Abstract
Pulmonary artery sarcoma is a rare malignancy with poor prognosis. Lack of specific clinical manifestations, some patients are even confirmed postoperatively or at autopsy, that leads to the delay in treatment. Early diagnosis and radical surgical resection provide the possibility of prolonged survival. We retrospectively enrolled 13 patients diagnosed with pulmonary artery sarcoma at our hospital between 2015 and 2019. Their clinical, laboratory, radiological, and histopathological data were collected and analyzed. Published case series were also reviewed. Results show that, the median age of the patients was 53 years, with 6 (46.2%) males. The most common symptom is exertional dyspnea. Erythrocyte sedimentation rate and C-reactive protein were increased in 76.9% and 69.2% of these patients, while D-Dimer remained normal or elevated slightly. Metastasis was present at diagnosis in eight (61.5%) patients. Ten patients were diagnosed histologically: three were diagnosed after pulmonary endarterectomy, four by endobronchial ultrasound-guided transbronchial needle aspiration, two by percutaneous lung biopsy, and one by endovascular aspiration biopsy. Four patients underwent surgery and one is waiting for surgery. Nine patients received chemotherapy; and three of them received targeted therapy with anlotinib after chemotherapy. Two patients received anti-PD-1 monoclonal antibody. One patient died during endobronchial ultrasound-guided transbronchial needle aspiration. Two patients died 9 and 13 months after diagnosis, respectively; one refused invasive diagnostic procedures and died three months after clinical diagnosis. In conclusion, the most appropriate approach to get tissue specimen needs to be tailored to every pulmonary artery sarcoma patient. Pulmonary endarterectomy combined with chemotherapy and targeted therapy has prolonged their survival time.
Keywords: pulmonary artery sarcoma, pulmonary thromboembolism, diagnosis, treatment, outcome
Introduction
Pulmonary artery sarcoma (PAS) is a rare malignancy which originates from pulmonary arteries with a poor prognosis. About 400 cases of PAS have been reported since Mandelstamm first reported it in 1923.1,2 The average survival time is approximately 1.5 months without surgical treatment.3 About 50% of PAS patients have lung and mediastinal metastases before diagnosis, and 16–19% of PAS patients develop distant metastases prior to diagnosis, which are associated with poor five-year survival.4
The etiology and origin of PAS remain to be elucidated. The multipotential stem cells in the intima of pulmonary artery are presumed as the origin of PAS. Pulmonary trunk (PT), right or left pulmonary arteries and their branches, right ventricular outflow tract (RVOT), pulmonary valve (PV), and tricuspid valve can be involved.
Due to the lack of specific clinical manifestations, most cases with PAS are misdiagnosed with pulmonary thromboembolism (PTE) or chronic thromboembolic pulmonary hypertension (CTEPH).5–9 In many cases, the diagnosis of PAS is not considered until the effect of anticoagulation or thrombolysis is far from satisfactory.8 Some patients are even confirmed postoperatively or at autopsy. The difficulty in the diagnosis leads to the delay in treatment. Early diagnosis and radical surgical resection provide the possibility of prolonged survival.10
Although an increased number of PAS cases have been reported, many cases are still misdiagnosed at the beginning.9,11 In order to identify the characteristics of PAS patients and help clinicians to diagnose PAS earlier, we analyzed the clinical and radiological data of patients who were diagnosed with PAS at our hospital.
Patients and methods
We retrospectively enrolled 13 patients who were diagnosed with PAS in China-Japan Friendship Hospital between November 2015 and November 2019. Their clinical, laboratory, radiological, and histological data were collected and analyzed. All the patients have written informed consent.
Ultrasonic cardiogram, CT pulmonary arteriography (CTPA), enhanced magnetic resonance imaging (MRI), and positron emission tomography-computed tomography (PET-CT) were used in the radiological assessment. In order to get the tissue specimen, pulmonary endarterectomy (PEA), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), percutaneous lung biopsy or endovascular aspiration biopsy through right heart catheterization were chosen based on the radiological characteristics and the patient's status.
Diagnosis was confirmed histologically. In the patients who refused invasive procedures, clinical diagnosis was made after the discussion by multidisciplinary team including experienced pulmonologists, oncologist, cardiothoracic surgeons, and radiologists.
Results
Demographic and clinical information
Thirteen patients were confirmed with the diagnosis of PAS, of whom six were males. The median age of these patients was 53 years (range 19–65 years). The most common symptom is exertional dyspnea, followed by cough, pleuritic chest pain, fever, hemoptysis, expectoration, anginal pain, and weight loss (Table 1). Seven patients were diagnosed with PTE. Only three patients were suspected with PAS at the first visit. The interval between the onset and confirmed diagnosis ranged from 1 to 24 months, and the majority of the patients had waited for 3–4 months to get diagnosed with PAS.
