Abstract
Morbidity and mortality from opioid use disorders (OUD) and other substance use disorders (SUD) is a major public health crisis, yet there are few medications to treat them. There is an urgency to accelerate SUD medication development. We present an integrated drug repurposing strategy that combines computational prediction, clinical corroboration using electronic health records (EHRs) of over 72.9 million patients and mechanisms of action analysis. Among top-ranked repurposed candidate drugs, tramadol, olanzapine, mirtazapine, bupropion, and atomoxetine were associated with increased odds of OUD remission (adjusted odds ratio: 1.51 [1.38–1.66], 1.90 [1.66–2.18], 1.38 [1.31–1.46], 1.37 [1.29–1.46], 1.48 [1.25–1.76], p value < 0.001, respectively). Genetic and functional analyses showed these five candidate drugs directly target multiple OUD-associated genes including BDNF, CYP2D6, OPRD1, OPRK1, OPRM1, HTR1B, POMC, SLC6A4 and OUD-associated pathways, including opioid signaling, G-protein activation, serotonin receptors, and GPCR signaling. In summary, we developed an integrated drug repurposing approach and identified five repurposed candidate drugs that might be of value for treating OUD patients, including those suffering from comorbid conditions.
Subject terms: Addiction, Drug discovery
Introduction
Substance use disorders (SUD) when manifested in their moderate or severe presentation are considered chronic diseases of the brain that in its most severe manifestation result in an escalating and an uncontrollable use of the drug despite its adverse consequences to the individual [1–3]. SUD are estimated to affect 10.8% of the adult population in the US [4] and account for 1.5% of global disease burden [5]. In the US overdose death associated with opioids were five times higher in 2016 than 1999, leading the US government to declare the opioid crisis a public health emergency [6]. As of now opioid overdose fatalities as well as drug fatalities in general have continue to rise in the US [7]. There are few medications for SUD and while effective they are limited by limited utilization and high relapse rates [8]. There are no approved medications to treat cocaine, marihuana, methamphetamine, benzodiazepine, or inhalant use disorders. The traditional drug discovery process for medication development is lengthy and costly [8, 9]. In addition, the very modest investment from the pharmaceutical sector in SUDs has limited the discovery of new medications to a greater extent than for other neuro-psychiatric disorders. Thus, novel strategies to evaluate the potential for repurposing existing drugs to treat SUD could accelerate access to additional medications [8, 10].
Drug repurposing is a strategy that can help identify potential new therapies for complex diseases, including SUD [11]. For example, lofexidine was approved for the treatment of hypertension and was recently approved for the treatment of acute opioid withdrawal [12]. With the accumulation of relevant data in machine-actionable formats, data-driven computational approaches have been developed to automate the drug repurposing process [13–18]. However, clinically validating a promising repurposed candidate drug generated by computational algorithms remains a challenge.
We propose an integrated drug repurposing strategy that combines computational-based drug prediction, patient Electronic Health Records (EHRs)-based clinical corroboration and mechanisms of action analysis. First, we developed phenome-driven network-based drug discovery system that prioritized repurposed anti-SUD candidate drugs. The phenotypic and genetic relationships among drugs, drug phenotypes (side effects or SEs), and genes were modeled using the novel context-sensitive network (CSN)-based modeling techniques that we previously developed [16, 19, 20, 22, 23]. Then phenome-driven network-based prioritization approaches, which we recently developed both for understanding disease mechanisms and for drug discovery [14, 16, 18, 19, 21–23], were used to prioritized repurposed candidate drugs based on their phenotypic and genetic relevance to the input disease (i.e., SUD). In our study, we significantly leveraged the context-sensitive drug side effect network that we constructed based on known drug side effects. Side effects are observable phenotypes of drugs manifested at the level of the whole-body system and are mediated by a drug interacting with its on- or off-targets through a cascade of downstream pathway perturbations. While mechanisms of action (on- and off-targets) of many drugs and the underlying molecular mechanisms of diseases remain largely unknown, we can infer novel connections between drugs and diseases (drug repurposing) based on the observed drug and disease phenotypes as well as known drug-targets and protein–protein connections [22]. Instead of directly identifying drugs that target SUD’s mechanisms, which remain largely unknown, the phenome-driven drug discovery system identifies drug candidates that share similar drug phenotypes (i.e., side effects) and/or common targets with drugs causing or treating SUD. Second, we then performed retrospective case-control studies to evaluate the clinical efficacy of promising repositioned candidate drugs using EHRs of 72.9 million patients (20% of the US population). Finally, we examined potential mechanisms of action of promising repurposed candidate drugs in targeting SUD by developing data-driven informatics approaches. The knowledge and data generated by our study (i.e., promising candidate drugs with both supporting clinical evidence and potential mechanism of actions) can set the foundation of experimental testing in animal models for SUD or for pilot testing in clinical trials.
Material and methods
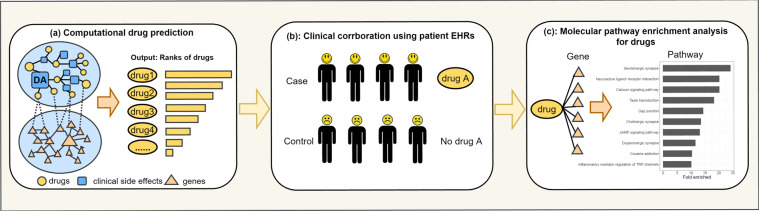
Our study entailed three steps (Fig. 1): (1) We constructed a drug side effect-gene (DSEG) computational drug prediction system to prioritize drugs to treat SUD. (2) We performed retrospectives case-control studies to evaluate top candidate drugs using patient EHR data. (3) We performed genetic and pathway enrichment analysis of top candidate drugs to understand their potential mechanisms of action.
