Abstract
Background
The recent pandemic by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global emergency. There is large number of asymptomatic cases of SARS-CoV-2 that are not reported. Hence, serological evidence of SARS-CoV2 antibodies is warranted for a better estimation of the actual number of infected patients to limit the disease spread and to get an idea of herd immunity.
Methods
This is a cross-sectional study conducted from May 2020 to July 2020 at National Institute of Blood Diseases at Pakistan. The study includes healthcare workers (HCWs), community and industrial workers. The anti-SARS-CoV-2 test was performed by electrochemiluminescence immunoassay analyzer.
Results
A total of 1675 samples have been received from three groups of population. The percentage positivity for industrial employees is high (50.3%) for HCW (13.2%) and community population (34%).Total percentage for positive antibodies result is ~36%.
Conclusion
Our seroprevalence is 36%, which still far from herd immunity that needs to be at least 60–70% in population. If we consider acquiring 60% seroprevalence in next few months, then herd immunity is not far from reality, provided the antibodies did not decline with time. Although the current study is based on a small sample of participants, the findings suggest a study with larger population to implement stronger and targeted interventions.
Keywords: infectious disease, public health
Introduction
New emergent strains of viruses have always remained a challenge to the healthcare system. There are various viral outbreaks reported worldwide, and coronaviruses are found to be the leading cause for these outbreaks. Coronaviruses are a group of enveloped viruses with non-segmented, single-stranded and positive-sense RNA genomes. Six different strains have been identified out of which severe acute respiratory syndrome coronavirus 1 and 2 (SARS-CoV-1 and 2) and Middle East respiratory syndrome coronavirus are zoonotic and highly pathogenic coronaviruses.1 The recent pandemic by SARS-CoV-2 started from Wuhan, China in December 2019, and later the community transmission lead to a global emergency. The World Health Organization (WHO) named the disease as coronavirus disease-19 (COVID-19) and the International Committee on Taxonomy of Virus named the novel coronavirus as SARS-CoV-2.2,3
The infection is transmitted largely via droplets from symptomatic and asymptomatic individuals or even the presymptomatic cases. COVID-19 can cause a wide spectrum of clinical manifestations including fever, dry cough, myalgia, diarrhea and dyspnea. In majority of cases SARS-CoV-2 leads to asymptomatic or mild self-limiting illness (81%).4 However, in 10% of patients, it can result in a severe or critical disease. A confirmed diagnosis of COVID-19 relies on specific molecular tests to detect viral RNA like polymerase chain reaction.2 Laboratory findings specific to COVID-19 include elevated prothrombin time, lactate dehydrogenase, D-dimer, alanine aminotransferase, C-reactive protein and creatine kinase and ferritin. Elevations in ferritin, neutrophil count, D-dimer, blood urea and creatinine levels have been associated with worst clinical outcome.5
There is no currently approved and recommended management for patients with COVID 19. The treatment is mainly supportive and symptomatic. Isolation of a suspected or diagnosed case is the first and crucial step in management, considering highly contagious nature of the disease. Patients with mild symptoms are preferably managed at home with antipyretics, adequate hydration and nutrition. For hypoxic patients, oxygen support via nasal prong, high-flow nasal cannula or noninvasive ventilation is recommended.6 Interleukin-6 inhibitor tocilizumab has been used for moderate to severe cases, but now its use has been stopped after finding unsuccessful phase 3 trial results. Trials concluded that it did not improve the clinical status of COVID patients with no reduction in patient mortality as well.7 Now, we are looking forward for promising results from convalescent plasma and antivirals like remdesivir being used in moderate to severely ill patients.