Table 1.
The symptoms of PAS patients.
| Symptoms | n (%) |
|---|---|
| Exertional dyspnea | 11 (84.6) |
| Fever | 6 (46.2) |
| Cough | 9 (69.2) |
| Expectoration | 4 (30.8) |
| Anginal pain | 4 (30.8) |
| Pleuritic chest pain | 7 (53.8) |
| Hemoptysis | 6 (46.2) |
| Weight loss | 4 (30.8) |
Clinical laboratory and imaging findings
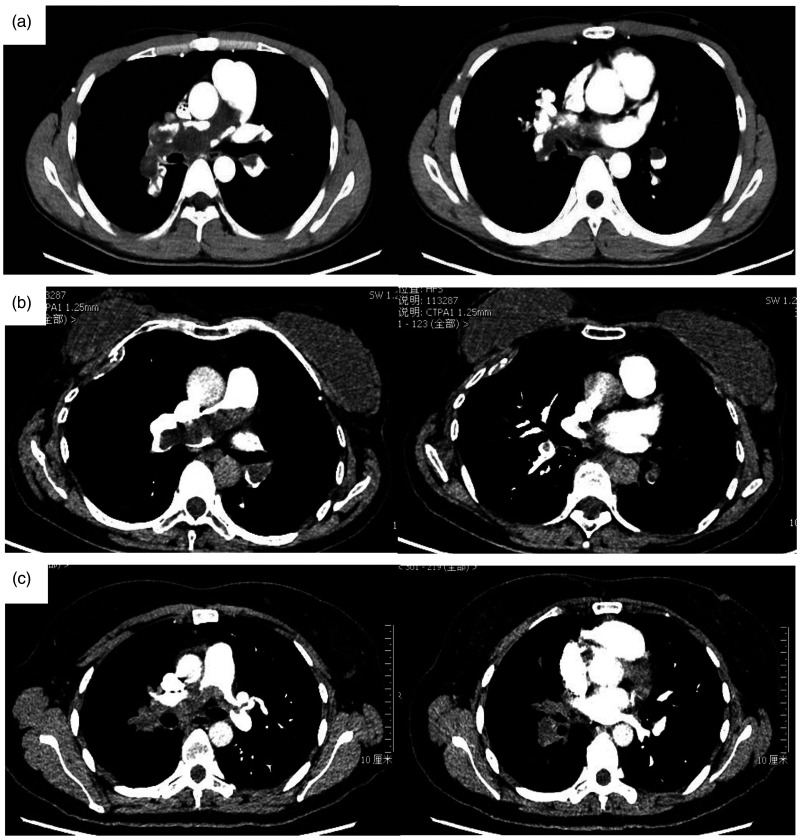
The serum biomarkers and imaging findings are presented in Table 2. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and lactate dehydrogenase were increased in 76.9%, 69.2%, and 23.1% of these patients, while D-Dimer remained normal or elevated slightly. Fig. 1 is CTPA of some patients, which shows extensive intraluminal mass in the central pulmonary arteries. The CTPA of one case during her course of disease is shown in Fig. 2a–c. She was first diagnosed with PTE and received anticoagulation therapy for 10 months before she came to our hospital. However, her condition aggravated and the intraluminal filling-defect in pulmonary arteries in CTPA also enlarged. We performed ultrasonic cardiogram and left heart contrast echocardiography (Fig. 2d and e). The development of contrast was seen in the mass in PT, implying that it is a tumor with blood supply inside, while thrombus does not. Fig. 2f is the PET-CT of this patient, showing the mildly increased uptake of F-18 fluorodeoxyglucose (FDG) in the mass in PT. Echocardiography demonstrated pulmonary hypertension in five patients (38.4%) and right ventricular dysfunction in two patients (15.4%). Fig. 3a and b shows PAS in right pulmonary artery (RPA) in the EBUS of patient No. 13.
Table 2.