Fig. 1. Flowcharts of the integrate SUD repurposing system.
a Computational drug prediction. b EHR-based clinical corroboration. c Mechanism of action analysis.
Computational drug prediction
Constructing drug side effect-gene prediction system
We constructed a DSEG drug prediction system that models the interconnections among drugs, side effects, and genes using the CSN-based modeling techniques that we previously developed [19–23]. The DSEG system included two networks (Fig. 1a): (1) a drug phenotypic (drug side effects) network (DPN) and (2) a protein–protein interaction network (PPIN). DPN was constructed using drug-SE pairs from the Side Effect Resource (SIDER) database [24] and consisted of 1430 drug nodes, 4251 SE nodes, and 145,321 drug-SE edges. PPIN was directly constructed from protein–protein interactions in STRING [25] and consisted of 17,906 gene nodes and 2,091,571 gene–gene edges. Drug nodes (899 drugs) on DPN were connected to gene nodes (1021 genes) on PPIN using drug-target associations from the DrugBank database [26].
Prioritize anti-SUD drug candidates
We prioritized candidate drugs using the network-based ranking algorithms that we previously used for drug repurposing, gene discovery, and gut microbial metabolite discovery for the disease [16–23, 27–29]. In brief, given an input or seeds (drug abuse, drug dependence, and drug withdrawal syndrome in our study), the ranking score for each drug on the entire network was iteratively updated by:
| 1 |
in which α (α = 0.15 in this study) denotes the probability of restarting from the seed nodes at each step. The algorithm was iterated until convergence ().
We used D and G to represent DPN and PPIN, respectively. T denotes the transition matrix of DSEG network:
| 2 |
In Eq. (2), the diagonal sub-matrices Txx (x ∈ {D, G}) were calculated through normalizing the adjacency matrix of D and G, the off-diagonal sub-matrices Txy (x, y ∈ {D, G}) were calculated through normalizing the adjacency matrix of the bipartite network connecting D and G.
Evaluation
We evaluated how the drug prediction algorithm ranked the four drugs (methadone, buprenorphine, naltrexone, and naloxone) approved for the treatment of OUD or opioid overdose reversal. Lofexidine, an alpha 2 adrenergic agonists that was recently approved for the treatment of acute opioid withdrawal [10], was not included since it was not in the SIDER database and on the DPN. The average ranking of these four drugs among all FDA-approved drugs was calculated.
Electronic health records-based large-scale clinical corroborations for predicted anti-SUD drug candidates
We performed a retrospective case-control study to evaluate top repurposed candidate drugs for treating OUD using de-identified population-level EHR data collected by the IBM Watson Health from 360 hospitals and 317,000 providers across 50 states from 1999 up to August, 2020, representing 20% of the US population [30]. The EHRs are de-identified according to the Health Insurance Portability and Accountability Act and the Health Information Technology for Economic and Clinical Health Act standards. After the de-identification process, the curation process normalizes the data by mapping key elements to widely-accepted biomedical terminologies [31]. Specifically, disease terms are coded using the Systematized Nomenclature of Medicine-Clinical Terms (SNOMED-CT), a global standard for health terms that provides the core general terminology for EHRs [32]. We have recently used this EHR database for drug repositioning for Alzheimer’s disease [23, 33] and for studying the risk and outcomes of COVID-19 in patients with SUD, mental disorders, and cancers [34–37]. The EHR data are de-identified and aggregated (not patient-level) and institutional review board review was exempt.
We used the odds of remission from OUD as the outcome measure. The status of OUD was based on the diagnosis of “Opioid dependence (disorder)” (SNOMED-CT concept code 75544000) and the outcome measure “remission of OUD” was based on the diagnosis of “Opioid dependence in remission (disorder)” (SNOMED-CT concept code 191821007). At the time of the study, there were 326,620 patients with OUD and 28,940 of whom had a record of OUD in remission. We investigated the associations between top-ranked drugs and the odds of remission from OUD.
For a candidate drug A for repurposing, we identified a study cohort of OUD patients diagnosed with drug A’s original indication. We obtained drug indication information from the National Library of Medicine DailyMed, a comprehensive and up-to-date resource of medication content and labeling as found in medication package inserts [38] and other authoritative health information sites. For example, “depressive disorder” is the initial treatment disease of citalopram. In the study cohort, the exposure group (drug group) was comprised of OUD patients with depressive disorder who were prescribed with drug A (citalopram) before remission of their OUD. The comparison group (no drug group) was comprised of OUD patients with depressive disorders but without drug A (citalopram). We compared the odds of remission from opioid dependence between the exposure group and the comparison group.
The EHR data are de-identified population-level (not patient-level) data, therefore we used odds ratios (OR) instead of regression analyses, as previously done by studies that used the Explorys EHR database [23–25, 34–37]. For a given input set of patient characteristics (e.g., age, gender, race, diagnosis, comorbidities), the Explorys Explore Cohort Discovery tool built a patient cohort by querying the EHR database for patients matching the inputs. Patients with missing values for the input queries were not included in the returned cohorts. The associations between candidate drugs and remission were estimated by an OR for remission comparing the exposure group versus the comparison group in which both groups suffered OUD. The adjusted odds ratios (AORs), 95% confidence intervals (CIs), and p values were calculated using Cochran–Mantel–Haenszel (CMH) method [39], controlling for age (adult: 18–64 years, seniors: >64 years), gender (male, female), race (Caucasian and non-Caucasian), original indication, and existing treatments for opioid dependence. A two-sided p value < 0.05 was considered as statistical significance. All analyses were done using R, version 3.6.3. Drugs with insufficient data (e.g., rarely used drugs in OUD patients) to perform the CMH analysis were excluded. Among the top 20 drugs, one drug was excluded.
