Abstract
Background
The clinical manifestations and natural history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related multisystem inflammatory syndrome in children (MIS-C) are poorly defined. Using a systematic review of individual cases and case series and collating elements of the clinical course, the objective of this study was to provide a detailed clinical description and natural history of MIS-C.
Methods
Case reports and series of MIS-C were recovered from repeated MEDLINE searches, a single EMBASE search, and table of contents reviews of major general medicine and pediatric journals performed between June 3 and July 23, 2020. Fever, evidence of inflammation, and evidence of organ dysfunction were required for inclusion.
Results
MEDLINE and EMBASE searches produced 129 articles, and 10 articles were identified from journal contents or article bibliographies; 16 reports describing 505 children with MIS-C comprise this review. Thirty-two children (14.7%) had negative results for SARS-CoV-2 by nucleic acid and/or antibody testing. The weighted median age was 9 years (6 months to 20 years). Clinical findings included fever (100%), gastrointestinal symptoms (88.0%), rash (59.2%), conjunctivitis (50.0%), cheilitis/ “strawberry tongue” (55.7%), or extremity edema/erythema (47.5%). Median serum C-reactive protein, ferritin, fibrinogen, and D-dimer concentrations were above the normal range. Intravenous gammaglobulin (78.1%) and methylprednisolone/prednisone (57.6%) were the most common therapeutic interventions; immunomodulation was used in 24.3% of cases. Myocardial dysfunction requiring ionotropic support (57.4%) plus extracorporeal membrane oxygenation (5.3%), respiratory distress requiring mechanical ventilation (26.1%), and acute kidney injury (11.9%) were the major complications; anticoagulation was used commonly (54.4%), but thrombotic events occurred rarely (3.5%). Seven (1.4%) children died.
Conclusions
MIS-C following SARS-CoV-2 infection frequently presents with gastrointestinal complaints and/or rash; conjunctivitis, cheilitis, and/or extremity changes also occur frequently. Serious complications occur frequently and respond to aggressive supportive therapy.
Keywords: MIS-C, SARS-CoV-2, systematic review
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–associated multisystem inflammatory syndrome in children is a serious complication of pediatric SARS-CoV-2 requiring intensive care in the majority of cases.
The earliest reports of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in children suggested a benign disease course. Dong et al described the clinical manifestations of SARS-CoV-2 in 2143 pediatric patients in China [1]. The majority of cases were diagnosed by clinical criteria alone. More than half of the children had asymptomatic or mild illnesses, the latter defined by symptoms characteristic of a viral upper respiratory tract infection. There was only 1 death in this series. A systematic review of pediatric cases had similar findings and identified 2 more pediatric deaths [2].
In mid-April 2020, Riphagen et al described 6 British children with SARS-CoV-2 infection associated with hyperinflammatory findings and hemodynamic shock; all of these children required intensive care and ionotropic support [3]. This report was quickly followed by another case from the United Kingdom (UK) and a case series of 10 older Italian children with a Kawasaki-like syndrome accompanied by hemodynamic instability [4, 5].
The purpose of this systematic review was to consolidate the individual case reports and case series of SARS-CoV-2 multisystem inflammatory syndrome in children (MIS-C) in order to provide a detailed picture of clinical and laboratory manifestations of this disorder, characterize its inflammatory nature, define and quantify its complications, describe the therapeutic interventions that have been employed, and determine its outcome.
METHODS
This was a systematic review performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Since the objective of this study was to provide a detailed clinical description and natural history of SARS-CoV-2–related MIS-C, no meta-analysis was performed.
Search Strategy and Selection Criteria
The MEDLINE database was searched twice (June 3, 2020 and June 8, 2020) using the terms “Covid 19,” ”inflammatory,” and “children”; the EMBASE database was searched once on June 22, 2020 (by S. C. A.). Because the topic was new and articles appeared frequently, the online first, coronavirus disease 2019 (COVID-19) specialty sections, and tables of contents of the JAMA Network journals, the Lancet Network journals, the New England Journal of Medicine, Pediatrics, BMJ, the Pediatric Infectious Disease Journal, and the Journal of the Pediatric Infectious Diseases Society were monitored weekly, beginning June 3, 2020 through July 23, 2020. The references from all included articles were also reviewed. No language limitations were imposed.
