To the Editor—
In response to the COVID-19 pandemic, many ambulatory practices in the United States delayed, canceled, or converted visits from in-person to telemedicine. Social distancing and masking decreased the transmission of many viral respiratory illnesses [1–3]. To assess the impact of these measures on ambulatory pediatric antibiotic prescribing, we performed a pre-post study comparing diagnoses and antibiotic prescription rates for children pre-pandemic (P1, March 1, 2019–May 15, 2019) and during the early pandemic (P2, March 1, 2020–May 15, 2020).
Encounters for children (≤18 years) in 4 ambulatory settings affiliated with Vanderbilt University Medical Center were included: the emergency department, urgent care clinics, primary care clinics, and retail health clinics (see Supplementary Methods). Diagnoses and electronic antibiotic prescriptions were extracted from the electronic medical record. Encounter diagnosis was defined as the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) diagnosis associated with the antibiotic prescription or the primary encounter diagnosis if no antibiotic was prescribed. Encounters with multiple diagnoses were assigned to the infectious group if any infectious diagnosis was made. Infectious diagnoses are listed in Supplementary Methods. Outcomes between P1 and P2 were compared using the Student’s t-test for continuous variables, χ 2 tests for categorical variables, and interrupted time series analysis was performed using Stata/IC version 15.1 for MAC (College Station, TX). The study was approved by the Vanderbilt University Insitutional Review Board (IRB).
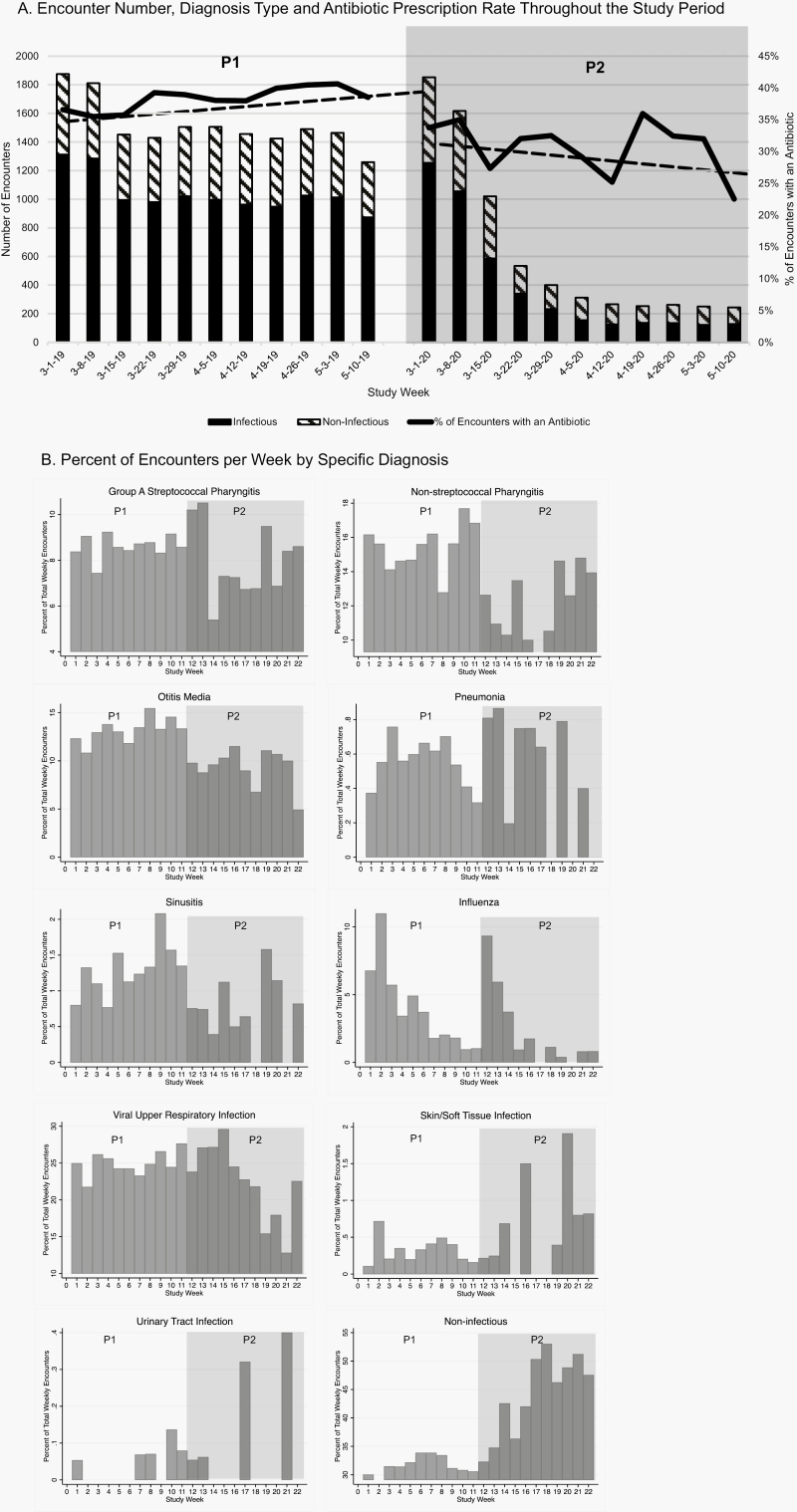
Overall encounter numbers decreased significantly in P2 compared with P1 (7010 vs 16671, P < .001, Figure 1A). The percent of encounters for infectious diagnoses was lower in P2 (4267/7010, 60.8%) vs P1 (11 412/16 671, 68.5%) (P < .001), especially for respiratory diagnoses (Figure 1B and Supplementary Figure). Conversely, the percent of encounters with noninfectious diagnoses was higher in P2 (2743/7010, 39%) than in P1 (5259/16 671, 31.5%), (P = .001, Figure 1B and Supplementary Figure). The percent of encounters with an antibiotic prescription was lower in P2 than P1 for all encounters (P2: 2240/7010 [32%]; P1 6373/16 671 [38.2%], P < .001, Figure 1A), and among encounters with infectious diagnoses (P2: 1324/2943 [45%]; P1: 3941/7471 [52.8%], P < .001).
Figure 1.
Infectious diagnoses = viral upper respiratory tract infection, pharyngitis, otitis media, sinusitis, pneumonia, abscess, skin/soft tissue infection, or urinary tract infection. All other diagnoses were considered noninfectious. (A) Encounter number, diagnosis type, and antibiotic prescription rate throughout the study period. Dark gray bars are number of encounters with infectious diagnoses, and striped bars are number of encounters with noninfectious diagnoses. Percent of encounters with an antibiotic prescribed is shown in the black line. Dashed line represents interrupted time series analysis trend line for percent of encounters with an antibiotic prescription in P1 and P2, respectively. P-value comparing slope in P1 vs P2 = 0.03. (B) Percent of encounters per week by specific diagnosis. Abbreviations: P1, pre-pandemic (March 1, 2019–May 15, 2019); P2, early pandemic (March 1, 2020–May 15, 2020), represented by the gray shaded area.