Analysis of top anti-SUD drug candidates in the context of OUD at the genetic and functional levels
Genetic-level analysis
We obtained 19 OUD-associated genes from the published literature [40–42], including APBB2, BDNF, CNIH3, COMT, CYP2D6, DRD2, DRD4, HTR1B, KCNC1, KCNG2, OPRD1, OPRK1, OPRM1, PDYN, PENK, POMC, RGMA, SLC6A4, and TPH2. For each repurposed candidate drug, we used the STITCH (Search Tool for Interactions of Chemicals) database to obtain its associated genes. STITCH contains data on the interactions between 500,000 small molecules and 9.6 million proteins from 2031 organisms [43]. In this study, we used chemical-gene associations found in the human body. The scores of chemical-gene associations ranged from 100 to 999 and we used the median score of 500 as the cutoff value. For example, at cutoff score of 500, olanzapine (a top repurposed candidate) is associated with 62 genes. We identified OUD genes that are directly targeted by a candidate drug by intersecting OUD-associated genes with drug-associated genes. For example, among 62 gene targets of olanzapine, seven are OUD-associated genes (BDNF, CYP2D6, DRD2, DRD4, HTR1B, POMM, and SLC6A4).
Functional-level analysis
We then investigated how the repurposed anti-OUD candidate drugs are functionally related to OUD by directly targeting OUD-associated pathways. We used rich pathway information from the Molecular Signatures Database (MSigDB), currently the most comprehensive resource of 17,779 annotated pathways and gene sets [44], to perform pathway enrichment analysis as in our previous studies [45, 46]. To identify genetic pathways significantly enriched for OUD, genetic pathways were first obtained from MSigDB for each OUD gene. For each pathway, we assessed its probability of being associated with the given set of 19 OUD-associated genes as compared to its probability of being associated with the same number of randomly selected genes. The random process was repeated 1000 times and a t-test was used to assess the statistical significance. We obtained a total of 53 significantly enriched pathways for 19 OUD genes, which include G-protein activation, opioid signaling, gap junction, and serotonin receptors, among others.
Similarly, we identified genetic pathways significantly enriched for each candidate drug. Genetic targets of each candidate drug were obtained from the STITCH database. For each drug-targeted gene, its associated pathways were obtained from the gene–pathway pairs form the MSigDB database. For each pathway, we assessed its probability of being associated with the given set of drug-associated genes as compared to its probability of being associated with the same number of randomly selected genes. For example, a total of 317 pathways were significantly enriched for the set of 62 olanzapine-associated genes, which include 41 of the 53 OUD-associated pathways. For each repurposed candidate drug, we identified OUD pathways that are also significantly enriched for the drug by intersecting drug-associated pathways with OUD-associated genes and pathways.
Results
Our drug prediction system ranked approved SUD treatments highly
Table 1 lists the ranks (in top percentage) for the four medications used clinically to treat OUD and opioid overdose reversal (naloxone). Our system prioritized these four drugs within the top 3.4% among 1430 FDA-approved drugs on the network.
Table 1.
Ranks for approved OUD medications generated by our prediction system.
| Drug name | Rank |
|---|---|
| Methadone | Top 3.2% |
| Buprenorphine | Top 2.7% |
| Naltrexone | Top 0.91% |
| Naloxone | Top 2.0% |
Table 2 shows the top 20 predicted drug candidates for SUD. Overall, 17 out of 20 are implicated as SUD treatments through different sources, including FDA drug labels, published literature, and clinical trials. In addition, 17 of the top 20 drugs were approved for treating central nervous system-related diseases particularly for depression and pain, which are frequently comorbid with SUD [47]. We noticed that top-ranked drugs include some opioid analgesics with high addiction risks (e.g., fentanyl). Since two of the approved OUD medications (e.g., methadone and buprenorphine) are opioid analgesics but with slower pharmacokinetics than fentanyl, it is not surprising that opioid analgesics were ranked high by the DSEG system.
Table 2.
Top 20-ranked repurposed drug candidates.
| Rank | Drug | Original Indication | Evidence |
|---|---|---|---|
| 1 | Pregabalin | Diabetic neuropathic pain | NCT00142883 |
| 2 | Fentanyl | Pain/acute pain | |
| 3 | Morphine | Pain/acute pain | |
| 4 | Oxycodone | Pain/acute pain | NCT00218374 |
| 5 | Hydromorphone | Pain/acute pain | NCT00218361 |
| 6 | Citalopram | Depression | NCT01535573 |
| 7 | Ziprasidone | Schizophrenia | PMID24628830 |
| 8 | Atomoxetine | ADHD | NCT01498549 |
| 9 | Tramadol | Pain | NCT00301210, NCT01188421, NCT00142896, NCT03365817 |
| 10 | Bupropion | Depression | NCT02111798 |
| 11 | Mirtazapine | Depression | NCT02541526, NCT00249444, NCT00322309, NCT00732901 |
| 12 | Cortisol | Rheumatoid arthritis | NCT00759005, NCT01718964 |
| 13 | Naltrexone | OUD | FDA-approved |
| 14 | Tapentadol | Pain | NCT00687713 |
| 15 | Amphetamine | ADHD | NCT00421603 |
| 16 | Methylprednisolone | Rheumatoid arthritis | |
| 17 | Triazolam | Insomnia | NCT00218166 |
| 18 | Olanzapine | Schizophrenia | NCT02643355 |
| 19 | Topiramate | Seizures | NCT00396734, NCT00421603 |
| 20 | Fluoxetine | Depression | NCT00142779 |
NCT: SUD drugs from clinical trials. PMID: SUD drugs from biomedical literature.