The WHO has reported >20 million confirmed cases of COVID-19 so far, with number of highest positive cases encountered in USA, followed by Brazil and India.8 It is assumed that the actual burden of COVID-19 is underestimated as only a small fraction of acute infections have been diagnosed and reported. There is large number of asymptomatic cases associated with SARS-CoV-2 that are not reported. Furthermore, there are limitations of testing and surveillance in healthcare settings. Identification of seroprevalence among asymptomatic individuals is essential for two major reasons. First, to recognize the individuals who were infected without any symptoms (asymptomatic carriers) to limit the disease transmission, and second, the idea of herd immunity could be an important indicator in a community for guiding future decisions regarding lifting the social restrictions yet preventing the subsequent epidemic outbreaks or second wave as well as the need of vaccination in that particular community. Hence, serological evidence of SARS-COV-2 antibodies is warranted for a better estimation of the actual number of infected patients.
Seroprevalence surveys in the USA and the Europe suggest that actual burden of disease is 10-folds or more of that is reported.9,10 As of 18 August 2020, there are 281 136 confirmed COVID-19 cases reported in Pakistan leading to 2.1% mortality (6190 deaths) as stated by official government website. Globally surveys are being conducted on large scale to know the actual prevalence of disease.11 Serology tests are used for seroprevalence surveys to identify people that have antibodies against SARS-CoV-2. Seroprevalences for anti-SARS-CoV-2 antibodies in different parts of world have shown disparity. The rate is low in developed world ranging from 5% in Spain,12 6.9% in the USA.10 However the developing countries are showing contrasting findings with high seroprevalence.
Therefore, we are performing this study to estimate the prevalence of anti SARS-CoV2 antibodies in different groups of population residing in Karachi.
Material and methods
This is a prospective cross-sectional study conducted from May 2020 to July 2020 at National Institute of Blood Diseases and Bone Marrow Transplantation Hospital (NIBD) at Karachi, Pakistan after approval from institution’s ethical review committee. The study included adult male and female participants with age range from 18 to 60 years. Participants have been recruited from different healthcare institutes and companies of Karachi, Pakistan. They have been categorized into three groups, including industrial workers, healthcare workers (HCWs) and subjects from the community comprised of walk-in patients for testing of anti-SARS-CoV-2 antibody test and healthy blood donors. Blood sample was collected in gel vacutainer (3–5 ml) from all subjects. The anti-SARS-CoV-2 test was performed by electrochemiluminescence immunoassay analyzer method on Cobas e-411 by Roche diagnostics International Ltd at Rotkreuz, Switzerland. It is a qualitative total antibody assay against SARS-CoV-2 (including IgG, IgM and IgA) that use double-antigen sandwich technique utilizing recombinant protein and detected antibodies against nucleocapsid (N) proteins of coronavirus. Result has been reported as reactive if the Cut-of-Index (COI) value is >1.0 and non-reactive if COI is <1.0 as per manufacture recommendations.
Statistical analysis
SPSS 23 has been used for the analysis of data; chi-square test has been applied to determine the significant difference among our three groups. P value of <0.05 is considered significant.
Results
This study has been conducted to assess the anti-SARS-CoV-2 antibodies levels in diverse group of residents to comprehend its prevalence in community. Samples have been received and tested for antibodies at pathology laboratory of NIBD, Karachi, Pakistan.
A total of 1675 samples have been received from three major groups of population including HCWs, industrial workers and people from community as shown in Figure 1. HCWs included from different hospitals. Industrial employees comprise of staff from pharmaceutical and hardware company. In addition, subjects from community included walk-in patients for testing of anti-SARS-CoV-2 antibody test and healthy blood donor. The pie chart below shows the breakdown of major sets of residents.
Fig. 1.
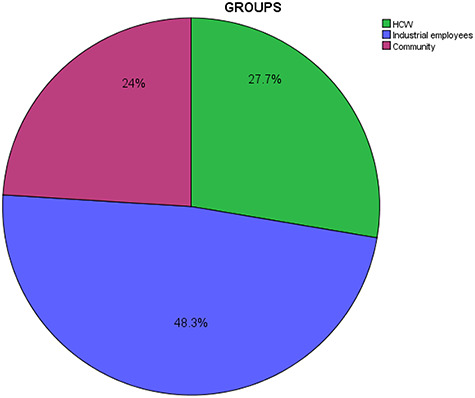
The figure showing the percentage inclusion of various residents including HCW, industrial employees and community population.
