Abstract
Multigram drug depot systems for extended drug release could transform our capacity to effectively treat patients across a myriad of diseases. For example, tuberculosis (TB) requires multimonth courses of daily multigram doses for treatment. To address the challenge of prolonged dosing for regimens requiring multigram drug dosing, we developed a gastric resident system delivered through the nasogastric route that was capable of safely encapsulating and releasing grams of antibiotics over a period of weeks. Initial preclinical safety and drug release were demonstrated in a swine model with a panel of TB antibiotics. We anticipate multiple applications in the field of infectious diseases, as well as for other indications where multigram depots could impart meaningful benefits to patients, helping maximize adherence to their medication.
INTRODUCTION
Lack of medication adherence is a worldwide problem. As many as 50% of patients experience difficulty following treatment recommendations (1). Whereas adherence is driven by many factors including the socioeconomic status of a patient and the quality of the health care team, drug regimen complexity also affects treatment outcomes (1). For example, adherence decreases as the number of pills per dose and the number of doses per day increases (1). For diseases where potent medications are available, depot formulations provide sustained drug release to simplify dosing. For diseases lacking potent compounds for treatment, there remains an unmet need for depot systems that could transform medication adherence (2).
Tuberculosis (TB) is one such disease with a high pill burden, where poor patient adherence to the treatment regimen is a major cause of treatment failure and contributes to the emergence of drug-resistant TB strains (1). For example, an average 60-kg patient with TB needs to take 3.3 g of antibiotics per day, which is a dose that exceeds the largest swallowable capsule and current depot systems (3–6). According to the World Health Organization (WHO), 10 million people developed TB in 2017 with a global economic burden amounting to $12 billion annually (7, 8). Furthermore, TB is the most serious pathogen in the global antimicrobial resistance crisis (9). Unless radical action is taken, drug-resistant strains of TB will account for 25% of antimicrobial resistance–related deaths and will cost the global economy $16.7 trillion by the year 2050 (9, 10).
There are multiple factors that influence adherence among people living with TB (11). These include a provider-focused system of care delivery, high pill burden, transport difficulties, and other competing daily priorities. In 1994, the WHO endorsed the directly observed treatment short course (DOTS) strategy, which is now accepted worldwide (12). DOTS involves administration of oral fixed-dose combination formulations of TB drugs at a designated clinic in the presence of a health care provider either daily or three times per week (3, 13). Although some studies have shown DOTS to be effective, it requires substantial infrastructure with adequately staffed health care personnel to achieve desired results (11, 14–20). Furthermore, a recent study found that patients who fully adhered to a dosing regimen 7 of 7 days per week had more favorable outcomes compared to patients who were fully adherent to a dosing regimen 6 of 7 days per week (21). Full adherence may not be easily achieved in resource-constrained environments, where DOTS is costly to provide and time consuming for both patients and caregivers (22, 23).
The WHO End TB strategy has patient-centered care as a central pillar, and achieving such care requires innovative methods of treatment support and drug delivery (24–26). Shorter and simplified regimens, electronic reminder systems, and incentive programs are being implemented to improve adherence (27–29). Yet, additional interventions will be necessary to eliminate TB. Technologies that enable extended drug release of medication have the potential to help patients adhere to long and frequent dosing regimens. For example, ingestible gastric resident devices for controlled release drug delivery of antimalarials and antiretrovirals have been demonstrated in large animal models (30, 31). Although easy to administer and capable of tunable drug release profiles, these systems have capacities limited to approximately <500 mg of drug, which is a fraction of the daily dose of treatment for a patient with TB (3).
The challenge with designing drug depot systems for diseases such as TB is to balance the ease and safety of administration with the accommodation of multigram-level quantities of drugs. During the intensive phase of treatment, a 60-kg patient with TB swallows almost 100 g of antibiotics in 1 month (3). Inspired by the recognized capacity of the stomach to hold large objects including bariatric balloons and bezoars, we reasoned that a gastric resident system (GRS) capable of prolonged gram-level dosing could help patients adhere to TB treatment (32, 33). Drug delivery via the gastrointestinal (GI) tract offers multiple advantages, including ease of administration, immunotolerance to a broad range of materials, and the ability to accommodate gram-level dosing in line with current regimens for TB. Here, we describe a proof-of-concept study demonstrating the capacity of a device to be administered through the nasogastric (NG) route, to safely reside in the gastric cavity of a large mammal, to hold a multigram drug load, to provide controlled release of the drug over several weeks, and to be retrieved via an NG tube. We investigated the potential for patient and practitioner acceptability using a field questionnaire distributed in TB clinics in India and demonstrated the potential economic advantages associated with the implementation of a GRS intervention.