The laboratory and imaging findings of PAS patients.
| The laboratory and imaging findings | Results/n (%) |
|---|---|
| ESR, mm/h | 31 (12.75, 60.5) |
| CRP, mg/L | 1.75 (1.18, 4.13) |
| LDH, U/L | 185 (150, 222) |
| D-Dimer, mg/L | 0.93 (0.56, 1.84) |
| NT-proBNP, pg/mL | 58.5 (35, 424.25) |
| Bulky mass in pulmonary trunk in CTPA (n = 12) | 9 (75.0) |
| Wall eclipsing sign in CTPA (n = 12) | 11 (91.7) |
| Heterogeneous enhancement in enhanced MRI (n = 7) | 7 (100.0) |
| Increased uptake of F-18 FDG in PET-CT (n = 9) | 9 (100.0) |
| Pulmonary hypertension in UCG | 5 (38.5) |
| Right ventricular dysfunction in UCG | 2 (15.4) |
ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; LDH: lactate dehydrogenase; NT-proBNP: N-terminal pro brain natriuretic peptide; CTPA: computed tomographic pulmonary angiography; MRI: magnetic resonance imaging; FDG: fluorodeoxyglucose; PET-CT: positron emission tomography-computed tomography; UCG: ultrasonic cardiogram.
Fig. 1.
CTPA of PAS patients.
Fig. 2.
CTPA, echocardiography, surgical sample, and histopathological finding of one patient. (a–c) The CTPA of patient No. 2 during her course of disease are shown. She was first diagnosed with pulmonary thromboembolism and received anticoagulation therapy for 10 months before she came to our hospital. However, her condition aggravated and the intraluminal filling-defect in pulmonary arteries in CTPA also enlarged. (d and e) The ultrasonic cardiogram and left heart contrast echocardiography. The development of contrast was seen in the mass in PT, implying that it is a tumor with blood supply inside. (f) The PET-CT of this patient, showing the mildly increased uptake of F-18 FDG in the mass in PT. (g) The surgical sample of pulmonary endarterectomy from patient No. 2. (h and i) Histopathological finding of the surgical sample. Many malignant cells with vacuole nucleus and multinucleated tumor giant cell can be seen in the Hematoxylin–Eosin staining.
Fig. 3.
EBUS of patient No. 13. (a) A mass in the right pulmonary artery can be seen. (b) Blood stream can be seen inside and around the mass in Doppler ultrasonography.
Involved sites
In this study, RPA is the most common site involved, followed by the PT and left pulmonary artery (LPA). Metastatic disease was present at diagnosis in eight (61.5%) patients; the most common metastatic site was lung, followed by hilar or mediastinal lymph nodes (Table 3).
Table 3.
Involved sites at diagnosis.
| Involved sites | n (%) |
|---|---|
| Pulmonary trunk | 9 (69.2) |
| Right pulmonary artery | 12 (92.3) |
| Left pulmonary artery | 8 (61.5) |
| Distal branches of right pulmonary artery | 5 (38.5) |
| Distal branches of left pulmonary artery | 4 (30.8) |
| Outflow tract of right ventricle | 3 (23.1) |
| Tricuspid valve | 1 (7.7) |
| Metastasis | 8 (61.5) |
| Intrathoracic metastasis | 8 (61.5) |
| Lung | 8 (61.5) |
| Hilar or mediastinal lymph nodes | 3 (23.1) |
| Pleura | 1 (7.7) |
| Extrathoracic metastasis | 3 (23.1) |
| Bone | 2 (15.4) |
| Adrenal gland | 1 (7.7) |
| Muscle | 1 (7.7) |
Histopathological and immunohistochemical findings
Histopathological diagnosis, treatment, and clinical outcome of all the patients are shown in Table 4. Ten patients were diagnosed histologically; of them, three patients were diagnosed after PEA, four were diagnosed by EBUS-TBNA, two by percutaneous lung biopsy, and one by endovascular aspiration biopsy through right heart catheterization. Based on histomorphologic features and immunohistochemical findings, four tumors were classified as spindle cell sarcoma, four as intimal sarcoma, one as undifferentiated pleomorphic sarcoma, and one as synovial sarcoma. Vimentin is the most common immunohistochemical maker positive in these patients (n = 9), followed by Desmin, KP-1, P53, and CD34. One patient refused to the attempt of invasive diagnostic procedures to get the histological diagnosis (patient No. 11).
Table 4.
