed] [Google Scholar]
- 30.Al-Naamani N, Paulus JK, Roberts KE, et al. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ 2017; 7: 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong MS, Abman S, Austin ED, et al. Racial and ethnic differences in pediatric pulmonary hypertension: an analysis of the Pediatric Pulmonary Hypertension Network Registry. J Pediatr 2019; 211: 63.e66–71.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NHS England. Commissioning policy: targeted therapies for use in pulmonary hypertension in adults. Reference: NHS England A11/P/c, www.england.nhs.uk/wp-content/uploads/2018/07/Targeted-therapies-for-use-in-pulmonary-hypertension-in-adults.pdf (2005, accessed 1 March 2020).
- 33.Escribano-Subias P, Blanco I, Lopez-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012; 40: 596–603. [DOI] [PubMed] [Google Scholar]
- 34.Pektas A, Pektas BM, Kula S. An epidemiological study of paediatric pulmonary hypertension in Turkey. Cardiol Young 2016; 26: 693–697. [DOI] [PubMed] [Google Scholar]
- 35.Mathai SC, Mathew S. Breathing (and coding?) a bit easier: changes to International Classification of Disease coding for pulmonary hypertension. Chest 2018; 154: 207–218. [DOI] [PubMed] [Google Scholar]
- 36.Gillmeyer KR, Lee MM, Link AP, et al. Accuracy of algorithms to identify pulmonary arterial hypertension in administrative data: a systematic review. Chest 2019; 155: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Food and Drug Administration. VELETRI prescribing information, www.veletri.com/pdf/veletri_full_prescribing_information_3rd_gen.pdf (accessed 1 March 2020).
- 38.Sitbon O, Gomberg-Maitland M, Granton J, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitbon O, Vonk Noordegraaf A. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur Respir Rev 2017; 26: 160055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Food and Drug Administration. ADEMPAS prescribing information, www.accessdata.fda.gov/drugsatfda_docs/label/2017/204819s006lbl.pdf (accessed 1 March 2020).
- 41.Jansa P, Jarkovsky J, Al-Hiti H, et al. Epidemiology and long-term survival of pulmonary arterial hypertension in the Czech Republic: a retrospective analysis of a nationwide registry. BMC Pulm Med 2014; 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adempas SmPC, www.ema.europa.eu/en/documents/product-information/adempas-epar-product-information_en.pdf (February 2019, accessed 1 July 2020).
- 43.European Medicines Agency. Trepulmix summary of product characteristics, www.ema.europa.eu/en/documents/product-information/trepulmix-epar-product-information_en.pdf (accessed 1 July 2020).
- 44.Baptista R, Meireles J, Agapito A, et al. Pulmonary hypertension in Portugal: first data from a nationwide registry. Biomed Res Int 2013; 2013: 489574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spronk I, Korevaar JC, Poos R, et al. Calculating incidence rates and prevalence proportions: not as simple as it seems. BMC Public Health 2019; 19: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.State of Peadiatric Medicines in the EU 10 years of the EU paediatric regulation report from the Commission to the European Parliament and Council COM (2017) 626, https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf (accessed 1 October 2020).
- 47.Miranda-Machado P, Baños-Alvarez I, Alvarez-Barrios A. Prevalence of pulmonary arterial hypertension in the Colombian Caribbean. Pulm Circ 2019; 9. DOI: 10.1177/2045894019847643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 49.Fruchter O, Yigla M. Underlying aetiology of pulmonary hypertension in 191 patients: a single centre experience. Respirology 2008; 13: 825–831. [DOI] [PubMed] [Google Scholar]
- 50.Dubroff J, Melendres L, Lin Y, et al. High geographic prevalence of pulmonary artery hypertension: associations with ethnicity, drug use, and altitude. Pulm Circ 2020; 10. DOI: 10.1177/2045894019894534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraisse A, Jais X, Schleich JM, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis 2010; 103: 66–74. [DOI] [PubMed] [Google Scholar]
- 52.Kwiatkowska J, Zuk M, Migdal A, et al. Children and adolescents with pulmonary arterial hypertension: baseline and follow-up data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J Clin Med 2020; 9. DOI: 10.3390/jcm9061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.del Cerro Marin MJ, Sabate Rotes A, Rodriguez Ogando A, et al. Assessing pulmonary hypertensive vascular disease in childhood. Data from the Spanish registry. Am J Respir Crit Care Med 2014; 190: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 54.van Loon RL, Roofthooft MT, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 2011; 124: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 55.Bohacekova M, Kaldararova M, Valkovicova T, et al. Risk factors detection in chronic thromboembolic pulmonary hypertension, a tool for risk quantification? Bratisl Lek Listy 2016; 117: 577–582. [DOI] [PubMed] [Google Scholar]
- 56.Martínez-Santos P, Velázquez-Martín MT, Barberá JA, et al. Chronic thromboembolic pulmonary hypertension in Spain: a decade of change. Rev Esp Cardiol (Engl Ed) Epub ahead of print 2020. DOI: 10.1016/j.rec.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Kramm T, Wilkens H, Fuge J, et al. Incidence and characteristics of chronic thromboembolic pulmonary hypertension in Germany. Clin Res Cardiol 2018; 107: 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cottin V, Avot D, Levy-Bachelot L, et al. Identifying chronic thromboembolic pulmonary hypertension through the French national hospital discharge database. PloS One 2019; 14: e0214649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Llanos-González AB, Martín Medina A, Juliá Serdá G, et al. Evolution and survival of chronic thromboembolic pulmonary hypertension in the Canary Islands. Eur Respir J 2019; 54: PA1441. [Google Scholar]
- 60.Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 61.Puengpapat S, Pirompanich P. Incidence of chronic thromboembolic pulmonary hypertension in Thammasat University Hospital. Lung India 2018; 35: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020977300 for Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review by Laurence Leber, Amélie Beaudet and Audrey Muller in Pulmonary Circulation