RESULTS
Design of a GRS for multigram dosing
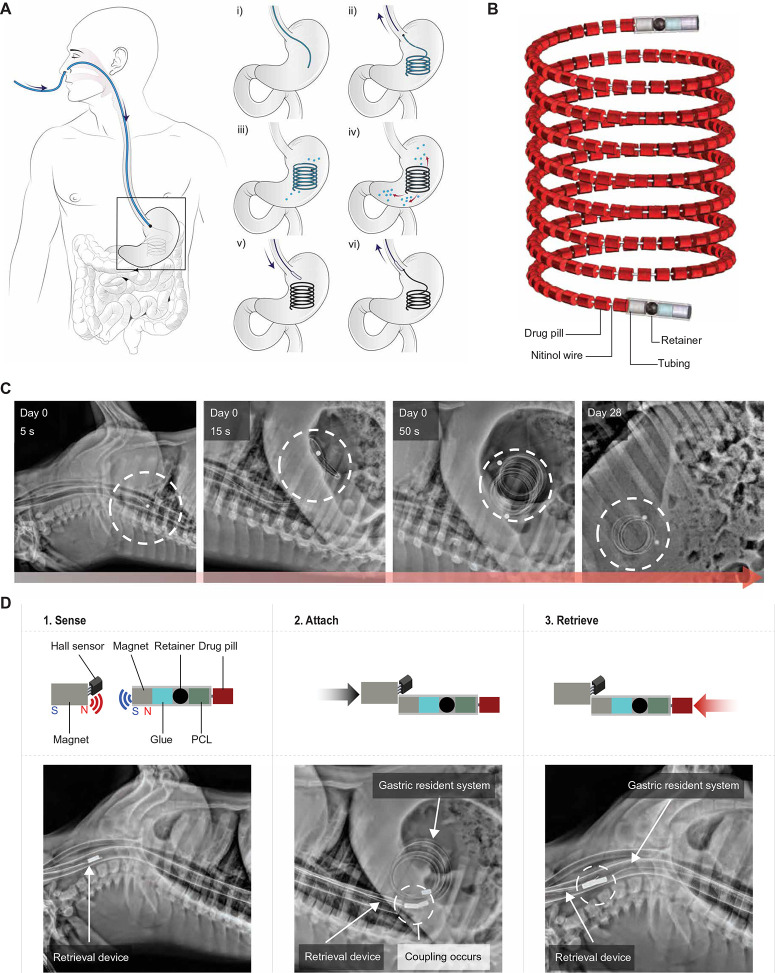
A large-dose GRS for long-term treatment should (i) have a size and shape that can fit through the esophagus of a patient to non-surgically access the stomach, (ii) have the ability to adopt an alternative conformation in the stomach that prevents passage through the pylorus, (iii) achieve high concentrations of drug loading, (iv) be composed of biocompatible materials that are stable for an extended duration in the acidic gastric environment, (v) have no potential for gastrointestinal obstruction or perforation, and (vi) either be able to degrade into forms that can safely pass or be retrieved after the drug has been released from the device (30). Inspired by the rapid deployment of a gastric balloon through similar means, we set out to design a GRS that could be administered through an NG tube, which is inserted via the nose to access the stomach (34). After reaching the stomach, the GRS forms a cylindrical coil and continually releases grams of drug over the course of weeks, whereupon the device is retrieved back through an NG tube (Fig. 1A). The assembled GRS consists of a superelastic nitinol wire as the retention frame upon which drug pills are strung with a retainer and tubing at the ends of the device (Fig. 1B) (35). To tailor the drug loading and duration of therapy, the length of the GRS and formulation of drug pills can be modified (fig. S1).
Fig. 1.
Design and in vivo evaluation of a large-dose GRS for drug delivery. (A) (i-ii) An NG tube is first placed as a conduit for the large-dose GRS to be non-surgically administered, and then the NG tube is removed from the patient. (iii-iv) The GRS resides in the gastric cavity while releasing drugs. (v-vi) An NG tube is again placed in the patient for deployment of a retrieval device to attach and remove the GRS from the gastric cavity. Black arrows indicate direction of movement of the NG tube and retrieval device, and red arrows indicate drug release. (B) The GRS consists of a series of drug pills on a coiled superelastic nitinol wire; the ends are protected with a retainer and tubing. (C) Representative radiographs of the GRS immediately after deployment and on day 28 in a swine model. Dashed circles indicate GRS location. (D) The retrieval device consists of a Hall effect sensor and a magnet that can detect and attach to the magnets on either end of the GRS. Representative stepwise radiographs of the retrieval process executed in a swine model are shown below. Dashed circles indicate coupling of retrieval device with GRS. The components of both ends of the GRS [glue, a retainer, and a poly(ε-caprolactone) (PCL) plug] are also shown.
We deployed a coiled nitinol wire inside tubing to the gastric cavity of 30-to 75-kg Yorkshire pigs to demonstrate transesophageal administration and safe gastric retention in vivo. Yorkshire pigs have similar gastric anatomy to humans and have been previously used to evaluate long-acting drug delivery platforms (30, 36). Representative serial abdominal radiographs during device deployment and month-long residence revealed the feasibility of the GRS to pass through the esophagus and form a coil in the stomach within 50 s (Fig. 1C). The GRS was able to curl back into its original coil shape in the gastric cavity after passing through the esophagus because of the superelasticity of nitinol (37). Safe long-term gastric residence was evaluated by serial radiographs obtained over the course of 1 month and through endoscopic evaluation (Fig. 1C and fig. S2). Even after prolonged gastric residence of these large devices, mucosal surfaces of the animals’ stomachs did not show injury, erosions, or ulcerations; in addition, the animals did not show any weight loss, evidence of GI obstruction, or limitation in the passage of food or liquid (fig. S3).