The diagnosis, treatment, and prognosis of PAS patients.
| No. | Sex/Age | Site | Histopathological diagnosis | Treatments | Clinical outcomes |
|---|---|---|---|---|---|
| 1 | F/39 | PT, RPA, LPA | Intimal sarcoma | PEA and chemotherapy | Alive, followed for 18 months |
| 2 | F/52 | PT, RPA, LPA, PV, RVOT, lung | Undifferentiated pleomorphic sarcoma | PEA and chemotherapy | PAS recurrence two months after PEA |
| 3 | M/54 | PT, LPA | Spindle cell sarcoma | PEA, chemotherapy and targeted therapy | Alive, followed for 10 months |
| 4 | F/53 | RPA, LPA, lung, bone | Spindle cell sarcoma | Chemotherapy, immunotherapy and targeted therapy | Alive, followed for nine months |
| 5 | F/53 | RPA, pleura | Spindle cell sarcoma | Chemotherapy and targeted therapy | Alive, followed for 10 months |
| 6 | F/62 | PT, RPA, LPA | – | Waiting for PEA | Alive, followed for 11 months |
| 7 | M/19 | PT, RPA, LPA, lung | Intimal sarcoma | Chemotherapy | Died after nine months |
| 8 | M/35 | PT, RPA, LPA, RVOT, lung | Intimal sarcoma | PEA | PAS recurrence after three months |
| 9 | M/65 | PT, RPA, LPA, lung, lymph nodes | – | – | Died during EBUS-TBNA |
| 10 | M/53 | PT, RPA | spindle cell sarcoma | Chemotherapy and immunotherapy | PAS recurrence after three months |
| 11 | F/61 | PT, RPA, RVOT | – | – | Died after three months |
| 12 | M/26 | RPA, lung, lymph nodes, bones, muscle | Synovial sarcoma | Chemotherapy | Died after 13 months |
| 13 | F/42 | RPA, lung, lymph nodes, adrenal gland | Intimal sarcoma | Chemotherapy | Alive, followed for five months |
PAS: pulmonary artery sarcoma; PT: pulmonary trunk; RPA: right pulmonary artery; LPA: left pulmonary artery; RVOT: right ventricular outflow tract; PV: pulmonary valve; PEA: pulmonary endarterectomy; EBUS-TBNA: endobronchial ultrasound-guided transbronchial needle aspiration.
Treatment and prognosis
Four patients underwent PEA surgery and one patient is waiting for PEA. Nine patients received chemotherapy, of whom three patients received chemotherapy after radical surgical resection. The chemotherapy regimens made up of ifosfamide and epirubicin was used in all these nine patients. Three of these nine patients received targeted therapy with anlotinib after 4–6 cycles of chemotherapy. One patient used gemcitabine and docetaxel for two cycles and then changed to ifosfamide and epirubicin. Three patients received targeted therapy with anlotinib. Two patients used immunotherapy with anti-PD-1 monoclonal antibody.
In all these patients, four patients died. One patient died during the procedure of EBUS-TBNA with laryngeal mask airway under intravenous anesthesia (patient No. 9). Three other patients died: two of them received chemotherapy and died 9 and 13 months after diagnosis, respectively; the other one (patient No. 11) refused invasive diagnostic procedures to get the histological diagnosis and died three months after clinical diagnosis. Three patients developed sarcoma recurrence during follow-up and two of them received chemotherapy before recurrence.
Discussion
Previous studies demonstrated that PAS typically affects middle-aged people.12,13 Early literature reported that 60% of patients with PAS were diagnosed at autopsy.13 More and more patients are diagnosed earlier with the improvement of clinicians' comprehension of this disease and the development of diagnostic techniques. In the present study, the median interval from the beginning of symptoms to the diagnosis and surgery was three months (range 1–24), comparable to the results of previous studies (range, 3–12 months).14–17
Published cohorts of PAS with detailed diagnostic, therapeutic, and follow-up information are listed in Table 5.5,15,16,18–23 Adult patients with all ages are involved, without significant gender propensity. Surgical treatment is the first choice for operable lesion, while chemotherapy and radiation therapy are adjuvant therapy after the operation and for recurrence, or necessary for unresectable PAS. The survival duration has been prolonged with the development of comprehensive treatment. There are still a lot of issues in suspense, such as the most appropriate diagnostic method to make the radiological and pathological diagnosis, type of surgical procedure, chemotherapy regimen, and the value of targeted therapy and immunotherapy.
Table 5.