EHR-based analysis provided evidence that top-ranked drug candidates have clinical effectiveness in OUD patients
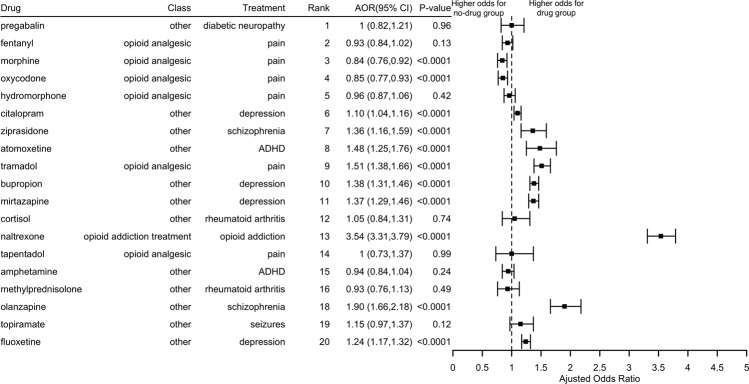
We performed case-control studies to evaluate top 20 repdurposed candidates. Figure 2 shows the AORs and 95% CIs of remission from OUD for the 19 top candidate drugs. Triazolam was excluded due to insufficient cases for patients with OUD. Naltrexone, an FDA-approved OUD treatment, has the highest effects on remission from OUD. Specifically, we studied OUD patients who were not prescribed methadone or buprenorphine. In this cohort, those who have prescribed naltrexone had odds of remission nearly 4.00 times as compared to those not prescribed naltrexone (AOR: 3.54, 95% CI: [3.31,3.69], p value < 0.0001), adjusting for age, gender, and race. This result supports the validity of the EHR-based evaluation of clinical efficacy of candidate drugs for treating OUD.
Fig. 2. Odds ratios of remission from opioid dependence and the corresponding 95% CI of 10 of top 20-ranked drugs.
Triazolam was excluded due to insufficient cases for patients with opioid dependence.
For the remaining 18 drugs, eight had an AOR of remission significantly higher than 1.0. Olanzapine, a drug approved to treat schizophrenia, has the highest AOR of remission from OUD. Specifically, we studied patients with OUD and schizophrenia but not prescribed methadone, buprenorphine, or naltrexone. In this cohort, OUD patients who were prescribed olanzapine had odds of OUD remission nearly 2.00 times compared to patients not prescribed olanzapine (AOR: 1.90; 95% CI [1.66,2.18], p value < 0.0001), adjusting for age, gender, and race. Three antidepressants bupropion, mirtazapine and atomoxetine had odds of OUD remission nearly 50% higher compared to patients with depression not prescribed these antidepressants (AOR = 1.38 [1.31–1.46], p value < 0.0001; 1.37 [1.29–1.46], p value < 0.0001; 1.48 [1.25–1.76], p value < 0.0001, respectively).
The prescription of opioid analgesics was negatively associated with OUD remission. For example, patients who were prescribed morphine had odds of remission 16% lower compared to patients with no prescription of morphine (AOR: 0.84; 95% CI [0.76,0.92], p value < 0.0001). These results show that opioid analgesics can aggravate OUD. Interestingly, we found that tramadol was the only opioid analgesic positively associated with OUD remission (AOR: 1.51; 95% CI [1.38,1.66], p value < 0.0001). Tramadol has a lower risk of addiction compared to other opioid analgesics [48] and previous studies support benefits in the management of opioid withdrawal symptoms and long-term OUD treatment [49, 50]. Our findings alongside the prior studies in the literature support further evaluation of tramadol for OUD treatment, including treatment of comorbid OUD in pain patients.
Analysis of repurposed candidate drugs in the context of OUD at the genetic and functional level
We analyzed how each of the five promising drugs (reducing OUD relapse) (Fig. 2) relates to OUD at the genetic and functional level. At the genetic level, each candidate drug directly targets multiple OUD-associated genes. For example, olanzapine targets a total of 62 genes, among which seven genes are implicated in OUD. Tramadol targets 16 genes, of which five are OUD genes, including three opioid receptors (OPRD1, OPRK1, and OPRM1) (Table 3). While each candidate drug is associated with higher odds of OUD remission based on EHR analysis, these drugs target different aspects of OUD and OUD genes as shown in Table 3. The complete list of OUD- and drug-associated genes are in Supplementary file S1.
Table 3.
OUD genes targeted by candidate drugs.
| Drug | Total target genes | Target OUD genes | OUD genes |
|---|---|---|---|
| Atomoxetine | 11 | 3 | BDNF, CYP2D6, SLC6A4 |
| Bupropion | 22 | 2 | CYP2D6, SLC6A4 |
| Mirtazapine | 30 | 5 | CYP2D6, DRD2, DRD4, OPRK1, SLC6A4 |
| Olanzapine | 62 | 7 | BDNF, CYP2D6, DRD2, DRD4, HTR1B, POMC, SLC6A4 |
| Tramadol | 16 | 5 | CYP2D6, OPRD1, OPRK1, OPRM1, SLC6A4 |
We then analyzed how these candidate drugs are functionally involved in OUD. We identified 53 genetic pathways that are significantly enriched for the 19 OUD-associated genes. The top enriched OUD pathways are opioid signaling, G-protein activation, gap junction, potassium channels (full list is in Supplementary file 2). We identified significantly enriched pathways for each candidate drug and the shared pathways between the drug and OUD. As shown in Table 4, each candidate drug targets multiple OUD-associated pathways. For example, 317 pathways are significantly enriched for olanzapine, among which 41 are also significantly enriched for OUD (77% of 53 OUD pathways) including G-protein activation, Serotonin receptors among others. A total of 42 pathways are significantly enriched for tramadol, among which 24 were OUD-associated pathways (45% of 53 OUD pathways) including G-protein activation, Serotonin receptors, opioid signaling among others. The full list of OUD-associated pathways targeted by each candidate drug is in Supplementary file S2.