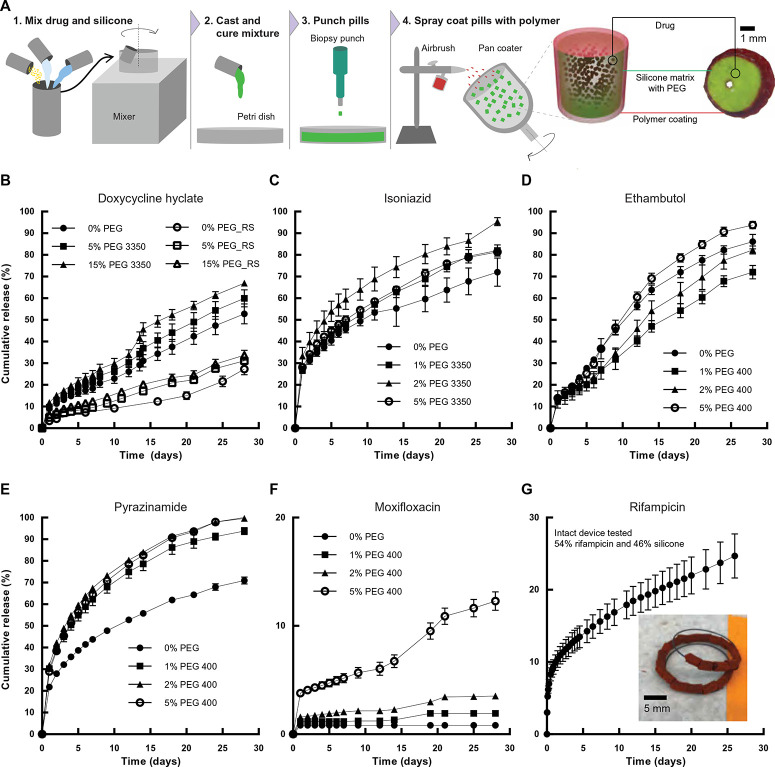
Fig. 2.
Fabrication and in vitro release of TB antibiotics from individual drug pills. (A) Coated drug pills are made by mixing drug with silicones and extracting individual pills from the homogeneous matrix using a biopsy punch before spray-coating pills in a pan coater. A schematic visualization and a cross-sectional image of the Eudragit RS 100–coated doxycycline hyclate pill are shown. (B) In vitro release of doxycycline hyclate from a drug pill in SGF with formulations including different concentrations of PEG and Eudragit RS 100 coatings. (C) In vitro release of isoniazid from a drug pill in water. (D) In vitro release of ethambutol from a drug pill in SGF. (E) In vitro release of pyrazinamide from a drug pill in SGF. (F) In vitro release of moxifloxacin from a drug pill in SGF. (G) In vitro release of rifampicin in water from devices with 2 g of drug and 0% PEG. Inset: Image of the rifampicin-loaded device. Error bars represent SD for n = 3 samples in each group.
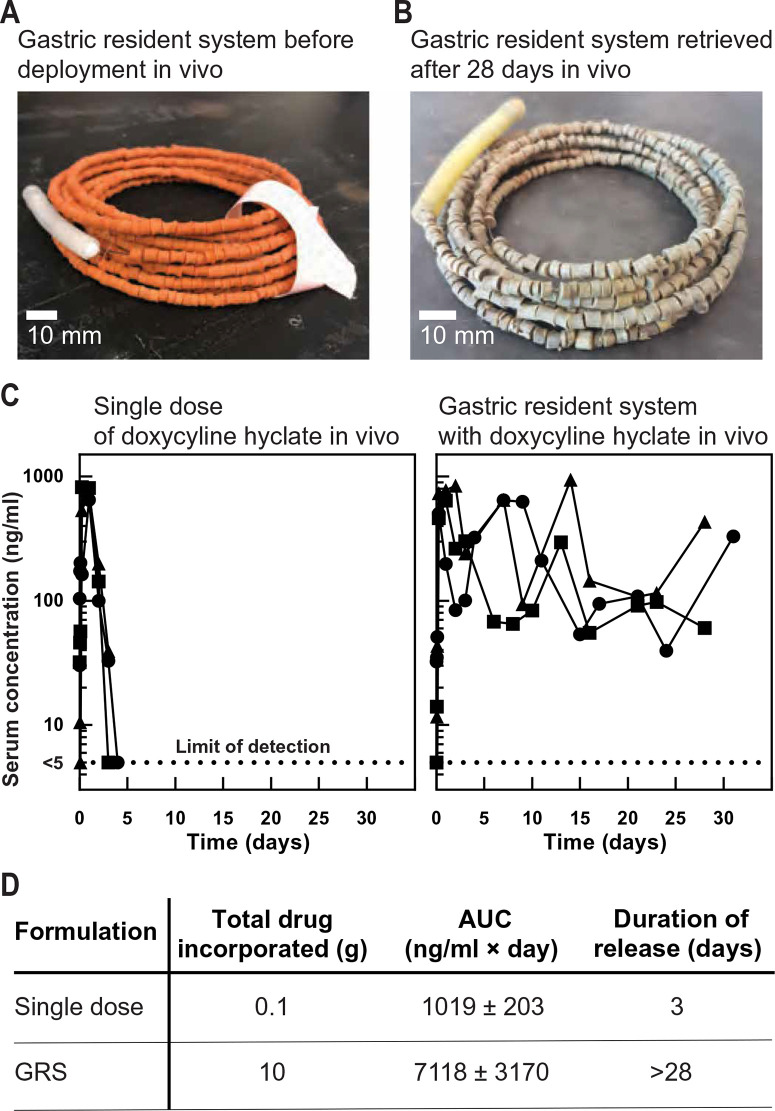
Fig. 3.
In vivo release of doxycycline hyclate from the GRS in a swine model. (A) Representative photograph of a GRS after assembly of drug pills along a nitinol wire before deployment in vivo. (B) Representative photo of a retrieved GRS after 28 days in vivo in a swine model. (C) Left: Concentration-time profiles of doxycycline hyclate in serum after administering a single dose of 100 mg (n = 3). Right: Concentration-time profiles of doxycycline hyclate in serum after administering the GRS, which had 10 g of drug across four formulations (n = 3; fig. S5). (D) Area under the curve (AUC) and the duration of drug release for a single dose compared to the formulations of the GRS administered in vivo, with the mean value and SD reported for n = 3 samples in each group.