The clinical definition of MIS-C was adapted from the guidance announcement provided by the Royal College of Paediatrics and Child Health describing pediatric multisystem inflammatory syndrome temporally associated with COVID-19: fever, evidence of inflammation, and evidence of single or multisystem organ injury [6]. Demonstration of serum antibody or nucleic acid sequences of SARS-CoV-2 in nasopharyngeal washings or stool was not required for inclusion. Study inclusion was limited to case series or case reports of children with suspected MIS-C. Epidemiology studies, case reports, and case series of SARS-CoV-2 infections among neonates, infants, children, and adolescents that did not include MIS-C; narrative reviews; and editorials were excluded. Discrepancies in eligibility were resolved by conference (S. C. A. and M. T. D. V.).
Studies were initially screened by title and abstract; those articles that were not case series or case reports of MIS-C were excluded. The full text of the remaining articles was reviewed to ensure that inclusion criteria were met and that the cases reported were not included in other studies.
Data Extraction
The specific features that were extracted from each study are detailed in Supplementary Table 1. Median age and age range, signs and symptoms at the time of presentation, common laboratory tests, inflammatory markers, echocardiographic and chest imaging results, evidence of SARS-CoV-2 infection (nucleic acid testing of nasal washings or stool, immunoglobulin G (IgG) antibody to the virus, and recent exposure to infected individuals), therapeutic interventions, and outcomes were recorded for each study by 1 author (S. C. A.) and checked by a second (A. H.). Every effort was made to include only those laboratory values, signs, and symptoms determined at the time of hospital admission.
Data Analysis
Analysis was limited to descriptive summary statistics. Discrete variables were recorded as the number positive for each study and summarized as percentages of the total number of children tested. Median, minimum, and maximum values for continuous variables were reported or calculated for each case series; individual values were reported for case reports. Continuous variables were summarized as the weighted median value from all of the studies that reported the variable; the minimum and maximum values identified across all included patients were also included in the data summary.
RESULTS
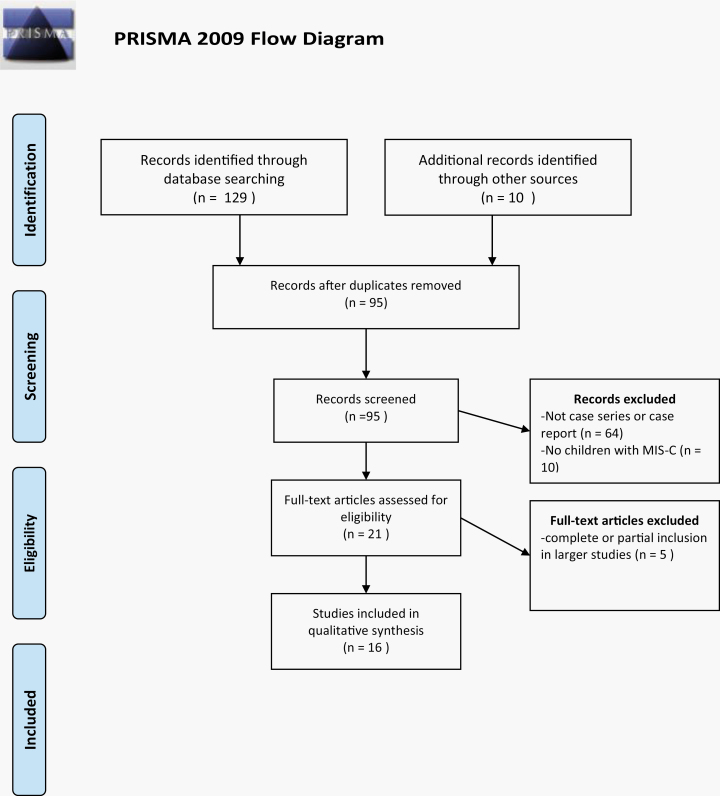
The initial MEDLINE search, performed on June 3, 2020, produced 37 articles; the search was repeated on June 8, 2020 and produced 85 articles. The EMBASE search performed on June 22, 2020 produced 7 articles. The final review of monitored journals was on July 23, 2020. An additional 10 reports were identified from the early access section or table of contents of the monitored journals or from the bibliographies of included references. The results of the data search are shown in Figure 1. The database searches yielded 129 articles. Ninety-five studies remained after duplicates were removed. Sixty-four studies were disqualified since they failed to meet the case report or case series criterion. Ten studies were case reports or case series but did not include children who met the definition of MIS-C. Twenty-one articles were reviewed in full; one study was excluded since it came from the same institution as an included article and the dates of hospitalization overlapped; the larger study was used [7, 8]. Two studies included in the multicenter study of Feldstein et al also were excluded [9–11]. One study from New York that covered the same time period as the multicenter New York report of Dufort et al was included since it was not identified as included in the larger report [8, 12]. The subjects of the original report by Riphagen et al were included among the 58 patients reported by Whittaker et al as well as the 78 subjects reported by Davies et al [3, 13, 14]. Twenty-nine of the 58 subjects reported by Whittaker et al were included in the large UK study. Since these cases could not be separated, the study of Whittaker et al was excluded as well. All 16 of the remaining studies were case reports or case series that met the inclusion criteria for MIS-C [4, 5, 8, 9, 12, 14–24]. Twenty-three patients met the case definition for MIS-C but failed to have SARS-CoV-2 infection demonstrated by nucleic acid or antibody test or to have a documented SARS-CoV-2 exposure within 4 weeks of onset of MIS-C symptoms [3, 5, 13, 17, 19, 21, 22]. Seven studies were isolated case reports, including one child with Crohn’s disease [4, 8, 10, 11, 13, 15–25]. The largest case series was from the United States (US) and included 186 children [9]. In the end, 505 children were included; the findings are detailed, by report, in Supplementary Table 1.
Figure 1.
Search flowchart. Abbreviations: MIS-C, multisystem inflammatory syndrome in children; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Clinical Presentation
The weighted median age was 9 years with an overall range of 6 months to 20 years. The clinical manifestations of MIS-C are shown in Table 1. Fever, required for inclusion, was present in all cases. Abdominal pain, vomiting, and/or diarrhea occurred in 442 of 502 children (88.0%). Clinical signs of Kawasaki syndrome occurred frequently: rash (298/503 [59.2%]), conjunctivitis or conjunctival injection (184/368 [50.0%]), oral changes defined as cheilitis and/or “strawberry tongue” (112/201 [55.7%]), extremity changes defined as edema or erythema of the hands and feet (19/40 [47.5%]), and cervical lymphadenopathy (37/87 [42.5%]). In the large, multicenter US study, 40% of subjects met clinical criteria for Kawasaki disease whereas 36% of subjects in the New York series met criteria for complete or incomplete Kawasaki disease [9, 12]. Periungual peeling was described in several children.
Table 1.
Summary of Clinical Presentation
| Clinical Presentation | No. Reported | Positive Subjects | Positive, % |
|---|---|---|---|
| Fever | 218 | 218 | 100.0 |
| Gastrointestinal symptomsa | 215 | 170 | 79.1 |
| Anosmia | 21 | 1 | 4.8 |
| Chest pain | 44 | 6 | 13.6 |
| Cough | 12 | 5 | 41.7 |
| Sore throat | 77 | 11 | 14.3 |
| Cervical lymphadenopathy | 155 | 47 | 30.3 |
| Rash | 216 | 130 | 60.2 |
| Conjunctivitis | 180 | 94 | 52.2 |
| Oral findingsb | 170 | 74 | 43.5 |
| Extremity findingsc | 108 | 32 | 29.6 |
| Tachycardia | 49 | 49 | 100.0 |
| Hypotension | 150 | 109 | 72.7 |
| Respiratory symptomsd | 119 | 51 | 42.9 |
aAbdominal pain, vomiting, diarrhea, hematochezia.
bChelitis, strawberry tongue.
cEdema, palmar, and/or solar erythema.
dTachypnea, retractions, increased work of breathing.
Diagnostic and Laboratory Testing
Nasopharyngeal washings and/or stool for nucleic acid testing were positive in 198 of 505 (39.2%) patients. Antibody testing was performed in 500 patients and was positive in 301(60.2%). For 22 of 314 patients (7.0%), both diagnostic tests were negative and the diagnosis was made on clinical grounds. A history of family contact with confirmed or suspected cases of SARS-CoV-2 was reported for 83 of 252 (32.9%) children.