Table 1 presents the results obtained from preliminary analysis of antibodies positivity from various groups of population. Out of 1675 samples, 809 have been received from industrial employees, in which 407 (50.3%) were positive for SARS-CoV-2-specific antibodies. While 464 samples were received from HCWs in which 62 (13.2%) were positive for antibodies. In our community population, 135 (34%) cases were positive for antibodies out of 402 cases. The percentage positivity for industrial employees (50.3%) was high as compared with HCWs (13.2%) and community population (34%) as shown in Figure 2. Total percentage for positive antibodies result is ~36%. The most surprising aspect of the data that can be seen from Table 1 is that the industrial employees group reported significantly more than other two groups.
Table 1.
Serological expression from different groups of population
| Groups | Results | Total | |
|---|---|---|---|
| Negative (%) | Positive (%) | ||
| HCW | 402 (87) | 62 (13) | 464 |
| Industrial employees | 402 (49.6) | 407(50.3) | 809 |
| Community | 267 (66) | 135(34) | 402 |
| Total | 1071 (64) | 604 (36) | 1675 |
Table shows the positivity for SARS-CoV-2-specific antibodies for HCW, industrial employees and community.
Fig. 2.
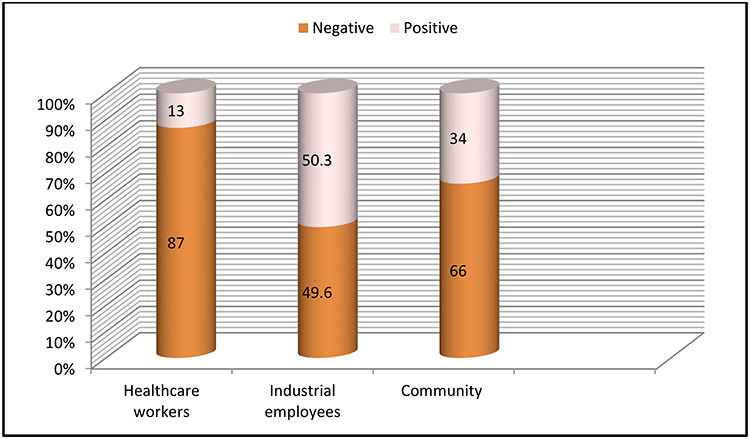
The above graph shows the percentage positivity for SARS-CoV-2 antibodies for HCW, industrial employees and community.
The correlation between HCWs, community people and industrial employees results were tested using chi-square test. The chi-square test showed significant differences between HCWs and industrial employee results. P-value of <0.05 has been considered as significant. There was also a difference in mean antibodies cutoff values among different groups as shown in Table 2. Mean antibodies cutoff values are high for HCW and community-based residents, which mostly comprise of healthy blood donors as compared with industrial employees. The mean antibodies cutoff values for HCW and community population were 20.59 and 28.9, respectively, whereas mean value for industrial employees was 18.8. In addition mean values for negative results were also noted that did not show significant difference. Together these results provide important insights into seroprevalence in diverse residents of Karachi.
Table 2.
Mean cut-off values for positive and negative test
| Groups | Mean cutoff value for positive test | Mean cutoff value for negative tests |
|---|---|---|
| HCWs | 20.594 | 0.237 |
| Industrial employees | 18.80 | 0.221 |
| Community | 28.93 | 0.091 |
Table shows the mean cutoff values for positive and negative results for all the groups.
Discussion
Main findings of the study
This study has been instigated to evaluate the seroprevalence of anti-SARS-CoV-2 antibodies in different healthcare and community population from Karachi and with the aim of assessing the importance of seroprevalence in these groups. The overall seroprevalence is found to be 36% with highest positivity in industrial employees (50.5%), whereas only 13% of HCW were tested positive. Moreover, the community that comprised of healthy blood donors and walk-in patients for antibody testing had 34% positivity rate. Seroprevalence is the incidence of a disease or illness within a distinct population at one time, as measured by serology tests. Therefore, serological recognition of specific antibodies against SARS-CoV-2 can better estimate the accurate number of infections. Different serology-based studies have been conducted worldwide, showing wide variations in positivity rate.