We designed the GRS to be retrieved through an NG tube after the release of the drug payload in the gastric cavity. The retrieval device consists of a Hall effect sensor to determine the distance between a magnet on the end of the GRS and a magnet at the end of retrieval device (Fig. 1D) (38). To ensure the stability of the Hall effect sensor in a low pH environment, we placed it in simulated gastric fluid (SGF) for 90 min; the measured voltage was comparable to the voltage measured in air before immersion in SGF (fig. S4A). A three-dimensional (3D) printed in vitro human stomach model was constructed to test the feasibility of the retrieval procedure (fig. S4B and data file S1). A magnet was placed on each end of the GRS to maximize likelihood of retrieval. In vivo demonstration of GRS retrieval was successful, as demonstrated by representative serial radiographs (Fig. 1D). Thus, we demonstrated the potential of the GRS to be safely administered, to reside safely in the gastric cavity for 1 month, and to be retrieved through the esophagus.
Controlled drug release with coated drug-matrix pills
We fabricated pills of a single drug mixed inside a silicone matrix and encapsulated each pill in a polymer coating to enable tailored dosing of each drug (Fig. 2A). Vinylpolysiloxane (VPS) was selected as a drug release matrix because of its flexibility, rapid curing time, and low-temperature mixing process with drug. Because of their mechanical and chemical properties, polysiloxanes have been extensively used for controlled drug delivery applications (39–42). We spray-coated a 300-µm-thick Eudragit RS 100 polymer coating to prevent the burst release of drug from the surface of the matrix (43–46). Each pill had a height and diameter of 4 mm with a 0.5-mm hole in the center through which to pass the nitinol wire and contribute to the assembled GRS (Fig. 2A).
We assembled drug-VPS pills for multiple antibiotics used for TB treatment including doxycycline hyclate, isoniazid, ethambutol, pyrazinamide, moxifloxacin, and rifampicin (3, 47, 48). As demonstrated with doxycycline hyclate, the drug release rate from the VPS matrix in SGF can be tuned by varying the amount of a hydrophilic polymer, poly(ethylene glycol) (PEG), mixed within the VPS (Fig. 2B). The PEG domains act as channels inside the hydrophobic VPS matrix that can dissolve and form pores for the doxycycline hyclate to release. Furthermore, formulations that were coated with Eudragit RS 100 showed a linear kinetic profile with limited burst release of doxycycline hyclate (Fig. 2B). The drug-VPS pills were also able to release isoniazid, ethambutol, pyrazinamide, moxifloxacin, and rifampicin in vitro, indicating that the VPS matrix is compatible with a wide variety of TB drugs (Fig. 2, C to G).
In vivo sustained delivery of antibiotic for 4 weeks
Having demonstrated controlled release with coated drug-matrix pills in vitro for 1 month, we prepared the GRSs loaded with 10 g of doxycycline hyclate as a model drug (Fig. 3A) and administered them in swine. The GRS was assembled to contain 600 pills using four different formulations—two each with Eudragit RS 100 or PCL coatings—which released drug simultaneously (fig. S5) (49–51). After 28 days of gastric residence in vivo, the GRS was safely retrieved (Fig. 3B). The serum concentration profile of a 100-mg single dose is shown in Fig. 3C. The drug was absorbed rapidly, and detectable concentrations were observed within 15 min. No drug was detectable after 3 days with the single-dose formulation. In contrast, drug was detectable for at least 28 days when doxycycline hyclate was dosed in the GRS. We also incorporated rifampicin into the GRS and achieved detectable serum concentrations for a week in vivo (fig. S6).
Preliminary end-user assessment and economic impact of the GRS
We surveyed 111 TB health care providers and 300 patients with TB at DOTS clinics in India and learned that a long-term drug delivery device administered through an NG tube was acceptable and feasible in the field (figs. S7 and S8, tables S1 and S2, and data file S2). An established model was used to evaluate the potential impact of a GRS on patients with TB, with savings estimated at more than $8000 per patient in New Delhi, India (table S3 and data file S3) (52, 53).
Discussion
Here, we report the development of a GRS capable of multigram-level dosing of a TB antibiotic over the course of 4 weeks. The GRS drug pills are compatible with all first-line TB antibiotics, and we anticipate that further formulation development and large-scale manufacturing with an array of polymer matrices and coatings will optimize a linear drug release profile in the gastric cavity to reduce variability in serum concentrations and match drug release kinetics across drugs. These macrodevices showed no evidence of GI obstruction or injury during gastric residence and retrieval, as supported by radiographic, endoscopic, and histopathologic evaluation in a swine model.
Adherence to TB treatment is challenging because of the long and frequent dosing regimen, and additional patient-centered interventions are necessary to supplement DOTS in resource-constrained environments (1, 20, 22). Technologies such as the GRS described here can improve the effectiveness of DOTS by ensuring that patients receive their medication over the course of extended periods of time, thereby reducing the frequency of clinic visits. Less frequent dosing visits would reduce the potential impact on daily life, specifically on productivity of individuals receiving treatment for TB (11). The ability of the GRS to contain and serve as a multigram drug depot in the gastric cavity supports further development of prolonged drug depots on the order of weeks and even months, which could mitigate the effects of poor adherence (54).
To establish a route for translation, we anticipate that the full development of these devices will include preclinical evaluation in an additional animal model such as the dog, which has gastric compressive forces and transit times similar to humans (55). Optimizing drug release kinetics is a critical next step, such that serum concentrations of the drug remain within the therapeutic window and do not increase the likelihood of drug resistance. Different diet conditions will also need to be tested to understand the effect on pharmacokinetic parameters across a broad spectrum of drugs. Ultimately, safety and efficacy of the GRS will need to be confirmed in humans.
In addition, we recognize the importance of amplifying training of health care workers to deploy NG tubes safely, so that the GRS can be implemented alongside DOTS interventions in the field where trained personnel are generally present (56, 57). Because patients will be conscious during the NG tube procedure, they will be able to speak to a health care worker to ensure correct placement of the tube (58). The cost of this additional intervention as part of DOTS will need to be assessed in further fieldwork.