Published studies of PAS.
| References | Total/male patients no. | Age (years) | Pathological results | Treatment | Clinical Outcomes |
|---|---|---|---|---|---|
| 18 | 43/23 | 15–75 | Pleomorphic-fascicular sarcoma (28), leiomyosarcoma (4), rhabdomyosarcoma (1), osteosarcomas (7), low-grade myofibroblastic sarcomas (3) | Surgery (36), chemotherapy (6), radiation therapy (4) | Median survival was 10 months for pleomorphic-fascicular sarcoma, 16 months for myogenic sarcoma, and 24 months for sarcomas with osteosarcomatous differentiation. Two patients with low-grade myofibroblastic sarcomas were alive with apparent cure at 10 years |
| 19 | 20/10 | 33–75 | Spindle cell sarcoma (8), intimal sarcoma (4), pleomorphic sarcoma (3), high grade sarcoma (2), chondroid differentiation (1), angiosarcoma (1), and smooth muscle differentiation (1) | Surgery (17), chemotherapy (6), radiation therapy (8), | Overall median survival 24 months |
| 20 | 8/3 | 32–75 | NA | Surgery (1), chemotherapy (2) | Survival time: 3–15 months |
| 21 | 5/3 | 34–70 | High grade spindle cell sarcoma (3), chondroid differentiation (1), malignant spindle cell tumor with extensive necrosis (1) | Surgery (4), chemotherapy (3) | Alive during the median follow-up duration of 128 days (range, 25–1589 days) |
| 16 | 31/16 | 26–78 | Malignant fibrous histiocytoma type pleomorphic fascicular sarcoma (18), leiomyosarcoma (4), high-grade sarcoma with prominent myxoid changes (2), poorly differentiated sarcoma with osteo-sarcomatous differentiation (1), sarcoma with chondrosarcomatous differentiation (1), leiomyosarcomas (2), spindle cell sarcomas (3) | Surgery (31), adjuvant therapy (18) | Four patients died in 1 month, 11 patients alive during the median follow-up duration of 19 months (range, 1–99 months) |
| 22 | 12/8 | 34–69 | Rhabdomyosarcoma (2), leiomyosarcoma (3), osteogenic sarcoma (1), epithelioid leiomyosarcoma (1), high-grade sarcoma (4), angiosarcoma (1), | Surgery (12), chemotherapy (7), radiation therapy (2) | Five patients died in 3–23 months, one patient had the longest survival of 68 months |
| 23 | 9/5 | 32–67 | Intimal sarcoma (4), leiomyosarcoma (3), undifferentiated sarcoma (1), pleomorphic rhabdomyosarcoma (1) | Surgery (9) | The mean postoperative survival period was 254 days. The longest postoperative survival period was 35.5 months. Two patients died on days 2 and 30 post-surgery, respectively. |
| 5 | 9/4 | 24–74 | High-grade spindle cell sarcoma (2), intimal sarcoma (2), intermediate-grade spindle-cell sarcoma (1), myxofibrosarcoma (1), intermediate-grade myofibroblastic sarcoma (1), high-grade undifferentiated pleomorphic spindle-cell sarcoma (1), angiosarcoma (1) | Surgery (8), chemotherapy (5), radiation therapy (4) | Five patients died after a mean duration of 2.1 years after diagnosis. Two patients are alive with recurrence and metastases of the disease 23 and 27 months after diagnosis, respectively; one patient is alive and disease-free 116 months after diagnosis |
| 15 | 13/6 | 31–77 | Intimal sarcoma (6), high-degree sarcoma (1), high-degree leiomyosarcoma (1), fibrosarcoma (1), mesenchymal high-degree sarcoma (1), angiosarcoma (1), malignant fibrous histiocytoma (1), myxofibrosarcoma (1) | Surgery (13, pneumonectomy in 5 patients and pulmonary endarterectomy in 8) | Two patients died in-hospital. The median survival was 26.8 months after pneumonectomy and 6.6 months after pulmonary endarterectomy. |
PAS is usually misdiagnosed as PTE because of their similar symptoms and similar appearance on CT scans as intraluminal filling defects in the pulmonary arteries.5 Patients with PAS present with an obstruction of centric pulmonary arteries, sometimes concomitant with pulmonary hypertension or right-ventricular dysfunction insidiously, whereas patients with PTE usually present with sudden onset symptoms as a result of a pulmonary embolism, including acute right-ventricular dysfunction. PAS usually has an insidious onset and nonspecific symptoms.23 The most common symptom is progressively exertional dyspnea. Some patients have fever, fatigue, and weight loss, which are less common in PTE and CTEPH and might be used as a distinguishing feature.23
There are no specific biomarkers used for the diagnosis of PAS. In our study, increased ESR and CRP were observed in 83.3% and 75.0% of our patients, respectively. Similar findings were also reported in previous studies.23–25 D-Dimer remains normal or slightly elevated in PAS patients, implying that it is a condition without significant thrombosis and thrombolysis. These results indicate that serum inflammatory markers like ESR and CRP may be useful for screening for PAS or differentiating it from pulmonary thromboembolic disease.