What is already known?
The seroprevalence rate identified in US population varies from 1.9 to 6.9%, which is very low as compared with our seroprevalence rate.13 Regarding European data, Stringhini et al.9 and Pollán et al.12 reported that the population-based serosurveillance from Switzerland and Spain composed of severely affected urban areas from April to early May 2020. The seropositivity rates reported were 10.8 and 5.0% from Switzerland and Spain, respectively. Surveillance report from France reported the prevalence rate of ~25.9%.14 Although our results are in contrast with western population findings, it is in consistent with the neighboring population results i.e. 22% for Iran and 22–33% for India.15,16 Very little was found in the literature on the question of increased seropositivity in Asian countries. However, there could be multiple reasons for this interesting finding and some of the points need more clarification and further research. There are different researches conducted globally emphasizing that increased exposures to different microorganisms including viruses, bacteria and parasites are playing an important role in providing the immunity to SARS-CoV-2 and showing the high seroprevalence rate in these Asian population.17 Moreover, in early days of pandemic, some studies have stated that population receiving BCG vaccinations is more protected against COVID-19 and has mild infection consequently.18 Likewise, increased temperature and high humidity is also considered to have defending role against severe SARS-CoV-2 infection and thus leading to asymptomatic and mild infection, which is resulting in high seroprevalence rate.19 Nevertheless, these hypotheses are not proven yet.
The most obvious finding to emerge from the analysis is that HCWs have shown the lower prevalence rate of 13% as compared with our two other groups, although it was hypothesized that participants with a history of healthcare work have highest prevalence. A possible explanation for this might be that our HCWs are majorly in low-risk category and are not directly involved in care of COVID-19-positive patients. There are unpublished data from one of the laboratories of Karachi that shows seroprevalence of ~32% in HCW.20 The seropositivity rate observed in this investigation is higher than those observed by Korth et al.21 from Germany. The study from Germany has categorized their healthcare population in three groups comprising of high risk, intermediate risk and low risk with 1.6, 5.4 and 0% positivity, respectively.21 Our results are in agreement with Chen et al.’s22 findings that showed the seroprevalence of 17% in HCWs of China.
The second important finding reported is the prevalence rate of 34% from community population including walk-in patients and healthy blood donors. If we analyze the breakdown of the community population, then our walk-in patients has 15% prevalence rate, whereas the blood donors have shown 36% of seropositivity. Bigg et al.23 has reported very low prevalence rate of 2.7% from Georgia counties as compared with our community group. Similarly, Stringhini et al.24 has reported the weekly prevalence from 3.1 to 9.7% in community population comprising of household persons from Geneva, Switzerland in June 2020. Their seroprevalence percentage is close to our findings. While if we observe our healthy blood donors results that is showing high seropositivity of 36% as compared with other walk-in patients group. The seroprevalence of 23% has been reported from Italy in healthy blood donors and 11.5% has been reported from Spain.25,26 Findings from Italy is in consistent with our results; however, very low prevalence 0.9% has been reported from another European country (Germany).27 Filho et al.28 from Brazil has reported the 4.0% prevalence among blood donors, which is again low as compared with our findings. So, there is variability worldwide in seroprevalence of blood donors.
The surprising finding was the highest seroprevalence rate of 50.3% in industrial employees. Jerkovic et al.29 has reported the seroprevalence of 1.27% in industrial workers that is far lower than our seroprevalence rate. This finding was unexpected and a possible explanation for this might be the delay in the implementation of lockdown measures. Investigation was done and found out that many of the cases were asymptomatic. The staff were quarantined for 2 weeks and advised to work from home. The average pay of general Pakistani staff is $150–200 per month, and this contradictory result may be due to the underprivileged living conditions/circumstances including small houses with poor ventilation. On the other hand, this also shows the higher percentage of mild and asymptomatic cases in our population that correlates with international data of developing countries.