A summary of the laboratory evaluation is shown in Table 2. Lymphopenia occurred commonly. The weighted median lymphocyte count was 700 cells/mL. Feldstein et al reported that 147 of 184 (80%) children with MIS-C were lymphopenic while Dufort et al noted lymphopenia in 59 of 89 (66%) children [9, 12]. The weighted median values for markers of general inflammation (C-reactive protein [CRP], interleukin 6 [IL-6], procalcitonin, ferritin, D-dimer, and fibrinogen) exceeded the upper boundary of the normal range (6 mg/mL [16], 8 pg/mL [16], 2 ng/mL [16], 140 ng/mL [13], 500 ng/mL [16], and 409 mg/L [13], respectively. The largest series reported serum concentrations of CRP ≥3 mg/dL in 156 of 172 (91%) children as well as elevated serum concentrations of ferritin (100/163 [61%]), D-dimer (79/118 [67%]), and fibrinogen 122/153 [80%]); the erythrocyte sedimentation rate was increased in 90 of 117 reported cases (77%) [9]. All of the subjects in the New York series had elevated serum CRP; serum procalcitonin was elevated in 60 of 65 children (92%) [12].
Table 2.
Summary of Laboratory Values
| Laboratory Test | Reference Range | No. of Studies Reporting | Weighted Median | Min | Max |
|---|---|---|---|---|---|
| White blood cell count | 4–13.5 cells × 1000/mLb | 8 | 10.4 | 3.95 | 42.8 |
| Absolute neutrophil count | 1.5–7 cells × 1000/mLb | 7 | 12 | 1.5 | 36.4 |
| Absolute lymphocyte count | 1.5–4 × 1000/mLb | 8 | 0.7 | 0.25 | 7.2 |
| Platelet count | 200–450 × 1000/mLb | 10 | 155 | 66 | 892 |
| Serum creatinine | Varies with age, mg/dL | 5 | 0.62 | 0.31 | 4.72 |
| ALT | 0–34 U/Lb | 10 | 50 | 6 | 733 |
| LDH | 125–243 U/Lb | 4 | 313 | 178 | 4087 |
| ESR | <40 mm/hc | 6 | 62 | 21 | 130 |
| C-reactive protein | <6 mg/La | 15 | 73 | 3 | 525 |
| Brain natriuretic peptide | <400 pg/mLc | 6 | 5112 | 16 | 19 013 |
| Troponin-1 | <26 ng/La | 10 | 157 | 3 | 6900 |
| D-dimer | <500 ng/mLa | 10 | 2400 | 350 | 38 710 |
| Fibrinogen | 199–409 mg/Lb | 5 | 624 | 313 | 924 |
| Procalcitonin | <2 ng/mLa | 5 | 6.2 | 0.1 | 448 |
| Ferritin | 7–140 ng/mLb | 8 | 522 | 199 | 3213 |
| IL-6 | <8 pg/mLa | 9 | 116 | 0 | 2220 |
Complications
Cardiac
Hypotension was reported in 162 of 251 (64.5%) children. The weighted median values for brain natriuretic peptide and troponin-1 exceeded the normal range, suggesting decreased cardiac function and myocardial injury. Coronary artery ectasia was demonstrated by 2-dimensional echocardiography in 66 of 499 (13.2%) patients; frank coronary artery aneurysms were identified in 27 of 499 (5.4%) patients. Left ventricular dysfunction, defined as a left ventricular ejection fraction of 60% or less, occurred in 214 of 420 patients (51.0%). Ionotropic support was given to 288 of 502 (57.6%) children. Extracorporeal membrane oxygenation (ECMO) was required in 26 of 490 (5.3%) cases.
Pulmonary
Respiratory distress defined as tachypnea, retractions, and/or increased work of breathing, occurred in 235 of 328 (71.6%) children. Pneumonia and/or pleural effusions were identified in chest radiographs of 182 of 326 (55.8%) children; mechanical ventilation was required for 128 of 490 (26.1%) patients.
