To the Editors,
The global spreading of severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection1–3 has led to a deep reorganization of hospital services affecting inflammatory bowel disease (IBD) units activity worldwide.4,5 In many cases, scheduled follow-up visits were cancelled or rescheduled, and access to outpatient clinics was limited to unstable and relapsing patients. A delay in scheduled therapeutic infusions was also reported due to patient’s decision or practical issues.6
Inflammatory bowel disease patients, especially those under biological and immunosuppressive therapies, are believed to have a higher risk to be infected by SARS-CoV-2. Moreover, early treatment of coronavirus disease 2019 (COVID-19) is associated with better outcomes.7 For these reasons, in a context of strongly limited interactions between IBD patients and IBD care providers, many referral centers started to screen patients for COVID-19-related symptoms by phone or video call interviews.
To quantify the capacity of such an approach to identify early stages of COVID-19, the SoCOVID-19 survey, which contained 11 questions related to the activity of remote screening for COVID-19-related symptoms and signs (Table 1) was sent by the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) to all Italian referral centers for the diagnosis and therapy of IBD.
TABLE 1.
List of Questions Sent to Participating Centers Related to the Activity of Remote Screening Activity for COVID-19
| No. | Questions |
|---|---|
| 1 | How many IBD patients in active follow-up at your center have been contacted by phone or video call in order to identify suspected cases of COVID19? |
| 2 | How many patients reported the following symptoms or signs? |
| Fever | |
| …Cough | |
| …Dyspnea? | |
| …Anosmia? | |
| …Dysgeusia? | |
| …Diarrhea? | |
| 3 | How many patients resulted suspected for COVID19 and received indication to perform SARS-CoV-2 RT-PCR test? |
| 4 | How many suspected patients who received SARS-CoV-2 RT-PCR resulted positive? |
| 5 | How many of the identified positive patients were admitted to the hospital? |
| 6 | How many of the identified positive patients were admitted to ICU? |
| 7 | How many of the identified positive patients died? |
| 8 | How many patients defined as suspected (confirmed and not confirmed) were contacted again after 2 weeks or more from the initial contact? |
| 9 | How many patients recovered? |
| 10 | In how many patients with suspicious symptoms or signs the therapy was self-suspended? |
| 11 | In how many patients with suspicious symptoms or signs the therapy was modified by the contacting IBD specialist? |
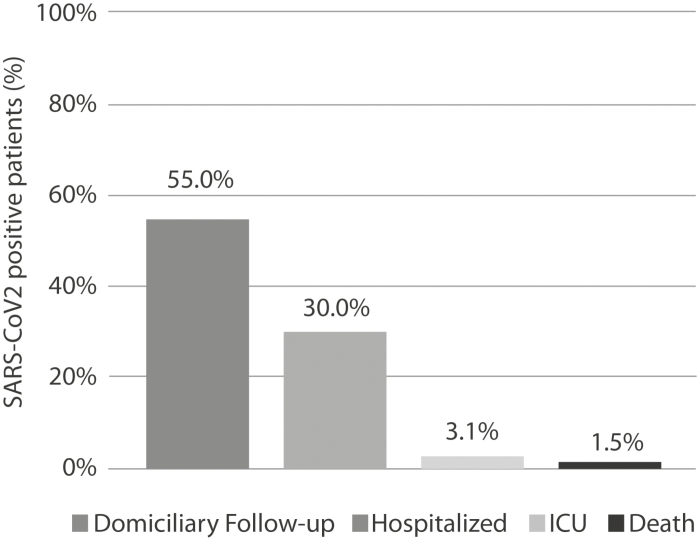
In the period between March 9 and April 14, 2020, 4304 IBD patients in active follow-up at 24 centers throughout Italy, 13 (52%) of which located in the northern Italy—the most affected area in the country—were contacted by phone or video calls. One hundred patients (2.3%) reported symptoms and/or signs compatible with COVID-19, defined as having at least 2 symptoms among fever, cough, dyspnea, anosmia, dysgeusia, and diarrhea. The most common symptom was diarrhea (63.0%), followed by fever (54.0%), cough (53.0%), anosmia (16.0%), dysgeusia (16.0%), and dyspnea (10.0%). Sixty-five (1.2%) patients with suspicious symptoms were judged eligible for SARS-CoV-2 nasopharyngeal swab by the local health care system authorities, and 20 patients (30.8%) resulted positive. Six positive patients (30.0%) were admitted to the hospital, and 2 (10.0%) were transferred to the intensive care unit (ICU). A single patient died from COVID-19 among those initially remotely identified. The remaining 11 patients (55.0%) did not require hospitalization and received domiciliary follow-up (Fig. 1). Seventy-seven patients (77.0%) among those with symptoms compatible with COVID-19 were re-interviewed after 2 weeks from the initial contact to check their health status, and 70 patients (90.9%) referred complete resolution of symptoms. During this period, none of the patients autonomously suspended the therapy prescribed by the IBD specialist, although for 24 patients (24.0%), therapy was modified by the contacting center due to suspected symptoms, reflecting the scarce awareness and uncertainty about the risk of concomitant therapies on COVID-19 clinical course at the beginning of the pandemic.