What this study adds?
The present study raises the possibility that if 36% of adult population of Karachi is supposed to be seropositive, then we can hypothesize that in next 2–3 months ~60% of general population will become seropositive. This assumption or theory if proven right then it will reduce the chance of second wave in Karachi and increase the possibility of acquiring herd immunity. Moreover, we need to assess the need of vaccine in seropositive individuals by having further research on neutralization ability and half-life of these antibodies.
Alternatively, there are studies conducted globally that stated the decline and waning of antibodies titer with time, which will pose a huge challenge for the people worldwide and overall in acquiring herd immunity for COVID 19.30 These findings raise intriguing questions regarding the nature and extent of antibodies-based protection and resulting seropositivity. Furthermore, it also warrants the need of vaccine as in this case second wave and subsequent outbreak is possible.
Currently, there are different phase trials for vaccines are in development in various countries of the world.31 Recently Russian company has started their first human clinical trials for their vaccine after having successful phase 1 results.32
Future recommendation and limitations of the study
The main goal of the current study was to determine the seroprevalence, and our results complement those earlier studies from Asian countries; however, they are distinct from developed world. There are still many unanswered questions about this disparity in results, and we suggest further research should be undertaken to investigate the virological and immunological aspects of SARS-CoV-2. Our seroprevalence is 36%, and it is still bit far from herd immunity that needs to be at least 60–70% in general population. In addition, if we consider acquiring 60% of seroprevalence in next couple of months, then herd immunity is not far from reality provided the antibodies did not decline with time. The principal theoretical implication of this study is that high seroprevalence occurrence will surely help us in deciding the national policies and preventive measures. Although the current study is based on a small sample of participants, the findings suggest a nationwide study with larger population to implement stronger and targeted interventions. We also recommend continuing the preventive procedures and robust efforts for vaccine development.
Conflict of interest
None.
Samreen Zaidi, Consultant Paediatrician and Paediatric ID Specialist
Faiza Rizwan, Consultant Clinical Microbiologist
Quratulain Riaz, Consultant Haematologist and Oncologist
Asma Siddiqui, Consultant Physician Internal Medicine
Shabnan Khawaja, Consultant Chemical Pathologist
Mehjabeen Imam, Consultant Immunologist
Arshi Naz, Assistant Professor and Scientific Manager
Samra Waheed, Consultant Haematologist
Tahir Shamsi, Dean, Postgraduate Studies, Chairman & Professor, Consultant Haematologist and Bone Transplant Physician
Contributor Information
Samreen Zaidi, Department of Paediatrics and Paediatric Infectious Diseases, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Faiza Rizwan, Department of Microbiology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Quratulain Riaz, Department of Haematology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Asma Siddiqui, Department of Internal Medicine, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Shabnam Khawaja, Department of Chemical Pathology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Mehjabeen Imam, Department of Immunology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Arshi Naz, Department of Haematology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Samra Waheed, Department of Haematology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
Tahir Shamsi, Department of Haematology, National Institute of Blood Diseases and Bone Marrow Transplant, Karachi 75300, Pakistan.
References
- 1. Unhale SS, Ansar QB, Sanap S et al. A review on corona virus (Covid-19). World J Pharm Life Sci 2020;6(4):109–15. [Google Scholar]
- 2. Rodriguez-Morales AJ, Bonilla-Aldana DK, Tiwari R et al. COVID-19, an emerging coronavirus infection: current scenario and recent developments-an overview. J Pure Appl Microbiol 2020;14:6150. [Google Scholar]
- 3. Cascella M, Rajnik M, Cuomo A et al. Features, Evaluation and Treatment Coronavirus (COVID-19) In Statpearls [internet] StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 4. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020;92(6):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Health Commission of China The guidelines for diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (the sixth edition draft) issued by the National Health Commission of China. http://www.gov.cn/zhengce/zhengceku/2020%2002/19/content_5480948.htm2020(accessed February, 2020).
