To the Editor—Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) drew concern early in the coronavirus disease 2019 (COVID-19) pandemic. This concern was rooted in prior observations that these medications were associated with increased expression of ACE2, the well-described receptor used by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This fear was amplified by the recognition that several disease states (hypertension, diabetes, and chronic kidney disease) associated with increased COVID-19 risk cause upregulation of ACE2; however, several cardiovascular medicine governing bodies have quelled anxieties by recommending that patients continue ACEIs and ARBs following evidence demonstrating absence of risk. The recent study by Lam et al [1] takes this one step further. They demonstrated that when ACEIs and ARBs were continued during in-hospital treatment for COVID-19, there was a reduction in intensive care unit admissions by approximately 50% and reduced mortality (6% vs 28%; P = .001; odds ratio = 0.215; 95% confidence interval [CI], .101–.455) compared with a non-ACEI/ARB group. This result likely sheds light on the central pathophysiology of this disease and supports the notion that SARS-CoV-2 exploits individuals with baseline nitric oxide (NO) deficiency.
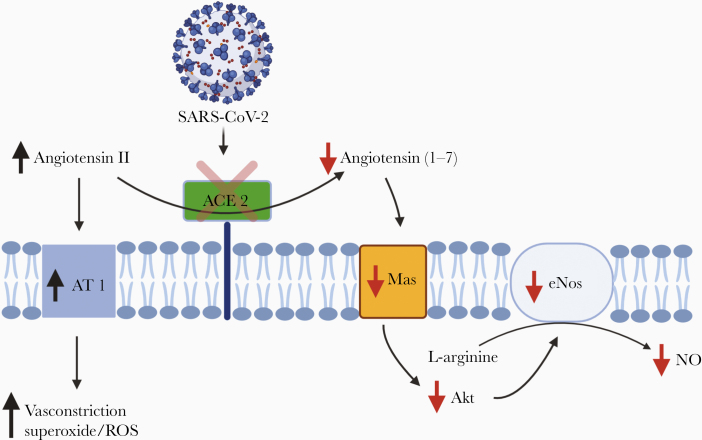
ACE2 functions as a counter-regulatory enzyme to ACE1. While ACE1 converts angiotensin I to the pro-oxidant and vasoconstrictor angiotensin II, ACE2 degrades angiotensin II via conversion to angiotensin (1–7). Angiotensin (1–7) stimulates a transmembrane receptor Mas, which cascades to Akt phosphorylation and activation of endothelial NO synthase (eNOS), thereby increasing NO production. This process is referred to as the ACE2-angiotensin (1–7)-Mas axis (Figure 1).
Figure 1.
Downstream effects of SARS-CoV-2 antagonism of ACE2. Abbreviations: ACE2, angiotensin-converting enzyme 2; Akt, protein kinase B; AT 1, angiotensin 1 receptor; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The resultant NO has pleiotropic functions including vessel dilation, anti-inflammation, anticoagulation, and immune system activation. In other respiratory diseases such as influenza, decreased ACE2 expression is associated with worse outcomes, and this effect is attributed to the downregulation of the ACE2-agniotensin (1–7)-Mas axis [2]. Additionally, NO has been shown to have direct antimicrobial activity against a range of viruses, fungi, helminths, bacteria, and protozoa [3]. More specifically, it has been demonstrated to be viricidal against SARS-CoV-1 in addition to decreasing its effectiveness to latch to the ACE2 receptor via depalmitoylation of the spike protein [4].
Both ACEIs and ARBs are thought to increase ACE2 expression and/or activity, and this alone would augment NO production via the mechanism described above; however, these medications increase NO independent of ACE2 as well. Blocking ACE1, for instance, increases bradykinin, which through a series of intracellular steps increases eNOS activity [5]. Meanwhile, the mechanism by which ARBs increase NO is less clear and may simply reflect decreased NO scavenging by radicals [6].
If ACE2 is protective, then why do those most at risk have increased ACE2 expression? It is important to recognize that the upregulation of ACE2 observed in pathology is compensatory for increased reactive oxygen species (ROS) and NO deficiency. Furthermore, it is equally important to recognize that this compensatory mechanism is insufficient to correct the underlying NO deficiency. SARS-CoV-2 likely acts as an ACE2 antagonist, thereby suppressing ACE2 activity, which has been demonstrated for SARS-CoV-1. This further inhibits NO formation via the ACE2-angiotensin (1–7)-Mas axis, and increases radical production via accumulating angiotensin II, which explains why this disease appears to exploit populations deficient in NO.
If SARS-CoV-2 inhibition of ACE2 facilitates the predominant pathology of COVID-19 via radical production and NO deficiency, then medications influencing NO should have predictable effects. The study by Lam et al is the third to date that demonstrates reduced mortality associated with in-hospital ACEI/ARB use, albeit the largest, consisting of 6235 patients [1]. Zhang et al also evaluated in-hospital use of both ACE-I/ARBs and found a significant reduction in mortality associated with the medications (adjusted hazard ratio, 0.37; 95% CI, .15–.89; P = .03) [7]. Meng et al demonstrated that the use of ACEIs/ARBs was associated with reduced viral load and inflammatory markers, and increased CD3 and CD8 counts [8].
Additionally, inhaled NO was effective for SARS-CoV-1, while historically this treatment has produced generally unsatisfactory results in non-SARS–induced acute respiratory distress syndrome [9]. The described pathology may explain this discrepancy. Statins are potent inducers of eNOS via multiple pathways and are also associated with improved outcomes. Meanwhile, proton pump inhibitors, which inhibit eNOS via an accumulation of asymmetric dimethylarginine, have recently been shown to substantially increase risk of COVID-19.
The results presented by Lam et al [1], as well as others, may reveal important pathophysiologic mechanisms of SARS-CoV-2. The targeting of ACE2 inhibition and subsequent downstream effects, combined with the vascular sequelae of COVID-19, is suggestive of a central pathology of NO deficiency.
Notes
Conflict of interests. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lam KW, Chow KW, Vo J, et al. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcomes. J Infect Dis 2020; 222:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang P, Gu H, Zhao Z, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep 2014; 4:7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martel J, Ko YF, Young JD, Ojcius DM. Could nasal nitric oxide help to mitigate the severity of COVID-19? Microbes Infect 2020; 22:168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akerström S, Gunalan V, Keng CT, Tan YJ, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology 2009; 395:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desideri G, Grassi D, Croce G, et al. Different effects of angiotensin converting enzyme inhibitors on endothelin-1 and nitric oxide balance in human vascular endothelial cells: evidence of an oxidant-sensitive pathway. Mediators Inflamm 2008; 2008:305087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mason RP, Jacob RF, Kubant R, et al. Effects of angiotensin receptor blockers on endothelial nitric oxide release: the role of eNOS variants. Br J Clin Pharmacol 2012; 74:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126:1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 2020; 9:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adusumilli NC, Zhang D, Friedman JM, Friedman AJ. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide 2020; 103:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]