The location and morphological characteristics are useful in the differential diagnosis of lesions in pulmonary arteries. Cox et al.26 reported that 85%, 71%, 65%, 32%, and 10% of PAS affect the PT, RPA, LPA, PV, and RVOT, respectively. Similar involvement was observed in the patients with PAS in our study, with most of the PASs occurring in the PT, RPA, and LPA. CT findings include a heterogeneous tumoral attenuation resulting in a filling defect that primarily involves the entire luminal diameter of the proximal PT, with variable intravascular extension in one or both pulmonary arteries. On CTPA, Gan et al.2 suggested that the wall eclipsing sign is pathognomonic for PAS, which is defined as: (i) almost full occupation of the lumen of the PT, LPA, or RPA by a low-density mass; (ii) protrusion of the proximal end of this mass toward the RVOT; (iii) eclipsing of one or both walls of the PT, LPA, or RPA by this lesion. Enhanced MRI, PET-CT, and left heart contrast echocardiography are emerging imaging approach to diagnose PAS. It is believed that heterogeneous enhancement after administration of gadolinium-diethylenetriamine pentaacetic acid on MRI favors pulmonary artery tumor over embolism.27 Liu et al. found that a hyperintense filling defect invading the main pulmonary arteries on fat-suppressed T2-weighted imaging, contrast enhancement, and grape-like appearance in distal pulmonary arteries were the major findings of PAS on MRI.28 Increased FDG uptake seen in the intraluminal lesion of the PT and its branches in F-18 FDG PET-CT, which is useful in the differential diagnosis of PAS. The metastatic sites could also be seen with increased FDG uptake. In our study, extensive intraluminal lesion of the central pulmonary arteries and the wall eclipsing sign were seen in CTPA in the majority of our patients. All the patients who received MRI had heterogeneous enhancement in the mass, as increased uptake of F-18 FDG in PET-CT. One patient had the development of contrast seen in the mass in PT in left heart contrast echocardiography, reminding us this method could be used in the differential diagnosis of lesions in proximal pulmonary arteries. PAS shows the development of contrast because they have blood supply by inside vessels, while thrombus does not.
Early diagnosis of PAS may improve its prognosis.29 Several diagnostic technics have been used to make the diagnosis preoperatively. Endovascular aspiration biopsy through right heart catheterization is an attempt; however, the diagnostic rate is limited because of insufficient tissue.11,30,31 EBUS-TBNA has also been reported with a successful diagnosis of PAS. However, the increasing complications of bleeding have been questioned.32–34 One patient in our series died during the procedure of EBUS-TBNA. Since we did not find active and major bleeding in the airway after biopsy, the possible reason was that the vasospasm of pulmonary artery and the clot formed after the aspiration of the mass obstructed the narrow access in the PT. Therefore, the diagnostic method to get tissue specimen should be tailored to every patient and the benefit–risk ratio of each method should be evaluated carefully.
The prognosis reported in earlier studies has been very poor, with survival of 1.5 months without surgical resection and 10 months with surgical resection.13 Surgical therapy is the first-line treatment for PAS with the effect of relieving clinical symptoms and lengthening survival.35 Radical surgical resection provides the possibility of prolonged survival. The surgical strategy included pneumonectomy, for possible radical resection, and palliative endarterectomy, to reduce symptoms and increase the life expectancy. Pneumonectomy could be performed in the case of a unilateral presentation and in the absence of pulmonary hypertension, with a potential curative goal.8 It has been reported that the median survival was 26.8 months after pneumonectomy and 6.6 months after PEA.15 The 1-, 3-, and 5-year survival was 63%, 29%, and 22%, respectively.16
Therapeutic strategy has seen great progress in recent years with the development of antineoplastic drugs. Postoperative chemotherapy and/or radiotherapy have been shown to improve the efficacy of the available treatments for PAS.36,37 Ifosfamide and epirubicin regimen, which is used in all the patients who received chemotherapy in our study, is the most classic chemotherapy for PAS. The combined therapy, including surgery, chemotherapy, and targeted therapy, improves the prognosis of PAS patients. Anlotinib, a multitargeted tyrosine kinase inhibitor, is used in non-small cell lung cancer, soft tissue sarcoma, and other types of malignancy. In our study, three patients received anlotinib as targeted therapy since progressive disease of PAS after chemotherapy and they survived till we drafted this manuscript. Its effect in PAS needs to be observed in future. Pazopanib, also a multitargeted tyrosine kinase inhibitor, is used to treat one patient with PAS in a case report, showing a partial response to treatment. However, hand-foot syndrome led to cessation of pazopanib. After cessation of pazopanib, the patient demonstrated a rebound effect.38 Pazopanib could be considered in patients with PAS that is unresectable or recurrent after surgery or chemotherapy.38
One of the limitations of this study is limited sample, which is inevitable since PAS is a rare disease. Another limitation is that not all the patients were diagnosed histologically since one patients refused to receive invasive procedures and one patient died during EBUS-TBNA.