Table 4.
Number of significantly enriched pathways for each drug, number of significantly enriched pathways shared between the drug and OUD, and top 10 OUD pathways targeted by each candidate drug.
| Drug | Total (n) | Shared (n) | Top 10 pathways |
|---|---|---|---|
| Atomoxetine | 42 | 16 |
Xenobiotics Voltage gated potassium channels Cytochrome P450—arranged by substrate type Phase 1—functionalization of compounds Neuroactive ligand–receptor interaction Biological oxidations Posttranslational regulation of adherens junction stability and disassembly Potassium channels SHP2 signaling Neurotrophic factor-mediated Trk receptor signaling |
| Bupropion | 305 | 21 |
Serotonin receptors Xenobiotics Amine ligand-binding receptors Cytochrome P450—arranged by substrate type Opioid Signaling Phase 1—functionalization of compounds Class A/1 (Rhodopsin-like receptors) Neuroactive ligand–receptor interaction Biological oxidations Posttranslational regulation of adherens junction stability and dissassembly |
| Mirtazapine | 93 | 24 |
Serotonin receptors Repression of pain sensation by the transcriptional regulator DREAM Xenobiotics Regulation of ck1/cdk5 by type 1 glutamate receptors Amine ligand-binding receptors G alpha (1) signaling events Cytochrome P450—arranged by substrate type G alpha (1) pathway Phase 1—functionalization of compounds Class A/1 (Rhodopsin-like receptors) |
| Olanzapine | 317 | 41 |
G-protein activation Androgen biosynthesis Serotonin receptors Repression of pain sensation by the transcriptional regulator DREAM Peptide hormone biosynthesis Endogenous sterols Xenobiotics Regulation of ck1/cdk5 by type 1 glutamate receptors Syndecan-3-mediated signaling events Amine ligand-binding receptors |
| Tramadol | 42 | 24 |
G-protein activation Serotonin receptors Repression of pain sensation by the transcriptional regulator DREAM Xenobiotics Amine ligand-binding receptors G alpha (1) signaling events Cytochrome P450—arranged by substrate type Opioid Signaling Peptide ligand-binding receptors Phase 1—functionalization of compounds |
Discussion
We developed an integrated drug repurposing strategy for OUD that is applicable to other SUDs. The approach combined computational drug candidate prediction, EHRs-based clinical corroboration, and mechanisms of action analysis using genetic, genomic, phenotypic, and patient EHR data. We identified one opioid analgesic (tramadol), one antipsychotic (olanzapine), two antidepressants (mirtazapine and bupropion), and one selective norepinephrine reuptake inhibitor (atomoxetine), that might be of value in improving outcomes in OUD patients, including those suffering from comorbid conditions.
Our study shows that patients with schizophrenia and OUD who were prescribed olanzapine had higher odds of OUD remission than individuals without olanzapine (AOR = 1.90). Olanzapine is an antipsychotic drug that acts as an antagonist at dopamine D1 and D2 receptors, 5H2A and 5H6 serotonin receptors, H1 histamine receptors, adrenergic alpha one receptor, and muscarinic M1–M5 receptors [51]. In our study, we showed that olanzapine directly targets 7 of 19 OUD-associated genes, including BDNF, CYP2D6, DRD2, DRD4, HTR1B, POMC, and SLC6A4. Based on pathway enrichment analysis, olanzapine significantly targets 41 of the 53 OUD-associated pathways (77%). Some of the pathways, such as G-protein activation, serotonin receptors, neuroactive ligand–receptor interaction, and GPCR signaling are known to be involved in opioid addiction [52, 53].
In our analysis, patients with pain and OUD who were prescribed with tramadol had higher odds of OUD remission than individuals without tramadol (AOR = 1.51). Tramadol is a prodrug whose metabolite (O-desmethyltramadol) acts as an opioid agonist and a serotonin-norepinephrine reuptake inhibitor [54]. Tramadol is widely used as an analgesic and is believed to be less addictive than other equipotent opioid analgesics. The increased odds of OUD remission, we observed when tramadol was prescribed to patients with comorbid pain identifies it as another opioid agonist potentially beneficial for OUD treatment. Consistent with this, studies have reported the benefit of Tramadol use for opioid detoxification [55]. Intriguingly whereas the prescription of opioid analgesics was associated with decreased risk for OUD remission, the prescription of tramadol showed the opposite association. In this respect, tramadol might be particularly useful in the management of pain patients suffering from OUD. Our analysis of genes and genetic pathways targeted by tramadol show that tramadol directly target OUD-associated genes and opioid receptors (CYP2D6, OPRD1, OPRK1, OPRM1, and SLC6A4). Among 42 pathways significantly enriched for tramadol, 24 were OUD-associated pathways (45% of 53 OUD pathways) including G-protein activation, serotonin receptors, opioid signaling, GPCR signaling among others. These potential mechanisms of action of tramadol suggest tramadol may help treat OUD in general and not only in OUD patients with pain.