FIGURE 1.
Outcomes of IBD patients with positive naso-pharyngeal swab testing remotely identified. ICU: intensive care unit.
Overall, data from our survey indicate that in order to identify one SARS-CoV-2 positive IBD patient, around 215 patients had to be contacted and interviewed. Diarrhea was overreported as compared with the COVID-19 general population, and it was probably related to IBD clinical activity.8,9 The proactive remote identification of positive patients did not seemingly change the clinical outcome. Indeed, the rate of hospitalization among the SARS-CoV-2 positive patients was in line with that recently reported in a prospective Italian cohort study of IBD patients affected by COVID-19.8 Yet, remote screening of IBD patients for suspicious signs and symptoms for COVID-19 might reduce the risk of contact between IBD care providers and potentially infected patients who are candidate to SARS-CoV-2 molecular testing.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has determined a so far never seen challenge for national health care systems. In-person follow-up visits and diagnostic procedures of patients affected by chronic diseases such as inflammatory bowel fisease (IBD) have been delayed and, in many cases, even canceled. At the same time, IBD patients are considered immunologically fragile subjects due to wide use of immunosuppressants and immunomodulators, and a tight monitoring of early COVID-19-related symptoms is advisable. For this reason, spontaneous activity of telemedicine started in many IBD centers around the world during the lockdown. Here we report results from the SoCOVID-19 survey exploring the detection rate and outcomes of COVID-19 IBD patients remotely identified telemedicine.
Conflicts of interest: MCF received consultancy fees from Abbvie, Takeda, Jannsen-Cilag, Pfizer, and Sandoz and research grants from Pfizer and Jannsen-Cilag. CB received lecture fees from Takeda, AbbVie, and Janssen. SS received lecture fees from Takeda Pharmaceuticals and Janssen Pharmaceuticals and served as a consultant and a member of advisory boards for AbbVie and Janssen Pharmaceuticals. AM received consultancy fees from Abbvie, Janssen, MSD, Mundipharma, and Takeda. DGR received consultancy fees from Janssen, Ferring, and Errekappa. FB received consultancy fees from Mundipharma, Abbvie, MSD, Takeda, Janssen, Biiogen, Celgene, and MSD. MA received consultancy fees from Nikkiso Europe, Mundipharma, Janssen, Abbvie, and Pfizer. ACP received consultancy fees from Mundipharma, Abbvie, MSD, Takeda, and Janssen; he received lecture fees from Abbvie. AV received consultancy fees from AbbVie, Janssen, and Pfizer. LG received consultancy fees from Abbvie, MSD Italia, and Takeda. ES received consultancy fees from Medtronic, Reckitt Benckiser, Takeda, Abbvie, Amgen, Novartis, Fresenius Kabi, Sandoz, Sofar, Malesci, Janssen, Grifols, Aurora Pharma, Innovamedica, Johnson&Johnson, SILA, Unifarco, Alfasigma, Shire, and EG Stada Group. RP received consultancy fees from Abbvie, Mundipharma, and Takeda. MD served as a speaker, consultant and advisory board member for AbbVie, Takeda, Janssen, Norgine, Pfizer, MSD, Celltrion, Roche, Gilead, Bioclinica, Ferring, SOFAR, Chiesi, and Zambon. FC served as consultant and a member of advisory board for Mundipharma, AbbVie, MS&D, Takeda, Janssen, Roche, and Celgene and received lecture fees from AbbVie, Amgen, Ferring, Takeda, and Allergy Therapeutics. MaC received consultancy fees from AbbVie, Ferring, Janssen, MSD, Takeda, and Shire. SF received consultancy fees from Takeda, SOFAR, Alfa-Wasserman, Ferring, Abbvie, and Zambon. PB received consultancy fees from Janssen, Abbvie, and MSD. AA received consultancy fees from AbbVie, Amgen, Biogen, Ferring, Gilead, Janssen, MSD, Mitsubishi-Tanabe, Nikkiso, Pfizer, Sandoz, Samsung Bioepis, and Takeda and research grants from MSD, Pfizer, and Takeda. SS received lecture fees from Takeda Pharmaceuticals and Janssen Pharmaceuticals and served as a consultant and a member of advisory boards for AbbVie and Janssen Pharmaceuticals. GF received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis, and Celltrion. All other authors have no conflicts of interest.
REFERENCES
- 1. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. World Health Organization. Rolling Updates on Coronavirus Disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen [Google Scholar]
- 4. Fiorino G, Allocca M, Furfaro F, et al. Inflammatory bowel disease care in the COVID-19 pandemic era: the Humanitas, Milan experience. J Crohn’s Colitis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. [DOI] [PubMed] [Google Scholar]
- 6. Scaldaferri F, Pugliese D, Privitera G, et al. Impact of COVID-19 pandemic on the daily management of biotechnological therapy in inflammatory bowel disease patients: reorganisational response in a high-volume Italian inflammatory bowel disease centre. United European Gastroenterol J. 2020:2050640620929133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020. [DOI] [PubMed] [Google Scholar]
- 8. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020. [DOI] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for COVID-19 Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]