In summary, CTPA, enhanced MRI, PET-CT, and left heart contrast echocardiography are useful imaging approaches to assist the diagnosis of PAS. The most appropriate procedure to get tissue sample and make a histological diagnosis for patients suspected with PAS still remains to be elucidated and need to be tailored for every single patient.30–34 Radical surgical resection is still the first-line therapy for PAS, providing the possibility of prolonged survival.35 Chemotherapy, radiotherapy, and other emerging antineoplastic drugs can be used in combination of surgery to improve clinical outcome of these patients.36–38
Footnotes
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81600036 and 81570049), the Fund of the National Key Research and Development Program of China (No. 2016YFC0905600) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2018-I2M-1-003)
ORCID iD: Min Liu https://orcid.org/0000-0003-1298-4441
References
- 1.Bandyopadhyay D, Panchabhai TS, Bajaj NS, et al. Primary pulmonary artery sarcoma: a close associate of pulmonary embolism-20-year observational analysis. J Thorac Dis 2016; 8: 2592–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gan HL, Zhang JQ, Huang XY, et al. The wall eclipsing sign on pulmonary artery computed tomography angiography is pathognomonic for pulmonary artery sarcoma. PLoS One 2013; 8: e83200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McConnell TH. Bony and cartilaginous tumors of the heart and great vessels. Report of an osteosarcoma of the pulmonary artery. Cancer 1970; 25: 611–617. [DOI] [PubMed] [Google Scholar]
- 4.Restrepo CS, Betancourt SL, Martinez-Jimenez S, et al. Tumors of the pulmonary artery and veins. Semin Ultrasound CT MR 2012; 33: 580–590. [DOI] [PubMed] [Google Scholar]
- 5.Srivali N, Yi ES, Ryu JH. Pulmonary artery sarcoma mimicking pulmonary embolism: a case series. QJM 2017; 110: 283–286. [DOI] [PubMed] [Google Scholar]
- 6.Kostic S, Guth S, Bachmann G, et al. Sarcoma of the pulmonary artery mimicking pulmonary artery embolism. Eur Heart J 2019; 40: 2824. [DOI] [PubMed] [Google Scholar]
- 7.Chen PW, Liu PY. Pulmonary artery sarcoma mimicking pulmonary embolism. BMJ Case Rep 2018. bcr2018226999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneeweis C, Magedanz A, Guth S, et al. Pulmonary artery sarcoma mimicking pulmonary embolism. Clin Res Cardiol 2016; 105: 962–963. [DOI] [PubMed] [Google Scholar]
- 9.Lu P, Yin BB. Misdiagnosis of primary intimal sarcoma of the pulmonary artery as chronic pulmonary embolism: a case report. World J Clin Cases 2020; 8: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Ballmoos MCW, Chan EY, Reardon MJ. Imaging and surgical treatment of primary pulmonary artery sarcoma. Int J Cardiovasc Imaging 2019; 35: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 11.Bhagwat K, Hallam J, Antippa P, et al. Diagnostic enigma: primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg 2012; 14: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendel EC, Maleszewski JJ, Araoz PA. Imaging sarcomas of the great vessels and heart. Semin Ultrasound CT MR 2011; 32: 377–404. [DOI] [PubMed] [Google Scholar]
- 13.Krüger I, Borowski A, Horst M, et al. Symptoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg 1990; 38: 91–95. [DOI] [PubMed] [Google Scholar]
- 14.Blackmon SH, Reardon MJ. Pulmonary artery sarcoma. Methodist DeBakey Cardiovasc J 2010; 6: 38–43. [DOI] [PubMed] [Google Scholar]
- 15.Grazioli V, Vistarini N, Morsolini M, et al. Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2014; 148: 113–118. [DOI] [PubMed] [Google Scholar]
- 16.Mussot S, Ghigna MR, Mercier O, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2013; 43: 787–793. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Hong T, Wang C. Clinical treatment for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2010; 38: 115–116. [DOI] [PubMed] [Google Scholar]