Two antidepressants (bupropion and mirtazapine) and one selective norepinephrine reuptake inhibitor (atomoxetine) that also has antidepressant properties were identified as anti-OUD candidates. Inasmuch as depression is frequently comorbid with OUD and contributes to relapse this could be a mechanism for improving outcomes in OUD. Most notable is bupropion, a blocker of dopamine and norepinephrine transporters and an antagonist at various nicotine receptors including the alpha 4 beta 2 receptor associated with the rewarding effects of nicotine [56], since it is an approved treatment for smoking cessation. Furthermore, preclinical studies have reported that bupropion prevented the development of tolerance to opioids and the emergence of withdrawal symptoms [57]. Our analysis shows that bupropion directly targets multiple OUD-associated pathways including serotonin receptors, opioid and GPCR signaling, neuroactive ligand–receptor interaction, which suggests that bupropion may also be beneficial in OUD treatment.
Mirtazapine enhances noradrenergic and presumably dopaminergic signaling through its antagonistic effects at serotonin receptors (5HT2 family) and alpha 2 adrenergic receptors. Preliminary evidence has shown a therapeutic benefit of mirtazapine in the treatment of patients with methamphetamine use disorder alone or when combined with naltrexone [58]. Our genetic and pathway analyses showed that mirtazapine directly target five OUD-associated genes (CYP2D6, DRD2, DRD4, OPRK1, and SLC6A4) and 24 OUD-associated pathways including serotonin receptors, G alpha signaling, GPCR signaling, and GAP junctions, among others.
Atomoxetine is a selective norepinephrine (noradrenaline) reuptake inhibitor for the treatment of attention-deficit hyperactivity disorder (ADHD) [59, 60]. It is a highly selective and potent inhibitor of the presynaptic noradrenalin transporter and could be of use in the treatment of depression associated with OUD as a monotherapy or as an augmentation agent [61]. Preclinical studies have shown that atomoxetine attenuated stimulant self-administration perhaps by modifying the reinforcing properties of stimulants [62]. Our EHR-based analysis showed that atomoxetine is associated with significant OUD remission in patients with ADHD and OUD. In addition, atomoxetine directly targets three OUD genes (BDNF, CYP2D6, and SLC6A4) and 16 of the 53 OUD pathways including potassium channels, neuroactive ligand–receptor interaction, and chemical synapsis.
One important finding is that all five candidate drugs directly target multiple OUD genes and pathways, suggesting that these drugs may treat OUD in the general population and might be particularly beneficial in patients with comorbid conditions (pain, depression, schizophrenia, ADHD). In addition, these drugs target different OUD genes and pathways, suggesting that combinations of these drugs may have synergistic efficacy in treating OUD.
Our study has several limitations. First, we used patient EHR data to corroborate top-ranked anti-OUD candidate drugs generated by the network-based prediction algorithm. Patient EHR data were collected for clinical convenience and billing, not for research purposes. Though EHR data have been widely used for research purposes, they have inherent limitations including under-diagnosis, over-diagnosis, or mis-diagnosis, limited information on time-series, timing, and adherence of medications and treatments, among others [63–66]. Second, disease diagnoses in the patient EHR data are coded using SNOMED-CT terminology and for SUD these differ from categories used by DSM-5. A previous study showed EHRs alone were inadequate in capturing mental health diagnoses, visits, specialty care, hospitalizations, and medications [67]. In our recent study, we showed that 10.3% of the study population in the Explorys EHR database had a diagnosis of SUD [34], which is similar to the reported prevalence of 10.8% among people aged 18 or older in the US according to the 2018 National Survey on Drug Use and Health [68]. However, the under-diagnosis of other mental disorders including depression, schizophrenia, and ADHD, some of which are the original indications of identified candidate drugs, may have impacts on the EHR-based corroboration in our study. Third, though patients in this EHR database represent 20% of US population, they represent patients who had encounters with healthcare systems and are not necessarily representative of the general US population. In addition, EHRs in the Explorys database are population-level (not patient-level) data, on which more advanced statistical testing could not be performed. The candidates identified by the computational prediction algorithm need to be evaluated in other databases and in other populations or to be investigated in a prospective manner to control for confounding factors. Because of the inherent limitations in EHR-based drug discovery, our drug discovery scheme comprised of three complementary components (computational drug prediction, hypothesis-driven EHR-based testing, and mechanisms of action analyses), in which EHR-based testing was not used for drug discovery but for gathering clinical evidence to further corroborate candidates output from the computational prediction system. Finally, another concern is that our current knowledge regarding genes and pathways involved in OUD is likely to be incomplete. In the future as new knowledge emerges one can investigate how newly identified OUD-related genes and pathways interact with the previously known OUD genes and pathways and determine how including them affects the prediction for recognizing therapeutic drugs for OUD or other SUD.
In summary, we developed an integrated drug repurposing system for OUD by combining computational prediction, clinical corroboration, and mechanisms of action studies. We identified one opioid analgesic (tramadol), that might be of particular value for the management of pain patients suffering from OUD; one antipsychotic (olanzapine), that might be of particular value for the management of bipolar or schizophrenia patients with OUD, two antidepressant (mirtazapine and bupropion) that might be of value in improving OUD outcomes in OUD patients in whom depression contributes to relapse and one norepinephrine transporter blocker (tomoxetine) that might be of particular values for ADHD patients with OUD. These medications might also be of value in OUD patients in general by themselves or in combination with approved medication for OUD. While our approach that seamlessly integrates computational drug prediction, patient EHR-based evaluation and bioinformatics-based mechanisms of action analysis is at the in-silico level it provides the foundation for future hypothesis-driven preclinical and clinical studies of these candidate drugs in treating OUD.
Supplementary information
Acknowledgements
We acknowledge support from Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under the NIH Director’s New Innovator Award number DP2HD084068, NIH National Institute on Aging R01 AG057557, R01 AG061388, R56 AG062272, National Institute on Drug Addiction UG1DA049435, American Cancer Society Research Scholar Grant RSG-16-049-01—MPC, and The Clinical and Translational Science Collaborative (CTSC) of Cleveland 1UL1TR002548-01.