To begin addressing the acceptability and feasibility of the NG tube approach, we conducted a preliminary field questionnaire of 300 patients with TB and 111 TB health care workers in TB clinics in India. Our survey results indicated that more than 90% of health care personnel have experience deploying NG tubes, and patients prefer the use of an NG tube for deployment of a month-long TB treatment as opposed to swallowing many capsules or drinking liters of water-drug mixture as potential alternative modes of generating large drug depots. We further demonstrated the potential impact of the implementation of our GRS to improve adherence in terms of lives saved and economic savings for patients suffering from TB.
One limitation of the field study is the incorporation of an NG tube description versus physical NG tube insertion into the questionnaire subjects. Although this questionnaire was administered to patients and health care providers with a comprehensive understanding of TB, ultimately, the physical discomfort of NG tube placement along with GRS retrieval requires further evaluation.
We believe that macrodevices consisting of multigram drug depots could have an impact across a range of diseases in addition to TB and could be coupled to other procedures such as endoscopy. For broad implementation, a range of chemical therapeutics will need to be studied and incorporated into the modular pill design of the GRS. Formulations will need to be optimized to ensure high drug loading efficiencies and controlled release profiles for efficacious treatment and controlled drug release. The GRS has potential as a platform technology for improving medication adherence and thereby also improve outcomes for patients suffering from a myriad of diseases.
Materials And Methods
Study design
We designed, fabricated, and tested devices for month-long drug delivery in the gastric cavity. This GRS contains a series of drug pills loaded onto a nitinol shape memory alloy wire. The device forms a coil shape after reaching the stomach. A retrieval device compatible with nasogastric administration uses a sensor and magnet to attach to a magnet on the GRS. Approvals were obtained from the Committee on Animal Care at the Massachusetts Institute of Technology to assess the safety and long-term drug release of the GRS, as well as the feasibility of the retrieval device in a swine model. Radiographic, endoscopic, and histopathologic evaluation were conducted.
We assessed the end-user acceptability and feasibility of NG tube placement through a questionnaire of 111 TB health care providers and 300 patients with TB at DOTS clinics in New Delhi, India. The field questionnaire study was approved by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects and all required ethical committees of the hospitals in India. Sample sizes were determined on the basis of a conservative method with a 90% confidence interval and 8% margin of error for the health care providers and 90% confidence interval and 5% margin of error for the patients (59). All health care providers who filled out more than 90% of the questionnaire were included in the analysis. All 300 patients who provided consent for the study were included in the analysis. We also applied an economic model to quantify the impact of the GRS on the Indian government and patients with TB. The data, assumptions, and economic calculations were derived from a previous model, and we conducted sensitivity analysis on several of our assumptions.
Statistical analysis
For all experimentation shown, the mean is plotted with error bars representing the SD of n = 3. Individual subject-level data for Figs. 2 (B to G) and 3 (C and D) and figs. S3A, S4A, S5B, S6, S7 (B, C, E, and F), and S8 are shown in table S4.
Supplementary Material
Acknowledgments
We thank the leadership and mentors from the MIT Tata Center, especially A. D. Rigos, C. Vaishnav, J. Prapas, and N. C. Hanumara, for helpful discussions with planning the field questionnaire study and conducting the economic analysis. We thank health care professionals at Operation ASHA, especially I. Gurha, A. Agarwal, and A. Negi, for conducting the patient questionnaire. We are grateful to the health team at the Tata Trusts for feedback on the field questionnaire results and facilitating interactions with collaborators in India. We thank V. P. Myneedu, A. R. Kansal, S. K. Munjal, and T. Ahluwahlia for support with the field questionnaire. We thank J. Haupt and M. Jamiel for help with the in vivo porcine work. We are grateful to all members of the Langer and Traverso Laboratories, especially S. McDonnell, L. Booth, M. Jimenez, and A. R. Kirtane for help with in vivo porcine work and discussion of pharmacokinetic analysis. We thank B. Herrmann from Flacktek Inc. for his help with the SpeedMixer used for drug-pill formulations. We thank A. Hupalowksa for help with illustrations.
Funding
This work in part was funded by the Bill and Melinda Gates Foundation Grants OPP1096734 and OPP1139927, the NIH Grant EB000244, and the MIT Tata Center Grant to R.L. and G.T. M.V. was supported in part by the MIT Tata Center Grant and the National Science Foundation Graduate Research Fellowship. C.S. was supported in part by the Alexander von Humboldt Foundation. G.T. was supported in part by the Division of Gastroenterology, Hepatology, and Endoscopy at Brigham and Women’s Hospital.
Author contributions
M.V., T.G., R.L., and G.T. conceived and designed the research. M.V., K.V., T.G., A.M.B., T.B., D.M., V.S., J.A.F.S., T.H., C.I., G.Z., E.M., S. Boominathan, and E.P. made prototypes of the GRS. T.B., D.M., and T.H. designed and fabricated the in vitro stomach model. M.V., D.J.F., D.L., Shelly Batra, S.A., M.B., U.A., J. Chowdhury, R. Stoner, A.H.S., J.F., and G.T. wrote and conducted the field questionnaire. Sonali Batra developed the software for the survey. R. Sarin, S.D.K., N.K.G., D.G., A.K.B., K.K.C., N.S., and A.K. were the principal investigators for the field questionnaire in India. M.V., K.V., N.R., C.S., J.A.F.S., M.J.C., R.L., and G.T. designed and performed the in vitro release experiments. M.V., K.V., C.C., A.H., J. Collins, and S.M.T. performed the in vivo pig experiments. H.M., A.L., and K.H. developed the methods and analyzed the data from the high-performance liquid chromatography and ultra-performance liquid chromatography–tandem mass spectrometry for all the drugs used. M.V., J.B.M., and D.C. performed the economic analysis. M.V., F.E., N.R., and M.C. designed and tested the retrieval device in vitro and in vivo. M.V., K.V., R.L., and G.T. analyzed the data. M.V., R.L., and G.T. wrote the manuscript with contributions from all authors.