- 18.Tavora F, Miettinen M, Fanburg-Smith J, et al. Pulmonary artery sarcoma: a histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. Am J Surg Pathol 2008; 32: 1751–1761. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Kim HJ, Yoon H, et al. Clinical characteristics and treatment outcomes of primary pulmonary artery sarcoma in Korea. J Korean Med Sci 2016; 31: 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie WM, Zhai ZG, Wang LF, et al. Endovascular catheter-guided forceps biopsy for the diagnosis of suspected pulmonary artery sarcoma: a preliminary study of fight cases. Chin Med J (Engl) 2016; 129: 2246–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon BS, Park JH, Gwon DI, et al. Feasibility of endovascular catheter biopsy as a diagnostic modality for patients with pulmonary artery sarcoma: case series of a single-center experience. Vasc Endovascular Surg 2018; 52: 636–640. [DOI] [PubMed] [Google Scholar]
- 22.Huo L, Moran CA, Fuller GN, et al. Pulmonary artery sarcoma: a clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol 2006; 125: 419–424. [PubMed] [Google Scholar]
- 23.Pu X, Song M, Huang X, et al. Clinical and radiological features of pulmonary artery sarcoma: a report of nine cases. Clin Respir J 2018; 12: 1820–1829. [DOI] [PubMed] [Google Scholar]
- 24.Parish JM, Rosenow EC, 3rd, Swensen SJ, et al. Pulmonary artery sarcoma. Clinical features. Chest 1996; 110: 1480–1488. [DOI] [PubMed] [Google Scholar]
- 25.Evison M, Crosbie P, Chaturvedi A, et al. Pulmonary artery sarcoma: a rare thoracic tumor frequently misdiagnosed at presentation. Thorac Cancer 2015; 6: 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox JE, Chiles C, Aquino SL, et al. Pulmonary artery sarcomas: a review of clinical and radiologic features. J Comput Assist Tomogr 1997; 21: 750–755. [DOI] [PubMed] [Google Scholar]
- 27.Rafal RB, Nichols JN, Markisz JA. Pulmonary artery sarcoma: diagnosis and postoperative follow-up with gadolinium-diethylenetriamine pentaacetic acid – enhanced magnetic resonance imaging. Mayo Clin Proc 1995; 70: 173–176. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Luo C, Wang Y, et al. Multiparametric MRI in differentiating pulmonary artery sarcoma and pulmonary thromboembolism: a preliminary experience. Diagn Interv Radiol 2017; 23: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaumann A, Bode-Lesniewska B, Zimmermann DR, et al. Exploration of the APC/beta-catenin (WNT) pathway and a histologic classification system for pulmonary artery intimal sarcoma. A study of 18 cases. Virchows Arch 2008; 453: 473–484. [DOI] [PubMed] [Google Scholar]
- 30.Yamada N, Kamei S, Yasuda F, et al. Primary leiomyosarcoma of the pulmonary artery confirmed by catheter suction biopsy. Chest 1998; 113: 555–556. [DOI] [PubMed] [Google Scholar]
- 31.Hu W, Xie Y, Zhang DD. Pulmonary artery intimal sarcoma diagnosed by percutaneous transcatheter aspiration. Chin Med J 2013; 126: 1590–1591. [PubMed] [Google Scholar]
- 32.Chan JW, Chu SY, Lam CH, et al. Pulmonary artery sarcoma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Hong Kong Med J 2014; 20: 152–155. [DOI] [PubMed] [Google Scholar]
- 33.Dusemund F, Schneider T, Zeisel C, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of an intravascular sarcoma metastasis. Respiration 2013; 86: 430–432. [DOI] [PubMed] [Google Scholar]
- 34.Caraway NP, Salina D, Deavers MT, et al. Pulmonary artery intimal sarcoma diagnosed using endobronchial ultrasound-guided transbronchial needle aspiration. Cytojournal 2015; 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattoo A, Fedullo PF, Kapelanski D, et al. Pulmonary artery sarcoma: a case report of surgical cure and 5-year follow-up. Chest 2002; 122: 745–747. [DOI] [PubMed] [Google Scholar]
- 36.Ayaram D, Bellolio MF, Murad MH, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med 2013; 20: 861–871. [DOI] [PubMed] [Google Scholar]
- 37.Hirose T, Ishikawa N, Hamada K, et al. A case of intimal sarcoma of the pulmonary artery treated with chemoradiotherapy. Intern Med 2009; 48: 245–249. [DOI] [PubMed] [Google Scholar]
- 38.Funatsu Y, Hirayama M, Shiraishi J, et al. Intimal sarcoma of the pulmonary artery treated with pazopanib. Intern Med 2016; 55: 2197–2202. [DOI] [PubMed] [Google Scholar]