Thrombosis and Acute Kidney Injury
As noted previously, the majority of children with MIS-C had elevated serum D-dimer and fibrinogen concentrations. Overall, 197 of 362 (54.4%) patients received anticoagulation therapy; deep vein thrombosis and/or pulmonary embolism was identified in 8 of 229 (3.5%). Acute kidney injury, defined by serum creatinine for age or creatinine clearance, was reported in 42 of 353 (11.9%) children. None of the reports suggested that long-term dialysis was required.
Treatment and Outcome
The therapeutic interventions employed and the clinical outcomes are shown in Table 3. Intravenous gammaglobulin (IVIG) and methylprednisolone or prednisone were administered to 389 of 498 (78.1%) and 288 of 500 (57.6%) children, respectively; 73 of 158 (46.2) children received aspirin. Feldstein et al reported that IVIG was administered between day 5 and 8 of illness in most cases, and that 21% of subjects received a second dose [9]. Anti–tumor necrosis factor–α, anti–interleukin 1 type 1 receptor, or anti–IL-6 receptor monoclonal antibodies were used in 24.3% of children. Of the 505 children with MIS-C included in this review, 415 (82.2%) survived and 7 (1.4%) children died; the remainder were still hospitalized at the time of the given report.
Table 3.
Summary of Interventions and Outcomes
| Intervention and Outcome | Positive, No. | Reported Cases, No. | % |
|---|---|---|---|
| Therapeutic intervention | |||
| Intravenous gammaglobulin | 389 | 498 | 78.1% |
| Aspirin | 73 | 158 | 46.2% |
| Methylprednisolone/prednisone | 288 | 500 | 57.6% |
| Anticoagulation | 197 | 362 | 54.4% |
| Infliximab | 8 | 133 | 6.0% |
| Anakinra | 45 | 375 | 12.0% |
| Tocilizumab | 24 | 380 | 6.3% |
| Ionotropic agents | 288 | 502 | 57.4% |
| Mechanical ventilation | 128 | 490 | 26.1% |
| Extracorporeal membrane oxygenation | 26 | 490 | 5.3% |
| Outcome | |||
| Survived | 415 | 505 | 82.2% |
| Died | 7 | 505 | 1.4% |
| Unresolved at time of report | 82 | 505 | 16.2% |
Discussion
While the incidence cannot be estimated from this study, these observations suggest that MIS-C is a serious complication of SARS-CoV-2 infection. Fever and gastrointestinal symptoms alone or accompanied by rash, conjunctival injection, cheilitis, and/or extremity changes in a school-aged child or adolescent from a SARS-CoV-2–endemic area should increase diagnostic suspicion. The evidence from this review supports the Centers for Disease Control and Prevention and Royal College definitions that a generalized inflammatory response is a hallmark of the syndrome and is required for diagnosis. Myocardial injury and dysfunction are the most common complications of SARS- CoV-2 MIS-C; more than half of the children in this review had left ventricular ejection fractions <60%, elevated serum concentrations of troponin-1 and brain natriuretic peptide, and required ionotropic support in an intensive care unit (ICU) setting. A third of patients required mechanical ventilation, and 5% of children required ECMO. Hypercoagulability in MIS-C is unclear, since elevated serum D-dimer and fibrinogen concentrations may indicate thrombotic risk or may occur as part of the inflammatory cascade. Overall, deep vein thrombosis or pulmonary embolism occurred in 3.5% of children. However, median serum concentrations of fibrinogen and D-dimer were above the normal range; 67% of the children in the multicenter US study had elevated serum D-dimer concentrations, and more than half of the patients received anticoagulation therapy [9].
At this point in time, specific therapy is based on expert opinion, not randomized controlled trials. More than three-quarters of patients received at least one dose of IVIG and more than half received methylprednisolone or prednisone. Immunomodulators (infliximab, anakinra, and tocilizumab) were used less frequently. Overall, 7 children died (1.4%), supporting the use of aggressive supportive care.
The molecular etiology of SARS-CoV-2–related MIS-C is unknown. The high prevalence of antibody positivity coupled with the lower rate of polymerase chain reaction positivity from nasopharyngeal washes and stool suggest that MIS-C is a late manifestation of infection and may relate to a specific rather than an innate host response to the virus.