- 7. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm (4 August 2020, date last accessed).
- 8. WHO, Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Avaiable from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (18 August 2020, date last accessed).
- 9. Stringhini S, Wisniak A, Piumatti G et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020 Jun;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Havers FP, Reed C, Lim T et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Government of Pakistan. Avaiable from: http://covid.gov.pk (18 August 2020, date last accessed).
- 12. Pollán M, Pérez-Gómez B, Pastor-Barriuso R et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020;396(10250):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19) [Internet]. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/geographic-seroprevalence-surveys.html (18 August 2020, date last accessed).
- 14. Fontanet A, Tondeur L, Madec Y et al. Cluster of COVID-19 in northern France: a retrospective closed cohort study. medRxiv Preprint posted online April 23, 2020. doi: 10.1101/2020.04.18.20071134. [DOI]
- 15. Shakiba M, Nazari S, Mehrabian F et al. Seroprevalence of COVID-19 virus infection in Guilan province. Iran. medRxiv 2020;26. doi: 10.1101/2020.04. [DOI] [Google Scholar]
- 16. Bhattacharyya R, Bhaduri R, Kundu R et al. Reconciling epidemiological models with misclassified case-counts for SARS-CoV-2 with seroprevalence surveys: a case study in Delhi, India. medRxiv 2020. [Google Scholar]
- 17. Riggioni C, Comberiati P, Giovannini M et al. A compendium answering 150 questions on COVID-19 and SARS-CoV-2. Allergy 2020 June;10–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abbas M, AbouBakr A, Bahaa N et al. The effect of BCG vaccine in the era of COVID-19 pandemic. Scand J Immunol 2020. doi: 10.1111/sji.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sajadi MM, Habibzadeh P, Vintzileos A et al. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19). JAMA Network Open 2020;3(6):e2011834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chughtai O. Preliminary results of the @ChughtaiLab survey of doctors / nurses / allied healthcare workers across Pakistan for #COVID antibodies. [Internet] 2020[cited 5 August 2020]: https://twitter.com/OmarChughtai/status/1290939416840736768 (5 August 2020, date last accessed).
- 21. Korth J, Wilde B, Dolff S et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol 2020;104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Tong X, Wang J et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81(3):420–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biggs HM, Harris JB, Breakwell L et al. Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia counties, April 28–May 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69(29):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stringhini S, Wisniak A, Piumatti G et al. Repeated seroprevalence of anti-SARS-CoV-2 IgG antibodies in a population-based sample from Geneva, Switzerland. medRxiv 2020. doi: org/ 10.1101/2020.05.02.20088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Percivalle E, Cambiè G, Cassaniti I et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi red zone in Lombardy, Italy, as at 06 April 2020. Eurosurveillance 2020;25(24):2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang L, Hou W, Zhao L et al. The prevalence of antibodies to SARS-CoV-2 among blood donors in China. medRxiv 2020. doi: 10.1101/2020.07.13.20153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fischer B, Knabbe C, Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Eurosurveillance 2020;25(28):2001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amorim Filho L, Szwarcwald CL, Mateos SD et al. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Revista de saude publica 2020;54:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jerkovic I, Ljubic T, Basic Z et al. SARS-CoV-2 antibody seroprevalence in industry workers in Split-Dalmatia and Sibenik-Knin County, Croatia. medRxiv 2020. doi: 10.1101/2020.05.11.20095158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crawford KH, Dingens AS, Eguia R et al. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. medRxiv 2020. doi: 10.1101/2020.08.06.20169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. JAMA 2020;323:2462–63. doi: 10.1101/2020.07.13.20153106. [DOI] [PubMed] [Google Scholar]
- 32. Clinical Trials Sputnikvaccine.com. [Internet] 2020[cited 18 August 2020]: https://sputnikvaccine.com/about-vaccine/clinical-trials/ (18 August 2020, date last accessed).