In our health system, the early COVID-19 pandemic was associated with significant declines in in-person encounters, infectious diagnoses, and overall antibiotic prescribing and a significant increase in visits for noninfectious diagnoses. Even among encounters with infectious diagnoses, we observed a reduction in antibiotic prescribing. This is likely because social distancing measures decreased the transmission of viral illnesses and secondary bacterial infections, as described elsewhere [1, 3]. Notably, we did not see a decline in diagnosis and prescription rates for nonrespiratory illnesses such as urinary tract infections and skin/soft tissue infections, supporting this theory.
Study strengths include the high volume of encounters, the variety of outpatient settings assessed, and the ability to match the 2 study periods by time of year to account for seasonal variation. Study limitations include the short P2 time period and lack of generalizability to communities with different demographics, infection prevalence, or mitigation strategies. Finally, we evaluated antibiotic prescribing at the visit level, not at the patient level.
In conclusion, early in the COVID-19 pandemic, we observed a significant reduction in outpatient infection-related visits and antibiotic prescribing for children. Whether this trend will continue in the face of relaxing social distancing measures, ongoing community transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the upcoming influenza season is unknown and warrants evaluation. Future studies should also investigate the relationship between fewer outpatient encounters and unintended consequences like delayed diagnoses [4].
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. S.K. received grant support from the Centers for Disease Control and Prevention as a recipient of the Leadership in Epidemiology, Antimicrobial Stewardship and Public Health (LEAP) fellowship, sponsored by the Society for Healthcare Epidemiology of America (SHEA), Infectious Diseases Society of America (IDSA), and Pediatric Infectious Diseases Society (PIDS).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. [Published online ahead of print September 1, 2020]. doi: 10.1542/peds.2020-006460. [DOI] [PubMed] [Google Scholar]
- 2. Jones N. How coronavirus lockdowns stopped flu in its tracks. Nature. [Published online ahead of print May 21, 2020]. doi: 10.1038/d41586-020-01538-8. [DOI] [PubMed] [Google Scholar]
- 3. Yang DD, Ouldali N, Gajdos V, et al. Common pediatric respiratory infectious diseases may serve as an early predictor for SARS-CoV-2 new wave of infections [Published online ahead of print September 7, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health 2020;4:e10–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.