Author contributions
RX conceived, designed, and supervised the study. MZ performed drug prediction and clinical evaluation. QW performed the mechanism of action study. RX, QW, MZ, and NDV drafted the manuscript. NDV, CLZ, and AR critically contributed to data interpretation and result discussion. All authors approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mengshi Zhou, QuanQiu Wang
Supplementary information
The online version of this article (10.1038/s41380-020-01011-y) contains supplementary material, which is available to authorized users.
References
- 1.Volkow ND, McLellan AT. Opioid abuse in chronic pain-misconceptions and mitigation strategies. N. Engl J Med. 2016;374:1253–63. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N. Engl J Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 4.National Survey on Drug Use and Health—SAMHSA. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. National Survey on Drug Use and Health. 2018. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf.
- 5.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services. HHS acting secretary declares public health emergency to address national opioid crisis. Department of Health and Human Services; 2017. p. 2016–7. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html.
- 7.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics. Mortality. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/nchs/data/health_policy/Provisional-Drug-Overdose-Deaths-by-Quarter-Demographic-Characteristics-Q1-2020.pdf.
- 8.Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology. 2012;37:290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullard A. New drugs cost US $2.6 billion to develop. Nat Rev Drug Discov. 2014;13:877. doi: 10.1038/nrd4507. [DOI] [Google Scholar]
- 10.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Forum on Neuroscience and Nervous System Disorders. Advancing Therapeutic Development for Pain and Opioid Use Disorders Through Public-Private Partnerships: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2018. [PubMed]
- 11.Ashburn TT, Thor KB. Drug repurposing: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava AB, Mariani JJ, Levin FR. New directions in the treatment of opioid withdrawal. Lancet. 2020;395:1938–48. doi: 10.1016/S0140-6736(20)30852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Zheng S, Chen B, Butte AJ, Swamidass SJ, Lu Z. A survey of current trends in computational drug repurposing. Brief Bioinform. 2016;17:2–12. doi: 10.1093/bib/bbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Xu R. Disease comorbidity-guided drug repurposing: a case study in schizophrenia. AMIA Annu Symp Proc. 2018;2018:1300–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaraj AB, Wang Q, Joseph P, Zheng CL, Chen Y, Kovalenko O, et al. Using a novel computational drug repurposing approach (DrugPredict) to rapidly identify potent drug candidates for cancer treatment. Oncogene. 2017. 10.1038/onc.2017.328. [DOI] [PMC free article] [PubMed]
- 16.Chen Y, Xu R. Context-sensitive network-based disease genetics prediction and its implications in drug discovery. Bioinformatics. 2017;33:1031–9. doi: 10.1093/bioinformatics/btw737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu R, Wang Q. A genomics-based systems approach towards drug repurposing for rheumatoid arthritis. BMC Genom. 2016;17:518. doi: 10.1186/s12864-016-2910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu R, Wang Q. PhenoPredict: a disease phenome-wide drug repurposing approach towards schizophrenia drug discovery. J Biomed Inf. 2015;56:348–55. doi: 10.1016/j.jbi.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Xu R. Context-sensitive network-based disease genetics prediction and its implications in drug discovery. Bioinformatics. 2017;33:1031–9. doi: 10.1093/bioinformatics/btw737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Xu R. Context-sensitive network analysis identifies food metabolites associated with Alzheimer’s disease: an exploratory study. BMC Med Genom. 2019;12:17. doi: 10.1186/s12920-018-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng C, Xu R. Large-scale mining disease comorbidity relationships from post-market drug adverse events surveillance data. BMC Bioinforma. 2018;19:500. doi: 10.1186/s12859-018-2468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Chen Y, Xu R. A drug-side effect context-sensitive network approach for drug target prediction. Bioinformatics. 2019;35:2100–7. doi: 10.1093/bioinformatics/bty906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Zheng C, Xu R. Combining phenome-driven drug-target interaction prediction with patients’ electronic health records-based clinical corroboration toward drug discovery. Bioinformatics. 2020;36:i436–i444. doi: 10.1093/bioinformatics/btaa451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn M, Letunic I, Jensen LJ, Bork P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44:D1075–9. doi: 10.1093/nar/gkv1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:808–15. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–82. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu R, Wang Q, Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015;16:S4. doi: 10.1186/1471-2164-16-S7-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu R, Wang Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol. 2016;10:63. doi: 10.1186/s12918-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Xu R. CoMNRank: an integrated approach to extract and prioritize human microbial metabolites from MEDLINE records [published online ahead of print, 2020 Aug 11] J Biomed Inf. 2020;109:103524. doi: 10.1016/j.jbi.2020.103524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IBM Explorys. IBM Watson Health. IBM Explorys. https://www.ibm.com/watson/health/explorys/. Accessed June–August 2020. Accessed June–Aug 2020.
- 31.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267–D270. doi: 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SNOMED International. The systematized nomenclature of medicine—Clinical Terms (SNOMED CT). SNOMED International. 2020. http://www.snomed.org/snomed-ct/why-snomed-ct.
- 33.Zhou M, Xu R, Kaelber DC, Gurney ME. Tumor necrosis factor (TNF) blocking agents are associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis. PLoS ONE. 2020;15:e0229819. doi: 10.1371/journal.pone.0229819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2020:1–10. 10.1038/s41380-020-00880-7. [DOI] [PMC free article] [PubMed]
- 35.Wang QQ, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2020. 10.1002/wps.20806. [DOI] [PMC free article] [PubMed]
- 36.Wang QQ, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes of cancers and coronavirus disease 2019 from electronic health 4 records in the US. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.6178.