Competing interests
A.M.B. and T.G. are employees of Lyndra Inc., a biotechnology company focused on the development of oral drug delivery systems for ultralong drug release. R.L., G.T., A.M.B., and T.G. have financial interest in Lyndra Inc. M.V., K.V., F.E., N.R., T.G., M.C., C.S., T.B., D.M., A.M.B., M.J.C., R.L., and G.T. are co-inventors on multiple patent applications describing large-dose gastric drug delivery and retrieval systems. Complete details of all relationships for profit and not for profit for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5ioq9AfT1IqIJAE-T5a?dl=0. Complete details for R.L. can be found at the following link: https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0. The rest of the authors declare that they have no competing financial interests.
Data and materials availability
All data associated with this study are present in the paper and/or Supplementary Materials.
References And Notes
- 1.Sabaté E., Adherence to Long-Term Therapy: Evidence for Action (World Health Organization, 2003); http://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf. [Google Scholar]
- 2.Kirtane A. R., Langer R., Traverso G., Past, present, and future drug delivery systems for antiretrovirals. J. Pharm. Sci. 105, 3471–3482 (2016). [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization , Treatment of Tuberculosis: Guidelines—4th Edition (2010); http://apps.who.int/iris/bitstream/handle/10665/44165/9789241547833_eng.pdf. [PubMed] [Google Scholar]
- 4.Tibbitt M. W., Dahlman J. E., Langer R., Emerging frontiers in drug delivery. J. Am. Chem. Soc. 138, 704–717 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Shaik M. R., Korsapati M., Panati D., Polymers in controlled drug delivery systems. Int. J. Pharma Sci. 2, 112–116 (2012). [Google Scholar]
- 6.Mc Caffrey C., Chevalerias O., O’Mathuna C., Twomey K., Swallowable-capsule technology. IEEE Pervasive Comput. 7, 23–29 (2008). [Google Scholar]
- 7.World Health Organization, Global Tuberculosis Report 2018 (2018); http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf.
- 8.Kim J. Y., Shakow A., Castro A., Vande C., Farmer P., Tuberculosis Control: The burden of tuberculosis: Economic burden (2) (World Health Organization, 2003); www.who.int/trade/distance_learning/gpgh/gpgh3/en/index7.html.
- 9.O’Neill J., Tackling Drug-Resistant Infections Globally: Final Report and Recommendations (Review on Antimicrobial Resistance, 2016); https://amr-review.org/sites/default/files/160518_Finalpaper_with cover.pdf. [Google Scholar]
- 10.The Price of a Pandemic: Counting the cost of MDR-TB (2015); https://docs.wixstatic.com/ugd/309c93_f0731d24f4754cd4a0ac0d6f6e67a526.pdf.
- 11.Kulkarni P. Y., Akarte S. V., Mankeshwar R. M., Bhawalkar J. S., Banerjee A., Kulkarni A. D., Non-adherence of new pulmonary tuberculosis patients to anti-tuberculosis treatment. Ann. Med. Health Sci. Res. 3, 67–74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Tuberculosis Programme: Framework for Effective Tuberculosis Control (World Health Organization, 1994); http://apps.who.int/iris/bitstream/handle/10665/58717/WHO_TB_94.179.pdf.
- 13.Karumbi J., Garner P., Directly observed therapy for treating tuberculosis. Cochrane Database Syst. Rev. CD003343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gninafon M., Tawo L., Kassa F., Monteiro G. P., Zellweger J.-P., Shang H., Lambregts K., Trébucq A., Outcome of tuberculosis retreatment in routine conditions in Cotonou, Benin. Int. J. Tuberc. Lung Dis. 8, 1242–1247 (2004). [PubMed] [Google Scholar]
- 15.Erhabor G. E., Adewole O., Adisa A. O., Olajolo O. A., Directly observed short course therapy for tuberculosis—A preliminary report of a three-year experience in a teaching hospital. J. Natl. Med. Assoc. 95, 1082–1088 (2003). [PMC free article] [PubMed] [Google Scholar]
- 16.Rutta E., Kipingili R., Lukonge H., Assefa S., Mitsilale E., Rwechungura S., Treatment outcome among Rwandan and Burundian refugees with sputum smear-positive tuberculosis in Ngara, Tanzania. Int. J. Tuberc. Lung Dis. 5, 628–632 (2001). [PubMed] [Google Scholar]
- 17.Lewis D. K., Peters R. P. H., Schijffelen M. J., Joaki G. R. F., Walsh A. L., Kublin J. G., Kumwenda J., Kampondeni S., Molyneux M. E., Zijlstra E. E., Clinical indicators of mycobacteraemia in adults admitted to hospital in Blantyre, Malawi. Int. J. Tuberc. Lung Dis. 6, 1067–1074 (2002). [PubMed] [Google Scholar]
- 18.Joseph M. R., Thomas R. A., Nair S., Balakrishnan S., Jayasankar S., Directly observed treatment short course for tuberculosis. What happens to them in the long term? Indian J. Tuberc. 62, 29–35 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Benbaba S., Isaakidis P., Das M., Jadhav S., Reid T., Furin J., Direct observation (DO) for drug-resistant tuberculosis: Do we really DO? PLOS ONE 10, e0144936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asuquo Otu A., Is the directly observed therapy short course (DOTS) an effective strategy for tuberculosis control in a developing country? Asian Pac. J. Trop. Dis. 3, 227–231 (2013). [Google Scholar]
- 21.Imperial M. Z., Nahid P., Phillips P. P. J., Davies G. R., Fielding K., Hanna D., Hermann D., Wallis R. S., Johnson J. L., Lienhardt C., Savic R. M., A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat. Med. 24, 1708–1715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fochsen G., Deshpande K., Ringsberg K. C., Thorson A., Conflicting accountabilities: Doctor’s dilemma in TB control in rural India. Health Policy 89, 160–167 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Steffen R., Menzies D., Oxlade O., Pinto M., de Castro A. Z., Monteiro P., Trajman A., Patients’ costs and cost-effectiveness of tuberculosis treatment in DOTS and non-DOTS facilities in Rio de Janeiro, Brazil. PLOS ONE 5, e14014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright C. M., Westerkamp L., Korver S., Dobler C. C., Community-based directly observed therapy (DOT) versus clinic DOT for tuberculosis: A systematic review and meta-analysis of comparative effectiveness. BMC Infect. Dis. 15, 210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toczek A., Cox H., du Cros P., Cooke G., Ford N., Strategies for reducing treatment default in drug-resistant tuberculosis: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 17, 299–307 (2012). [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization, Implementing the End TB Strategy: The Essentials (2015); www.who.int/tb/publications/2015/end_tb_essential.pdf.