There are several sources of potential bias in this study. First, the newness of this disorder was a major obstacle to a complete literature search. The first reports of SARS-CoV-2–related MIS-C are <4 months old as of this writing. Some of the newest series appeared as early releases during the preparation of this manuscript and several large case series appeared between the time this review was submitted and revised. It is reasonable to expect additional studies to appear.
Variability of data collection and reporting is another limitation. Only 8 studies reported absolute lymphocyte counts, 10 reported troponin-1 concentrations, 8 reported serum ferritin concentrations, and 9 reported serum IL-6 concentrations. In some reports, clinical descriptions lacked specific pertinent negatives such as exposure to infected family contacts and specific denials of significant underlying diseases. Nevertheless, the size, scope, and diversity of this review are reassuring that the patients included comprise a representative sample.
Both of the case definitions used by later series to identify patients included severe illness as a criterion. Not surprisingly, the majority of reported cases were from pediatric ICUs. In the course of clinical practice, the authors have seen several patients with gastrointestinal complaints, elevated serum concentrations of inflammatory markers, and positive serum IgG antibody to SARS-CoV-2. These personal observations and the reporting bias from pediatric critical care units raise the question as to whether or not a forme fruste of the SARS-CoV-2 inflammatory syndrome exists. Case series and surveys of ambulatory centers and non-ICU settings would be helpful in identifying these cases.
Conclusions
In summary, MIS-C due to SARS-CoV-2 is a serious illness in school-aged children and adolescents and manifests with fever, abdominal pain, vomiting, and diarrhea; many patients present with rash, conjunctival injection, cheilitis, and extremity changes. Elevated serum concentrations of inflammatory markers are an integral feature of this disorder. Myocardial injury, respiratory distress hypercoagulability, and acute renal injury are major complications of the syndrome but appear to respond to aggressive, supportive therapy. First-line therapy with IVIG and methylprednisolone or prednisone was employed in a majority of cases but empiric, comparative data are lacking. Prospective studies are needed to further define the biochemical and inflammatory changes associated with this syndrome as well as effective therapies.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in china. Pediatrics 2020; 145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2. Mustafa NM, A Selim L. Characterisation of COVID-19 pandemic in paediatric age group: a systematic review and meta-analysis. J Clin Virol 2020; 128:104395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pain CE, Felsenstein S, Cleary G, et al. Novel paediatric presentation of COVID-19 with ARDS and cytokine storm syndrome without respiratory symptoms. Lancet Rheumatol 2020; 2:e376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19 2020. Available at: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims. Accessed 8 June 2020.
- 7. Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020; 324:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller J, Cantor A, Zachariah P, et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases [manuscript published online ahead of print June 4, 2020]. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldstein LR, Rose EB, Horwitz SM, et al. Overcoming COVID-19 Investigators and the CDC COVID-19 Response Team Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc 2020; 9:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waltuch T, Gill P, Zinns LE, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department [manuscript published online ahead of print May 23, 2020]. Am J Emerg Med 2020. doi: 10.1016/j.ajem.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dufort EM, Koumans EH, Chow EJ, et al. New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team Multisystem inflammatory syndrome in children in New York state. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whittaker E, Bamford A, Kenny J, et al. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health 2020; 4:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr 2020; 57:681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic [manuscript published online ahead of print May 17, 2020]. Circulation 2020. doi: 10.1161/circulationaha.120.048360. [DOI] [PubMed] [Google Scholar]
- 17. Cabrero-Hernández M, García-Salido A, Leoz-Gordillo I, et al. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr Infect Dis J 2020; 39:e195–8. [DOI] [PubMed] [Google Scholar]
- 18. Deza Leon MP, Redzepi A, McGrath E, et al. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc 2020; 9:407–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 2020; 10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oberweis ML, Codreanu A, Boehm W, et al. Pediatric life-threatening coronavirus disease 2019 with myocarditis. Pediatr Infect Dis J 2020; 39:e147–9. [DOI] [PubMed] [Google Scholar]
- 21. Rauf A, Vijayan A, John ST, et al. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr 2020; 87:745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020; 10:537–40. [DOI] [PubMed] [Google Scholar]
- 24. Dolinger MT, Person H, Smith R, et al. Pediatric Crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr 2020; 71:153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review [manuscript published online ahead of print April 22, 2020]. JAMA Pediatr 2020. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.