- 37.Wang QQ, Berger NA, Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death and disparities. Blood Rev. 10.1016/j.blre.2020.100775. [DOI] [PMC free article] [PubMed]
- 38.The National Library of Medicine (NLM). DailyMed. The National Library of Medicine. https://healthdata.gov/dataset/dailymed. Accessed June–Aug 2020.
- 39.Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Annu Rev Public Health. 1988;9:123–160. doi: 10.1146/annurev.pu.09.050188.001011. [DOI] [PubMed] [Google Scholar]
- 40.Wang SC, Chen YC, Lee CH, Cheng CM. Opioid addiction, genetic susceptibility, and medical treatments: a review. Int J Mol Sci. 2019;20:4294. doi: 10.3390/ijms20174294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19:499–514. doi: 10.1038/s41583-018-0028-x. [DOI] [PubMed] [Google Scholar]
- 42.Crist RC, Reiner BC, Berrettini WH. A review of opioid addiction genetics. Curr Opin Psychol. 2019;27:31–35. doi: 10.1016/j.copsyc.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szklarczyk D, Santos A, von Mering C, Jensen LJ, Bork P, Kuhn M. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016;44:D380–D384. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–40. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Xu R. Data-driven multiple-level analysis of gut-microbiome-immune-joint interactions in rheumatoid arthritis. BMC Genom. 2019;20:124. doi: 10.1186/s12864-019-5510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Li L, Xu R. A systems biology approach to predict and characterize human gut microbial metabolites in colorectal cancer. Sci Rep. 2018;8:6225. doi: 10.1038/s41598-018-24315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institute on Drug Abuse. Common comorbidities with substance use disorders research report. National Institute on Drug Abuse. 2018. https://www.drugabuse.gov/publications/drugfacts/comorbidity-substance-use-disorders-other-mental-illnesses. [PubMed]
- 48.Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18:395–400. doi: 10.36076/ppj.2015/18/395. [DOI] [PubMed] [Google Scholar]
- 49.Lofwall MR, Babalonis S, Nuzzo PA, Siegel A, Campbell C, Walsh SL. Efficacy of extended-release tramadol for treatment of prescription opioid withdrawal: a two-phase randomized controlled trial. Drug Alcohol Depend. 2013;133:188–97.. doi: 10.1016/j.drugalcdep.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarkar S, Varshney M, Patil V, Lal R. Maintainence treatment of opioid dependence with tramadol. J Neurosci Rural Pr. 2017;8:S98. doi: 10.4103/jnrp.jnrp_422_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bymaster FP, Rasmussen K, Calligaro DO, Nelson DL, DeLapp NW, Wong DT, et al. In vitro and in vive biochemistry of olanzapine: a novel, atypical antipsychotic drug. J Clin Psychiatry. 1997;58:28–36. [PubMed] [Google Scholar]
- 52.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–81. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 55.Radcliff JA, Vellanki SC, Moore PS, Weisensell BJ, Cather JK. A retrospective chart review of outcomes resulting from a three-day tramadol taper for acute opioid withdrawal. J Addict Dis. 2018;37:252–8. doi: 10.1080/10550887.2019.1668743. [DOI] [PubMed] [Google Scholar]
- 56.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159–66. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamdy MM, Elbadr MM, Barakat A. Bupropion attenuates morphine tolerance and dependence: Possible role of glutamate, norepinephrine, inflammation, and oxidative stress. Pharm Rep. 2018;70:955–62. doi: 10.1016/j.pharep.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pr. 2011;24:541–50. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- 59.Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharm Exp Ther. 1982;222:61–5. [PubMed] [Google Scholar]
- 60.Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paed Drugs. 2009;11:203–26. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 61.Carpenter LL, Milosavljevic N, Schecter JM, Tyrka AR, Price LH. Augmentation with open-label atomoxetine for partial or nonresponse to antidepressants. J Clin Psychiatry. 2005;66:1234–8. doi: 10.4088/JCP.v66n1005. [DOI] [PubMed] [Google Scholar]
- 62.Lofwall MR, Walsh SL, Bigelow GE, Strain EC. Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology. 2007;194:381–393. doi: 10.1007/s00213-007-0847-3. [DOI] [PubMed] [Google Scholar]
- 63.Cowie MR, Blomster JI, Curtis LH, Duclaux S, Ford I, et al. Electronic health records to facilitate clinical research. Clin Res Cardiol. 2017;106:1–9. doi: 10.1007/s00392-016-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coorevits P, Sundgren M, Klein GO, Bahr A, Claerhout B, Daniel C, et al. Electronic health records: new opportunities for clinical research. J Intern Med. 2013;274:547–560. doi: 10.1111/joim.12119. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad FS, Chan C, Rosenman MB, Post WS, Fort DG, Greenland P, et al. Validity of cardiovascular data from electronic sources: the Multi-Ethnic Study of Atherosclerosis and HealthLNK. Circulation. 2017;136:1207–1216. doi: 10.1161/CIRCULATIONAHA.117.027436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safran C, Bloomrosen M, Hammond WE, Labkoff S, Markel-Fox S, Tang PC, et al. Toward a national framework for the secondary use of health data: an American Medical Informatics Association White Paper. J Am Med Inf Assoc. 2007;14:1–9. doi: 10.1197/jamia.M2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madden JM, Lakoma MD, Rusinak D, Lu CY, Soumerai SB. Missing clinical and behavioral health data in a large electronic health record (EHR) system. J Am Med Inform Assoc. 2016; 10.1093/jamia/ocw021. [DOI] [PMC free article] [PubMed]
- 68.The Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf. Accessed 12 Nov 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.