- 27.Prasad R., Gupta N., Banka A., Rapid diagnosis and shorter regimen for multidrug-resistant tuberculosis: A priority to improve treatment outcome. Lung India 34, 1–2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q., Abba K., Alejandria M. M., Sinclair D., Balanag V. M., Lansang M. A. D., Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment. Cochrane Database Syst. Rev. CD006594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutge E. E., Wiysonge C. S., Knight S. E., Sinclair D., Volmink J., Incentives and enablers to improve adherence in tuberculosis. Cochrane Database Syst. Rev., CD007952 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellinger A. M., Jafari M., Grant T. M., Zhang S., Slater H. C., Wenger E. A., Mo S., Lee Y.-A. L., Mazdiyasni H., Kogan L., Barman R., Cleveland C., Booth L., Bensel T., Minahan D., Hurowitz H. M., Tai T., Daily J., Nikolic B., Wood L., Eckhoff P. A., Langer R., Traverso G., Oral, ultra–long-lasting drug delivery: Application toward malaria elimination goals. Sci. Transl. Med. 8, 365ra157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirtane A. R., Abouzid O., Minahan D., Bensel T., Hill A. L., Selinger C., Bershteyn A., Craig M., Mo S. S., Mazdiyasni H., Cleveland C., Rogner J., Lee Y.-A. L., Booth L., Javid F., Wu S. J., Grant T., Bellinger A. M., Nikolic B., Hayward A., Wood L., Eckhoff P. A., Nowak M. A., Langer R., Traverso G., Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 9, 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips M. R., Zaheer S., Drugas G. T., Gastric trichobezoar: Case report and literature review. Mayo Clin. Proc. 73, 653–656 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Palmisano S., Silvestri M., Melchioretto B., Giuricin M., Giudici F., Lucchetta A., Barbieri V. P, Osenda E., Urban F., Simeth C., Monica F., de Manzini N., Intragastric balloon device: Weight loss and satisfaction degree. Obes. Surg. 26, 2131–2137 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Machytka E., Chuttani R., Bojkova M., Kupka T., Buzga M., Stecco K., Levy S., Gaur S., Elipse, a procedureless gastric balloon for weight loss: A proof-of-concept pilot study. Obes. Surg. 26, 512–516 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Lee H., Cima M. J., An intravesical device for the sustained delivery of lidocaine to the bladder. J. Control. Release 149, 133–139 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Tarnoff M., Shikora S., Lembo A., Acute technical feasibility of an endoscopic duodenal-jejunal bypass sleeve in a porcine model: A potentially novel treatment for obesity and type 2 diabetes. Surg. Endosc. Other Interv. Tech. 22, 772–776 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Duerig T., Pelton A., Stöckel D., An overview of nitinol medical applications. Mater. Sci. Eng. A 273–275, 149–160 (1999). [Google Scholar]
- 38.Hall E. H., On a new action of the magnet on electric currents. Am. J. Math. 2, 287–292 (1879). [Google Scholar]
- 39.Golomb G., Fisher P., Rahamim E., The relationship between drug release, particle size, and swelling of silicone matrices. Scan. Electron Microsc. 12, 121–132 (1990). [Google Scholar]
- 40.Gao Z., Nahrup J. S., Mark J. E., Sakr A., Poly(dimethylsiloxane) coatings for controlled drug release. III. Drug release profiles and swelling properties of the free-standing films. J. Appl. Polym. Sci. 96, 494–501 (2005). [Google Scholar]
- 41.Mashak A., Rahimi A., Silicone polymers in controlled drug delivery systems: A review. Iran. Polym. J. 18, 279–295 (2009). [Google Scholar]
- 42.Brannon-Peppas L., Novel vaginal drug release applications. Adv. Drug Deliv. Rev. 11, 169–177 (1993). [Google Scholar]
- 43.Thakral S., Thakral N. K., Majumdar D. K., Eudragit: A technology evaluation. Expert Opin. Drug Deliv. 10, 131–149 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Singh, Neelam S., Arora S., Singla Y. P., An overview of multifaceted significance of eudragit polymers in drug delivery systems. Asian J. Pharm. Clin. Res. 8, 1–6 (2015). [Google Scholar]
- 45.Huang X., Brazel C. S., On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 73, 121–136 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Akhgari A., Tavakol A., Prediction of optimum combination of Eudragit RS/Eudragit RL/ ethyl cellulose polymeric free films based on experimental design for using as a coating system for sustained release theophylline pellets. Adv. Pharm. Bull. 6, 219–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalo X., Combination of amikacin and doxycycline against multidrug-resistant and extensively drug-resistant tuberculosis. Int. J. Antimicrob. Agents 45, 406–412 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Fox G. J., Dobler C. C., Marais B. J., Denholm J. T., Preventive therapy for latent tuberculosis infection—The promise and the challenges. Int. J. Infect. Dis. 56, 68–76 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Hanas T., Sampath Kumar T. S., Perumal G., Doble M., Tailoring degradation of AZ31 alloy by surface pre-treatment and electrospun PCL fibrous coating. Mater. Sci. Eng. C Mater. Biol. Appl. 65, 43–50 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Suharti N., Sulaiman, Febriyenti S., Zaini E., Suardi M., Ben E. S., Djamaan A., Effect of bioblend polystyrene/polycaprolactone and polystyrene/starch utilization toward coating thickness and release of active substance from urea granule. Der Pharma Chem. 8, 83–87 (2016). [Google Scholar]
- 51.Kim Y.-K., Lee K.-B., Kim S.-Y., Jang Y.-S., Kim J. H., Lee M.-H., Improvement of osteogenesis by a uniform PCL coating on a magnesium screw for biodegradable applications. Sci. Rep. 8, 13264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins D., Lam H., Hafidz F., Antipolo J., Mangao P., Economic Cost of Non-Adherence to TB Medicines Resulting from Stock-Outs and Loss to Follow-Up in the Philippines (Management Sciences for Health, 2016); http://apps.who.int/medicinedocs/documents/s23230en/s23230en.pdf. [Google Scholar]
- 53.Collins D., Njuguna C., The Economic Cost of Non-adherence to TB Medicines Resulting from Stock-outs and Loss to Follow-up in Kenya (Management Sciences for Health, 2016); http://siapsprogram.org/publication/altview/the-economic-cost-of-non-adherence-to-tb-medicines-resulting-from-stock-outs-and-loss-to-follow-up-in-kenya/english/. [Google Scholar]
- 54.Kishimoto H., Maehara M., Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: Analysis of data from the CISA. Arch. Osteoporos. 10, 231 ( 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laulicht B., Tripathi A., Schlageter V., Kucera P., Mathiowitz E., Understanding gastric forces calculated from high-resolution pill tracking. Proc. Natl. Acad. Sci. U.S.A. 107, 8201–8206 ( 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gai R., Xu L., Wang X., Liu Z., Cheng J., Zhou C., Liu J., Zhang H., Li H., Kuroiwa C., The role of village doctors on tuberculosis control and the DOTS strategy in Shandong Province, China. Biosci. Trends 2, 181–186 (2008). [PubMed] [Google Scholar]
- 57.Awofeso N., Schelokova I., Dalhatu A., Training of front-line health workers for tuberculosis control: Lessons from Nigeria and Kyrgyzstan. Hum. Resour. Health 6, 1–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomsen T. W., Shaffer R. W., Setnik G. S., Nasogastric intubation. N. Engl. J. Med. 354, e16 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Bartlett J. E., Kotrlik J. W., Higgins C. C., Organizational research: Determining appropriate sample size in survey research. Inf. Technol. Learn. Perform. J. 19, 43–50 (2001). [Google Scholar]
- 60.Evonik Nutrition & Care GmbH, EUDRAGIT RL 100 and/or RS 100: Organic Sustained Release Coating (2016); https://studylib.net/doc/18093309/eudragit-rl-100-and-or-rs-100-organic-sustained-release. [Google Scholar]
- 61.Zhang S., Bellinger A. M., Glettig D. L., Barman R., Lee Y.-A. L., Zhu J., Cleveland C., Montgomery V. A., Gu L., Nash L. D., Maitland D. J., Langer R., Traverso G., A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 14, 1065–1071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J., Pang Y., Zhang S., Cleveland C., Yin X., Booth L., Lin J., Lucy Lee Y.-A., Mazdiyasni H., Saxton S., Kirtane A. R., von Erlach T., Rogner J., Langer R., Traverso G., Triggerable tough hydrogels for gastric resident dosage forms. Nat. Commun. 8, 124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mintz Y., Horgan S., Savu M. K., Cullen J., Chock A., Ramamoorthy S., Easter D. W., Talamini M. A., Hybrid natural orifice translumenal surgery (NOTES) sleeve gastrectomy: A feasibility study using an animal model. Surg. Endosc. 22, 1798–1802 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Singh S., Mariappan T. T., Sharda N., Singh B., Degradation of rifampicin, isoniazid and pyrazinamide from prepared mixtures and marketed single and combination products under acid conditions. Pharm. Pharmacol. Commun. 6, 491–494 (2000). [Google Scholar]
- 65.Benetton S. A., Kedor-Hackmann E. R. M., Santoro M. I. R. M., Borges V. M., Visible spectrophotometric and first-derivative UV spectrophotometric determination of rifampicin and isoniazid in pharmaceutical preparations. Talanta 47, 639–643 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Jiang Z., Wang H., Locke D. C., Determination of ethambutol by ion-pair reversed phase liquid chromatography with UV detection. Anal. Chim. Acta 456, 189–192 (2002). [Google Scholar]
- 67.Osterberg L., Blaschke T., Adherence to medication. N. Engl. J. Med. 353, 487–497 (2005). [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization , Global Tuberculosis Report 2017 (2017); http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.